Abstract
Background
A novel swine-origin influenza A (H1N1) virus was identified in March 2009 and subsequently caused worldwide outbreaks. The San Diego region was an early focal point of the emerging pandemic. We describe the clinical and epidemiologic characteristics of this novel strain in a military population to assist in future outbreak prevention and control efforts.
Methods
We performed an epidemiologic evaluation of novel H1N1 virus infections diagnosed in San Diego County among 96,258 local US military beneficiaries. The structured military medical system afforded the ability to obtain precise epidemiologic information on the impact on H1N1 virus infection in a population. The novel H1N1 virus was confirmed using real-time reverse transcriptase polymerase chain reaction (rRT-PCR).
Results
From 21 April through 8 May 2009, 761 patients presented with influenza-like illness and underwent rRT-PCR testing. Of these patients, 97 had confirmed novel H1N1 virus infection, with an incidence rate of 101 cases per 100,000 persons. The median age of H1N1 patients with H1N1 virus infection was 21 years (interquartile range, 15–25 years). Fever was a universal symptom in patients with H1N1 virus infection; other symptoms included cough (present in 96% of patients), myalgia or arthralgia (57%), and sore throat (51%). Sixty-eight (70%) of our patients had an identifiable epidemiologic link to another confirmed patient. The largest cluster of cases of H1N1 virus infection occurred on a Navy ship and involved 32 (8%) of 402 crew members; the secondary attack rate was 6%–14%. The rapid influenza testing that was used during this outbreak had a sensitivity of 51% and specificity of 98%, compared with rRT-PCR. Only 1 patient was hospitalized, and there were no deaths.
Conclusions
A novel H1N1 influenza A virus caused a significant outbreak among military beneficiaries in San Diego County, including a significant cluster of cases onboard a Navy ship. The outbreak described here primarily affected adolescents and young adults and resulted in a febrile illness without sequelae.
Swine influenza was first isolated in 1930 [1] and has caused periodic outbreaks among humans [2-7]. These cases were uniform in the lack of sustained human-to-human transmission, but were clinically indistinguishable from seasonal influenza. In March 2009, a novel swine origin influenza A virus (2009 H1N1) was isolated from 2 children in Southern California [8, 9] and was recognized as having caused a large outbreak in Mexico [10], with subsequent cases in the United States and multiple other countries [11]. Genetic characterization suggested that introduction into humans involved a single event, followed by multiple human-to-human transmissions [12]. San Diego County experienced a rapid increase in cases, notably involving the regional military community. In response, a systematic screening and testing program for influenza-like illness (ILI) was initiated at Naval Medical Center San Diego (NMCSD) and local Navy clinics. We report the results of that effort and describe the clinical characteristics of this novel influenza strain.
MATERIALS AND METHODS
Patient population and setting
We performed an epidemiologic evaluation of H1N1 infection among the US military and their beneficiaries in San Diego County who were evaluated at NMCSD or 1 of 16 affiliated Navy clinics. We investigated cases from 21 April 2009, immediately after notification of the first known case in a military beneficiary, until 8 May 2009, when ILI screening was reduced. The NMCSD system provides care for 96,258 locally enrolled beneficiaries (50,668 male and 45,590 female beneficiaries). Of those with documented ethnicity within the hospital database (39,666; 41%), 23,718 (60%) were white, 7128 (18%) were Pacific Islander, 5564 (14%) were black, and 3256 (8%) were other. Active duty members comprised 24,437 (25%) of beneficiaries; the remainder were family members and military retirees. The median age of our population was 29 years (range, 1 day to 111 years).
Patients with ILI were considered for H1N1 testing on the basis of Centers for Disease Control and Prevention (CDC) recommendations. Patients were tested if they reported fever (>38°C) and 2 of the following symptoms: cough, sore throat, headache, myalgia, and rhinorrhea [13-15]. Because our investigation was based in a clinical setting, influenza testing was also performed at the discretion of the health care provider. We established a liaison with local county health officials for daily patient contact tracking to ensure robust follow-up of patients during the outbreak.
Specimen collection
We employed a dual-testing strategy for patients presenting with ILI: a nasopharyngeal (NP) swab sample was obtained for rapid influenza antigen test (RIAT; .QuickVue Influenza A+B test [Quidel]), and a second NP swab sample was obtained for real-time reverse transcriptase polymerase chain reaction testing (rRT-PCR). This dual-testing strategy was implemented in waves; despite the intent to perform testing for all ILI subjects with both modalities, some patients received only partial testing. Specimens were sent to the Naval Health Research Center (NHRC) for diagnostic testing. From 21 April to 29 April, samples were confirmed as H1N1 by the CDC. On 30 April, NHRC began confirming cases using the CDC rRT-PCR assay [16]. All novel H1N1 cases in this report were confirmed by CDC and/or NHRC.
