Abstract
OBJECTIVE: To assess maintenance of efficacy and tolerability of gabapentin enacarbil in patients with moderate to severe primary restless legs syndrome (RLS).
PATIENTS AND METHODS: This study (conducted April 18, 2006, to November 14, 2007) comprised a 24-week, single-blind (SB) treatment phase (gabapentin enacarbil, 1200 mg) followed by a 12-week randomized, double-blind (DB) phase. Responders from the SB phase (patients with improvements on the International Restless Legs Scale [IRLS] and investigator-rated Clinical Global Impression–Improvement scale at week 24 and stable while taking a gabapentin enacarbil dose of 1200 mg for at least 1 month before randomization) were randomized to gabapentin enacarbil, 1200 mg, or placebo once daily at 5 pm with food. The primary end point was the proportion of patients experiencing relapse (worse scores on the IRLS and investigator-rated Clinical Global Impression of Change scale on 2 consecutive visits at least 1 week apart or withdrawal because of lack of efficacy) during the DB phase.
RESULTS: A total of 221 of 327 patients completed the SB phase, 194 (96 in the gabapentin enacarbil group and 98 in the placebo group) were randomized to DB treatment, and 168 (84 in the gabapentin enacarbil group and 84 in the placebo group) completed the DB phase. A significantly smaller proportion of patients treated with gabapentin enacarbil (9/96 [9%]) experienced relapse compared with the placebo-treated patients (22/97 [23%]) (odds ratio, 0.353; 95% confidence interval, 0.2-0.8; P=.02). Somnolence and dizziness were the most common adverse events. One death occurred (unintentional choking during the SB phase) and was judged as being unrelated to the study drug. No clinically relevant changes were observed in laboratory values, in vital signs, or on electrocardiograms.
CONCLUSION: Gabapentin enacarbil, 1200 mg, maintained improvements in RLS symptoms compared with placebo and showed long-term tolerability in adults with moderate to severe primary RLS for up to 9 months of treatment.
Gabapentin enacarbil at 1200 mg maintained improvements in restless legs syndrome symptoms compared with placebo and showed long-term tolerability in adults with moderate to severe primary restless legs syndrome for up to 9 months of treatment; no clinically relevant changes were observed in laboratory values, in vital signs, or on electrocardiograms.
AE = adverse event; AMTD = adjusted mean treatment difference; CGI-C = Clinical Global Impression of Change; CGI-I = Clinical Global Impression–Improvement; CI = confidence interval; DB = double blind; IRLS = International Restless Legs Scale; ITT = intent to treat; LOCF = last observation carried forward; MOS = Medical Outcomes Study; OR = odds ratio; PIVOT = Patient Improvements in Vital Outcomes following Treatment; PSQ = Post-Sleep Questionnaire; QoL = quality of life; RLS = restless legs syndrome; SB = single blind
The sensorimotor disorder restless legs syndrome (RLS) is characterized by an urge to move the legs, usually accompanied or caused by uncomfortable sensations in the legs. Symptoms typically begin or worsen at rest, are worse in the evening or at night, and are temporarily relieved by movement.1 Patients with RLS often report sleep disturbance that results in daytime fatigue, decreased alertness, and emotional distress.2-4
Dopamine agonists are currently the only approved treatment for RLS in the United States, but prolonged use may be associated with augmentation (a worsening of symptoms while receiving treatment)5 that can limit long-term use. Other dopaminergic-related adverse events (AEs), such as nausea, vomiting, orthostatic hypotension, syncope, and impulse control disorders, are reported to be treatment limiting in some patients.6-11 Therefore, additional therapies for RLS are needed.
Small studies have indicated that the nondopaminergic agent gabapentin may be an effective treatment of RLS.12-14 However, gabapentin displays dose-dependent exposure because of saturable absorption and high interpatient variability,15,16 resulting from absorption via low-capacity nutrient transporters located in a narrow region of the small intestine that start to become saturated at clinically relevant doses.17-19 In studies of patients with epilepsy, these pharmacokinetic limitations have been shown to lead to varied treatment effectiveness and the need for more frequent daily dosing.15,20 Gabapentin enacarbil is a transported prodrug of gabapentin that is being investigated for the treatment of RLS. After administration, gabapentin enacarbil is actively absorbed via high-capacity nutrient transporters throughout the small and large intestine and is rapidly converted to gabapentin, providing dose-proportional exposure and low interpatient variability.21-23 Gabapentin enacarbil provides sustained gabapentin exposure and once-daily dosing for the treatment of RLS.23,24
For editorial comment, see page 508
In a large placebo-controlled, 12-week study, gabapentin enacarbil, 1200 mg once daily, significantly improved RLS symptoms compared with placebo in adults with moderate to severe primary RLS and was generally well tolerated,24 with an AE profile consistent with that of gabapentin.12 The current study (PIVOT [Patient Improvements in Vital Outcomes following Treatment] RLS Maintenance) assessed the maintenance of efficacy and tolerability of gabapentin enacarbil in patients with moderate to severe primary RLS for up to 9 months.
PATIENTS AND METHODS
Study Design
This long-term maintenance study (XenoPort Inc, protocol XP060, ClinicalTrials.gov identifier NCT00311363) comprised a 24-week, single-blind (SB), active treatment phase (gabapentin enacarbil, 1200 mg) followed by a 12-week, randomized, double-blind (DB), parallel-group comparison of gabapentin enacarbil, 1200 mg, and placebo. The study was conducted from April 18, 2006, to November 14, 2007, in 27 US centers. Clinic visits occurred on day −7 (screening) and weeks 0 (baseline), 1, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24 (randomization), 26, 28, 30, 32, 34, and 36 (end of treatment).
