Abstract
Previous assays in pregnant animals have demonstrated the effect of different host factors and timing of infection on the outcome of neosporosis during pregnancy. However, the influence of Neospora caninum isolate itself has been poorly investigated. Here, we compared the effects on clinical outcome and vertical transmission observed in a pregnant mouse model following infection with 10 different N. caninum isolates. The isolates in our study included the Nc-Liv isolate and nine N. caninum isolates obtained from calves. Female BALB/c mice were inoculated with 2 × 106 tachyzoites at day 7 of pregnancy. Morbidity and mortality, in both dams and offspring during the course of infection, and transmission to progeny at day 30 postpartum were evaluated. The serum IgG1 and IgG2a production in dams were also examined. All dams showed elevated IgG1 and IgG2a responses, confirming N. caninum infection, although signs of disease were only exhibited in dams infected with 4 of the 10 isolates (Nc-Spain 4H, Nc-Spain 5H, Nc-Spain 7 and Nc-Liv). In neonates, clinical signs were observed in all N. caninum-infected groups, and neonatal mortality rates varied from greater than 95% with the isolates mentioned above to less than 32.5% with the other isolates. Vertical transmission rates, as assessed by parasite PCR-detection in neonate brains, also varied from 50% to 100% according to the isolate implicated. These results confirm the wide pathogenic and transmission variability of N. caninum. The intra-specific variability observed herein could help us explain the differences in the outcome of the infection in the natural host.
Keywords: Neospora caninum, bovine isolate, pathogenic characterisation, vertical transmission, pregnant mouse model
1. INTRODUCTION
Neospora caninum infection has been recognised as a major cause of abortion in cattle. Abortion may occur either following a primary infection by consumption of oocysts shed by the definitive canine host (exogenous transplacental transmission) or following the reactivation of a pre-existing chronic infection during pregnancy (endogenous transplacental transmission) and the subsequent transmission of N. caninum to the foetus [8, 9, 34]. Experimental infections in pregnant cattle have demonstrated a relationship between the occurrence of abortion and the time during pregnancy when foetal infection occurs, the maternal immune response generated to control the parasite and the relative immunocompetence of the foetus at the time of infection [8, 10, 11]. However, the role of the biological diversity of N. caninum isolates in bovine abortion has been poorly investigated. Foetal infections have been primarily studied in pregnant cattle with the N. caninum Nc-1 and Nc-Liv isolates originating from affected dogs, although the variety of breeds, inoculation routes, timing of inoculation during pregnancy and tachyzoite doses used in these studies limit meaningful comparisons (see review [8]). In one study, the ability to induce foetal death in a pregnant bovine model by two different isolates, Nc-Spain 1H and the Nc-1 was evaluated. These infections were compared, and noticeable differences in clinical, immunological and pathological responses were revealed [29]. Interestingly, 3 of 5 dams infected with Nc-1 tachyzoites at day 70 of gestation aborted 3–4 weeks post-inoculation (p.i.), whereas cattle infected with Nc-Spain 1H tachyzoites, which have previously demonstrated a low virulence in mouse models, did not result in foetal death until the end of the experiment at 45 days p.i. [29, 30]. Testing a broad panel of isolates in a pregnant cattle model to investigate pathogenic diversity of N. caninum would be difficult due to its high economic cost.
Alternative animal models based on the use of susceptible mouse strains such as BALB/c and C57BL/6 [13, 15, 16, 18, 19, 26] or clinically resistant out-bred Qs mice [24] have been developed to study N. caninum infection. Thus, non-pregnant mouse models have constituted one of the first approaches to investigate and confirm the N. caninum-isolate pathogenic-diversity [1, 7, 14, 20]. Furthermore, pregnant models with these types of mice not only offer a suitable bioassay to examine the virulence of different N. caninum isolates during pregnancy, but they also allow us to evaluate the potential differences existing among N. caninum isolates regarding the transmission rate of neosporosis to progeny [24, 25, 30].
Recently, new studies have been performed to investigate the pathogenic diversity of a representative number of N. caninum isolates obtained from asymptomatic calves using a non-pregnant BALB/c model [22]. Differences in temporal dissemination of the parasite, parasite burdens and histological lesions in the target organs (brain and lungs) were described for the eight isolates analysed. Here, we employed a pregnant BALB/c mouse model in order to examine the influence of intra-species diversity existing among nine Spanish N. caninum isolates, including those isolates previously studied in the non-pregnant model, and the Nc-Liv isolate in the outcome of infection during pregnancy.
2. MATERIALS AND METHODS
2.1. Cultures and preparation of bovine N. caninum isolates for BALB/c mice inoculation
The Nc-Liv and the Spanish N. caninum isolates (Tab. I) were maintained in a monolayer culture of the MARC-145 cell line, as described previously [27]. The Nc-Liv isolate, which had been maintained by an undetermined number of successive culture passages, was previously passaged by nude mouse and re-isolated in MARC-145 cell cultures as described previously [27] with the purpose of minimising its potential changes in virulence due to prolonged maintenance in vitro [3, 23].
Table I.
Summary of name, host and geographical origin, and passage number in cell culture of isolates included in this study.
| Isolate | Host origin | Geographical origin* | Passage number** |
|---|---|---|---|
| Nc-Spain 2H | 2-day-old healthy calf | Zaragoza | 15 |
| Nc-Spain 3H | 52-day-old healthy calf | Navarra | 11 |
| Nc-Spain 4H | 22-day-old healthy calf | Navarra | 11 |
| Nc-Spain 5H | 14-day-old healthy calf | León | 11 |
| Nc-Spain 6 | 30-day-old healthy calf | País Vasco | 11 |
| Nc-Spain 7 | 57-day-old healthy calf | Navarra | 16 |
| Nc-Spain 8 | 2-day-old healthy calf | Navarra | 17 |
| Nc-Spain 9 | 7-day-old healthy calf | Navarra | 19 |
| Nc-Spain 10 | 2-day-old affected calfa | Madrid | 13 |
| Nc-Liverpool | 4-week-old affected dog | UK* | 13Rb |
* Geographical origin of Spanish isolates is noted according to province. Nc-Liverpool was isolated in the United Kingdom.