Epidemiologic investigation
With use of the NMCSD and NHRC databases, we identified patients who underwent RIAT and/or rRT-PCR for ILI encounters during the study period. Electronic medical records (EMRs) was accessed for demographic data, duty status, tobacco use, influenza vaccinations, and medical history, including pregnancy. History of contact with an individual with H1N1 virus infection or ILI, recent travel to Mexico, symptoms, vital signs, and medical management were recorded. Data were recorded on case report forms for standardized data collection.
Information on the residence of each patient was obtained, and the geographic area of the outbreak was divided into 6 areas by zip code on the basis of the San Diego Public Health Maps for disease surveillance [17]. To establish epidemiologic linkages between cases, the proximity of addresses (determined with local maps) and shared military work areas were investigated.
Statistical analyses
Statistical analyses included descriptive statistics with median values and interquartile ranges (IQRs). Comparisons between groups used Fisher’s exact and Wilcoxon rank sum tests for categorical and continuous variables, respectively. Age-adjusted rates of cases of H1N1 virus infection were calculated on the basis of demographic categories of beneficiaries. P values were 2-sided and considered to be statistically significant if <.05 (Stata software, version 10.0; Stata).
RESULTS
Epidemiologic investigation
From 21 April through 8 May 2009, a total of 995 patients presented with ILI and underwent testing for influenza (Figures 1 and 2A); 761 had rRT-PCR testing performed, and 234 underwent RIAT only. Among those with an rRT-PCR test result, 97 (12.7%) were confirmed to have H1N1 virus infection, whereas 641 had negative results. Twenty-six additional cases (3 diagnosed by RIAT only) of seasonal influenza were diagnosed: 21 cases of influenza B and 5 cases of A/H3 virus infection.
Figure 1.

Flow diagram of influenza testing performed. rRT-PCR, real-time reverse transcriptase polymerase chain reaction.
Figure 2.

A, Epidemiologic curve of influenza-like illness (ILI) cases and cases of H1N1 virus infection among US military beneficiaries, San Diego, California, 21 April–8 May 2009 B, Epidemiologic curve of ILI cases and cases of H1N1 virus infection onboard ship A. rRT-PCR, real-time reverse transcriptase polymerase chain reaction.
Demographic and clinical characteristics of novel H1N1 virus infections
The median age of patients with H1N1 virus infection was 21 years (IQR, 15–25 years; range, 6 months to 78 years); 73 (75%) were male. Of those with race or ethnicity recorded (60 patients), 23 (38%) were white, 16 (27%) were black, 10 (16%) were Pacific Islander/Asian, 9 (15%) were Hispanic, and 2 (3%) were other (Table 1). Age-adjusted rates for H1N1 virus infection were 54 cases per 100,000 persons <2 years of age, 110 cases per 100,000 persons 2–17 years of age, 345 cases per 100,000 persons 18–24 years of age, 73 cases per 100,000 persons 25–49 year of age, and 5 cases per 100,000 persons ≥50 years of age (Table 2). Sixty cases (62%) occurred among active duty members, 36 (37%) occurred among family members, and 1 (1%) occurred in a retiree. Of note, 68% of those who underwent rRT-PCR testing were family members or retirees, and 32% were active duty members of the military.
Table 1.