Study Participants
Men and women, at least 18 years of age, diagnosed as having moderate to severe primary RLS (International RLS Study Group criteria)1 were recruited. Eligible patients had RLS symptoms on at least 15 nights during the month before screening (or, if undergoing treatment, similar symptom frequency before treatment initiation), symptoms on at least 4 nights during the 7-day screening period, an International Restless Legs Scale (IRLS)25 total score of at least 15 points at the beginning and end of the baseline period, and creatinine clearance of at least 60 mL/min. If patients were receiving treatment for RLS or a sleep disorder, use of medication was to be discontinued at least 2 weeks before baseline. All patients provided written informed consent before study participation. The study was conducted in accordance with good clinical practice guidelines and the 1996 version of the Declaration of Helsinki.26 The protocol was reviewed and approved by a local or regional institutional review board, depending on center requirements.
Patients were excluded if they were pregnant or breastfeeding; had evidence of secondary RLS; had a body mass index (calculated as weight in kilograms divided by height in meters squared) of more than 34; were currently experiencing a moderate or severe major depressive disorder (Diagnostic and Statistical Manual of Mental Disorders [Fourth Edition, Text Revision] criteria)27; had primary sleep disorders, neurologic disease, or movement disorders other than RLS; or had a history of RLS symptom augmentation or end-of-dose rebound with previous RLS treatment. Although the presence of daytime (10 am to 6 pm) RLS symptoms for at least 2 days during the week before baseline was originally an exclusion criterion, this restriction was removed after enrollment of approximately 10% of the total study population.
Interventions
Patients were instructed to take study medication once daily at 5 pm with food.
SB Phase. Treatment was initiated on days 1 to 3 with one 600-mg extended-release tablet of gabapentin enacarbil. From day 4, patients received gabapentin enacarbil, 1200 mg (two 600-mg tablets). Patients who successfully completed the SB phase were considered responders if they had an IRLS total score of less than 15 points at week 24 that had decreased by at least 6 points compared with baseline, an assessment of “much improved” or “very much improved” on the investigator-rated Clinical Global Impression–Improvement (CGI-I) scale at week 24,28 and were stable while taking gabapentin enacarbil, 1200 mg, for at least 1 month before the DB phase. Responders were randomized 1:1 to receive gabapentin enacarbil, 1200 mg, or placebo for 12 weeks (DB phase), using a blocked randomization schedule (block size of 4), stratified by study site.
DB Phase. Patients randomized to placebo received one 600-mg tablet of gabapentin enacarbil and 1 placebo tablet once daily in a 2-week taper from weeks 24 to 26, followed by 2 placebo tablets from weeks 26 to 36. Blinding was maintained using matching placebo and gabapentin enacarbil tablets and by switching from a single bottle of tablets to 2 bottles with identical packaging 1 month before randomization so that patients did not know when placebo treatment was initiated. Patients randomized to gabapentin enacarbil continued to receive gabapentin enacarbil, 1200 mg once daily, during weeks 24 to 36. At the end of the study or after early withdrawal, patients received one 600-mg tablet of gabapentin enacarbil or 1 placebo tablet, according to their treatment schedule, during a 7-day taper.
Outcome Measures
The primary end point was the proportion of patients who experienced relapse during the DB phase. Relapse was defined as the worsening of RLS symptoms (an increase of at least 6 points in the IRLS total score from DB baseline [week 24, randomization] to a score of at least 15 points and a rating of “much worse” or “very much worse” on the investigator-rated Clinical Global Impression of Change [CGI-C] scale on 2 consecutive visits at least 1 week apart) or withdrawal because of lack of efficacy.
Secondary outcome end points during the DB phase included the time to relapse (the first date on which relapse criteria were met or the date of withdrawal because of lack of efficacy) for weeks 24 to 36; change from randomization in IRLS total score at week 36; proportion of patients rated as responders on the investigator-rated CGI-C scale (response since randomization defined as “very much improved,” “much improved,” “minimally improved,” or “no change”) and patient-rated CGI-I (response since randomization defined as “much improved” or “very much improved”) at week 36; change from randomization to week 36 in domains of the Medical Outcomes Study (MOS) Sleep Scale29,30; responses on the investigator-designed Post-Sleep Questionnaire (PSQ) at week 3624; change from randomization to week 36 in the RLS Quality of Life (QoL) questionnaire overall life-impact score31; and the onset and severity of RLS symptoms at week 36 recorded by a 24-hour RLS diary (beginning at 8 am daily).
Efficacy during the SB phase was assessed using the change from baseline in IRLS total score at week 24 and response to treatment since baseline on the investigator- and subject-rated CGI-I at week 24. The IRLS was assessed at every visit. The investigator-rated CGI-I was assessed at weeks 4, 8, 12, 16, 20, and 24 and the investigator-rated CGI-C at weeks 24, 26, 28, 30, 32, 34, and 36 (end of treatment). The patient-rated CGI-I was assessed at weeks 4, 8, 12, 16, 20, 24, 26, 28, 30, 32, 34, and 36 (end of treatment). The MOS Sleep Scale, PSQ, RLS QoL questionnaire, and 24-hour RLS diary were assessed at week 0 (baseline) and every 4 weeks thereafter.
The incidence and intensity of AEs were recorded at each visit. The AEs were considered treatment emergent in the DB phase if onset or worsening occurred after randomization. Vital signs, electrocardiograms, and clinical laboratory assessments (hematology, biochemistry, and urinalysis) were obtained at screening and weeks 0 (baseline), 1, 4, 8, 12, 16, 20, 24 (randomization), 28, 32, and 36 (or early termination). All outcome measures scheduled for the week 36 visit were to be performed if the patient attended an early termination visit.
