Abstract
Gap junctions are specialized membrane domains composed of collections of channels that directly connect neighboring cells providing for the cell-to-cell diffusion of small molecules, including ions, amino acids, nucleotides, and second messengers. Vertebrate gap junctions are composed of proteins encoded by the “connexin” gene family. In most cases examined, connexins are modified post-translationally by phosphorylation. Phosphorylation has been implicated in the regulation of gap junctional communication at several stages of the connexin “lifecycle”, such as the trafficking, assembly/disassembly, degradation, as well as, the gating of gap junction channels. Since connexin43 (Cx43) is widely expressed in tissues and cell lines, we understand the most about how it is regulated, and thus, connexin43 phosphorylation is a major focus of this review. Recent reports utilizing new methodologies combined with the latest genome information have shown that activation of several kinases including protein kinase A, protein kinase C, p34cdc2/cyclin B kinase, casein kinase 1, mitogen-activated protein (MAP) kinase and pp60src kinase can lead to phosphorylation at 12 of the 21 serine and two of the six tyrosine residues in the C-terminal region of connexin43. In several cases, use of site-directed mutants of these sites have shown that these specific phosphorylation events can be linked to changes in gap junctional communication.
Keywords: Connexin, Gap junction, Phosphorylation, v-Src, MAP kinase, Cell signaling, PKC, PKA, Casein kinase
1. Introduction
Gap junctions are collections of plasma membrane channels that enable adjacent cells to exchange cytoplasmic components directly without transit through the extracellular space. These channels allow passage of small molecules (generally less than 1000 Da) such as ions, individual amino acids or short peptides, second messengers (e.g. Ca2+, IP3, cAMP) and other metabolites. In vertebrates, gap junctions are composed of proteins from the connexin family, which is comprised of approximately 20 members in humans. Connexins are designated with numerical suffixes referring to the molecular weight of the deduced sequence in kilodaltons (e.g. connexin43 or Cx43) (Goodenough, Goliger, & Paul, 1996) or an α/β nomenclature (Kumar & Gilula, 1996). Connexins are differentially expressed in tissues with some being significantly expressed in only a few tissues and some, like Cx43, being more widespread.
Deficient or improper gap junction function has recently been associated with a variety of diseases, including some forms of neuropathy, hereditary deafness, cataracts, skin disease, heart disease, and cancer (Willecke et al., 2002). Specifically, Cx26, Cx30 and Cx31 gene mutations have been implicated by genetic linkage studies in some forms of non-syndromic deafness in humans, and defects in Cx26, Cx30.3 and Cx31 have been linked to the skin disease erythroker-atoderma (Kelsell et al., 1997). Mutations within the Cx32 gene are associated with the X-linked peripheral nerve disorder Charcot–Marie tooth syndrome, which results from the demyelination of Schwann cells that surround peripheral neurons (Bergoffen et al., 1993). Cx32-deficient mice also can show neuropathy at older age and are 25-fold more susceptible to carcinogen-induced liver cancer (Willecke et al., 2002). Junctional communication appears to be critical in development as Cx26 and Cx45 knockout mice die in utero, Cx37 knockout female mice are infertile, and Cx43-deficient mice die shortly after birth from malformation of the conotruncal region of the right ventricle (Gabriel et al., 1998; Reaume et al., 1995; Simon, Goodenough, & Paul, 1998; Willecke et al., 2002). These disparate phenotypes not only show the diversity of the expression pattern of connexins, but they also illustrate that gap junctions play different roles in different tissues.
Recent work has also illustrated that the complexity of the connexin family may have evolved to serve different functions via transfer of molecules specific to a particular connexin isotype and through functions not directly connected to the exchange of small molecules. For example, when Cx43 was replaced by Cx32 or Cx40 in a “knock-in” experiment, the mice survived past birth but the developmental defect was only somewhat moderated in the case of Cx32 and several additional defects became apparent in both groups of mice (Plum et al., 2000). In another example, knockout of either the lens fiber cell Cx46 or Cx50 leads to cataract formation and reduced lens growth for Cx50. Targeted replacement of Cx50 with Cx46 in a knock-in experiment corrected defects in differentiation and prevented cataract formation but did not restore growth control (White, 2002). In a study designed to determine what molecules pass through gap junction channels, cells expressing Cx43 were shown to exchange ATP 300-fold and ADP and AMP 8-fold better than Cx32-expressing cells (Goldberg, Moreno, & Lampe, 2002). Cells expressing Cx32 exchanged adenosine 12-fold better than those containing Cx43 in a manner not easily explained by size and charge differences of the permeant molecules (Goldberg et al., 2002).
Connexin proteins possess four hydrophobic membrane-spanning domains, two conserved, extracellular domains involved in docking with a connexin in the adjacent membrane, and three cytoplasmic domains corresponding to the amino (N)-terminal region, a loop between transmembrane domains 2 and 3, and the carboxy (C)-terminal tail region (Fig. 1) (Willecke et al., 2002). During intercellular channel formation, six connexin proteins oligomerize into a hemi-channel or connexon, followed by connexon trafficking to the plasma membrane. The intact channel is formed when one hemi-channel docks with a second in an opposing cell (Fig. 2). Once assembled, groups of these intercellular channels (termed gap junctional plaques) allow coordinated function in post-mitotic cells. Formation and degradation of gap junctions is a very dynamic process with reports of half-lives of less than 2 h in cultured cells and tissues (Beardslee, Laing, Beyer, & Saffitz, 1998; Crow, Beyer, Paul, Kobe, & Lau, 1990; Laird, Puranam, & Revel, 1991; Lampe, 1994; Musil, Beyer, & Goodenough, 1990a). Therefore, the regulation of gap junction assembly and turnover is likely to be critical in the control of intercellular communication. Gap junction channels can also be acutely regulated in response to various stimuli, including changes in voltage, pH, and connexin phosphorylation.
Fig. 1.
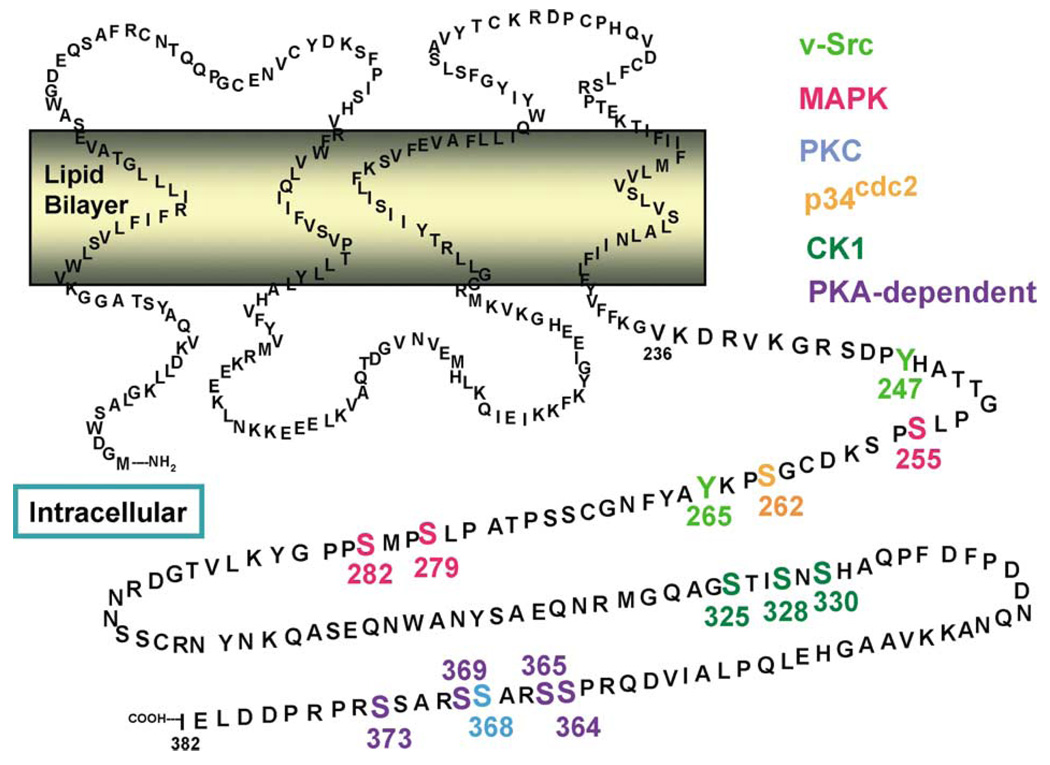
Phosphorylation sites in Cx43. Phosphorylation sites targeted by known kinases are indicated by different colors (Src, lime green; MAPK, red; PKC, blue; p34cdc2, orange; CK1, dark green; PKA-dependent, purple). The primary references for these assignments are listed in Table 1
Fig. 2.