Patients with H1N1 Virus Infection (H1N1 Group) Compared with Patients Presenting with an Influenza-Like Illness who had Negative Real-Time Reverse-Transcriptase Polymerase Chain Reaction Results for Influenza (Influenza-Negative Group) and Patients with H3/B Seasonal Influenza (H3/B Group)
| Characteristic | H1N1 group (n = 97) | Influenza negative group (n = 641) | Pa | Influenza H3/B group (n = 26) | Pb |
|---|---|---|---|---|---|
| Demographic characteristic | |||||
| Age | |||||
| Median years (IQR) | 21 (15–25) | 20 (4–30) | .15 | 16 (10–20) | .003 |
| <2 years | 2/97 (2) | 78/641 (12) | <.01 | 0/26 (0) | .001 |
| 2–17 years | 25/97 (26) | 210/641 (33) | 17/26 (65) | ||
| 18 to 24 years | 43/97 (44) | 117/641 (18) | 6/26 (23) | ||
| 25–49 years | 26/97 (27) | 173/641 (27) | 2/26 (8) | ||
| ≥50 years | 1/97 (1) | 63/641 (10) | 1/26 (4) | ||
| Male sex | 73/97 (75) | 317/641 (49) | <.01 | 15/26 (58) | .09 |
| Race/ethnicity | <.01 | .02 | |||
| White | 23/60 (38) | 155/331 (47) | 10/18 (56) | ||
| Black | 16/60 (27) | 39/331 (12) | 3/18 (17) | ||
| Hispanic | 9/60 (15) | 16/331 (5) | 0/18 (0) | ||
| Pacific Islander | 10/60 (16) | 46/331 (14) | 1/18 (6) | ||
| Other | 2/60 (3) | 75/331 (23) | 4/18 (22) | ||
| Duty status | <.01 | <.01 | |||
| Active duty | 60/97 (62) | 174/641 (27) | 5/26 (19) | ||
| Family member | 36/97 (37) | 440/641 (69) | 19/26 (73) | ||
| Retiree | 1/97 (1) | 27/641 (4) | 2/26 (8) | ||
| Exposure history | |||||
| Travel to Mexico | 1/97 (1) | 15/641 (2) | .71 | 0/26 (0) | >.99 |
| Contact with H1N1 virus infection | 50/97 (52) | 40/641 (6) | <.01 | 1/26 (4) | <.01 |
| Contact with ILI | 56/97 (58) | 143/641 (22) | <.01 | 8/26 (31) | .04 |
| Current tobacco use | 15/82 (18) | 60/499 (12) | .15 | 2/22 (9) | .52 |
| Medical history | |||||
| Asthma | 17/94 (18) | 94/602 (16) | .55 | 2/26 (8) | .36 |
| COPD | 0/94 (0) | 4/602 (1) | >.99 | 0/26 (0) | … |
| Heart disease | 1/94 (1) | 13/602 (2) | .71 | 0/26 (0) | >.99 |
| Diabetes | 2/94 (2) | 29/602 (5) | .42 | 0/26 (0) | … |
| Pregnancy | 4/97 (4) | 1/613 (0.2) | <.01 | 0/26 (0) | .58 |
| Influenza vaccination history | |||||
| History of vaccination within the previous 12 months | 63/95 (66) | 259/641 (40) | <.01 | 8/26 (31) | .002 |
| LAIV used for most recent vaccination | 38/73 (52) | 96/340 (28) | <.01 | 4/9 (44) | .74 |
| Clinical symptoms | |||||
| Time to presentation, median days (IQR) | 2 (1–3) | 2 (1–4) | .02 | 3 (2–6) | .002 |
| Setting of initial influenza testing | <.01 | .01 | |||
| Outpatient clinic | 33/97 (34) | 320/641 (50) | 17/26 (65) | ||
| Emergency department | 26/97 (27) | 284/641 (44) | 5/26 (29) | ||
| Ship/training Depot | 38/97 (39) | 19/641 (3) | 4/26 (15) | ||
| Hospital ward | 0/97 (0) | 18/641 (3) | 0/26 (0) | ||
| Fever | 91/91 (100) | 465/606 (77) | <.01 | 22/23 (96) | .20 |
| Cough | 87/91 (96) | 449/606 (74) | <.01 | 20/23 (87) | .15 |
| Rhinorrhea | 40/91 (44) | 259/606 (43) | .82 | 18/23 (78) | .005 |
| Myalgia or arthralgia | 52/91 (57) | 223/606 (37) | <.01 | 10/23 (43) | .25 |
| Sore throat | 46/91 (51) | 288/606 (48) | .65 | 17/23 (74) | .06 |
| Eye symptoms | 9/91 (10) | 38/606 (6) | .26 | 1/23 (4) | .68 |
| Nausea | 23/91 (25) | 148/606 (24) | .90 | 6/23 (26) | >.99 |
| Vomiting | 17/91 (19) | 134/606 (22) | .50 | 3/23 (13) | .76 |
| Diarrhea | 6/91 (7) | 117/606 (19) | <.01 | 1/23 (4) | >.99 |
| Headache | 45/91 (49) | 225/606 (37) | .03 | 11/23 (48) | >.99 |
| Temperature | |||||
| Median °F (IQR) | 101.0 (100.2–102.0) | 99.6 (98.2–101.6) | <.01 | 101.0 (98.7–102.1) | .39 |
| ≥38°C | 66/89 (74) | 243/595 (41) | <.01 | 15/24 (63) | .31 |
NOTE. Data are proportion (%) of patients, unless otherwise indicated. COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LAIV, live attenuated influenza vaccine.