** Total passages in cell culture prior to mice inoculation.
Nc-Spain 10 was isolated from a calf with clinical signs, although they could not be attributed to Neospora infection [27].
Total passages after re-isolation in cell culture from BALB/c nu/nu mice.
Inocula were prepared as described previously [22]. Tachyzoite number was determined by Trypan blue exclusion followed by counting in a Neubauer chamber, and the organisms were resuspended in PBS at the required dose of 2 × 106 tachyzoites in a final volume of 200 μL per mouse. Mice were inoculated within an hour of tachyzoite collection. All isolates used to infect mice were passaged in cell culture between 11 and 19 times (Tab. I).
2.2. Mating and pregnancy of mice
Six-week-old inbred BALB/c mice were purchased from a commercial supplier (Harlan Iberica, Barcelona, Spain). Female mice were housed in groups of 10 mice in a controlled environment with 12 h-light and 12 h-dark cycles and provided with rodent feed and water ad libitum. At 8 weeks of age, female mice were synchronised using the Whitten effect [36], after which two female mice were placed with a male for 4 nights. The last day that females were housed with males was determined as day 0 of pregnancy. Female mice were randomly assigned into 11 experimental groups of 22–24 mice.
2.3. Experimental design, samples from dams and progeny, and data collection
Infection studies were performed using a pregnant BALB/c mouse model as described previously [18, 19]. At day 7 of pregnancy, female mice in all groups were subcutaneously (s.c.) inoculated with different N. caninum isolates included in this study (Tab. I) and the uninfected group was subcutaneously injected with sterile PBS (control group). Pregnant mice were identified on day 14 of pregnancy by weighing and were individually housed. All mice were allowed to give birth, and neonates were housed with dams until day 30 post-partum (PP), when both neonates and dams were sacrificed by CO2 inhalation. Only pregnant mice continued until the end of the experiment, and non-pregnant infected mice were sacrificed by CO2 inhalation. Pregnant mice and neonates were examined daily for clinical signs compatible with neosporosis (delayed hair coat development, rough hair coat, and neurological signs) from their inoculation, and the progeny were weighed every 2–3 days, but only from day 15 PP to avoid excessive handling of the pups and their consequent rejection by the dams. Severe clinically affected mice were also sacrificed by CO2 inhalation. All animal-handling procedures complied with EU legislation.
Blood samples were collected from dams by cardiac puncture at necropsy, and the sera recovered were preserved at −80 °C for ELISA. Brains from neonates and dams were also collected under aseptic conditions and were frozen at −80 °C until DNA extraction and PCR analysis. Some samples from progeny could not be collected due to cannibalism by the dams.
Litter size was considered to be the number of pups delivered per dam. Hebdomadal mortality was defined as the number of full-term dead pups at the time of birth and those dead from birth to day 2 PP. Neonatal mortality was considered to be the number of pups sacrificed from days 2 to 30 PP. The vertical transmission rate was determined in this study by the presence of the parasite DNA in the brain of neonates, as detected by nested-PCR.
2.4. DNA extraction and nested-PCR
Genomic DNA was extracted from 20 mg of brain tissues from mice using the BioSprint 96 workstation and BioSprint 96 DNA blood kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Detection of parasite DNA was performed by a nested-PCR on the ITS-1 region of N. caninum under experimental procedures as described previously [7, 22].
2.5. Serum IgG1 and IgG2a analysis
Neospora-specific IgG2a and IgG1 isotypes were quantified by ELISA as described previously [7]. Briefly, an ELISA was developed with soluble N. caninum tachyzoite antigen (0.5 μg in 100 μL/well) using diluted serum samples (1:100) and anti-mouse IgG2a or IgG1 antibody (1:5 000; Southern Biotechnology, Birmingham, AL, USA). Serum samples were analysed in duplicate, and the average O.D. value was converted into a relative index percent (RIPC) using the following formula: RIPC = (O.D. sample − O.D. negative control)/(O.D. positive control − O.D. negative control) × 100. The serum isotype balance was evaluated by the IgG1/IgG2a ratio.
2.6. Statistical analysis
Differences in pregnancy, hebdomadal mortality, neonatal mortality and vertical transmission rates were analysed using the Chi-square (χ2) or Fisher exact F-test. In addition, neonatal morbidity and mortality were analysed by the Kaplan–Meier survival method [5] to estimate the percentage of healthy or surviving animals at each time point (days PP). To compare the health and survival curves between infected groups, the logrank statistical test was applied [6]. The median health time and median survival time or the day when 50% of the neonates had been clinically affected or had died, respectively, was calculated [5]. Statistical significance for all analysis was established at p < 0.05. When significant differences were found, a multiple comparison test was used to examine all possible pairwise comparisons in the χ2 and Kaplan–Meier tests. A value of p < 0.05/(k × [k − 1]/2) was considered statistically significant for pairwise comparisons, with k corresponding to the group number. In addition, Spearman’s rank correlation coefficient (ρ) was applied to analyse the association between the parasite detection rate in dams and their progeny (vertical transmission rate). Finally, a one-way ANOVA test followed by Duncan’s multiple range test was employed to compare litter size, neonate body weight by day and serum anti-N. caninum IgG1 and IgG2a responses. All statistical analyses were carried out using Statgraphics Plus v.5.1 (StatPoint, Inc., Herndon, VA, USA) and GraphPad Prism 5 v.5.01 (San Diego, CA, USA) software.
3. RESULTS
3.1. Evaluation of N. caninum infection in dams
3.1.1. Pregnancy rate
Pregnancy rates in this study varied from 33% of females inoculated with the Nc-Liv isolate to 81% of females inoculated with the Nc-Spain 5H isolate or PBS (Tab. II), although no statistical differences were found between groups (p > 0.9; χ2 = 30.93). The average pregnancy rate in this study was approximately 59%.
Table II.