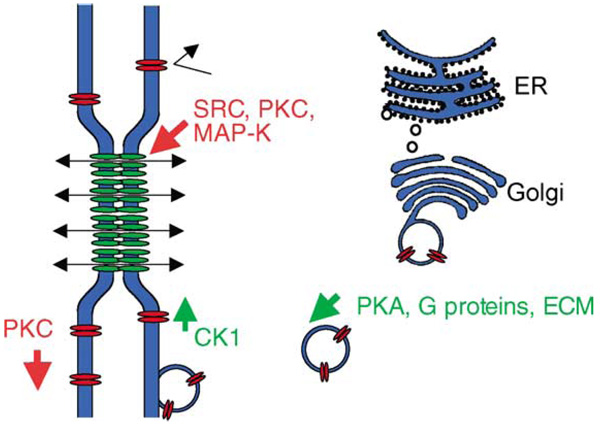
Protein kinases act at different steps in the life cycle of Cx43. Cx43 produced in the endoplasmic reticulum assembles into a hexameric connexon as it is transported through the Golgi and trans-Golgi network (not to scale relative to the plasma membrane). After export and fusion with the plasma membrane, randomly dispersed Cx43 connexons or hemi-channels will accumulate at pre-existing or newly formed gap junctions. Cell communication (indicated by double headed arrows) occurs upon gap junction formation. Presumably hemi-channels are not normally open (indicated by the bent-back arrow). The possible subcellular sites of action of different protein kinases are shown and kinases that lead to increased communication are indicated in green and inhibitors in red. PKA may stimulate gap junctional communication by upregulating connexin trafficking and assembly. Gap junction channel assembly may be promoted by CK1 under basal cell conditions. PKC may inhibit gap junction channels by inhibiting gap junction assembly and channel gating. The effects of Src and MAPK appear to inhibit Cx43 channel gating by effecting channel closure.
This review starts by discussing what is known about phosphorylation of all members of the connexin gene family, but then focuses on Cx43 because it accounts for the majority of the literature related to connexin phosphorylation. Connexin phosphorylation has been previously reviewed (Lampe & Lau, 2000; Saez, Martinez, Branes, & Gonzalez, 1998; Stagg & Fletcher, 1990) and, thus, this review concentrates on the most recent developments. The total number of references has been limited by the journal and, thus, we apologize in advance for the curtailed citations and the use of reviews.
2. General aspects of connexin phosphorylation
Although the completion of the human genome sequence has increased the number of known human connexins from a dozen or so to approximately 20, we have little direct experimental information on many of these newly discovered family members. Many of the connexins not only contain protein kinase “consensus phosphorylation sequences”, but have also been demonstrated to be phosphorylated by kinases in vitro and in some cases in cell culture or tissues. In addition, many connexins (Cx31, Cx32, Cx37, Cx40, Cx43, Cx45, Cx46, Cx50 and Cx56) have been shown to be phosphoproteins by either a shift in their electrophoretic mobility or direct incorporation of 32P (Lampe & Lau, 2000; Saez et al., 1998), and we expect this list to grow as recently discovered family members are investigated. Functional data related to phosphorylation has been reported for Cx32, Cx43, Cx45 and Cx56. The C-terminal region of the connexin proteins appears to be the primary region that becomes phosphorylated. However, Cx56 can be phosphorylated within the cytoplasmic loop region, in addition to its C-terminal domain (Berthoud, Beyer, Kurata, Lau, & Lampe, 1997). Cx43 does not contain serine residues in its intracellular loop region. No reports of phosphorylation of the N-terminal region of connexins have been presented.
Cx26 is the only connexin that has been reported to not be phosphorylated (Traub et al., 1989) which may be due to the fact that it is the shortest connexin and only has a few C-terminal tail amino acids after the fourth transmembrane domain that could interact with cytoplasmic signaling elements. Since Cx26 can form functional channels, connexin phosphorylation clearly is not necessary for the formation of gap junction channels. Analogous to Cx26, truncated Cx43 mutants that lack most of the cytoplasmic, C-terminal portion can nevertheless form functional channels, but they exhibit different permeability and electrophysiological properties than those formed by wild-type Cx43 (Fishman, Moreno, Spray, & Leinwand, 1991).
The importance of the C-terminal domain in the modulation was evident in experiments where independent expression of the C-terminal region (amino acids 246–382) of Cx43 restored the ability of the v-Src tyrosine kinase and cytoplasmic acidification to disrupt the activity of channels formed by a truncated Cx43 mutant in Xenopus oocytes (Calero, Kanemitsu, Taffet, Lau, & Delmar, 1998; Zhou, Kasperek, & Nicholson, 1999).
3. Phosphorylation of Cx32, Cx40, Cx45, Cx46 and Cx50
Cx32 is phosphorylated in vitro by cAMP-dependent protein kinase (PKA) at S233, by protein kinase C (PKC) at S233, and by Ca2+/calmodulin-dependent kinase II and epidermal growth factor receptor at unidentified residues (Diez, Elvira, & Villalobo, 1998; Saez et al., 1986, 1990; Takeda, Saheki, Shimazu, & Takeuchi, 1989). Activation of PKA has been correlated temporally with increased junctional conductance in multiple cell types expressing either Cx32 or Cx40 (Chanson, White, & Garber, 1996; Saez et al., 1986; Van Rijen, Van Veen, Hermans, & Jongsma, 2000). Cx45 has been shown to be phosphorylated in cells incubated with 32P-orthophosphate. Deletion of the last 26 amino acids of Cx45 (nine of which are serines) or substitution of the serines to glycine or alanine led to a 90% reduction in phosphorylation and a dramatic loss of communication in former case (Hertlein, Butterweck, Haubrich, Willecke, & Traub, 1998). Cx45 channels have also been shown to be modulated by phosphorylation (van Veen, van Rijen, & Jongsma, 2000).
The vertebrate ocular lens fiber cell expresses high levels of Cx46 and Cx50 and lens gap junctions have been shown to change communication properties in a manner correlated with changes in connexin phosphorylation. The sheep homologue of human Cx50 can be phosphorylated by casein kinase I, and inhibition of this kinase led to an increase in communication (Cheng & Louis, 2001). The chicken homologue of human Cx50 can be phosphorylated at S363 by casein kinase II, and this phosphorylation event inhibits cleavage of the connexin via a caspase protease (Yin, Gu, & Jiang, 2001). The chicken homologue of Cx46 has been shown to be phosphorylated at S118 and S493 in cells and activation of PKC led to increased phosphorylation at S118 and decreased communication in a manner dependent on PKCγ (Berthoud et al., 1997, 2000).
4. The functional consequences of phosphorylation of Cx43
4.1. The life cycle of Cx43 in homeostatic cells
Gap junctions are dynamic plasma membrane structures with rapid turnover rates. Although there is some controversy as to whether all connexins follow the conventional pathway of synthesis in the endoplasmic reticulum (ER), transport through the Golgi and export to the plasma membrane where they accumulate into gap junction structures (see Fig. 2), essentially all data indicates that Cx43 follows this pathway. However, whether Cx43 oligomerizes into a connexon in the endoplasmic reticulum, Golgi or trans-Golgi network is not as clear. Several reports have shown that Cx43 has a half-life in the range of 1–3 h in cultured cells or in tissues (Beardslee et al., 1998; Crow et al., 1990; Laird et al., 1991; Lampe, 1994; Musil et al., 1990a). A fast turnover rate could imply a high level of post-translational regulation. Indeed, Cx43 is differentially phosphorylated throughout its life cycle in homeostatic cells (Fig. 2) (Laird, Puranam, & Revel, 1993; Musil & Goodenough, 1991; Solan, Fry, TenBroek, & Lampe, 2003). Evidence for the acute regulation of gap junction assembly/disassembly can be found in the myometrium where gap junctions are necessary for the synchronization of electrical and metabolic activities of smooth muscle contractions during delivery (Garfield, Sims, & Daniel, 1977). A 5–10-fold increase in Cx43 mRNA and protein levels was detected immediately prior to and during labor. Cx43 protein accumulates within cytoplasmic membranes until parturition, when it is rapidly transported to the plasma membrane and assembled into gap junctions, followed by a rapid loss of the protein after delivery (e.g. Lye, Nicholson, Mascarenhas, Macenzie, & Petrocelli, 1993).
Cx43 demonstrates multiple electrophoretic isoforms when analyzed by sodium dodecylsulfate– polyacrylamide gel electrophoresis (SDS–PAGE), including a faster migrating, non-phosphorylated (P0 or NP) form, and at least two slower migrating forms, commonly termed P1 and P2. Both P1 and P2 co-migrate with NP following alkaline phosphatase treatment, suggesting that phosphorylation is the primary covalent modification detected in SDS–PAGE analysis (Crow et al., 1990; Musil, Cunningham, Edelman,&Goodenough, 1990b). Phosphoamino acid analysis indicates the majority of the phosphorylation events occur on serines (Lampe, Kurata,Warn-Cramer, & Lau, 1998; Musil et al., 1990b; Warn-Cramer et al., 1996), although tyrosine phosphorylation has also been observed in the presence of activated pp60src (Crow et al., 1990; Swenson, Piwnica-Worms, McNamee, & Paul, 1990). Pulse-chase studies indicate some Cx43 phosphorylation occurs prior to reaching the plasma membrane (Crow et al., 1990; Laird, Castillo, & Kasprzak, 1995). In addition, studies investigating phosphorylation in normal rat kidney cells show that Cx43 acquires resistance to Triton X-100 once it has been phosphorylated to the P2 form and assembled into gap junction plaques (Musil & Goodenough, 1991). Based on several studies, Cx43 undergoes multiple phosphorylation events, as at least five phosphorylated serines have been detected on Cx43 isolated from unstimulated cells (Cooper, Solan, Dolejsi, & Lampe, 2000) and several more in growth factor or kinase activator treated cells (see Fig. 1). These mostly uncharacterized phosphorylation events have been correlated with changes in assembly, acquisition of Triton X-100 insolubility, and degradation of Cx43 gap junction channels, and hence, could play critical roles in regulating gap junctional communication.
