Abstract
Nutcracker phenomenon refers to compression of the left renal vein, most commonly between the aorta and the superior mesenteric artery, with impaired blood outflow often accompanied by distention of the distal portion of the vein. The nutcracker syndrome (NCS) is the clinical equivalent of nutcracker phenomenon characterized by a complex of symptoms with substantial variations. Depending on specific manifestations, NCS may be encountered by different medical specialists. Although it may be associated with substantial morbidity, the diagnosis of NCS is often difficult and is commonly delayed. Diagnostic and treatment criteria are not well established, and the natural history of NCS is not well understood. We performed an initial review of the literature through MEDLINE, searching from 1950 to date and using the keywords nutcracker syndrome, nutcracker phenomenon, and renal vein entrapment. We performed additional reviews based on the literature citations of the identified articles. We attempted to elucidate clinical relevance of these conditions and their prominent features and to summarize professional experience.
AMA = aortomesenteric angle; BMI = body mass index; CT = computed tomography; DD = diameter of the distended portion; DN = diameter of the narrowed portion; DUS = Doppler ultrasonography; IVC = inferior vena cava; LRV = left renal vein; MR = magnetic resonance; NCP = nutcracker phenomenon; NCS = nutcracker syndrome; PV = peak velocity; SMA = superior mesenteric artery
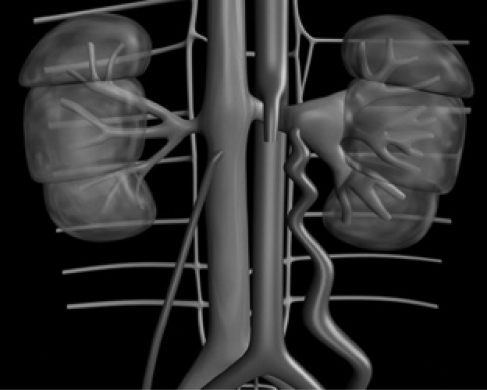
Nutcracker phenomenon (NCP), also known as left renal vein entrapment,1-3 is characterized by impeded outflow from the left renal vein (LRV) into the inferior vena cava (IVC) due to extrinsic LRV compression, often accompanied by demonstrable lateral (hilar) dilatation and medial (mesoaortic) narrowing (schematic representation of NCP/nutcracker syndrome [NCS] in Figure 1). Although the terms nutcracker syndrome and nutcracker phenomenon are sometimes used interchangeably in the literature, Shin and Lee4 emphasize that the nutcracker anatomy is not always associated with clinical symptoms and that some of the anatomic findings suggestive of nutcracker may represent a normal variant or be accounted for by other conditions. Therefore, the term nutcracker syndrome should be reserved for patients with characteristic clinical symptoms associated with demonstrable nutcracker morphologic features. No consensus exists on what symptoms are severe enough to warrant the designation of a clinical syndrome or to what extent various findings may simply reflect different evolutionary stages of the process. Because of these uncertainties, some authors focus on the characteristic anatomic and hemodynamic findings, referring to them as NCP rather than NCS.5
FIGURE 1.
Schematic representation of nutcracker phenomenon. Hilar portion of the left renal vein and the gonadal vein are distended. Distended lumbar and azygous collaterals may be seen in some cases.