Effect of Neospora caninum infection on dams and litter size.
| N. caninum infected mice group (and control group) | No. of pregnant dams at day 14 of gestation (%) | No. of clinically affected pregnant dams (nervous signs) | No. of PCR-positive dams (%) | Litter size (average ± SD) |
|---|---|---|---|---|
| Nc-Spain 2H | 11/24 (45.8)a | Hb | 3/11 (27.2)d | 4.9 ± 1.8 |
| Nc-Spain 3H | 14/22 (63.6) | H | 11/14 (78.5) | 6 ± 1.6 |
| Nc-Spain 4H† | 12/22 (54.5) | 12/12 (100%)c | 11/12 (91.6)1 | 5.5 ± 1.3 |
| Nc-Spain 5H | 18/22 (81.8) | 5/18 (27.7%) | 18/18 (100)1 | 4.8 ± 2.3 |
| Nc-Spain 6 | 12/22 (54.5) | H | 3/12 (25) | 4.9 ± 2.1 |
| Nc-Spain 7 | 13/22 (59) | 4/13 (30.7%) | 9/13 (69.2) | 5.6 ± 1.3 |
| Nc-Spain 8 | 19/24 (79.1) | H | 5/19 (26.3)2 | 5.2 ± 1.5 |
| Nc-Spain 9 | 10/24 (41.6) | H | 1/10 (10)2 | 5.1 ± 1.2 |
| Nc-Spain 10 | 13/22 (59) | H | 11/13 (84.6) | 5.9 ± 1.6 |
| Nc-Liverpool | 8/24 (33.3) | 5/8 (62.5%) | 8/8 (100)1 | 4.8 ± 2.4 |
| Control | 18/22 (81.8) | H | 0/10 (0)e | 5.3 ± 1.7 |
† Nc-Spain 4H data were collected at day 20 PP when dams were sacrificed.
Number of pregnant dams, number of clinically affected dams, and number of PCR-positive dams, according to superscript/number of total female mice.
Healthy. No clinical signs were detected in pregnant mice.
Ten female mice and their offspring were randomly selected as a control group to evaluate the presence of parasites in the dams’ brain and vertical transmission.
Percentages determined for infected groups followed by unlike superscripts differ significantly by χ2 multiple-comparison test.
3.1.2. Morbidity and mortality
The development of skin lesions consisting of dermal nodules at the site of parasite inoculation (interscapular region) were observed in some dams from day 7 to 14 p.i. The most affected group was inoculated with the Nc-Spain 4H isolate, which resulted in 75% of pregnant dams developing ulcerative or non-ulcerative dermal nodules measuring approximately 5 to 8 mm in diameter. Groups infected with the Nc-Spain 5H and Nc-Liv isolates resulted in 11% and 37.5% of dams affected with non-ulcerative dermal nodules measuring 2 to 3 mm in diameter, respectively. Skin lesions were not detected in mice infected with the other N. caninum isolates. Affected dams with ulcerative nodules were individually located.
Clinical signs such as rough hair coat, rounded back, and inactivity followed by nervous signs (ataxia, paralysis and walking in circles) were more prevalent in dams infected with the Nc-Spain 4H and Nc-Liv isolates from day 15 PP and in those infected with the Nc-Spain 5H and Nc-Spain 7 isolates from day 22 PP. All dams and their offspring from the Nc-Spain 4H group were sacrificed prior to day 20 PP due to the severity of nervous signs displayed. No clinical signs were observed in dams from the control and the other N. caninum-infected groups throughout the experiment (Tab. II).
3.1.3. Parasite DNA detection in dams’ brain
The frequency of N. caninum DNA detection in brain tissue from dams is also shown in Table II. The parasite detection frequencies were elevated in dams infected with 6 of the 10 isolates (from 69% to 100% of dams), including those infected with isolates that induced clinical signs in dams, whereas the other 4 isolates varied from 10% and 27.2%. Significant differences in parasite detection frequency in dams were found among all inoculated groups (p < 0.0001; χ2 = 81.63), although they were only significant between groups with the highest (> 90%) parasite detection frequencies (Nc-Spain 4H, Nc-Spain 5H and Nc-Liv) compared to some with the lowest (Nc-Spain 8, Nc-Spain 9), less than 27% (p ≤ 0.0006; Fisher exact test) (Tab. II).
3.1.4. Humoral immune response analysis in dams
Serum IgG1 and IgG2a levels were significantly increased at day 30 PP for all mice inoculated with the parasite when compared to the uninfected control group. The highest significant serum IgG1 levels were stimulated in dams by the Nc-Spain 5H and Nc-Spain 7 isolates, and the lowest were stimulated by the Nc-Spain 3H and Nc-Spain 6 isolates (p < 0.0017; 1-way ANOVA, followed by Duncan’s test). Furthermore, the highest significant serum IgG2a production was detected in dams infected with the Nc-Spain 5H and Nc-Spain 7 isolates, together with the Nc-Spain 4H and Nc-Liv isolates, and the lowest was found in dams infected with the Nc-Spain 3H isolate, in addition to the Nc-Spain 2H and Nc-Spain 9 isolates (p < 0.0001; 1-way ANOVA followed by Duncan’s multiple range test). All infected groups had a higher concentration of IgG1 than IgG2a (IgG1/IgG2a ratios > 1), and no significant differences among N. caninum-infected groups were found (data not shown).
3.2. Evaluation of N. caninum infection in the offspring
3.2.1. Litter size and hebdomadal mortality
The smallest average number of pups born per litter was observed in those groups where the frequency of parasite detection in the dams was 100% (Nc-Spain 5H and Nc-Liv), although no significant differences in litter size were found among all inoculated groups (p = 0.7151; 1-way ANOVA followed by Duncan’s multiple range test) (Tab. II).
With regards to hebdomadal mortality, the highest number of dead neonates was observed in the progeny of dams infected with the Nc-Spain 4H isolate, the most clinically affected group (100%), although significant differences among groups were not found (p = 0.18, χ2 = 15.01) (Tab. III).
Table III.