4.2. Changes in Cx43 phosphorylation during the cell cycle
Transient changes in gap junctional communication, probably regulated by signaling cascades, have been observed and may be necessary for normal cell cycling. For example, a decrease in gap junctional communication as cells transit from Go to S was correlated with Cx43 phosphorylation changes and was dependent on PKC activity (Koo, Kim, Park, Kang, & Joe, 1997). Phosphorylation of Cx43 increases as cells proceed through the cell cycle (Kanemitsu, Jiang, & Eckhart, 1998). Gap junctional communication was reported to be moderate during G1/S, increased through S, and dramatically decreased in G2/M (Bittman & LoTurco, 1999). The downregulation of junctional communication during G2/M has been correlated with increased, p34cdc2 kinase-dependent phosphorylation of Cx43 (Lampe et al., 1998; Kanemitsu et al., 1998) and redistribution of Cx43 from gap junctions to the cytoplasm (Lampe et al., 1998; Xie, Laird, Chang, & Hu, 1997). Phosphorylation at serines 255 and 262 and other undefined sites (Fig. 1 and Table 1) appears to be increased during mitosis either through direct and indirect activation of p34cdc2 (Lampe et al., 1998; Kanemitsu et al., 1998). Increased Cx43 phosphorylation at S368 (Fig. 1 and Table 1) during S and G2/M phases has also been correlated with decreased gap junction assembly (Solan et al., 2003). Thus, gap junctional communication and Cx43 phosphorylation appear to be linked and highly regulated during the cell cycle.
Table 1.
Cx43 residues phosphorylated by activated protein kinases
| Residue phosphorylateda |
Kinase or signaling pathway responsible |
Reference |
|---|---|---|
| Y247 | pp60src | Lin et al. (2001) |
| S255b | MAP-K and cyclin B/p34cdc2 | Kanemitsu et al. (1998), Lampe et al. (1998), Warn-Cramer et al. (1996) |
| S262b | Cyclin B/p34cdc2 | Kanemitsu et al. (1998); Lampe et al. (1998) |
| Y265 | pp60src | Lin et al. (2001) |
| S279 | MAP-K | Warn-Cramer et al. (1996) |
| S282 | MAP-K | Warn-Cramer et al. (1996) |
| S325 | CK1 | Cooper and Lampe (2002) |
| S328 | CK1 | Cooper and Lampe (2002) |
| S330 | CK1 | Cooper and Lampe (2002) |
| S364 | PKA or PKA-dependent kinases | Shah et al. (2002), TenBroek et al. (2001), Warn-Cramer et al. (1996) |
| S365 | FSH stimulation (PKA?) | Yogo et al. (2002) |
| S368 | PKC | Lampe et al. (2000) |
| S369 | FSH stimulation (PKA?) | Yogo et al. (2002) |
| S373 | FSH stimulation (PKA?) | Yogo et al. (2002) |
See also Fig. 1.
Phosphorylation induced by p34cdc2 may be direct or indirect.
4.3. Phosphorylation of Cx43 by tyrosine protein kinases
4.3.1. Non-receptor protein tyrosine kinases
The phosphorylation of Cx43 by the v-Src tyrosine protein kinase represents a well investigated example of the regulation of gap junctional communication by a protein kinase. In earlier work, several laboratories showed that activated non-receptor tyrosine kinases, such as pp60v-Src and p130gag-fps, phosphorylated Cx43 on tyrosine residues, which was generally correlated with a marked disruption of intercellular junctional communication (Crow et al., 1990; Filson, Azarnia, Beyer, Loewenstein, & Brugge, 1990; Kurata & Lau, 1994; Swenson et al., 1990). Pp60v-Src appeared to phosphorylate Cx43 directly in vitro and in vivo (Loo, Berestecky, Kanemitsu, & Lau, 1995). Consistent with this demonstrated catalytic activity, Cx43 not only co-localized with the v-Src tyrosine kinase to plasma membrane regions of the cell (Loo, Kanemitsu, & Lau, 1999), but also appeared to interact directly with v-Src (Kanemitsu, Loo, Simon, Lau, & Eckhart, 1997). Tyr265 in Cx43 was identified as a critical site that appeared to be targeted by the v-Src kinase (Fig. 1 and Table 1). An Y265F site-directed mutant of Cx43 produced functional gap junction channels in Xenopus oocytes that were no longer disrupted by the presence of active pp60src kinase (Swenson et al., 1990). The interaction was dependent upon the SH3 and SH2 domains of v-Src and a proline-rich region (P274–P284) and phosphorylated Y265 in Cx43 (Kanemitsu et al., 1997). Indeed, a Y265F Cx43 mutant not only failed to interact with v-Src, but was no longer phosphorylated by the v-Src kinase (Fig. 1 and Table 1) (Kanemitsu et al., 1997). The corresponding Y247F mutant was still able to bind v-Src, indicating that this site was not likely involved in the interaction.
More recent studies not only confirmed Y265 as a site phosphorylated by pp60v-Src, but also identified Y247 as a second v-Src site in Cx43 (Lin, Warn-Cramer, Kurata, & Lau, 2001). Interestingly, phosphorylation of the Y265 site alone was insufficient to induce channel closure, raising the possibility that Cx43 phosphorylation occurs in a processive fashion with phosphorylation of Y265 occurring first followed by phosphorylation at Y247 and channel closure. The MEK inhibitor, PD98059, did not block the ability of v-Src to induce channel closure, suggesting that the activation of MAP kinase and possible serine phosphorylation of Cx43 were not involved in v-Src’s actions. An activated mutant of c-Src has also been reported to interact with, and phosphorylate, Cx43 on Y265 (Giepmans, Hengeveld, Postma, & Moolenaar, 2001; Toyofuku et al., 2001). These actions were associated with the disruption of gap junctional communication as described below. The critical importance of phosphorylation of the tyrosine sites in Cx43 by v-Src was underscored by a recent report measuring electrical coupling (Cottrell, Lin, Warn-Cramer, Lau, & Burt, 2003). Moreover, the actions of v-Src on Cx43 most likely provoked channel closure by decreasing channel open probability, rather than diminishing channel unitary conductance, which is characteristic of actions of PKC on Cx43 (Kwak, Van Veen, Analbers, & Jongsma, 1995; Lampe et al., 2000). This study also detected possible differences in the selectivity profiles of phosphotyrosine-containing Cx43 channels induced by v-Src.
Taken together, these data on the phosphotyrosine sites of Cx43 targeted by v-Src and the domains necessary for their molecular association suggested a possible model for the interaction and the phosphorylation events leading to the disruption of gap junctional communication (Kanemitsu et al., 1997; Lin et al., 2001). This model hypothesizes that the SH3 domain of v-Src may initially bind to a proline-rich region in Cx43 (P274–P284) bringing the molecules in close physical proximity that is necessary for phosphorylation of Y265 in Cx43 by the Src kinase domain. Binding between the two partners may be stabilized further by a SH2 domain-phospho-Y265 (P-Y265) interaction. Phosphorylation of Y247 and the observed disruption of gap junctional communication may then ensue. These data offered the impression that the phosphotyrosine sites may have distinct roles: P-Y265 may be important in the interaction between Cx43 and v-Src, whereas P-Y247 may provoke the closure of Cx43 gap junction channels. These data and the working model raise numerous intriguing questions, one of which is the precise manner by which the P-Y265 and P-Y247 sites cooperate to close gap junctions.
Work from Bruce Nicholson’s laboratory challenges this model for the direct regulation of Cx43 channel activity by v-Src (Zhou et al., 1999). In this work, junctional conductance established by similar Y265F or Y247F site-directed Cx43 mutants in paired Xenopus oocytes, was disrupted by v-Src expressed from injected RNA (Zhou et al., 1999). Moreover, the MEK inhibitor, PD98059, blocked the ability of v-Src to disrupt gap junctional communication in fibroblasts expressing temperature-sensitive v-Src, suggesting that phosphorylation of Cx43 on serine, not tyrosine, by an enzyme, such as MAP kinase, may be involved in v-Src’s action (Zhou et al., 1999). At the present time, there is no simple resolution of these experimental differences, however, they may relate to the acute presence of kinase-active Src produced either by transcription of Src RNA microinjected into oocytes or activation of Src by temperature shift in cells containing temperature-sensitive Src (Zhou et al., 1999) versus the chronic presence of non-conditionally active v-Src in mouse fibroblasts (Lin et al., 2001).
This work also examined the ability of the C-terminal tail of Cx43, introduced separately into oocytes, to permit the closure of mutant Cx43 (truncated at D245) channels by v-Src (Zhou et al., 1999). These critical observations supported the concept that Cx43 channel closure induced by v-Src phosphorylation may be mediated through a ball and chain mechanism, as proposed for pH- and insulin-induced channel gating (Homma et al., 1998; Morley, Taffet, & Delmar, 1996). The specific details underlying the mechanisms for either pH- or phosphorylation-induced channel closure have yet to be illuminated satisfactorily.