Statistical Analyses
A sample size of 90 patients per treatment group (180 total) was considered sufficient to provide 90% power to detect a difference in relapse rate of 25% (40% for the gabapentin enacarbil group vs 65% for the placebo; odds ratio [OR], 0.359) at the .05 significance level using a 2-group continuity-corrected χ2 test.
The SB and DB safety populations comprised all patients who took at least 1 dose (or partial dose) of SB and DB study medication, respectively. The SB intent-to-treat (ITT) population comprised all patients who took at least 1 dose (or partial dose) of SB study medication and had at least 1 IRLS and CGI-I assessment during the SB phase. The DB phase efficacy analyses were based on the DB ITT population, comprising all patients who took at least 1 dose (or partial dose) of DB study medication and had at least 1 postrandomization IRLS and CGI-C assessment.
The primary end point was analyzed using logistic regression adjusted for treatment, IRLS total score at randomization, and pooled site. Analyses excluding taper period relapses were conducted to ensure that the relapse assessment was not measuring the effect of abrupt withdrawal of active drug (ie, RLS symptom rebound). Time to relapse and time to first onset of RLS symptoms were analyzed using survival analysis; treatment groups were compared using a 2-sided log-rank test, the median time to relapse was estimated using product-limit estimation, and time to relapse was presented as Kaplan-Meier survival curves. Patients who did not meet the relapse criteria but withdrew from the DB phase for reasons other than lack of efficacy were assessed at the date of last dose of study drug. Treatment differences with respect to continuous change from randomization variables were analyzed using an analysis of covariance model adjusted for treatment, pooled site, and score at randomization; binary variables were analyzed using logistic regression adjusted for treatment and pooled site. Multicategorical response variables were analyzed using the Cochran-Mantel-Haenszel test with equally spaced scores stratified by pooled site. The last observation carried forward (LOCF) method was used for continuous, binary, and multicategorical secondary efficacy end points. Study sites were pooled regionally into 5 larger consolidated sites that were defined before treatment allocation was unmasked.
RESULTS
Patient Disposition
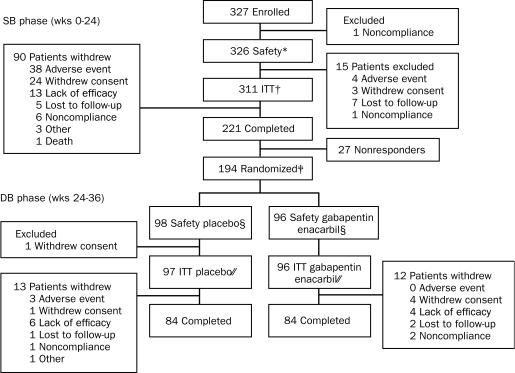
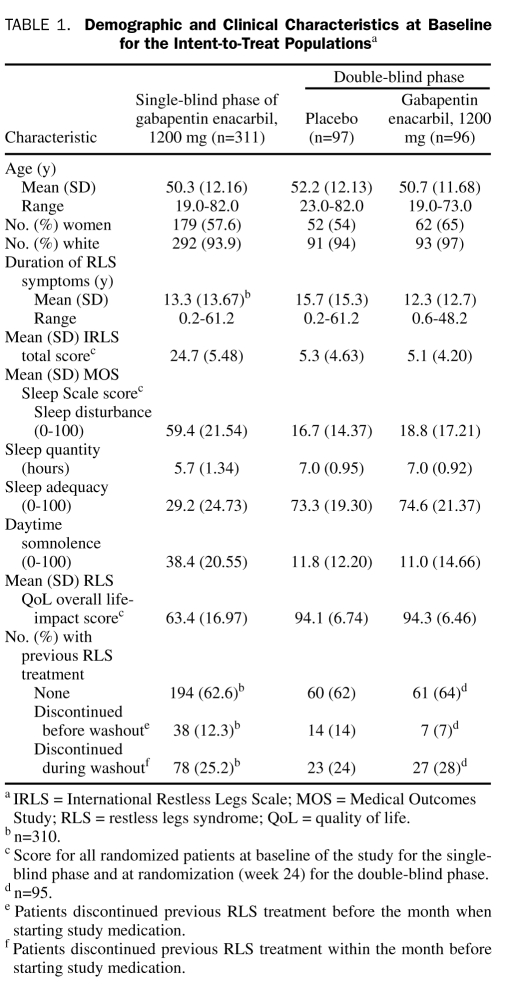
A total of 326 of 327 enrolled patients were eligible for the SB safety population; 221 (67.6%) of 327 completed the SB phase, and 194 (59.3%) were rated as responders and entered the DB phase (96 in the gabapentin enacarbil group and 98 in the placebo group; Figure 1). The participation of 1 site was discontinued during the study because of protocol noncompliance, and their data were included in the analyses. A post hoc analysis that excluded patients from the discontinued site demonstrated that their data did not influence the overall study outcome. Overall, DB phase completion rates were similar for the gabapentin enacarbil and placebo groups. Patient demographics and baseline disease characteristics were similar for both treatment groups (Table 1). Baseline responses on the PSQ are presented in eTable 1 (online linked to this article).
FIGURE 1.
Patient disposition.
* Single-blind (SB) safety population defined as all patients who received at least 1 dose of SB study drug.
† SB intent-to-treat (ITT) population defined as all patients who received at least 1 dose of SB study drug and for whom at least 1 SB visit, International Restless Legs Scale (IRLS) total score, and Clinical Global Impression–Improvement (CGI-I) assessment were available.
‡ Patients entering the randomized double-blind (DB) phase were responders who had an IRLS total score reduced by at least 6 points compared with baseline and an IRLS total score of less than 15 at week 24, who had a “much improved” or “very much improved” rating on the investigator-rated CGI-I at week 24, who were stable while taking 1200 mg of gabapentin enacarbil for at least 1 month before week 24, and who successfully completed the entire 24-week SB phase.