P value compares H1N1 group to influenza negative group.
P value compares H1N1 group to H3/B group.
Table 2.
Age-Specific Incidence Rates for Novel H1N1 Infection among U.S. Military Beneficiaries
| Age, years | No. (%) of total population served (n = 96,258) | No. (%) of patients with H1N1 virus infection (n = 97) | Incidence rate, no. of cases per 100,000 population |
|---|---|---|---|
| <2 | 3719 (3.9) | 2 (2.1) | 53.8 |
| 2–17 | 22,691 (23.6) | 25 (25.8) | 110.2 |
| 18–24 | 12,471 (13.0) | 43 (44.3) | 344.8 |
| 25–49 | 35,463 (36.8) | 26 (26.8) | 73.3 |
| ≥50 | 21,914 (22.8) | 1 (1.0) | 4.6 |
| Overall | 96,258 (100) | 97 (100) | 100.9 |
Fifty-six patients (58%) reported contact with an individual with ILI, and 50 (52%) reported contact with a patient with known H1N1 virus infection. Thirty-two H1N1 virus infection cases occurred among active duty members stationed on a single ship (“ship A”). Seventeen (18%) of 94 patients with medical history data had asthma, 1 (1%) had heart disease, 2 (2%) had diabetes, and none had chronic obstructive pulmonary disease. Four (4%) were pregnant at diagnosis; there were no observed obstetric complications. Vaccine status was available for 95 of 97 patients; 63 (66%) had received influenza vaccination in the previous 12 months, and in 38 (52%) of 73, the type of vaccine was the live attenuated influenza vaccine (LAIV).
Median time from symptom onset to presentation was 2 days (IQR, 1–3 days). Clinical symptoms were available for 91 (94%) of patients: all patients reported fever, and 96% had cough (Table 1). The median temperature was 38.3°C. Chest radiographs were obtained from 23% of patients—2 patients had concurrent pneumonia. Oseltamivir was administered to 71 (73%) of 91 patients; none received zanamivir. Information on the duration of illness was available for 30 patients; the median time from symptom onset to resolution was 7.0 days (IQR, 3–10 days) for those who received oseltamavir and 8.5 days (IQR, 6–11 days) for those who were untreated (P = .41). Only 1 patient (1%) required hospitalization—a 72-year-old man with diabetes, heart disease, and a prior stroke. He had concurrent bacterial pneumonia and respiratory failure and was discharged from the hospital after 9 days. There were no deaths or known long-term morbidity due to H1N1 virus.
Geographic distribution and clustering of cases
We investigated the residences of military beneficiaries, patients with ILI who underwent rRT-PCR testing, and persons with confirmed cases of H1N1 virus infection (Figure 3). The overall incidence rate of novel H1N1 virus infection was 101 cases per 100,000 persons, but the incidence rate varied by geographic location. Seventy percent of patients with H1N1 infection had an identifiable epidemiologic link to another confirmed case. Most linkages occurred on ship A (described below), whereas others included military training sites, as well as family or neighborhood clusters involving spouses and children.
Figure 3.
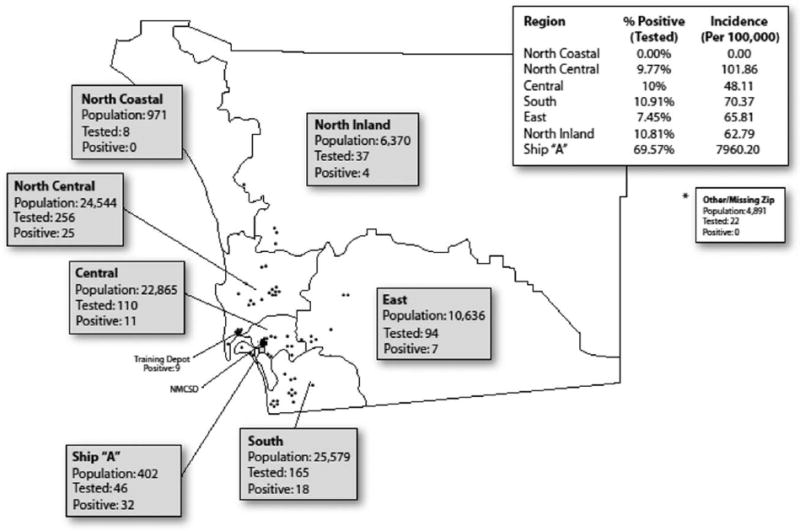
Geographic locations of novel H1N1 testing by real-time reverse-transcriptase-polymerase chain reaction and confirmed cases among US military beneficiaries, San Diego County, 21 April–8 May 2009.