The c-Src tyrosine kinase has been implicated in the ability of G protein-coupled receptors to induce the disruption of Cx43-mediated gap junctional communication (Postma et al., 1998). In this work, downstream signaling initiated by G protein-coupled receptors activated by lysophosphatidic acid and thrombin were independent of Ca2+ mobilization and the activation of PKC, MAP kinase, Rho, or Ras. Instead, activated G protein-coupled receptors appeared to rely upon the activity of c-Src to effect the observed changes in channel activity. The actions of wild-type c-Src in this experimental system may be indirect because phosphorylation of Cx43 on tyrosine could not be demonstrated. In related studies, an activated c-Src kinase mutant (c-SrcY527A) was capable of phosphorylating the Y265 site in the C-terminal tail of Cx43 in vitro and in Rat-1 fibroblasts (Giepmans et al., 2001). A Y265F Cx43 mutant was not phosphorylated by, and no longer interacted with the activated c-Src mutant. As proposed in the model described previously, phosphorylation of Y265 was envisioned to stabilize the interaction between Cx43 and c-Src. In related, but independent studies, a similar activated c-Src mutant was reported to inhibit the endogenous interaction between Cx43 and the tight junction-associated protein, ZO-1, in rat neonatal cardiac myocytes (Toyofuku et al., 2001). The disruption of ZO-1 binding to Cx43 appeared to depend upon the competitive binding of activated c-Src through its SH2 domain. Interestingly, these changes in phosphorylation and binding activities correlated with a downregulation of total cell and plasma membrane-bound Cx43 and an inhibition of gap junction conductance.
Lipopolysaccharide (LPS) treatment of primary microvascular endothelial cells isolated from rat skeletal muscle stimulated a protein tyrosine kinase-dependent reduction in junctional coupling (Lidington, Tyml, & Ouellette, 2002). This functional alteration was accompanied by the phosphorylation of Cx43 on tyrosine, as demonstrated by immunoblotting with Ptyr antibody and direct phosphoamino acid analysis, with little change in the content of phosphoserine (Lidington et al., 2002). Although the tyrosine protein kinase involved in these effects of LPS was not definitively identified, data derived from the use of chemical inhibitors suggested the actions of a Src family member.
4.3.2. Receptor protein tyrosine kinases
In many cases, activation of the EGF and PDGF receptor tyrosine kinases by ligand binding resulted in the rapid and transient disruption of gap junctional communication and a marked increase in the phosphorylation of Cx43, not on tyrosine, but serine amino acids (Hossain, Ao, & Boynton, 1998a; Kanemitsu & Lau, 1993; Lau, Kanemitsu, Kurata, Danesh, & Boynton, 1992; Rivedal & Opsahl, 2001). The disruption of gap junctional communication appeared to occur independently of observable changes in gap junction plaques (Lau et al., 1992). In the case of the EGF receptor, the disruption of Cx43 function was independent of PKC activity, but dependent upon the activation of the downstream mitogen-activated protein (MAP) kinase (Kanemitsu & Lau, 1993). MAP kinase appeared to phosphorylate Cx43 directly in EGF-treated cells (Kanemitsu & Lau, 1993). S255, S279, and S282 were targeted by the activated MAP kinase in in vitro kinase reactions and in vivo (Fig. 1 and Table 1) (Warn-Cramer et al., 1996, 1998). A triple phosphorylation site Cx43 mutant (S255A, S279A, S282A), introduced into cells lacking wild-type Cx43, retained the ability to establish functional gap junctions, but, interestingly, was resistant to the disruptive effects of MAP kinase activated by the EGF receptor (Warn-Cramer et al., 1998). In a distinctly different experimental approach, Cx43 channels reconstituted into lipid vesicles were phosphorylated by purified MAP kinase, which resulted in the reduction in the permeability of reconstituted liposomes (Kim, Kam, Koo, & Joe, 1999). These data confirmed Cx43 and, particularly these three serine sites, as targets of EGF-activated MAP kinase in vivo and strongly supported the concept that phosphorylation of these sites was sufficient for the ability of the activated EGF receptor to disrupt gap junctional communication. The ability of the activated EGF receptor to induce gap junction channel closure through phosphorylation of Cx43 at these serine sites has been attributed to the possible reduction in P0 open channel probability (Cottrell et al., 2003).
Vascular endothelial growth factor (VEGF) binds to and activates the VEGF receptor 2 (Flk-1 or KDR) tyrosine kinase. In a manner reminiscent of EGF stimulation of epithelial cells (Kanemitsu & Lau, 1993; Lau et al., 1992), VEGF-A treatment of endothelial cells resulted in a transient reduction in gap junctional communication, which correlated with the phosphorylation of Cx43, probably on serine or threonine residues (Suarez & Ballmer-Hofer, 2001). Alterations in localization of Cx43 at the plasma membrane were not noted in this study. The effects on Cx43 phosphorylation and channel activity were dependent upon the activation of both the c-Src tyrosine kinase and MAP kinase.
Growth factors may also modulate gap junctional communication through mechanisms independent of phosphorylation of Cx43. Treatment of a kidney epithelial cell line with EGF for 2–3 h was reported to stimulate the upregulation of gap junctional communication, which may be related to the synthesis or transport of Cx43 (Vikhamar, Rivedal, Mollerup, & Sanner, 1998). EGF was also found recently to increase the expression of Cx43 protein in porcine granulosa cells by two to five-fold (Bolamba, Floyd, McGlone, & Lee, 2002). This change occurred in the absence of changes in Cx43 phosphorylation and may be involved in the regulation of early folliculogenesis.
Disruption of gap junctional communication induced by the activated PDGF receptor appears to be more complex in terms of possible downstream effectors because both PKC and MAPK were reported to be necessary for the disruption of Cx43 activity (Hossain, Ao, & Boynton, 1998b). In addition, recent reports suggested that the PDGF-induced disruption of Cx43 function did not rely solely on phosphorylation of Cx43 or activation of MAP kinase, but may require additional signaling pathways (Hossain, Jagdale, Ao, & Boynton, 1999a,b). The site(s) of serine phosphorylation induced by the activated PDGF receptor and other potential regulatory element(s) have not been identified. To contribute to the complexity of this experimental system, PDGF induced a rapid, transient disruption of gap junctional communication in mesangial cells of the kidney glomerulus, which correlated with the activation of ERK1/2 and phosphorylation of Cx43 on tyrosine (Yao, Morioka, & Oite, 2000). PI3-kinase may be involved in the signaling pathways because wortmanin and LY294002 blocked both the elevated tyrosine phosphorylation of Cx43 and the activation of ERK. The tyrosine kinase responsible for the PDGF-induced accumulation of phosphotyrosine in Cx43 in these cells was not demonstrated. Additional studies aimed at clarifying the molecular mechanism of action of the activated PDGF receptor on Cx43 function would be welcomed.
4.3.3. MAP kinase phosphorylation of Cx43
The phosphorylation of Cx43 by MAP kinase-activated downstream of receptor tyrosine kinases and v-Src has been discussed in the preceding paragraphs. In addition, several recent reports have described the abilities of diverse stimuli to activate MAP kinase and stimulate the phosphorylation of Cx43. Treatment of WB-F344 rat liver epithelial cells with 12-O-tetradeconylphorbol-13-acetate (TPA) activated Erk1/2, induced Cx43 phosphorylation, and blocked dye coupling (Ruch, Trosko, & Madhukar, 2001). The depressive effect of TPA on dye coupling correlated with the apparent loss of gap junction plaques at the plasma membrane, rather than an effect on gating alone. Similar results were obtained by TPA treatment of the IAR6.1 rat liver epithelial cell line (Rivedal & Opsahl, 2001). The TPA-induced loss of gap junctional communication in this system correlated with phosphorylation of Cx43. However, the effects on Cx43 internalization from plasma membrane regions were not reported. Finally, Vitamin K3 (menadione) was reported to decrease gap junctional intercellular communication in WB-F344 rat liver epithelial cells through a mechanism involving the activation of the EGF receptor, MAP kinase and phosphorylation of Cx43 (Klotz et al., 2002). These effects of Vitamin K3 occurred in the absence of any detectable changes in the distribution of Cx43 to the plasma membrane.