§ The DB safety population was defined as all patients who received at least 1 dose of DB study drug.
// The DB ITT population was defined as all patients who received at least 1 dose of DB study drug and for whom at least 1 postrandomization visit IRLS total score and Clinical Global Impression of Change assessment were available.
TABLE 1.
Demographic and Clinical Characteristics at Baseline for the Intent-to-Treat Populationsa
Efficacy During the SB Phase
Patients in the SB ITT population reported improvements in IRLS total score with a mean (SD) change from baseline to week 24 (LOCF) of −15.5 (9.16). After 24 weeks of gabapentin enacarbil treatment, 78 (25.1%) of 311 and 170 (54.7%) of 311 patients (LOCF) received a score of “much improved” or “very much improved,” respectively, on the investigator-rated CGI-I scale; 82 (26.6%) of 308 and 163 (52.9%) of 308 patients (LOCF) reported their symptoms as “much improved” or “very much improved,” respectively, on the patient-rated CGI-I.
Primary End Point
A significantly smaller proportion of gabapentin enacarbil–treated patients experienced relapse during the DB phase compared with placebo-treated patients (9% [9/96] vs 23% [22/97]; OR, 0.35; 95% confidence interval [CI], 0.2-0.8; P=.02). Of the 9 gabapentin enacarbil–treated patients who experienced relapse, 5 met the criteria for worsening RLS symptoms and did not withdraw because of lack of efficacy; 4 withdrew because of lack of efficacy without meeting the criteria for worsening RLS symptoms. Patients who met the definition of relapse were allowed to remain in the study or withdraw. Among placebo-treated patients, 18 met the criteria for worsening RLS symptoms; of these, 2 withdrew because of lack of efficacy. Four patients withdrew because of lack of efficacy without meeting the criteria for worsening RLS symptoms.
Secondary End Points
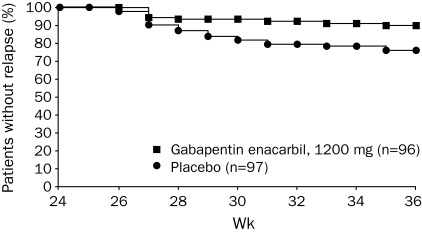
During the DB phase, gabapentin enacarbil–treated patients reported a significantly longer time to relapse compared with placebo, whether data from the taper period (weeks 25 and 26) were included in the analyses (P=.01; Figure 2) or not (weeks 27-36; P=.03). The median time to relapse could not be estimated for either treatment group because less than 50% of patients in each treatment group experienced a relapse. At the end of the DB 2-week taper (week 26), during which patients randomized to placebo received gabapentin enacarbil, 600 mg, 5 (5%) of 96 of the gabapentin enacarbil–treated patients experienced relapse compared with 9 (9%) of 97 of the placebo-treated patients (OR, 0.55; 95% CI, 0.2-1.7; P=.30).
FIGURE 2.
Time to relapse during double-blind treatment by week (double-blind intent-to-treat population). Log-rank test for treatment difference during week 24 to week 36 (P=.01). Relapse is defined as the worsening of restless legs syndrome symptoms (an increase of ≥6 points in the International Restless Legs Scale total score from double-blind baseline [week 24, randomization] to a score of ≥15 and a rating of “much worse” or “very much worse” on the investigator-rated Clinical Global Impression of Change on 2 consecutive visits ≥1 week apart) or withdrawal due to lack of efficacy during the double-blind phase.
At DB randomization, mean (SD) IRLS total scores were similar for the gabapentin enacarbil (5.1 [4.20]) and placebo (5.3 [4.63]) treatment groups (Table 1). At week 36 (LOCF), the mean (SD) change from randomization (week 24) in IRLS total score was significantly smaller with gabapentin enacarbil (1.9 [7.01]) compared with placebo (3.9 [6.49]; adjusted mean treatment difference [AMTD], −2.1; P=.03).
At week 36 (LOCF), 72 (75%) of 96 gabapentin enacarbil–treated patients were classified by investigators as CGI-C responders compared with 65 (67%) of 97 placebo-treated patients (OR, 1.47; 95% CI, 0.8-2.8; P=.24). A higher proportion of patients in both treatment groups classified themselves as responders on the patient-rated CGI-I at week 36 (LOCF) (88% [84/96] in the gabapentin enacarbil group compared with 79% [77/97] in the placebo group; OR, 1.8; 95% CI, 0.8-3.9; P=.15), although this difference was not statistically significant.
Patients randomized to gabapentin enacarbil during the DB phase reported significantly smaller mean (SD) changes from DB baseline in 2 MOS Sleep Scale domains at week 36 (LOCF) compared with placebo: sleep disturbance (2.3 [18.32] vs 10.2 [19.02]; AMTD, −7.0; P=.007) and sleep adequacy (−4.3 [22.28] vs −11.6 [24.01]; AMTD, 7.7; P=.02). Differences between gabapentin enacarbil and placebo in daytime somnolence (1.5 [11.67] vs 3.8 [13.33]; AMTD, −2.4; P=.18) and sleep quantity (−0.1 [0.92] vs −0.2 [0.90] hours; AMTD, 0.1; P=.72) were not statistically significant.
At week 36 (LOCF), a significantly greater proportion of gabapentin enacarbil–treated patients reported fewer nights with RLS symptoms (P=.05), fewer night-time awakenings (P=.04), and fewer hours awake per night due to RLS symptoms (P=.02) on the PSQ compared with placebo (Table 2), and a greater proportion of gabapentin enacarbil–treated patients reported higher overall quality of sleep (P=.15) and ability to function during the daytime in the past week (P=.54), although these differences were not statistically significant.