Effect of Neospora caninum infection on hebdomadal mortality, neonatal morbidity, and neonatal mortality.
| N. caninum infected mice group (and control group) | Hebdomadal mortality (%) | Median health time (days) (average ± SD) | Neonatal morbidity (%) | Median survival time (days) (average ± SD) | Neonatal mortality (%) |
|---|---|---|---|---|---|
| Nc-Spain 2H | 5/54 (9.2)a | 26.4 ± 0.761,2 | 18/39 (46.1)b,1 | 28.5 ± 0.471 | 10/49 (20.4)c,1 |
| Nc-Spain 3H | 7/84 (8.3) | 28.8 ± 0.422 | 8/75 (10.6)1,2 | 29.4 ± 0.271,2 | 6/77 (7.7)1,2 |
| Nc-Spain 4H | 15/66 (22.7) | 11 ± 0.43 | 51/51 (100)3 | 11.1 ± 0.43 | 51/51 (100)3 |
| Nc-Spain 5H | 12/88 (13.6) | 15.3 ± 0.494 | 74/75 (98.6)3 | 17.1 ± 0.594 | 73/76 (96)3 |
| Nc-Spain 6 | 2/59 (3.3) | 25.3 ± 0.961 | 19/55 (34.5)1 | 26.6 ± 0.81 | 17/57 (29.8)1 |
| Nc-Spain 7 | 7/68 (10.2) | 16.1 ± 0.584 | 59/60 (98.3)3 | 17.4 ± 0.614 | 58/61 (95)3 |
| Nc-Spain 8 | 11/99 (11.1) | 29.4 ± 0.282 | 4/85 (4.7)2 | 29.9 ± 02 | 1/88 (1.1)2 |
| Nc-Spain 9 | 8/51 (15.6) | 24.8 ± 1.11 | 16/41 (39)1 | 26.4 ± 0.891 | 14/43 (32.5)1 |
| Nc-Spain 10 | 10/77 (12.9) | 26.1 ± 0.961,2 | 12/47 (25.5)1 | 27 ± 0.871 | 12/67 (17.9)1 |
| Nc-Liverpool | 3/39 (7.6) | 12.1 ± 0.73 | 36/36 (100)3 | 12.5 ± 0.673 | 36/36 (100)3 |
| Control | 11/91 (12) | 30 ± 0 | 0/80 (0) | 30 ± 0 | 0/80 (0) |
Number of stillborn and dead pups at day 2 PP (% hebdomadal mortality), number of clinically affected neonates from day 2 PP onwards (% neonatal morbidity), and number of dead neonates from day 2 PP onwards (% neonatal mortality), according to superscript/number of total pups born.
Median and percentages determined for infected groups followed by unlike superscripts differ significantly by logrank and χ2 multiple-comparison test, respectively.
3.2.2. Morbidity and analysis of body weights
The clinical signs observed in neonates were detected along the first week and were characterised by a delay in body and hair coat development. Additional clinical signs such as rough hair coat, rounded back, and inactivity followed by nervous signs (ataxia, paralysis of hind limbs and forelimbs, head tilting and walking in circles) were observed from the second week PP onwards. No clinical signs were observed in the offspring of the uninfected group throughout the experiment. The majority of mice exhibiting clinical signs succumbed to infection at day 30 PP, and significant differences were detected when the number of affected neonates or the median health times were compared among the inoculated mice groups (p < 0.0001, χ2 = 469.2, Fisher exact test; p < 0.0001, χ2 = 909, logrank test, respectively). Subsequent pairwise analysis showed similar comparative results concerning neonatal mortality rates and the median survival times between mouse groups (Tab. III).
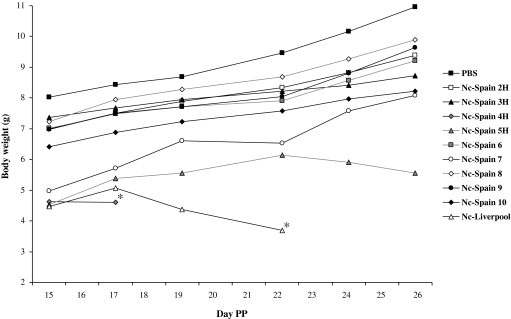
With regards to weight analysis, the average body weights of neonates infected with N. caninum were lower than those of the control group throughout the experiment. Body weights of offspring from all mouse groups gradually increased from day 15 PP, with the exception of those neonates born from the clinically affected Nc-Spain 4H, Nc-Spain 5H and Nc-Liv dam groups, in which average weights progressively decreased from days 15, 22 and 17 PP, respectively (Fig. 1). A decreasing average body weight was also detected at day 22 PP in offspring infected with the Nc-Spain 7 isolate, although the body weights of surviving mice increased after day 22 PP until the end of the experiment (Fig. 1). Offspring from clinically affected dam groups (Nc-Spain 4H, Nc-Spain 5H, Nc-Spain 7, and Nc-Liv) together with the progeny of the Nc-Spain 10 group showed a significantly lower body weight than offspring from the control group throughout the experiment (p < 0.0001; 1-way ANOVA test, followed by Duncan’s multiple range test). When we compared weight data from N. caninum-infected groups, significant differences were only found between the three groups with the lowest average body weights (Nc-Spain 4H, Nc-Spain 5H, and Nc-Liv) and the other six infected groups (Nc-Spain 10, Nc-Spain 2H, Nc-Spain 3H, Nc-Spain 6, Nc-Spain 8 and Nc-Spain 9). The neonates from the Nc-Spain 7 group showed significantly lower average weights than the neonates infected with the Nc-Spain 2H, Nc-Spain 9 or Nc-Spain 8 isolate, which had the highest body weights (p < 0.0001; 1-way ANOVA test, followed by Duncan’s multiple range test).
Figure 1.
Body weight progression of neonates born from dams infected on day 7 of pregnancy with 2 × 106 tachyzoites from each N. caninum isolate included in this study and the uninfected group (see graphic legend). Each point represents the average body weight of all animals per group (an asterisk denotes data obtained from a sole neonate that had not succumbed to infection and not included in the statistical analysis).
3.2.3. Neonatal mortality
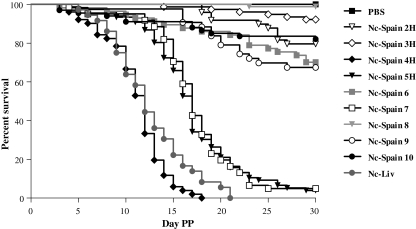
Neonatal mortality rate varied in all infected mouse groups according to the N. caninum isolate (Tab. III and Fig. 2). At day 30 PP, the highest mortality rate was observed in neonates from clinically affected dam groups (Nc-Spain 4H, Nc-Spain 5H, Nc-Spain 7 and Nc-Liv), whereas the remainder of the N. caninum-infected groups had a mortality rate lower than 32.5% (Tab. III).