4.4. Phosphorylation of Cx43 in response to increased cAMP levels
Agents that elevate intracellular cAMP have been shown to increase Cx43 phosphorylation and Cx43-mediated communication (Darrow, Laing, Lampe, Saffitz, & Beyer, 1995), the size and number of GJs (Atkinson et al., 1995), and the assembly of new GJs (Paulson et al., 2000). Such enhancement has been shown to result from increased synthesis (Mehta, Yamamoto, & Rose, 1992) and/or the increased trafficking of connexons to the plasma membrane (Burghardt, Barhoumi, Sewall, & Bowen, 1995; Holm, Mikhailov, Jillson, & Rose, 1999; Paulson et al., 2000). Cyclic AMP-enhanced GJ assembly appears to mediated by PKA (Paulson et al., 2000), and microinjected PKA catalytic subunit can have rapid effects on GJ communication (Britz-Cunningham, Shah, Zuppan, & Fletcher, 1995; Godwin et al., 1993). S364 is a major phosphorylation site in cellular Cx43 and phosphorylation of this site appears to be critical for cAMP-enhanced GJ assembly (TenBroek, Lampe, Solan, Reynhout, & Johnson, 2001). However, Cx43 is a relatively poor substrate for PKA compared to PKC or MAPK (Shah, Martinez, & Fletcher, 2002; TenBroek et al., 2001; Warn-Cramer et al., 1996). Whether PKA activates another kinase that actually phosphorylates Cx43 at S364 or PKA inefficiently directly phosphorylates this site implying some physiologic advantage to Cx43 being a poor substrate (Shah et al., 2002) is unknown. There is significant biological interest associated with S364. S364 resides in the first of a tandem RXSSR repeat found from R362–R374 (Fig. 1). A serine to proline conversion at S364 (S364P) was first identified as a mutation in a subset of patients with visceral atrial heterotaxia, and transfected cells expressing the S364P mutant Cx43 displayed altered gap junctional properties in response to PKC or PKA activity (Britz-Cunningham et al., 1995). In spite of the fact that Cx43 mutations have not been identified in other populations of visceral atrial heterotaxia patients (Debrus et al., 1997), the S364P mutation significantly affects left–right patterning in the early Xenopus embryo when misexpressed dorsally, resulting in a significant increase in heterotaxia (Levin & Mercola, 1998). The S364P mutation also alters the pH sensitivity of Cx43 gating in the Xenopus oocyte expression system (Ek-Vitorin et al., 1996).
4.5. Protein kinase C (PKC)
In the sustained absence of connexin expression, tumorigenesis is enhanced (Laird et al., 1999; Moennikes, Buchmann, Ott, Willecke, & Schwarz, 1999). The correlation between neoplastic transformation and reduced gap junctional communication has led to the hypothesis that reduced cell–cell communication is a critical step in multistage carcinogenesis. PKC has received considerable attention because PKC activators, which promote tumorigenesis, both increase Cx43 phosphorylation and decrease gap junction communication in a number of different cell types (Berthoud, Ledbetter, Hertzberg, & Saez, 1992; Brissette, Kumar, Gilula, & Dotto, 1991; Lampe, 1994; Reynhout, Lampe, & Johnson, 1992). In addition, TPA treatment has been shown to dramatically decrease gap junction assembly (Lampe, 1994), potentially causing a gradual decrease in communication over time followed by resumption of normal communication after PKC downregulation occurs. However, in neonatal rat cardiomyocytes, activation of PKC has been reported to cause an increase in junctional conductance (Kwak et al., 1995) or no change (Spray & Burt, 1990). The reasons for these differences within and particularly between cell types are not clear. It has been suggested that the variable response to TPA stems from differences: (i) inherent to cell lines vs. differentiated cells (Chanson, Bruzzone, Spray, Regazzi, & Meda, 1988; Munster & Weingart, 1993); (ii) in the connexin’s phosphorylation state prior to TPA exposure (Saez et al., 1997); (iii) in the PKC isotypes expressed by the cells (Munster & Weingart, 1993); or (iv) in experimental conditions (Kwak et al., 1995; Munster & Weingart, 1993). Nevertheless, it is interesting to note that even in an experiment where junctional conductance increased, a lower conductance state of the channel (~50 rather than ~100 pS conductance) was still favored and dye coupling was still reduced following TPA treatment (Kwak et al., 1995). Those data suggest that in addition to regulating the channel’s conductance state (and consequently permeability), PKC activation can lead to changes in other parameters that affect gap junctional communication.
Given that several hundred reports have shown that treatment of cells with tumor promoters can lead to decreases in gap junctional communication and changes in Cx43 mobility in SDS–PAGE, we focus here only on recent reports specifying specific residues that could be phosphorylated by PKC and specific PKC isozymes that might be involved in this process. PKC has been shown to phosphorylate Cx43 at S368 (Lampe et al., 2000; Saez et al., 1997; Shah et al., 2002) and S372 (Saez et al., 1997) in vitro. S368 has been shown to underlie the TPA-induced reduction in intercellular communication and alteration of single channel behavior (Lampe et al., 2000). However, other potential PKC sites might be involved in regulating assembly or degradation of gap junctions and channel behavior.
The introduction of several inhibitors and activators of PKC that are specific for certain isotypes has led to investigations of the roles that these PKC isotypes may play in the downregulation of communication. For example, overexpression of PKCγ or TPA treatment of lens epithelial cells caused a reduction in cell surface Cx43 and allowed co-immunoprecipiation of PKCγ and Cx43 (Wagner, Saleh, Boyle, & Takemoto, 2002). Inhibition of gap junctional communication was found to be dependent on PKCα, β and δ to different extents in various fibroblast systems (Cruciani, Sanner, & Mikalsen, 2001). PKCα and ε were found to associate with Cx43 in cardiomycytes (Bowling et al., 2001). Fibroblast growth factor-2, which decreases cardiomyocyte gap junctional permeability and increases Cx43 phosphorylation, increased colocalization of PKCε with Cx43 (Doble, Ping, & Kardami, 2000). Thus, it appears that several members of the conventional and novel PKC families influence gap junctional communication. One confounding factor is that different cells express distinct isotypes and respond differently to these agents, thus generalizing about the importance of specific isotypes in regulating gap junctional communication may be somewhat premature. Understanding the actual sites of phosphorylation and the consequences of these events will probably be necessary before we fully understand the importance of different PKC isozymes in regulating gap junctional communication.
4.6. Casein kinase 1 (CK1) and other kinases
CK1, particularly the δ isoform, has been shown to interact with and phosphorylate Cx43 on serine(s) 325, 328 or 330 in vitro (Fig. 1 and Table 1) (Cooper & Lampe, 2002). Cx43 may also be a direct substrate for CK1 in vivo as these residues are major phosphorylation sites of cellular Cx43 (Cooper & Lampe, 2002). Immunofluorescence and cell surface biotinylation experiments in the presence of a CK1 inhibitor showed increases in Cx43 plasma membrane localization, but reduced gap junction formation implying these sites are important in gap junction assembly.
Certainly other kinases phosphorylate Cx43. Several residues in the tandem serine repeat region can be phosphorylated (i.e. serines 365, 368, 369 and 373) in response to follicle-stimulating hormone (FSH), probably at least partially through a PKA mechanism (Fig. 1 and Table 1) (Yogo, Ogawa, Akiyama, Ishida, & Takeya, 2002). As shown in Table 1, there is experimental evidence that Cx43 can be phosphorylated on at least 12 of the 21 serines and two of the tyrosines of the cytoplasmic tail region (amino acids 250–382). Certainly, it is possible that multiple kinases may phosphorylate the same residue as noted in Table 1 for serine 255. Thus, considerable evidence indicates that Cx43 is a highly phosphorylated and a highly regulated protein. The challenge is to link these specific phosphoregulatory events with the different steps in the Cx43 lifecycle.
4.7. Phosphatases
Given the large number of phosphorylation sites on Cx43, a distinct role for phosphoprotein phosphatases (PP) can be logically envisioned. In fact, phosphatase inhibitors such as okadaic acid, an inhibitor of PP1 and PP2B, have been shown to have significant effects when cells are recovering from growth factor or other treatments that affect Cx43 phosphorylation. In astrocytes subjected to hypoxia, inhibitors of PP2B reduced hypoxia-induced Cx43 dephosphorylation and junctional uncoupling (Wei & Nagy, 2000). Okadaic acid retarded the resumption of intercellular communication and the associated dephosphorylation of Cx43 normally observed after EGF treatment of rat liver epithelial cells (Lau et al., 1992). Dephosphorylation of Cx43 induced by the junctional inhibitor 18-β-glycyrrhetinic acid was inhibited by okadaic acid (Guan, Wilson, Schlender, & Ruch, 1996). PP1 inhibitors partially preserved channel activity in voltage-clamped cardiomyocyte pairs in the absence of ATP (Duthe, Plaisance, Sarrouilhe, & Herve, 2001). PP2B was implicated in the preservation of phosphorylated Cx43 induced by TPA, but had less activity than PP2A on Cx43 immunoprecipitated from TPA treated cells indicating it may have an indirect effect (Cruciani, Kaalhus, & Mikalsen, 1999). Finally, in an interesting in vivo model, transgenic mice with cardiac-specific expression of an active form of calcineurin (PP2B) showed reduced Cx43 expression and phosphorylation, which correlated with a reduction in redistribution of Cx43 from the intercalated disc region to the long axis of the plasma membrane (Chu et al., 2002). These data implied a role for PP2B-sensitive phosphorylation sites in Cx43 localization and stability. However, in many untreated cell types, okadaic acid had little effect on either Cx43 mobility in SDS–PAGE or gap junctional communication (Berthoud et al., 1992; Guan et al., 1996; Husoy, Mikalsen, & Sanner, 1993; Lau et al., 1992; Saez, Nairn, Czernik, Spray, & Hertzberg, 1993). Certainly different phosphatases could play multiple roles in the regulation of gap junctional communication, but detailed investigation of these roles will depend on a better understanding of how phosphorylation at specific sites regulates the function or behavior of Cx43.