TABLE 2.
Post-Sleep Questionnaire Responses at Week 36 (Last Observation Carried Forward) for the Double-Blind Intent-to-Treat Populationa
Gabapentin enacarbil–treated patients reported a smaller mean (SD) change in the RLS QoL overall life-impact score from DB baseline to week 36 (LOCF) compared with placebo (−2.2 [7.86] vs −4.2 [11.53]); however, this difference was not statistically significant (AMTD, 1.9; P=.19).
At week 24, the estimated median time to onset of RLS symptoms during a 24-hour period could not be estimated for patients ultimately randomized to placebo because 48 (50%) of 96 had not reported RLS symptoms; for patients ultimately randomized to gabapentin enacarbil, the estimated median time to onset of RLS symptoms was 20.0 hours (95% CI, 13.0, upper limit could not be estimated).
At week 36, the estimated median time to onset of RLS symptoms was 14.5 hours (95% CI, 13.5-17.5 hours) for placebo-treated patients but could not be estimated for the gabapentin enacarbil–treated patients (gabapentin enacarbil vs placebo, P=.04). At week 36, 48 (54%) of 89 gaba pentin enacarbil–treated patients were symptom free during the 24-hour assessment period compared with 33 (38%) of 87 placebo-treated patients (eFigure 1 [online linked to this article]).
Safety and Tolerability
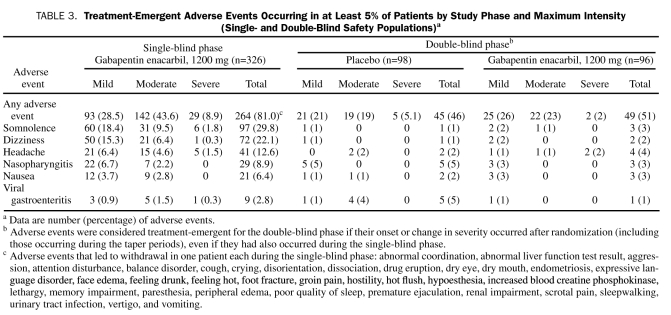
SB and DB Phases. Overall, somnolence and dizziness were the most commonly reported treatment-emergent AEs (Table 3); among patients reporting a first occurrence of somnolence or dizziness, most did so during the first 2 weeks of study participation. Most patients who reported somnolence (78% [29/37] in the gabapentin enacarbil group vs 75% [21/28] in the placebo group) or dizziness (89% [25/28] in the gabapentin enacarbil group vs 77% [17/22] in the placebo group) reported a single AE with no recurrence. The median duration throughout the entire study was 42.0 and 29.5 days of somnolence and 13.0 and 26.0 days for dizziness for the gabapentin enacarbil– and placebo-treated patients, respectively. There were no clinically relevant changes in laboratory values, vital signs, or electrocardiograms for either phase or treatment group.
TABLE 3.
Treatment-Emergent Adverse Events Occurring in at Least 5% of Patients by Study Phase and Maximum Intensity (Single- and Double-Blind Safety Populations)a
SB Phase. Treatment-emergent AEs were reported by 264 (81.0%) of 326 patients, most of which were mild or moderate in intensity (Table 3). Of 326 patients, 42 (12.9%) reported at least 1 AE that led to withdrawal; in 32 of these patients, investigators considered these AEs to be treatment related. Adverse events that led to the withdrawal of more than 1 patient were somnolence (n=6), dizziness (n=5), headache (n=5), constipation (n=3), fatigue (n=3), insomnia (n=3), blurred vision (n=2), decreased libido (n=2), diarrhea (n=2), feeling abnormal (n=2), and nausea (n=2). Adverse events that led to withdrawal in one patient each are presented in the footnote of Table 3.
One death occurred in the SB phase due to unintentional choking on food. This event was judged by the investigator not to be related to treatment. Two other patients reported serious AEs: one developed acute angina pectoris and another experienced chest pain. Neither event was judged to be treatment related, both resolved, and the patients continued in the study. One pregnancy was reported (the patient withdrew from the study), which resulted in the birth of a healthy newborn, with normal examination and developmental assessment results at 1 month of age.
DB Phase. Treatment-emergent AEs were reported by 49 (51%) of 96 gabapentin enacarbil–treated patients and 45 (46%) of 98 placebo-treated patients. Most AEs were mild or moderate in intensity (Table 3). There were no reports of severe somnolence or dizziness.
During the 2-week taper (weeks 25 and 26), 18 (19%) of 96 gabapentin enacarbil–treated patients and 28 (29%) of 98 placebo-treated patients (who received gabapentin enacarbil, 600 mg, during this period) reported at least 1 AE (eTable 2 [online linked to this article]). One patient (placebo) withdrew from the study during this period after an AE of tarsal tunnel syndrome, judged not to be treatment related. After week 36, a total of 4 patients in each group reported at least 1 AE during the 1-week taper at the end of the study: increased monocyte count (n=1), increased weight (n=1), lymphadenopathy (n=1), and convulsion (n=1, serious AE) for the gabapentin enacarbil group and allergic cough (n=1), erythematous rash (n=1), hyporeflexia (n=1), increased blood glucose level (n=1), increased monocyte count (n=1), and muscular weakness (n=1) for the placebo group.
Serious AEs were reported in 3 patients. One patient (placebo) experienced an anaphylactic reaction that led to withdrawal, and a second (placebo) patient developed diverticulitis, which resolved, and the patient continued in the study. Neither event was considered treatment related. Another patient (gabapentin enacarbil group) experienced convulsion (2 generalized seizures within approximately 7 hours) that occurred and resolved during the 1-week taper after week 36. This event was considered possibly treatment related and led to study withdrawal. An initial electroencephalographic assessment, performed 2 days after the seizures resolved, demonstrated left greater than right temporal slowing. The results of a subsequent electroencephalographic assessment, performed almost 2 months after resolution of the seizures, were abnormal and indicated probable left temporal sharp waves, suggestive of a possible underlying epileptic focus.