Comparisons of patients with H1N1 virus infection to those with ILI without influenza and to those with seasonal influenza
We compared the characteristics of persons with H1N1 virus infection (97 patients) to those who were negative for influenza by rRT-PCR (641 patients) (Table 1). Patients with H1N1 virus infection, compared with those with noninfluenza illnesses, were more likely to be 18–24 years of age (44% vs 18%; P < .001), black (27% vs 12%; P = .004), male (75% vs 49%; P < .01), on active duty (62% vs 27%; P < .01), to have reported contact with someone with H1N1 virus infection (52% vs 6%; P < .01) or ILI (58% vs 22%; P < .01), and were more likely to be pregnant (4% vs 0.2%; P < .01). Patients with H1N1 virus infection were also more likely to have received influenza vaccination within the previous year (66% vs 40%; P < .01) and to have received LAIV (52% vs 28%; P < .01). Active duty members of the military were examined separately from family members and retirees, and the findings for the 2 groups were similar, except that there were no associations with prior influenza vaccination or male sex among non–active duty patients (data not shown).
At presentation, patients with H1N1 virus infection were more likely to report fever (100% vs 77%; P < .01), cough (96% vs 74%; P < .01), myalgia and/or arthralgia (57% vs 37%; P < .01), and headache (49% vs 37%; P = .03), but they were less likely to report diarrhea (7% vs 19%; P < .01). On physical examination, patients with H1N1 virus infection were more likely to be febrile but had no other significant differences in vital signs.
Cases of novel H1N1 virus infection were compared with cases of seasonal influenza (Table 1). H1N1 virus infection occurred among slightly older persons (median age, 21 vs 16 years; P = .003); H1N1 virus infection preferentially occurred in the 18–24-year-old age group, whereas seasonal influenza cases occurred more often among the 2–17-year-old age group. Cases of H1N1 virus infection were more likely among active duty members of the military than they were among family members and retirees (62% vs 19%; P < .01), and patients with H1N1 virus infection were more likely to have contact with an individual with H1N1 virus infection (52% vs 4%; P < .01) or ILI (58% vs 31%; P = .04). Regarding previous influenza vaccination, 66% of the H1N1 group versus 31% of the H3/B group had received a recent influenza vaccination (P = .002). The clinical presentations were similar, except that rhinorrhea was more common in the H3/B group (78% vs 44%; P = .005), with a trend for sore throat (74% vs 51%; P = .06). There were no long-term adverse health outcomes in either group.
Outbreak on ship A
Forty-six (11%) of the 402 crew members onboard ship A presented with ILI between 26 April and 7 May 2009, of whom 32 (8%) had confirmed H1N1 virus infection (Figure 2B). In early April, the ship was pulled ashore for maintenance, and the crew had extensive contact with civilian maintenance personnel, some of whom resided in Mexico. No crew member reported recent travel to Mexico. While in port, many crewmembers worked onboard but returned to local residences daily.
There were no significant differences with respect to age or sex between patients with H1N1 virus infection and patients with test results negative for influenza; however, the ship’s personnel were demographically homogenous. More persons with H1N1 virus infection had received recent influenza vaccination, compared with those with negative test results (93% vs 64%; P = .03). Fever was the only symptom or sign that differed between the groups.
For the 32 H1N1 cases that occurred on the ship, the secondary attack rate among family members who resided in the same household was 6% (2 of 34 individuals), with cases occurring in a crew member’s spouse and a crew member’s 4-year-old child (Figure 4). Among the family members of the 356 crew members who did not present with ILI and were therefore not tested for H1N1 virus, 3 developed H1N1 virus infection during the study period. Including these cases, the attack rate was 14%.
Figure 4.

Flow diagram of patients with H1N1 virus infection and family members among the crew of ship A. yo, year old.
Because of the high number of cases onboard, fleet medical staff administered prophylactic oseltamivir (75 mg daily for 10 days) to the entire crew on 7 May; family members did not receive prophylaxis. No further cases associated with the ship have been diagnosed (Figure 2B).
RIAT compared with rRT-PCR test results
Of 761 persons undergoing rRT-PCR testing, 571 (75%) had concurrent RIAT performed (594 total patients, excluding 23 with seasonal influenza). Both rRT-PCR and RIAT results were negative in 483 cases, and both were positive in 40 cases. Nine patients with positive RIAT results had negative rRT-PCR results, and 39 patients with negative RIAT results had positive rRT-PCR (Table 3). Assuming rRT-PCR as the “gold standard”, the sensitivity of RIAT was 51%, the specificity 98%, the positive predictive value (PPV) was 82%, and the negative predictive value (NPV) was 93%.