4.8. New methodologies
Two relatively recent tools hold considerable promise to assist in the discovery of the biological roles of specific connexin phosphorylation events. First, the application of the power of tandem mass spectrometry to peptide sequencing and identification of sites of protein modification has greatly assisted the discovery of mechanisms of protein regulation in general. This powerful approach has been utilized to discover multiple sites of Cx43 phosphorylation targeted by CK1 (Cooper & Lampe, 2002) and PKA (TenBroek et al., 2001). Second, the investigation of protein phosphorylation events in a number of signaling pathways has also been greatly facilitated by the development of antibodies that only recognize proteins phosphorylated at specific sites. They can elucidate the temporal changes in phosphorylation during dynamic events such progression through the cell cycle or phosphorylation/dephosphorylation cycles. Phosphorylation site-specific antibodies also hold great promise because they can potentially detect phosphorylation events in situ via immunolabeling of tissue. An antibody specific for phosphorylated S368 of Cx43 was used to show that phosphorylation increases at this site as cells progress through the cell cycle (Solan et al., 2003). The phosphospecific S368 Cx43 antibody illustrated that in some cell types, TPA treatment did not lead to any shift in Cx43 mobility but phosphorylation of S368 increased by eight-fold confirming that a shift in SDS–PAGE mobility is only a crude measure of phosphorylation (Solan et al., 2003). This could help to explain why TPA treatment of some cell types showed no mobility shift of Cx43 by SDS–PAGE despite changes in gap junctional communication (e.g. Rivedal & Opsahl, 2001).
Acknowledgements
The work in the authors’ laboratories was supported in part by grants from the National Institutes Health (GM55632 to P.D.L. and CA52098 to A.F.L.) and the American Heart Association, Hawaii Affiliate.
Abbreviations
- Cx
connexin
- PKA
cAMP-dependent protein kinase
- MAPK
mitogen-activated protein kinase
- PKC
protein kinase C
- CK1
casein kinase 1
- PP
protein phosphatase
- VEGF
vascular endothelial growth factor
- TPA
12-O-tetradeconylphorbol-13-acetate
- FSH
follicle-stimulating hormone
- NP
non-phosphorylated
- SDS–PAGE
sodium dodecylsulfate–polyacrylamide gel electrophoresis
- LPS
lipopolysaccharide
References
- Atkinson MM, Lampe PD, Lin HH, Kollander R, Li X-R, Kiang DT. Cyclic AMP modifies the cellular distribution of connexin 43 and induces a persistent increase in the junctional permeability of mouse mammary tumor cells. Journal of Cell Science. 1995;108:3079–3090. doi: 10.1242/jcs.108.9.3079. [DOI] [PubMed] [Google Scholar]
- Beardslee M, Laing J, Beyer E, Saffitz J. Rapid turnover of connexin43 in the adult rat heart. Circulation Research. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- Bergoffen J, Scherer SS, Wang S, Oronzi Scott M, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fishbeck KH. Connexin mutations in X-linked Charcot-Marie-tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Ledbetter MLS, Hertzberg EL, Saez JC. Connexin43 in MDCK cells: Regulation by a tumor-promoting phorbol ester and calcium. European Journal of Cell Biology. 1992;57:40–50. [PubMed] [Google Scholar]
- Berthoud VM, Beyer EC, Kurata WE, Lau AF, Lampe PD. The gap junction protein connexin 56 is phosphorylated in the intracellular loop and the carboxyterminal region. European Journal of Biochemistry. 1997;244:89–97. doi: 10.1111/j.1432-1033.1997.00089.x. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Westphale EM, Grigoryeva A, Beyer EC. PKC isoenzymes in the chicken lens and TPA-induced effects on intercellular communication. Investigative Ophthalmology & Visual Science. 2000;41:850–858. [PubMed] [Google Scholar]
- Bittman KS, LoTurco JJ. Differential regulation of connexin 26 and 43 in murine neocortical precursors. Cerebral Cortex. 1999;9:188–195. doi: 10.1093/cercor/9.2.188. [DOI] [PubMed] [Google Scholar]
- Bolamba D, Floyd AA, McGlone JJ, Lee VH. Epidermal growth factor enhances expression of connexin 43 protein in cultured procine preantral follicles. Biological Reproduction. 2002;67:154–160. doi: 10.1095/biolreprod67.1.154. [DOI] [PubMed] [Google Scholar]
- Bowling N, Huang X, Sandusky GE, Fouts RL, Mintze K, Esterman M, Allen PD, Maddi R, McCall E, Vlahos CJ. Protein kinase C-alpha and -epsilon modulate connexin-43 phosphorylation in human heart. Journal of Molecular and Cellular Cardiology. 2001;33:789–798. doi: 10.1006/jmcc.2000.1349. [DOI] [PubMed] [Google Scholar]
- Brissette JL, Kumar NM, Gilula NB, Dotto GP. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Molecular and Cellular Biology. 1991;11:5364–5371. doi: 10.1128/mcb.11.10.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz-Cunningham SH, Shah MM, Zuppan CW, Fletcher WH. Mutations of the connexin43 gap junction gene in patients with heart malformations and defects of laterality. New England Journal of Medicine. 1995;332:1323–1329. doi: 10.1056/NEJM199505183322002. [DOI] [PubMed] [Google Scholar]
- Burghardt RC, Barhoumi R, Sewall TC, Bowen JA. Cyclic AMP induces rapid increases in gap junction permeability and changes in the cellular distribution of connexin43. Journal of Membrane Biology. 1995;148:243–253. doi: 10.1007/BF00235042. [DOI] [PubMed] [Google Scholar]
- Calero G, Kanemitsu M, Taffet SM, Lau AF, Delmar M. A 17mer peptide interferes with acidification-induced uncoupling of connexin43. Circulation Research. 1998;82:929–935. doi: 10.1161/01.res.82.9.929. [DOI] [PubMed] [Google Scholar]
- Chanson M, Bruzzone R, Spray DC, Regazzi R, Meda P. Cell uncoupling and protein kinase C: Correlation in a cell line but not in a differentiated tissue. American Journal of Physiology. 1988;255:C699–C704. doi: 10.1152/ajpcell.1988.255.5.C699. [DOI] [PubMed] [Google Scholar]
- Chanson M, White MM, Garber SS. cAMP promotes gap junctional coupling in T84 cells. American Journal of Physiology. 1996;271:C533–C539. doi: 10.1152/ajpcell.1996.271.2.C533. [DOI] [PubMed] [Google Scholar]
- Cheng H-L, Louis CF. Functional effects of casein kinase I-catalyzed phosphorylation on lens cell-to-cell coupling. Journal of Membrane Biology. 2001;181:21–30. doi: 10.1007/s0023200100055. [DOI] [PubMed] [Google Scholar]
- Chu G, Carr AN, Young KB, Lester JW, Yatani A, Sanbe A, Colbert MC, Schwartz SM, Frank KF, Lampe PD, Robbins J, Molkentin JD, Kranias EG. Enhanced myocyte contractility and Ca2+ handling in a calcineurin transgenic model of heart failure. Cardiovascular Research. 2002;54:105–116. doi: 10.1016/s0008-6363(02)00230-4. [DOI] [PubMed] [Google Scholar]
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. Journal of Biological Chemistry. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- Cooper CD, Solan JL, Dolejsi KK, Lampe PD. Analysis of connexin phosphorylation sites. Methods. 2000;20:196–204. doi: 10.1006/meth.1999.0937. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM. Mechanism of v-Src and mitogen-activated protein kinase-induced reduction of gap junction communication. American Journal of Physiology. 2003;284:C511–C520. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Molecular and Cellular Biology. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani V, Kaalhus O, Mikalsen SO. Phosphatases involved in modulation of gap junctional intercellular communication and dephosphorylation of connexin43 in hamster fibroblasts: 2B or not 2B? Experimental Cell Research. 1999;252:449–463. doi: 10.1006/excr.1999.4650. [DOI] [PubMed] [Google Scholar]
- Cruciani V, Sanner T, Mikalsen SO. Pharmacological evidence for system-dependent involvement of protein kinase C isoenzymes in phorbol ester-suppressed gap junctional communication. Carcinogenesis. 2001;22:221–231. doi: 10.1006/excr.2001.5275. [DOI] [PubMed] [Google Scholar]
- Darrow BJ, Laing JG, Lampe PD, Saffitz JE, Beyer EC. Expression of multiple connexins in cultured neonatal rat ventricular myocytes. Circulation Research. 1995;76:381–387. doi: 10.1161/01.res.76.3.381. [DOI] [PubMed] [Google Scholar]
- Debrus S, Tuffery S, Matsuoka R, Galal O, Sarda P, Sauer U, Bozio A, Tanman B, Toutain A, Claustres M, Le Paslier D, Bouvagnet P. Lack of evidence for connexin 43 gene mutations in human autosomal recessive lateralization defects. Journal of Molecular and Cellular Cardiology. 1997;29:1423–1431. doi: 10.1006/jmcc.1997.0380. [DOI] [PubMed] [Google Scholar]
- Diez JA, Elvira M, Villalobo A. The epidermal growth factor receptor tyrosine kinase phosphorylates connexin32. Molecular and Cellular Biochemistry. 1998;187:201–210. doi: 10.1023/a:1006884600724. [DOI] [PubMed] [Google Scholar]
- Doble BW, Ping P, Kardami E. The epsilon subtype of protein kinase C is required for cardiomyocyte connexin-43 phosphorylation. Circulation Research. 2000;86:293–301. doi: 10.1161/01.res.86.3.293. [DOI] [PubMed] [Google Scholar]
- Duthe F, Plaisance I, Sarrouilhe D, Herve JC. Endogenous protein phosphatase 1 runs down gap junctional communication of rat ventricular myocytes. American Journal of Physiology. 2001;281:C1648–C1656. doi: 10.1152/ajpcell.2001.281.5.C1648. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorin JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M. pH regulation of connexin43: Molecular analysis of the gating particle. Biophysical Journal. 1996;71:1273–1284. doi: 10.1016/S0006-3495(96)79328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filson AJ, Azarnia R, Beyer EC, Loewenstein WR, Brugge JS. Tyrosine phosphorylation of a gap junction protein correlates with inhibition of cell-to-cell communication. Cell Growth & Differentation. 1990;1:661–668. [PubMed] [Google Scholar]