DISCUSSION
As an investigational treatment, it is important to study the long-term treatment effects of gabapentin enacarbil in patients with RLS. Almost 60% of patients in this study met response criteria after 6 months of SB treatment with gabapentin enacarbil, 1200 mg, reporting sustained improvements in IRLS total score and investigator-rated impressions of global improvement. However, patients who did not complete the 24-week SB phase were not eligible to be considered responders. After DB randomization, patients who continued receiving gabapentin enacarbil, 1200 mg, demonstrated significantly lower rates of RLS symptom relapse after 36 weeks of treatment compared with those who received placebo. The time to onset of RLS symptoms was also significantly delayed in gabapentin enacarbil–treated patients during a 24-hour assessment period at the end of treatment. In addition to relapse rates, measures of RLS symptoms (eg, IRLS total scores, investigator- and patient-rated CGI-I ratings, MOS Sleep Scale scores, and PSQ outcomes) indicated that placebo-treated patients had significantly more RLS symptoms than gabapentin enacarbil–treated patients during the DB phase. Importantly, more than half of the gabapentin enacarbil–treated patients were symptom free during a 24-hour assessment period at the end of treatment, supporting the findings of the 12-week PIVOT RLS I study.24 These data indicate that gabapentin enacarbil efficacy is maintained for up to 9 months of treatment.
The overall design of the current study was similar to that of 2 studies that investigated the efficacy of the dopamine agonists ropinirole and pramipexole in long-term maintenance treatment of RLS.32,33 However, the definition of relapse applied herein is different from those studies and is arguably more stringent, making comparison of relapse rates across the 3 trials difficult. Overall, fewer patients experienced relapse during treatment withdrawal in the current study because patients were required to have an increase of at least 6 points to achieve an IRLS total score of at least 15 points and a rating of “much worse” or “very much worse” on the investigator-rated CGI-C at 2 consecutive visits at least 1 week apart or to have withdrawn because of lack of efficacy. The pramipexole trial required that patients had a worsening of CGI-I and IRLS scores at only one study visit.33 The ropinirole trial applied only the IRLS criteria at one study visit or withdrawal because of lack of efficacy.32 The occurrence of RLS symptom rebound reported with interruption of dopaminergic therapy34 may have contributed to the higher relapse rates in the dopamine agonist trials. Adverse events of symptom rebound have not been observed with gabapentin enacarbil in RLS, perhaps because of the different mechanism of action and the sustained duration of drug exposure.
A relatively high proportion of placebo-treated patients did not experience relapse in the DB phase of the current study. This could be in part due to perceived therapeutic benefits of clinical trial participation.35 Alternatively, gabapentin enacarbil may provide a durable clinical effect not previously observed with dopamine agonists.
Responders showed improvements in IRLS total score during the SB phase (−5.5) comparable with that observed in the ropinirole long-term maintenance study (−12.8).32 However, given the differences in trial design and drug class, direct comparisons of efficacy between dopamine agonists and gabapentin enacarbil should be made with caution.
Sleep disturbance is one of the primary morbidities of RLS and is often the main reason that patients seek medical attention.1 In the current study, patients treated with gabapentin enacarbil across 9 months had significant improvements in sleep disturbance and sleep adequacy relative to baseline and compared with placebo-treated patients. Gabapentin enacarbil–treated patients reported significantly fewer nights with symptoms, fewer nighttime awakenings, and fewer hours awake per night due to symptoms compared with placebo. The improvements on these subjective sleep measures are consistent with those reported in the PIVOT RLS I study24 and with subjective and objective reported or observed changes in a small, crossover polysomnography study of patients with primary RLS who reported improvements in sleep architecture after 14 days of treatment with gabapentin enacarbil at 1800 mg.36
Symptoms of RLS have been shown to have a considerable negative impact on the QoL of patients. In a population-based study, patients with moderate to severe RLS symptoms requiring treatment reported lower QoL scores on the 36-item Short Form Health Survey than the general US population and similar scores to those of patients with common chronic medical conditions.2 In the current study, gabapentin enacarbil–treated patients showed improvements in QoL relative to baseline, likely a consequence of the reduction in RLS symptoms and improvements in sleep, supporting the findings from the PIVOT RLS I study.24
Gabapentin enacarbil was generally well tolerated across 9 months of treatment, with a pattern of AEs consistent with that observed with short-term gabapentin enacarbil and gabapentin treatment.12,24,36 The frequency of significant central nervous system AEs during the taper periods was low. Fewer AEs were reported during the DB phase than the SB phase, possibly a result of early patient withdrawal due to AEs during the SB phase and also because only new-onset AEs (including change in severity) were recorded in the DB phase. The lower rate of somnolence and dizziness in the DB phase is also likely to be due to resolution of these AEs in the SB phase. The AEs reported during the DB phase included those from the 2-week dose taper during which the placebo group received 600 mg of gabapentin enacarbil.
A strength of the current study was the stringent definition of relapse, which provided a rigorous measure of maintenance of efficacy and not simply a reflection of transient worsening of RLS symptoms or of the natural course (eg, worsening) of this disorder.32,33 Furthermore, the masked downward dose titration of gabapentin enacarbil for patients randomized to placebo after the SB phase likely reduced the chance of a transient RLS symptom rebound effect mimicking treatment relapse. This masked transition may explain the gradual increase in the number of placebo-treated patients experiencing relapse over time because they would have been unaware when they had switched from gabapentin enacarbil to placebo. A limitation of the study design is that it may have enriched the DB population for responders to active treatment by allowing only those meeting the SB response criteria to enter the DB phase. However, because all treatment responders were randomized at the end of the SB phase to continue to take gabapentin enacarbil or to receive placebo, this potential for “enrichment” was balanced across both treatment arms in the DB phase.