Table 3.
Comparison of Rapid Influenza Antigen Test (RIAT) Results with Real-Time Reverse-Transcriptase Polymerase Chain Reaction (rRT-PCR) Results for the Diagnosis of H1N1 Virus Infection
| rRT-PCR result |
|||
|---|---|---|---|
| RIAT result | Negative | Positive | Total |
| Negative | 483 | 39 | 522 |
| Positive | 9 | 40 | 49 |
| Total | 492 | 79 | 571 |
We examined the relationship between the accuracy of RIAT and patient age: for those patients <18 years old, the sensitivity of RIAT was improved to 77.3% (17 of 22 patients) (95% confidence interval [CI], 54.2%–91.1%), with a specificity of 98% (200 of 204), PPV of 81% (17 of 21), and NPV of 98% (200 of 205). There were no statistically significant differences between patients <6 years of age and patients from 6 through <18 years of age, although the numbers for the former group were small. RIAT had a lower sensitivity 40% (23 of 57 patients) (95% CI, 27.8%–54.1%) among persons ≥18 years of age, but had similar results for specificity (283 [98%] of 288 patients), PPV (23 [82%] of 28), and NPV (283 [89%] of 317).
DISCUSSION
We report an outbreak of 97 H1N1 cases among military beneficiaries in San Diego County from 21 April through 8 May 2009. The outbreak involved persons of all age groups, but it mostly affected young adults. Active duty members of the military were also preferentially affected; 25% of the beneficiary population is on active duty, yet this group accounted for 62% of cases of H1N1 virus infection.
A unique outbreak occurred on a docked Navy ship (ship A), in which 8% of the 402 crew members developed confirmed H1N1 virus infection. Using detailed data on the crew and their family members (crew members returned home each night to their families), we estimated the secondary attack rate as 6%–14%. Although this number may underestimate the secondary attack rate because of the possibility of cases not presenting for care, medical care was readily available onboard the ship, and ill beneficiaries were encouraged to receive care. Compared with previous pandemic influenza strains [18, 19], this novel H1N1 strain may have lower or similar rates of transmissibility; however, unlike the swine flu outbreak that occurred at Fort Dix in 1976 [20], it has resulted in sustained human-to-human transmission.
Fever and cough were more commonly seen in patients with H1N1 virus infection than in those with noninfluenza diagnoses. These symptoms are highly predictive for the diagnosis of seasonal influenza during outbreaks [13, 21]. Both symptoms are part of the CDC case definition for ILI; however, H1N1 testing was performed in a nondifferential fashion among persons presenting with an ILI. Nonetheless, it is possible that cases without case-defining symptoms, such as cases of nonfebrile H1N1 virus infection, were missed. Patients with H1N1 virus infection were also more likely to report myalgia or arthralgia and headache, but they were less likely to have diarrhea, compared with individuals with noninfluenza ILI. The clinical course of H1N1 virus infection was generally mild: the percentage of patients with concurrent pneumonia was 2%, the percentage with hospitalization was 1%, and there were no fatalities, suggesting that this novel influenza strain usually does not lead to severe disease, similar to seasonal strains [22, 23], notwithstanding recent unfortunate reports of fatal H1N1 virus infections. Although our population was similar to the local community, it consisted of slightly younger persons (median age, 29 vs 33 years; 41% vs 37% were <25 years of age) [24] and may have included healthier individuals, potentially contributing to the low rates of severe disease in our cohort.
Medical conditions, such heart disease or diabetes, were not associated with H1N1 virus infection. This may be attributable to the low number of H1N1 virus infections among older age groups. We found that pregnancy was associated with H1N1 virus infection, although our numbers were small. Reports suggest that pregnant women with H1N1 virus infection are at higher risk for hospitalization, obstetric complications, and death [25, 26], as are those with seasonal influenza [22, 27]. These data emphasize the importance of assessing the safety and efficacy of H1N1 vaccines among pregnant women.
H1N1 virus infection preferentially affected adolescents and young adults. These trends differ from those for seasonal influenza viruses, which have the greatest impact on individuals at the extremes of age [22]. Of note, the 1918–1919 strain also primarily affected young adults [28, 29]. Some of the predisposition of young age groups may have been influenced by cohorting of young active duty members onboard ships or at training depots; however, the lower age-specific incidence rates among individuals <2 years of age and >50 years of age suggests that H1N1 virus infection preferentially affects adolescents and young adults.