- Fishman GI, Moreno AP, Spray DC, Leinwand LA. Functional analysis of human cardiac gap junction channel mutants. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3525–3529. doi: 10.1073/pnas.88.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel HD, Jung D, Butzler C, Temme A, Traub O, Winterhager E, Willecke K. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. Journal of Cell Biology. 1998;140:1453–1461. doi: 10.1083/jcb.140.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield RE, Sims S, Daniel EE. Gap junctions: Their presence and necessity in myometrium during parturition. Science. 1977;198:958–960. doi: 10.1126/science.929182. [DOI] [PubMed] [Google Scholar]
- Giepmans NG, Hengeveld T, Postma FR, Moolenaar WH. Interaction of c-Src with gap junction protein connexin-43. Journal of Biological Chemistry. 2001;276:8544–8549. doi: 10.1074/jbc.M005847200. [DOI] [PubMed] [Google Scholar]
- Godwin AJ, Green LM, Walsh MP, McDonald JR, Walsh DA, Fletcher WJ. In situ regulation of cell-cell communication by the cAMP dependent protein kinase and protein kinase C. Molecular and Cellular Biochemistry. 1993;127–128:293–307. doi: 10.1007/BF01076779. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. Heterotypic and homotypic gap junction channels mediate the selective transfer of endogenous molecules between cells. Journal of Biological Chemistry. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annual Review of Biochemistry. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Guan X, Wilson S, Schlender KK, Ruch RJ. Gap junction disassembly and connexin 43 dephosphorylation induced by 18b-glycyrrhetinic acid. Molecular Carcinogenesis. 1996;16:157–164. doi: 10.1002/(SICI)1098-2744(199607)16:3<157::AID-MC6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hertlein B, Butterweck A, Haubrich S, Willecke K, Traub O. Phosphorylated carboxy terminal serine residues stabilize the mouse gap junction protein connexin45 against degradation. Journal of Membrane Biology. 1998;162:247–257. doi: 10.1007/s002329900362. [DOI] [PubMed] [Google Scholar]
- Holm I, Mikhailov A, Jillson T, Rose B. Dynamics of gap junctions observed in living cells with connexin43-GFP chimeric protein. European Journal of Cell Biology. 1999;78:856–866. doi: 10.1016/S0171-9335(99)80087-9. [DOI] [PubMed] [Google Scholar]
- Homma N, Alvarado JL, Coombs W, Stergiopoulos K, Taffet SM, Lau AF, Delmar M. A particle-receptor model for the insulin-induced closure of connexin43 channels. Circulation Research. 1998;83:27–32. doi: 10.1161/01.res.83.1.27. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Ao P, Boynton AL. Rapid disruption of gap junctional communication and phosphorylation of connexin43 by platelet-derived growth factor in T51B rat liver epithelial cells expressing platelet-derived growth factor receptor. Journal of Cellular Physiology. 1998a;174:66–77. doi: 10.1002/(SICI)1097-4652(199801)174:1<66::AID-JCP8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Ao P, Boynton AL. Platelet-derived growth factor-induced disruption of gap junctional communication and phosphorylation of connexin43 involves protein kinase C and mitogen-activated protein kinase. Journal of Cellular Physiology. 1998b;176:332–341. doi: 10.1002/(SICI)1097-4652(199808)176:2<332::AID-JCP11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Jagdale AB, Ao P, Boynton AL. Mitogen-activated protein kinase and phosphorylation of connexin43 are not sufficient for the disruption of gap junctional communication by platelet-derived growth factor and tetradecanoylphorbol acetate. Journal of Cellular Physiology. 1999a;179:87–96. doi: 10.1002/(SICI)1097-4652(199904)179:1<87::AID-JCP11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Jagdale AB, Ao P, Kazlauskas A, Boynton AL. Disruption of gap junctional communication by the platelet-derived growth factor is mediated via multiple signaling pathways. Journal of Biological Chemistry. 1999b;274:10489–10496. doi: 10.1074/jbc.274.15.10489. [DOI] [PubMed] [Google Scholar]
- Husoy T, Mikalsen SO, Sanner T. Phosphatase inhibitors, gap junctional intercellular communication and [125I]-EGF binding in hamster fibroblasts. Carcinogenesis. 1993;14:2257–2265. doi: 10.1093/carcin/14.11.2257. [DOI] [PubMed] [Google Scholar]
- Kanemitsu MY, Lau AF. Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-O-tetradecanoyl 13-acetate-sensitive protein kinase C: The possible involvement of mitogen-activated protein kinase. Molecular Biology of the Cell. 1993;4:837–848. doi: 10.1091/mbc.4.8.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu MY, Loo LW, Simon S, Lau AF, Eckhart W. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. Journal of Biophysical Chemistry. 1997;272:22824–22831. doi: 10.1074/jbc.272.36.22824. [DOI] [PubMed] [Google Scholar]
- Kanemitsu MY, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth & Differentation. 1998;9:13–21. [PubMed] [Google Scholar]
- Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Laing JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- Kim DY, Kam Y, Koo SK, Joe CO. Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. Journal of Biological Chemistry. 1999;274:5581–5587. doi: 10.1074/jbc.274.9.5581. [DOI] [PubMed] [Google Scholar]
- Klotz L-O, Patak P, Ale-Agha N, Buchczyk DP, Abdelmohsen K, Gerber PA, von Monfort C, Sies H. 2-Methyl-1,4-naphthoquinone, Vitamin K3, decreases gap-junctional intercellular communication via activation of the epidermal growth factor receptor/extracellular signal-regulated kinase cascade. Cancer Research. 2002;62:4922–4928. [PubMed] [Google Scholar]
- Koo SK, Kim DY, Park SD, Kang KW, Joe CO. PKC phosphorylation disrupts gap junctional communication at G0/S phase in clone 9 cells. Molecular and Cellular Biochemistry. 1997;167:41–49. doi: 10.1023/a:1006831114120. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Kurata WE, Lau AF. p130gag-fps disrupts gap junctional communication and induces phosphorylation of connexin43 in a manner similar to that of pp60v-Src. Oncogene. 1994;9:329–335. [PubMed] [Google Scholar]
- Kwak BR, Van Veen TAB, Analbers LJS, Jongsma HJ. TPA increases conductance but decreases permeability in neonatal rat cardiomyocyte gap junction channels. Experimental Cell Research. 1995;220:456–463. doi: 10.1006/excr.1995.1337. [DOI] [PubMed] [Google Scholar]
- Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochemistry Journal. 1991;273:67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW, Puranam KL, Revel JP. Identification of intermediate forms of connexin43 in rat cardiac myocytes. Progress in Cell Research. 1993;3:263–268. [Google Scholar]
- Laird DL, Castillo M, Kasprzak L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in Brefeldin A-treated rat mammary tumor cells. Journal of Cell Biology. 1995;131:1193–1203. doi: 10.1083/jcb.131.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW, Fistouris P, Batist G, Alpert L, Huynh HT, Carystinos GD, Alaoui-Jamali MA. Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Research. 1999;59:4104–4110. [PubMed] [Google Scholar]
- Lampe PD. Analyzing phorbol ester effects on gap junction communication: A dramatic inhibition of assembly. Journal of Cell Biology. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Archives of Biochemistry and Biophysics. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Kurata WE, Warn-Cramer B, Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. Journal of Cell Science. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. Journal of Cell Biology. 2000;126:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AF, Kanemitsu MY, Kurata WE, Danesh S, Boynton AL. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Molecular Biology of the Cell. 1992;3:865–874. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left–right asymmetry. Developmental Biology. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Lidington D, Tyml K, Ouellette Y. Lipopolysaccharide-induced reductions in cellular coupling correlate with tyrosine phosphorylation of connexin 43. Journal of Cellular Physiology. 2002;193:373–379. doi: 10.1002/jcp.10179. [DOI] [PubMed] [Google Scholar]
- Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on tyr247 and tyr265 disrupts gap junctional communication. Journal of Cell Biology. 2001;154:815–827. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo LWM, Berestecky JM, Kanemitsu MY, Lau AF. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. Journal of Biological Chemistry. 1995;270:12751–12761. doi: 10.1074/jbc.270.21.12751. [DOI] [PubMed] [Google Scholar]
- Loo LW, Kanemitsu MY, Lau AF. In vivo association of pp60v-Src and the gap-junction protein connexin 43 in v-Src-transformed fibroblasts. Molecular Carcinogenesis. 1999;25:187–195. [PubMed] [Google Scholar]
- Lye SJ, Nicholson BJ, Mascarenhas M, Macenzie L, Petrocelli T. Increased expression of connexin-43 in the rat myometrium during labor is associated with an increase in the plasma estrogen-progesterone ratio. Endocrinology. 1993;132:22380–22386. doi: 10.1210/endo.132.6.8389279. [DOI] [PubMed] [Google Scholar]