CONCLUSION
These findings provide clinical insight into the long-term management of RLS and the maintenance of efficacy and tolerability of gabapentin enacarbil in adults with moderate to severe primary RLS.
Supplementary Material
Footnotes
The study was funded by XenoPort Inc, Santa Clara, CA. Research funding for the design and conduct of this study and the collection, management, analysis, and interpretation of the data were sponsored by XenoPort. Preparation, review, and approval of the submitted manuscript were sponsored by GlaxoSmithKline, Research Triangle Park, NC.
This study was presented in part as a poster (P286) at the 62nd Annual Meeting of the American Academy of Neurology; April 10-17, 2010; Toronto, Ontario, Canada; and will be presented in part as a poster (265) at the 24th Annual Meeting of the Associated Professional Sleep Societies, San Antonio, TX, June 5-9 2010.
All listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors.
We wish to acknowledge the following individuals for their contributions: Barbara Wilson, MEd (GlaxoSmithKline, Research Triangle Park, NC), for editorial coordination and editorial suggestions to draft versions of the submitted paper. Editorial support in the form of the development of a draft outline and the manuscript first draft, assembling tables and figures, and collating author comments was provided by Phillippa Curran, PhD, and Sarah White, MSc, at Caudex Medical Ltd, Oxford, England, and was funded by GlaxoSmithKline. Statistical support was provided by Daniel Bonzo, PhD (XenoPort Inc, Santa Clara, CA), Nicola Williams, MSc (GlaxoSmithKline, Harlow, England), Robin White (GlaxoSmithKline, Research Triangle Park, NC), Ben Stein, PhD (Premier Research Int Ltd, San Diego, CA), and Mark Jaros, PhD (Premier Research Group Ltd, Estes Park, CO).
Members of the XP060 Study Group are as follows: Mansoor Ahmed, MD, Cleveland Sleep Research Center Inc, Middleburg Heights, OH; Michael P. Bilber, MD, Neurocare Inc, Newton, MA; Richard K. Bogan, MD, SleepMed, Columbia, SC; James L. Borders, MD, Central Kentucky Research Associates, Lexington, KY; Michel A. Cramer Bornemann, MD, Minnesota Regional Sleep Disorders Center, Hennepin County Medical Center, Minneapolis, MN; Joseph David, MD, Charlottesville Medical Research, Charlottesville, VA; Gustavo DuBois, MD, PMA Research, Birmingham, AL; Philip Emrie, MD, Rocky Mountain Center for Clinical Research, Wheat Ridge, CO; Juan B. Espinosa, MD, TuKoi Clinical Research, Miami, FL; Brandon Essink, MD, Meridian Clinical Research, Omaha, NE; Mark A. Fisher, MD, Lynn Health Science Center, Oklahoma City, OK; Darrell N. Fiske, MD, Radiant Research, Stuart, FL; June Fry MD, Center for Sleep Medicine, Lafayette Hill, PA; J. Brevard Haynes, MD, Sleep Medicine of Middle Tennessee, Nashville, TN; William J. Keating, MD, Dawsonville Family Medicine, Dawsonville, GA; Louis C. Kirby II, MD, Pivotal Research, Peoria, AZ; Timothy Ladner, MD, Tenaya Family Practice/Lovelace Scientific Resources Inc, Las Vegas, NV; Joseph M. Pittard, MD (previously was James Igleburger, MD,), Four Rivers Clinical Research, Paducah, KY; George Rederich, MD, South Bay Neurology Research Center, Redondo Beach, CA; Vernon D. Rowe III, MD, MidAmerica Neuroscience Institute Consultants in Neurology, Lenexa, KS; David J. Seiden, MD, Broward Research Center, Pembroke Pines, FL; Todd A. Teague, MD, Jackson Clinic, Jackson, TN; Joseph A. Tornabene, MD, BC, Wenatchee Valley Medical Center, Wenatchee, WA; Navin K. Varma, MD, Center for Neurological Services, South Ogden, UT; Paul Wylie, MD, Arkansas Center for Sleep Medicine, Little Rock, AR; James P. Wymer, MD, PhD, Upstate Clinical Research, Albany, NY; Lixin Zhang, MD, PhD, Dent Neurologic Institute, Amherst, NY.