Figure 2.
Kaplan–Meier survival curves for neonates born from dams infected on day 7 of pregnancy with 2 × 106 tachyzoites from the different N. caninum isolates included in this study and the uninfected group (see graphic legend). Each point represents the percentage of survival animals at that day.
When the median survival times were compared, significant differences were found among the groups (p < 0.0001, χ2 = 897.7, logrank test). The median survival times determined in the progeny infected with the isolates that provoked the highest neonatal mortality rates (Nc-Spain 4H, Nc-Spain 5H, Nc-Spain 7 and Nc-Liv isolates) were significantly lower than those exhibited by all other infected groups (p ≤ 0.0001, logrank test). Furthermore, the low median survival times detected in neonates infected with the Nc-Spain 4H and Nc-Liv isolates (11–12 days) were also significantly different from those determined for the other groups that showed a reduced median survival time (Nc-Spain 5H and Nc-Spain 7; 17 days). In addition, the Nc-Spain 8-infected group, which had the highest median survival time (29.9 days), was also significantly different (p ≤ 0.0002, logrank test) to those infected groups with a high median survival time (Nc-Spain 2H, Nc-Spain 6, Nc-Spain 9, and Nc-Spain 10; 26–29 days) (Tab. III).
3.3. Vertical transmission
Vertical transmission rates were determined by the percentage of PCR-positive neonates at day 30 PP (Tab. IV). All dams transmitted the infection to at least one of the pups in the litter, with the exception of 1 dam from 4 of the infected groups (Nc-Spain 2H, Nc-Spain 6, Nc-Spain 7, and Nc-Spain 8), where transmission to progeny was not detected (Tab. IV). Parasite DNA was detected in almost all the infected neonates that succumbed to infection at day 30 PP (> 80.9%), confirming that clinical signs were produced by parasite infection. Parasite detection rates determined in the surviving neonates at day 30 PP varied from 34.6% to 100% with the inoculated isolate (Tab. IV).
Table IV.
Assessment of Neospora caninum vertical transmission by parasite PCR-detection in neonatal brains.
| N. caninum infected mice group (and control group) | Litter transmission (% litters with at least one PCR-positive pup) | Parasite detection rate in dead neonates (% PCR-positive neonates that succumbed to infection from day 2 PP) | Parasite detection rate in alive neonates (% PCR-positive neonates that overcame infection at day 30 PP) | Vertical transmission rate (%)* |
|---|---|---|---|---|
| Nc-Spain 2H | 90.9 | 7/7 (100)a | 20/37 (54)c | 27/44 (61.3)f,1,2 |
| Nc-Spain 3H | 100 | 2/2 (100) | 63/71 (88.7) | 65/73 (89)3 |
| Nc-Spain 4H | 100 | 37/38 (97.3) | –d | 37/38 (97.3)3 |
| Nc-Spain 5H | 100 | 39/39 (100) | 3/3 (100) | 42/42 (100)3 |
| Nc-Spain 6 | 91.6 | 12/13 (92.3) | 18/39 (46.1) | 30/52 (57.6)1 |
| Nc-Spain 7 | 91.6 | 17/21 (80.9) | 2/3 (66.6) | 19/24 (79.1) |
| Nc-Spain 8 | 94.7 | –b | 48/85 (56.4) | 48/85 (56.4)1,2 |
| Nc-Spain 9 | 100 | 11/12 (91.6) | 9/26 (34.6) | 20/38 (52.6)1 |
| Nc-Spain 10 | 100 | 7/7 (100) | 33/54 (61.1) | 40/61 (65.5) |
| Nc-Liverpool | 100 | 22/23 (95.6) | –d | 22/23 (95.6)2,3 |
| Control | 0 | –b | 0/30 (0)e | 0/30 (0)e |
* Vertical transmission determined by parasite detection in brains from all neonates.
Number of infected neonates/number of neonates that succumbed to infection, number of neonates that overcame the infection at day 30 PP, and number of total animals analysed, according to superscript.
No neonates succumbed to infection or brain samples were not collected due to cannibalism by the dams.
All neonates succumbed to infection.
Brain samples from 10 female mice and their offspring were selected as the control.
Percentages determined for infected groups followed by unlike superscripts differ significantly by χ2 multiple-comparison test.
The vertical transmission rate, considered as the percentage of parasite detection in all neonates (clinically affected or not), showed significant differences between all mouse groups (p < 0.0001, χ2 = 175.3). Overall, parasite DNA was detected in more than 50% of the neonates from infected groups, and significant differences were found between the groups with a parasite detection rate ≥ 89% (Nc-Spain 4H, Nc-Spain 5H and Nc-Spain 3H) and those with a parasite detection rate ≤ 61% (Nc-Spain 2H, Nc-Spain 6, Nc-Spain 8, and Nc-Spain 9) (p ≤ 0.0006, χ2 with Yates’ correction or Fisher exact tests). Differences in the vertical transmission rate detected in the Nc-Liv group were only statistically significant as compared to the Nc-Spain 6 and Nc-Spain 9 groups (p ≤ 0.0008, χ2 with Yates’ correction or Fisher exact tests) (Tab. IV).
Finally, a significant correlation between the percentage of parasite detection in brains from dams and neonates (vertical transmission) at day 30 PP was established according to the Spearman’s rho coefficient (p = 0.0011; ρ coefficient = 0.8675).
4. DISCUSSION
Comparative studies using pregnant models focussing on the investigation of the influence of the intra-species diversity of N. caninum on the outcome of infection during pregnancy are very limited. The majority of experimental infections performed in pregnant mouse models have investigated the ability to produce pathology and the immune response induced by a limited population of isolates, most of which were obtained from clinically affected animals and abortions [24, 25, 29]. In this study, we approached the pathogenic characterisation of 10 N. caninum isolates using a well-established pregnant BALB/c model [18, 19]. Almost all the isolates included in this study were recently isolated from healthy calves and genetically characterised using microsatellite markers [27]. Furthermore, in contrast to the Qs model, the BALB/c model not only allows us to evaluate the transmission of the parasite to their progeny but also offers an advantage since they exhibit clinical signs of the N. caninum infection, making them well suited for virulence isolate studies [19, 24].