We noted trends in an increased number of cases among black individuals. We are unaware of data suggesting that any influenza preferentially involves specific ethnic groups. The nature of this association in our study is unknown, but it may reflect a statistical anomaly, clustering among black individuals in workplaces and neighborhoods, or unknown host genetic factors [30]. Of note, both age and racial trends remained even when active duty members were excluded, among whom case clustering is more likely to occur.
Seasonal influenza vaccination was not protective against H1N1 virus; this was expected, because recent data showed that seasonal influenza vaccination did not induce protective antibody against H1N1 virus, and there was no significant serologic cross-reactivity [31]. Active duty members of the military with H1N1 virus infection (but no other group) were more likely to have received influenza vaccination (particularly LAIV) than were those without H1N1 virus infection. This finding is likely explained by vaccination of nearly all military members, and it is likely not a true association. A vaccine specifically effective against H1N1 virus infection is currently under production [32].
Regarding the diagnosis of novel H1N1 virus infections, the RIAT used in this study had low sensitivity but similar specificity for detecting H1N1, compared with its sensitivity and specificity for detecting seasonal influenza strains [33]. The RIAT performed best among individuals <18 years of age; this may be related to increased viral shedding among children, compared with that among adults, as seen with seasonal influenza [34]. Our results emphasize the importance of rRT-PCR in diagnosing novel H1N1 virus infection. Potential limitations of our investigation included the fact that not all ILI cases may have presented for care or received rRT-PCR testing, leading to an underestimation of the number of cases. Viral culture was not performed, because the virus does not grow well in standard respiratory tissue cultures; culture of H1N1 virus was initially restricted; and rRT-PCR appears to be more sensitive than viral culture [13, 34, 35]. We only evaluated individuals with H1N1 virus infection who were eligible for care in our system; consequently, we may have missed linkages between our beneficiaries and other community cases. Conversely, some beneficiaries may have received a diagnosis in the community, particularly those individuals >65 years of age; however, medical services are at no cost for beneficiaries at our facilities. Although a large number of H1N1 virus infections among active duty members could potentially bias some of our comparisons between H1N1 virus infection and noninfluenza cases, we analyzed our data stratified by duty status and found similar associations except for prior influenza vaccination and male sex. Finally, despite a robust EMR, some clinical data (eg, physicians’ handwritten notes and immunization records) were not available during our investigation.
Strengths included our well-defined population, comprising all age groups, with a diverse racial composition. We used detailed electronic information regarding demographic data, residency, medical encounters, and vaccinations to comprehensively describe the characteristics of the novel H1N1 strain. In addition, our affiliated laboratory center (NHRC) had rapid throughput rRT-PCR capability. Finally, our active duty population has unique exposure environments, such as ships and training depots, which illustrated the impact of H1N1 virus infection.
In summary, we report the clinical and epidemiologic features of an H1N1 virus infection outbreak among military beneficiaries in San Diego, California. The strain appears to preferentially affect adolescents and young adults, with pregnant women also having a greater risk of clinical infection. Although most cases resulted in a mild, febrile illness without sequelae, our investigation demonstrates the rapid spread of this novel H1N1 strain in a well-defined population, including an outbreak on a military vessel.
Acknowledgments
We thank all of the medical and laboratory personnel who provided clinical care during this outbreak and who collected much of the information used in this report, and we thank Judy Christensen for the medical graphics used in this article.
Financial support. US Department of Defense Global Emerging Infections Surveillance and Response System; the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences; and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (interagency agreement Y1-AI-5072).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
Presented in part: 47th Infectious Diseases Society of America Meeting (Philadelphia, PA), 29 October–1 November 2009 (abstract LB1).