REFERENCES
- 1.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology: a report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101-119 [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286-1292 [DOI] [PubMed] [Google Scholar]
- 3.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5(3):237-246 [DOI] [PubMed] [Google Scholar]
- 4.Kushida CA, Allen RP, Atkinson MJ. Modeling the causal relationships between symptoms associated with restless legs syndrome and the patient-reported impact of RLS. Sleep Med. 2004;5(5):485-488 [DOI] [PubMed] [Google Scholar]
- 5.Winkelman JW, Johnston L. Augmentation and tolerance with long-term pramipexole treatment of restless legs syndrome (RLS). Sleep Med. 2004;5(1):9-14 [DOI] [PubMed] [Google Scholar]
- 6.Bogan RK, Fry JM, Schmidt MH, Carson SW, Ritchie SY. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006;81(1):17-27 [DOI] [PubMed] [Google Scholar]
- 7.Driver-Dunckley ED, Noble BN, Hentz JG, et al. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol. 2007;30(5):249-255 [DOI] [PubMed] [Google Scholar]
- 8.Ferini-Strambi L, Aarskog D, Partinen M, et al. Effect of pramipexole on RLS symptoms and sleep: a randomized, double-blind, placebo-controlled trial. Sleep Med. 2008;9(8):874-881 [DOI] [PubMed] [Google Scholar]
- 9.Trenkwalder C, Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry 2004;75(1):92-97 [PMC free article] [PubMed] [Google Scholar]
- 10.Walters AS, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K. Ropinirole is effective in the treatment of restless legs syndrome: TREAT RLS 2: a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord 2004;19(12):1414-1423 [DOI] [PubMed] [Google Scholar]
- 11.Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology 2006;67(6):1034-1039 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology 2002;59(10):1573-1579 [DOI] [PubMed] [Google Scholar]
- 13.Happe S, Klosch G, Saletu B, Zeitlhofer J. Treatment of idiopathic restless legs syndrome (RLS) with gabapentin. Neurology 2001;57(9):1717-1719 [DOI] [PubMed] [Google Scholar]
- 14.Happe S, Sauter C, Klosch G, Saletu B, Zeitlhofer J. Gabapentin versus ropinirole in the treatment of idiopathic restless legs syndrome. Neuropsychobiology 2003;48(2):82-86 [DOI] [PubMed] [Google Scholar]
- 15.Gidal BE, DeCerce J, Bockbrader HN, et al. Gabapentin bioavailability: effect of dose and frequency of administration in adult patients with epilepsy. Epilepsy Res. 1998;31(2):91-99 [DOI] [PubMed] [Google Scholar]
- 16.Gidal BE, Radulovic LL, Kruger S, Rutecki P, Pitterle M, Bockbrader HN. Inter- and intra-subject variability in gabapentin absorption and absolute bioavailability. Epilepsy Res. 2000;40(2-3):123-127 [DOI] [PubMed] [Google Scholar]
- 17.Kriel RL, Birnbaum AK, Cloyd JC, Ricker BJ, Jones Saete C, Caruso KJ. Failure of absorption of gabapentin after rectal administration. Epilepsia 1997;38(11):1242-1244 [DOI] [PubMed] [Google Scholar]
- 18.Stevenson CM, Kim J, Fleisher D. Colonic absorption of antiepileptic agents. Epilepsia 1997;38(1):63-67 [DOI] [PubMed] [Google Scholar]
- 19.Stewart BH, Kugler AR, Thompson PR, Bockbrader HN. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993;10(2):276-281 [DOI] [PubMed] [Google Scholar]
- 20.Beydoun A, Fakhoury T, Nasreddine W, bou-Khalil B. Conversion to high dose gabapentin monotherapy in patients with medically refractory partial epilepsy. Epilepsia 1998;39(2):188-193 [DOI] [PubMed] [Google Scholar]
- 21.Cundy KC, Annamalai T, Bu L, et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II, improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311(1):324-333 [DOI] [PubMed] [Google Scholar]
- 22.Cundy KC, Branch R, Chernov-Rogan T, et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I, design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J Pharmacol Exp Ther. 2004;311(1):315-323 [DOI] [PubMed] [Google Scholar]
- 23.Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48(12):1378-1388 [DOI] [PubMed] [Google Scholar]
- 24.Kushida CA, Becker PM, Ellenbogan AL, Canafax DM, Barrett RW, XP052 Study Group A randomized, double-blind, placebo-controlled trial of XP13512/GSK1838262 in patients with RLS. Neurology 2009;72(5):439-446 [DOI] [PubMed] [Google Scholar]
- 25.Hening WA, Allen RP. Restless legs syndrome (RLS): the continuing development of diagnostic standards and severity measures. Sleep Med. 2003;4(2):95-97 [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. http://files.hpci.ch/hh/documents/guidelines/hh_gl_helsinki.pdf. http://files.hpci.ch/hh/documents/guidelines/hh_gl_helsinki.pdf Adopted 1964. Revised 1996. Accessed February 3, 2010.
- 27.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR 4th ed, text revised Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 28.National Institute of Mental Health (NIMH) Early clinical drug evaluation unit (ECDEU). clinical global impressions. In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology Revised ed Rockville, MD: NIMH; 1976:218-222 [Google Scholar]
- 29.Allen RP, Kosinski M, Hill-Zabala CE, Calloway MO. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep). Sleep Med. 2008;10(5):531-539 [DOI] [PubMed] [Google Scholar]
- 30.Hays RD, Stewart AL. Sleep measures. In: Stewart AL, Ware JEJ, eds. Measuring Functioning and Well-being: The Medical Outcomes Study Approach Durham, NC: Duke University Press; 1992:235-259 [Google Scholar]
- 31.Abetz L, Vallow SM, Kirsch J, Allen RP, Washburn T, Earley CJ. Validation of the Restless Legs Syndrome Quality of Life questionnaire. Value Health 2005;8(2):157-167 [DOI] [PubMed] [Google Scholar]
- 32.Montplaisir J, Karrasch J, Haan J, Volc D. Ropinirole is effective in the long-term management of restless legs syndrome: a randomized controlled trial. Mov Disord 2006;21(10):1627-1635 [DOI] [PubMed] [Google Scholar]
- 33.Trenkwalder C, Stiasny-Kolster K, Kupsch A, Oertel WH, Koester J, Reess J. Controlled withdrawal of pramipexole after 6 months of open-label treatment in patients with restless legs syndrome. Mov Disord 2006;21(9):1404-1410 [DOI] [PubMed] [Google Scholar]
- 34.Guilleminault C, Cetel M, Philip P. Dopaminergic treatment of restless legs and rebound phenomenon. Neurology 1993;43(2):445 [DOI] [PubMed] [Google Scholar]
- 35.Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008;336(7651):999-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kushida CA, Walters AS, Becker P, et al. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep 2009;32(2):159-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.