Clear differences in the ability to produce pathology, vertical transmission and immunological effects were demonstrated by the 10 N. caninum isolates included in this study, confirming the extensive variability in virulence of N. caninum species. Clinical signs attributed to N. caninum were exhibited from day 15 PP onwards by a variable proportion of dams infected with 4 of the 10 analysed isolates (Nc-Spain 4H, Nc-Spain 5H, Nc-Spain 7 and Nc-Liv), whereas the remaining isolates were unable to produce disease in dams. Furthermore, these clinically affected dam groups showed the smallest average litter sizes (Nc-Spain 5H and Nc-Liv), likely due to a high number of foetal resorptions, or the offspring showed the highest hebdomadal mortality (Nc-Spain 4H), although these differences were not significant. Neonates infected with any of these 4 isolates also displayed significantly low average body weights during the course of infection, an earlier appearance of nervous signs, median health and survival times lower than 18 days, and the largest percentage of clinically affected neonates (> 98%). In contrast, offspring infected with the Nc-Spain 3H and Nc-Spain 8 isolates showed higher median health and survival times and the lowest morbidity and mortality rates (≤ 10%). This pregnant BALB/c model has been recently employed to compare the virulence of the Nc-Spain 1H and the Nc-1 isolates, and significant variations in their capacity to produce disease were also determined. Offspring from Nc-1-infected dams showed significantly lower body weight and higher morbidity and mortality rates (76.8%) compared to Nc-Spain 1H-infected dams’ offspring, which remained clinically normal and had a survival rate of almost 100% [30]. These results indicate that Nc-Spain 1H is a potential low virulence isolate, which was later confirmed in pregnant cattle since Nc-Spain 1H was unable to induce abortion after their inoculation at day 70 of gestation [29]. Interestingly, in this and other bioassays in pregnant BALB/c mice, if pregnant dams showed clinical signs it was not mentioned [13, 15, 19, 21, 30]. Non-exhibition of clinical signs in pregnant dams may not be solely attributed to characteristics of the isolate implicated in the infection, but it may be due to the earlier sacrifice of dams after birth prior to clinical sign presentation or to other factors, including attenuation suffered by prolonged maintaining of the isolate in vitro, experimental conditions used for preparation of inocula, the inoculation route employed (intraperitoneal versus subcutaneous) and the inoculum size (106 versus 2 × 106), which may influence the outcome of infection in dams.
The brain has been recognised as the target organ for N. caninum chronic infection in mice. Brain pathological lesions and brain parasite loads have been associated with disease severity in mice and the appearance of neurological signs, since parasite number and the severity of histological lesions increase with tachyzoite doses and in mice with clinical symptoms [7, 16, 18]. In this study, when the parasite DNA detection rates in mouse brains from infected groups were compared, the highest percentages (> 69%) were detected in dams and neonates infected with the N. caninum isolates that provoked the earliest appearance and the most severe clinical signs (Nc-Spain 4H, Nc-Spain 5H, Nc-Spain 7 and Nc-Liv). The Nc-Liv isolate also induced more severe brain pathology in mice than Nc-SweB1 when they were comparatively investigated using pregnant Qs [24]. Moreover, significantly higher parasite DNA detection was also observed in dams and the offspring infected with Nc-1 than in mice infected with the low-virulence Nc-Spain 1H isolate [30]. High frequencies of parasite detection in the clinically affected groups suggest that those more pathogenic isolates spread more widely and reached higher burdens in infected mouse brains and consequently produced more pathological lesions, which could explain the differences in the isolate virulence exhibited in mice.
Nevertheless, it is important to bear in mind that neonatal morbidity and mortality are also affected by the occurrence and efficiency of vertical transmission for each isolate in BALB/c mice. Parasite DNA detection rates in neonate brains varied from 52% to 100% according to the isolate implicated, leading to observed differences in neonatal mortality rates over time. In fact, isolates that produced the more severe clinical signs in dams and neonatal mortality rates also showed high vertical transmission rates (> 79%) (Nc-Spain 4H, Nc-Spain 5H, Nc-Spain 7 and Nc-Liv). Those neonates infected with the 6 other isolates that succumbed to infection displayed vertical transmission rates higher than 90%. Similarly, notable differences were also established between the Nc-1 and the low-virulence Nc-Spain 1H isolates, which showed a vertical transmission rate of 92.8% and 5%, respectively [30]. In addition, the transmission rate for Nc-Liv (95.6%) was comparable to the rate determined in a previous study for the Nc-Liv isolate (91%) in out-bred Qs mice infected with a lower dose of 106 tachyzoites [24], confirming the high capacity for vertical transmission of this isolate. Even so, the Nc-Spain 3H isolate displayed transmission and pathogenic capacities clearly differentiated in mice, since a very efficient transmission of 89% but also a survival rate higher than 92% was observed in neonates, suggesting a distinctive behaviour for this isolate in mice with a high capacity of transmission but a reduced ability to produce pathology.
Biological characteristics of these isolates may explain the differences in pathogenicity and vertical transmission demonstrated by N. caninum in this study. Different studies of the closely related apicomplexa Toxoplasma gondii have defined the association between parasite motility, migratory capacity across biological barriers, intracellular growth and reinvasion rates in vitro with the differences in virulence shown by type I, type II and type III strains [2, 31, 33]. With regards to N. caninum, previous studies on its in vitro behaviour have described the existence of differences in intracellular growth [23, 32] and tachyzoite-bradyzoite conversion rates [28, 35]. Furthermore, a lower tachyzoite yield and a slower tachyzoite-bradyzoite conversion for the Nc-Spain 1H have been associated with their low virulence [29, 30]. Therefore, a higher efficiency of spread, invasion or proliferation rates in host cells, together with a better rate of bradyzoite conversion, facilitating the brain invasion, the persistence of the parasite in nervous tissues and the evasion of the maternal immune response, could explain the higher capacity to produce pathology for the more virulent N. caninum isolates. Similarly, a high parasite motility and migratory capacity may be related to a more rapidly and higher quantity of parasites reaching the placenta, increasing the chance of transmission to offspring and pathology in neonates, as demonstrated when the tachyzoite dose used to inoculate pregnant BALB/c was increased [19]. In fact, a significant correlation could be established between the parasite detection rates in the immunoprivileged brain from dams and the success of transmission to progeny in this study, suggesting an association between dissemination capacity to nervous and placental tissues. Further studies focussing on the evaluation of potential differences in migration capacity, invasion, proliferation and tachyzoite-bradyzoite conversion rates in vitro may help to explain the differences observed among N. caninum isolates and their behaviour in vivo.