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the National Institutes of Health or the Department of Health and Human Services, the Department of Defense, or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
References
- 1.Shope RE. Swine influenza: filtration experiments and etiology. J Exp Med. 1931;54:373–85. doi: 10.1084/jem.54.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–25. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 3.Wells DL, Hopfensperger DJ, Arden NH, et al. Swine influenza virus infections: transmission from ill pigs to humans at a Wisconsin agricultural fair and subsequent probable person-to-person transmission. JAMA. 1991;265:478–81. doi: 10.1001/jama.265.4.478. [DOI] [PubMed] [Google Scholar]
- 4.Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis. 2007;44:1084–8. doi: 10.1086/512813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman AP, Reisdorf E, Beinemann J, et al. Human case of swine influenza A (H1N1) triple reassortant virus infection, Wisconsin. Emerg Infect Dis. 2008;14:1470–2. doi: 10.3201/eid1409.080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowdle WR, Hattwick MA. Swine influenza virus infections in humans. J Infect Dis. 1977;136:S386–9. doi: 10.1093/infdis/136.supplement_3.s386. [DOI] [PubMed] [Google Scholar]
- 7.Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses a North American perspective. Adv Virus Res. 2008;72:127–54. doi: 10.1016/S0065-3527(08)00403-X. [DOI] [PubMed] [Google Scholar]
- 8.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Swine influenza A (H1N1) infection in two children—Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:400–2. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March–April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–70. [PubMed] [Google Scholar]
- 11.Influenza A (H1N1)— update 44. Geneva: World Health Organization; 2009. [6 June 2009]. Available at: http://www.who.int/csr/don/2009_06_05/en/index.html. [Google Scholar]
- 12.Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin G, Hardy I, Tellier G, Maziade J. Predicting influenza infections during epidemics with use of a clinical case definition. Clin Infect Dis. 2000;31:1166–9. doi: 10.1086/317425. [DOI] [PubMed] [Google Scholar]
- 14.Navarro-Mari JM, Perez-Ruiz M, Cantudo-Munoz P, et al. Influenza-like illness criteria were poorly related to laboratory-confirmed influenza in a sentinel surveillance study. J Clin Epidemiol. 2005;58:275–59. doi: 10.1016/j.jclinepi.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson KG. Clinical features of influenza. Semin Respir Infect. 1992;7:26–37. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention protocol of realtime RTPCR for influenza A (H1N1) [11 June 2009]; Available at: http://www.euro.who.int/Document/INF/CDC_realtime_RTPCR_H1N1.pdf.
- 17.County of San Diego community profiles by region and subregional area. [5 June 2009]; Available at: http://www.sdcounty.ca.gov/hhsa/programs/phs/documents/CHS-CommunityProfile_County_12-07.pdf.
- 18.White LF, Pagano M. Transmissibility of the influenza virus in the 1918 pandemic. PLoS ONE. 2008;3:e1498. doi: 10.1371/journal.pone.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viboud C, Tam T, Fleming D, Handel A, Miller MA, Simonsen L. Transmissibility and mortality impact of epidemic and pandemic influenza, with emphasis on the unusually deadly 1951 epidemic. Vaccine. 2006;24:6701–7. doi: 10.1016/j.vaccine.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 20.Lessler J, Cummings DA, Fishman S, Vora A, Burke DS. Transmissibility of swine flu at Fort Dix, 1976. J R Soc Interface. 2007;4:755–62. doi: 10.1098/rsif.2007.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–7. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 22.Monto AS. The risk of seasonal and pandemic influenza: prospects for control. Clin Infect Dis. 2009;48:S20–5. doi: 10.1086/591853. [DOI] [PubMed] [Google Scholar]
- 23.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 24.San Diego region demographic and economic characteristics. [15 September 2009]; Available at: http://www.sandag.org/index.asp?classidp=26&fuseaction=home.classhome.
- 25.Centers for Disease Control and Prevention. Hospitalized patients with novel influenza A (H1N1) virus infection—California, April-May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–41. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Novel influenza A (H1N1) virus infections in three pregnant women—United States, April-May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:497–500. [PubMed] [Google Scholar]
- 27.Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008;8:44–52. doi: 10.1016/S1473-3099(07)70311-0. [DOI] [PubMed] [Google Scholar]
- 28.Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018–28. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 29.Brundage JF. Cases and deaths during influenza pandemics in the United States. Am J Prev Med. 2006;31:252–6. doi: 10.1016/j.amepre.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Trammell RA, Toth LA. Genetic susceptibility and resistance to influenza infection and disease in humans and mice. Expert Rev Mol Diagn. 2008;8:515–29. doi: 10.1586/14737159.8.4.515. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58:521–4. [PubMed] [Google Scholar]
- 32.Vaccines for the new influenza A (H1N1) [8 June 2009]; Available at: http://www.who.int/csr/disease/swineflu/frequently_asked_questions/vaccine _preparedness/en/index.html.
- 33.Hurt AC, Alexander R, Hibbert J, Deed N, Barr IG. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J Clin Virol. 2007;39:132–5. doi: 10.1016/j.jcv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Harper SA, Bradley JS, Englund JA, et al. Expert panel of the Infectious Diseases Society of America: seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–2. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis JS, Fleming Dm, Zambon MC. Multiplex reverse transcriptase-PCR for surveillance of influenza A and B viruses in England and Wales in 1995 and 1996. J Clin Microbiol. 1997;35:2076–82. doi: 10.1128/jcm.35.8.2076-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