- Mehta PP, Yamamoto M, Rose B. Transcription of the gene for the gap junctional protein connexin43 and expression of functional cell-to-cell channels are regulated by cAMP. Molecular Biology of the Cell. 1992;3:839–850. doi: 10.1091/mbc.3.8.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moennikes O, Buchmann A, Ott T, Willecke K, Schwarz M. The effect of connexin32 null mutation on hepatocarcinogenesis in different mouse strains. Carcinogenesis. 1999;20:1379–1382. doi: 10.1093/carcin/20.7.1379. [DOI] [PubMed] [Google Scholar]
- Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophysics Journal. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster PN, Weingart R. Effects of phorbol ester on gap junctions of neonatal rat heart cells. Pflugers Archiv. 1993;423:181–188. doi: 10.1007/BF00374392. [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport phosphorylation and assembly into gap junctional plaques. Journal of Cell Biology. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: Molecular cloning, ultrastructural localization, and post-translational phosphorylation. Journal of Membrane Biology. 1990a;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of gap junction protein connexin43 in junctional communication-competent and-deficient cell lines. Journal of Cell Biology. 1990b;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson AF, Lampe PD, Meyer RA, Atkinson MA, Walseth TF, Johnson RG. Cyclic AMP and LDL trigger a rapid enhancement in gap junction assembly through a stimulation of connexin trafficking. Journal of Cell Science. 2000;113:3037–3049. doi: 10.1242/jcs.113.17.3037. [DOI] [PubMed] [Google Scholar]
- Plum A, Hallas G, Magin T, Dombrowski F, Hagendorff A, Schumacher B, Wolpert C, Kim J, Lamers WH, Evert M, Meda P, Traub O, Willecke K. Unique and shared functions of different connexins in mice. Current Biology. 2000;10:1083–1091. doi: 10.1016/s0960-9822(00)00690-4. [DOI] [PubMed] [Google Scholar]
- Postma FR, Hengeveld T, Alblas J, Giepmans BN, Zondag GC, Jalink K, Moolenaar WH. Acute loss of cell–cell communication caused by G protein-coupled receptors: A critical role for c-Src. Journal of Cell Biology. 1998;140:1199–1209. doi: 10.1083/jcb.140.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Reynhout JK, Lampe PD, Johnson RG. An activator of protein kinase C inhibits gap junction communication between cultured bovine lens cells. Experimental Cell Research. 1992;198:337–342. doi: 10.1016/0014-4827(92)90388-o. [DOI] [PubMed] [Google Scholar]
- Rivedal E, Opsahl Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis. 2001;22:1543–1550. doi: 10.1093/carcin/22.9.1543. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Trosko JE, Madhukar BV. Inhibition of connexin43 gap junctional intercellular communication by TPA requires ERK activation. Journal of Cellular Biochemistry. 2001;83:163–169. doi: 10.1002/jcb.1227. [DOI] [PubMed] [Google Scholar]
- Saez JC, Spray DC, Nairn AC, Hertzberg E, Greengard P, Bennett MVL. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principle gap junction polypeptide. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Nairn AC, Czernik AJ, Spray DC, Hertzberg EL, Greengard P, Bennett MVL. Phosphorylation of connexin 32, a hepatocyte gap-junction protein, by cAMP-dependent protein kinase, protein kinase C and calcium/calmodulin-dependent protein kinase. European Journal of Biochemistry. 1990;192:263–273. doi: 10.1111/j.1432-1033.1990.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Saez JC, Nairn AC, Czernik AJ, Spray DC, Hertzberg EL. Rat connexin43: Regulation by phosphorylation in heart. Progress in Cell Research. 1993;3:275–281. [Google Scholar]
- Saez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. Journal of Molecular and Cellular Cardiology. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- Saez JC, Martinez AD, Branes MC, Gonzalez HE. Regulation of gap junctions by protein phosphorylation. Brazilian Journal of Medical and Biological Research. 1998;31:593–600. doi: 10.1590/s0100-879x1998000500001. [DOI] [PubMed] [Google Scholar]
- Shah MM, Martinez A-M, Fletcher WH. The connexin43 gap junction protein is phosphorylated by protein kinase A and protein kinase C: In vivo and in vtitro studies. Molecular and Cellular Biochemistry. 2002;238:57–68. doi: 10.1023/a:1019902920693. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Current Biology. 1998;8:295–298. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- Solan JL, Fry MD, TenBroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. Journal of Cell Science. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- Spray DC, Burt JM. Structure–activity relationships of the cardiac gap junction channel. American Journal of Physiology. 1990;258:C195–C205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- Stagg RB, Fletcher WH. The hormone-induced regulation of contact-dependent cell–cell communication by phosphorylation. Endocrinological Reviews. 1990;11:302–325. doi: 10.1210/edrv-11-2-302. [DOI] [PubMed] [Google Scholar]
- Suarez S, Ballmer-Hofer K. VEGF transiently disrupts gap junctional communication in endothelial cells. Journal of Cell Science. 2001;114:1229–1235. doi: 10.1242/jcs.114.6.1229. [DOI] [PubMed] [Google Scholar]
- Swenson KI, Piwnica-Worms H, McNamee H, Paul DL. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regulation. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Saheki S, Shimazu T, Takeuchi N. Phosphorylation of the 27-kDa gap junction protein by protein kinase C in vitro and in rat hepatocytes. Journal of Biochemistry. 1989;106:723–727. doi: 10.1093/oxfordjournals.jbchem.a122923. [DOI] [PubMed] [Google Scholar]
- TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. Journal of Cell Biology. 2001;155:1307–1318. doi: 10.1083/jcb.200102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. Journal of Biological Chemistry. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- Traub O, Look J, Dermietzel R, Brummer F, Hulser D, Willecke K. Comparative characterization of the 21-kDa and 26-kDa gap junction proteins in murine liver and cultured hepatocytes. Journal of Cell Biology. 1989;108:1039–1052. doi: 10.1083/jcb.108.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijen HV, Van Veen TA, Hermans MM, Jongsma HJ. Human connexin40 gap junction channels are modulated by cAMP. Cardiovascular Research. 2000;45:941–951. doi: 10.1016/s0008-6363(99)00373-9. [DOI] [PubMed] [Google Scholar]
- van Veen TAB, van Rijen HvM, Jongsma HJ. Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation. Cardiovascular Research. 2000;46:496–510. doi: 10.1016/s0008-6363(00)00047-x. [DOI] [PubMed] [Google Scholar]
- Vikhamar G, Rivedal E, Mollerup S, Sanner T. Role of Cx43 phosphorylation and MAP kinase activation in EGF induced enhancement of cell communication in human kidney epithelial cells. Cell Adhesion and Communication. 1998;5:451–460. doi: 10.3109/15419069809005603. [DOI] [PubMed] [Google Scholar]
- Wagner LM, Saleh SM, Boyle DJ, Takemoto DJ. Effect of protein kinase C-gamma on gap junction disassembly in lens epithelial cells and retinal cells in culture. Molecular Vision. 2002;8:59–66. [PubMed] [Google Scholar]
- Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF. Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. Journal of Biological Chemistry. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. Journal of Biological Chemistry. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- Wei L, Nagy JI. Connexin43 phosphorylation state and intercellualr communication in cultured astrocytes following hypoxia and protein phosphatase inhibition. European Journal of Neuroscience. 2000;12:26444–26450. doi: 10.1046/j.1460-9568.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–320. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biological Chemistry. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Xie H, Laird DW, Chang T-H, Hu VW. A mitosis-specific phosphorylation of the gap junction protein connexin43 in human vascular cells: Biochemical characterization and localization. Journal of Cell Biology. 1997;137:203–210. doi: 10.1083/jcb.137.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Morioka T, Oite T. PDGF regulates gap junction communication and connexin 43 phosphorylation by PI 3-kinase in mesangial cells. Kidney International. 2000;57:1915–1926. doi: 10.1046/j.1523-1755.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- Yin X, Gu S, Jiang JX. The development-associated cleavage of lens connexin 45.6 by caspase-3-like protease is regulated by casein kinase II-mediated phosphorylation. Journal of Biological Chemistry. 2001;276:34567–34572. doi: 10.1074/jbc.M106073200. [DOI] [PubMed] [Google Scholar]
- Yogo K, Ogawa T, Akiyama M, Ishida N, Takeya T. Identification and functional analysis of novel phosphorylation sites in Cx43 in rat primary granulosa cells. FEBS Letters. 2002;531:132–136. doi: 10.1016/s0014-5793(02)03441-5. [DOI] [PubMed] [Google Scholar]
- Zhou L, Kasperek EM, Nicholson BJ. Dissection of the molecular basis of pp60(v-Src) induced gating of connexin 43 gap junction channels. Journal of Cell Biology. 1999;144:1033–1045. doi: 10.1083/jcb.144.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]