Different studies using mice have demonstrated that immunity against N. caninum involves a predominant Th1-type immune response to control the infection [4, 16]. However, during pregnancy, immunity is biased towards a Th2-type response, which produces the down-regulation of protective type 1 cytokine activity, which likely contributes to a more efficient spread and multiplication of the parasite in host tissues, leading to a higher transmission to progeny and an increase in parasite burdens compromising the health of dams [12, 17, 25]. Thus, the Th2-type response may also contribute to the exacerbation of the disease exhibited by mice infected with some isolates in this study, since these isolates were unable to produce clinical signs in non-pregnant mice intraperitoneally inoculated with 106 tachyzoites [22]. The analysis of IgG1 and IgG2a isotypes showed a significant N. caninum-specific antibody production in all infected dams, providing evidence of infection. Moreover, the IgG1 and IgG2a antibody levels also varied with the isolate administered, as reported in previous studies [24, 30]. Despite this, no significant differences in IgG1/IgG2a ratios were observed between infection groups, hampering to establish a predominant Th1 or Th2 response bias for each isolate.
Interestingly, the Nc-Spain 3H and Nc-Spain 4H isolates induced a different pathology in mice, although they were previously identified as genetically identical and were isolated from the same herd [27]. Both isolates displayed a high capacity of vertical transmission, although marked differences regarding the severity of clinical signs and mortality rates in dams and their offspring were observed as well as differences in their immunogenic capacities. According to these data and since both isolates were obtained from different calves, they could be considered to be different isolates, ostensibly with genetic variations in other loci not examined.
To summarise, this paper describes the pathogenic characterisation of 10 isolates in a pregnant BALB/c model. Neospora exposure was confirmed by specific antibody production in all N. caninum-inoculated dams and infection by parasite DNA detection in brain from dams and their progenies. Wide ranges of morbidity, mortality and vertical transmission rates were observed, suggesting the relevant role of the implicated isolate in the outcome of infection in pregnant mice. Nevertheless, further investigations are needed to determine the influence of this intra-species diversity in N. caninum and the actual virulence inherent to these isolates in the natural host, as well as its implications about the differences observed in the pathogenicity, clinical presentation and epidemiology of N. caninum infection in cattle.
Acknowledgments
This work was financed by the INIA project RTA04-047-C2. We thank Diana Williams (Liverpool School of Tropical Medicine, Liverpool, UK), who kindly provided us with the Nc-Liv isolate. We express our gratitude to Eduardo Cirera, Marta María Alonso and Carmen Cuevas for their excellent technical assistance and to all members of the SALUVET group for their excellent collaboration. We also thank Marta Gónzalez Warleta (Centro de Investigaciones Agrarias de Mabegondo, Spain) for her helpful contribution.
References
- 1.Atkinson R., Harper P.A., Ryce C., Morrison D.A., Ellis J.T., Comparison of the biological characteristics of two isolates of Neospora caninum , Parasitology (1999) 4:363–370 [DOI] [PubMed] [Google Scholar]
- 2.Barragan A., Sibley L.D., Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence, J. Exp. Med. (2002) 12:1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartley P.M., Wright S., Sales J., Chianini F., Buxton D., Innes E.A., Long-term passage of tachyzoites in tissue culture can attenuate virulence of Neospora caninum in vivo, Parasitology (2006) 4:421–432 [DOI] [PubMed] [Google Scholar]
- 4.Baszler T.V., Long M.T., McElwain T.F., Mathison B.A., Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice, Int. J. Parasitol. (1999) 10:1635–1646 [DOI] [PubMed] [Google Scholar]
- 5.Bland J.M., Altman D.G., Survival probabilities (the Kaplan–Meier method), BMJ (1998) 7172:1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bland J.M., Altman D.G., The logrank test, BMJ (2004) 7447:1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collantes-Fernandez E., Lopez-Perez I., Alvarez-Garcia G., Ortega-Mora L.M., Temporal distribution and parasite load kinetics in blood and tissues during Neospora caninum infection in mice, Infect. Immun. (2006) 4:2491–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey J.P., Buxton D., Wouda W., Pathogenesis of bovine neosporosis, J. Comp. Pathol. (2006) 4:267–289 [DOI] [PubMed] [Google Scholar]
- 9.Dubey J.P., Schares G., Ortega-Mora L.M., Epidemiology and control of neosporosis and Neospora caninum , Clin. Microbiol. Rev. (2007) 2:323–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innes E.A., Wright S., Bartley P., Maley S., Macaldowie C., Esteban-Redondo I., Buxton D., The host-parasite relationship in bovine neosporosis, Vet. Immunol. Immunopathol. (2005) 1–2:29–36 [DOI] [PubMed] [Google Scholar]
- 11.Innes E.A., The host-parasite relationship in pregnant cattle infected with Neospora caninum , Parasitology (2007) 13:1903–1910 [DOI] [PubMed] [Google Scholar]
- 12.Kano R., Masukata Y., Omata Y., Kobayashi Y., Maeda R., Saito A., Relationship between type 1/type 2 immune responses and occurrence of vertical transmission in BALB/c mice infected with Neospora caninum , Vet. Parasitol. (2005) 1–2:159–164 [DOI] [PubMed] [Google Scholar]
- 13.Liddell S., Jenkins M.C., Dubey J.P., Vertical transmission of Neospora caninum in BALB/c mice determined by polymerase chain reaction detection, J. Parasitol. (1999) 3:550–555 [PubMed] [Google Scholar]
- 14.Lindsay D.S., Lenz S.D., Cole R.A., Dubey J.P., Blagburn B.L., Mouse model for central nervous system Neospora caninum infections, J. Parasitol. (1995) 2:313–315 [PubMed] [Google Scholar]
- 15.Long M.T., Baszler T.V., Fetal loss in BALB/C mice infected with Neospora caninum , J. Parasitol. (1996) 4:608–611 [PubMed] [Google Scholar]
- 16.Long M.T., Baszler T.V., Mathison B.A., Comparison of intracerebral parasite load, lesion development, and systemic cytokines in mouse strains infected with Neospora caninum , J. Parasitol. (1998) 2:316–320 [PubMed] [Google Scholar]
- 17.Long M.T., Baszler T.V., Neutralization of maternal IL-4 modulates congenital protozoal transmission: comparison of innate versus acquired immune responses, J. Immunol. (2000) 9:4768–4774 [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Perez I.C., Risco-Castillo V., Collantes-Fernandez E., Ortega-Mora L.M., Comparative effect of Neospora caninum infection in BALB/c mice at three different gestation periods, J. Parasitol. (2006) 6:1286–1291 [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Perez I.C., Collantes-Fernandez E., Aguado-Martinez A., Rodriguez-Bertos A., Ortega-Mora L.M., Influence of Neospora caninum infection in BALB/c mice during pregnancy in post-natal development, Vet. Parasitol. (2008) 3–4:175–183 [DOI] [PubMed] [Google Scholar]
- 20.Miller C.M., Quinn H.E., Windsor P.A., Ellis J.T., Characterisation of the first Australian isolate of Neospora caninum from cattle, Aust. Vet. J. (2002) 10:620–625 [DOI] [PubMed] [Google Scholar]
- 21.Omata Y., Nidaira M., Kano R., Kobayashi Y., Koyama T., Furuoka H., et al., Vertical transmission of Neospora caninum in BALB/c mice in both acute and chronic infection, Vet. Parasitol. (2004) 3–4:323–328 [DOI] [PubMed] [Google Scholar]
- 22.Pereira García-Melo D., Regidor-Cerrillo J., Collantes-Fernandez E., Aguado-Martinez A., Del Pozo I., Minguijón E., et al., Pathogenic characterisation in mice of Neospora caninum isolates obtained from asymtomatic calves, Parasitology (2010) 17:1–12 [DOI] [PubMed] [Google Scholar]
- 23.Perez-Zaballos F.J., Ortega-Mora L.M., Alvarez-Garcia G., Collantes-Fernandez E., Navarro-Lozano V., Garcia-Villada L., Costas E., Adaptation of Neospora caninum isolates to cell-culture changes: an argument in favor of its clonal population structure, J. Parasitol. (2005) 3:507–510 [DOI] [PubMed] [Google Scholar]
- 24.Quinn H.E., Miller C.M., Ryce C., Windsor P.A., Ellis J.T., Characterization of an outbred pregnant mouse model of Neospora caninum infection, J. Parasitol. (2002) 4:691–696 [DOI] [PubMed] [Google Scholar]
- 25.Quinn H.E., Miller C.M., Ellis J.T., The cell-mediated immune response to Neospora caninum during pregnancy in the mouse is associated with a bias towards production of interleukin-4, Int. J. Parasitol. (2004) 6:723–732 [DOI] [PubMed] [Google Scholar]
- 26.Ramamoorthy S., Duncan R., Lindsay D.S., Sriranganathan N., Optimization of the use of C57BL/6 mice as a laboratory animal model for Neospora caninum vaccine studies, Vet. Parasitol. (2007) 3–4:253–259 [DOI] [PubMed] [Google Scholar]
- 27.Regidor-Cerrillo J., Gomez-Bautista M., Pereira-Bueno J., Aduriz G., Navarro-Lozano V., Risco-Castillo V., et al., Isolation and genetic characterization of Neospora caninum from asymptomatic calves in Spain, Parasitology (2008) 14:1651–1659 [DOI] [PubMed] [Google Scholar]
- 28.Risco-Castillo V., Fernandez-Garcia A., Ortega-Mora L.M., Comparative analysis of stress agents in a simplified in vitro system of Neospora caninum bradyzoite production, J. Parasitol. (2004) 3:466–470 [DOI] [PubMed] [Google Scholar]
- 29.Rojo-Montejo S., Collantes-Fernandez E., Blanco-Murcia J., Rodriguez-Bertos A., Risco-Castillo V., Ortega-Mora L.M., Experimental infection with a low virulence isolate of Neospora caninum at 70 days gestation in cattle did not result in foetopathy, Vet. Res. (2009) 5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rojo-Montejo S., Collantes-Fernandez E., Regidor-Cerrillo J., Alvarez-Garcia G., Marugan-Hernandez V., Pedraza-Diaz S., et al., Isolation and characterization of a bovine isolate of Neospora caninum with low virulence, Vet. Parasitol. (2009) 1:7–16 [DOI] [PubMed] [Google Scholar]
- 31.Saeij J.P., Boyle J.P., Boothroyd J.C., Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host, Trends Parasitol. (2005) 10:476–481 [DOI] [PubMed] [Google Scholar]
- 32.Schock A., Innes E.A., Yamane I., Latham S.M., Wastling J.M., Genetic and biological diversity among isolates of Neospora caninum , Parasitology (2001) 123:13–23 [DOI] [PubMed] [Google Scholar]
- 33.Taylor S., Barragan A., Su C., Fux B., Fentress S.J., Tang K., et al., A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii , Science (2006) 5806:1776–1780 [DOI] [PubMed] [Google Scholar]
- 34.Trees A.J., Williams D.J., Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii , Trends Parasitol. (2005) 12:558–561 [DOI] [PubMed] [Google Scholar]
- 35.Vonlaufen N., Muller N., Keller N., Naguleswaran A., Bohne W., McAllister M.M., et al., Exogenous nitric oxide triggers Neospora caninum tachyzoite-to-bradyzoite stage conversion in murine epidermal keratinocyte cell cultures, Int. J. Parasitol. (2002) 10:1253–1265 [DOI] [PubMed] [Google Scholar]
- 36.Whitten M.K., Effect of exteroceptive factors on the oestrous cycle of mice, Nature (1957) 4599:1436. [DOI] [PubMed] [Google Scholar]