The first clinical report of this phenomenon was by El-Sadr and Mina6 in 1950. The term nutcracker is usually credited to de Schepper7 (1972), although it was first used by Chait et al8 (1971); the earliest pathologic description belongs to the anatomist Grant9 (1937). Most typical nutcracker morphologic features imply compression of the LRV between the aorta and the superior mesenteric artery (SMA), known as anterior nutcracker. Less often, the third portion of the duodenum courses in front of the LRV between the aorta and the SMA.10 Therefore, anterior nutcracker is analogous to and may co-occur with compression of the duodenum by the SMA, known as the superior mesenteric artery syndrome (Wilkie syndrome).11-16 The retroaortic or circumaortic renal vein may be compressed between the aorta and the vertebral body, which is called posterior nutcracker.17-20 Theories of causes of NCP include posterior renal ptosis, an abnormally high course of the LRV, and an abnormal SMA branching from the aorta.21-24 Compression of the LRV may also be produced by pancreatic neoplasms, para-aortic lymphadenopathy, retroperitoneal tumors, overarching testicular artery, or strangulating fibrolymphatic tissue between the SMA and the aorta.1,15,25-27 Wendel et al22 described tethering of the left kidney with tight draping of the LRV over the aorta in a patient with prominent lumbar lordosis but no apparent impingement by the SMA. Right-sided NCP due to compression of large veins by the gravid uterus has been described.28
DEMOGRAPHIC CHARACTERISTICS
Because of the variability of symptoms and absence of consensus on diagnostic criteria, the exact prevalence of NCP is unknown but may be slightly higher in females.1,29-31 Patients' age can range from childhood to the seventh decade of life, but most symptomatic patients are in their second or third decade of life; there may be a second peak of NCS in middle-aged women.3 Nutcracker phenomenon is not a hereditary phenomenon, although coincidental cases in siblings have been described.32
CLINICAL FEATURES
The frequency and severity of the syndrome vary from asymptomatic microhematuria to severe pelvic congestion.30,33 Although some patients have severe and persistent symptoms, many, especially children, are asymptomatic.30,34,35 Symptoms are often aggravated by physical activity22 and commonly include hematuria, pain or gonadal vein syndrome,36 varicocele,8,22,36,37 orthostatic proteinuria,19,38-46 and orthostatic intolerance.5,47
Hematuria is the most commonly reported symptom and is attributed to rupture of thin-walled varices, due to elevated venous pressure, into the collecting system.48-50 It varies from microhematuria to macrohematuria, occasionally with resultant anemia that requires blood transfusions.3,50-55 Cystoscopy may identify a left ureteral origin.22,24,50,56,57 In a study by Shin et al,30 the causes of isolated hematuria could not be identified by routine methods in 69% of pediatric cases. Of those, 40% were found to have NCP by renal Doppler ultrasonography (DUS); although microhematuria in these patients was 4 times more common than macrohematuria, there were no differences in peak renal vein systolic velocities.
Pain is the next most common symptom. It is sometimes described as part of the gonadal vein pain syndrome, which is characterized by abdominal or flank pain that occasionally radiates to the posteromedial thigh and buttock. The pain is exacerbated by sitting, standing, walking, or riding in a vehicle that shakes.36,58,59 Left flank pain can also be due to left ureteral colic, from the passing of blood clots down the left ureter.
Varicoceles almost always occur on the left side and affect up to 9.5% of men. Considering the frequency of incompetency or absence of spermatic vein valves, Zerhouni et al25 contend that this finding is irrelevant and that LRV hypertension is the usual cause of varicoceles. The LRV was compressed in 50% to 100% of all patients with varicocele,8,60 although not all patients with varicocele have a distended LRV.10,37 Venous varicosities may be seen internally around the renal pelvis, upper ureter,22 and calyx20,57 and sometimes externally at the buttocks or in the vulvar area.33 Painful symptoms seen during venography were positively correlated with pelvic varices.3
Collateral veins may be evident on pelvic and abdominal DUS or venography.5,61 Positionality of symptoms is a hallmark of NCS and must be correlated with DUS and physical findings.5,62-65 Nutcracker phenomenon is more prominent in upright and supine positions because of visceral proptosis52 and changing aortomesenteric angle (AMA) (the angle between the aorta and the SMA).
Takebayashi et al61 clinically differentiate NCS into 3 subtypes: idiopathic renal bleeding, massive orthostatic proteinuria (protein level >400 mg/dL), and severe orthostatic intolerance that markedly impairs activities of daily living.5 Severe orthostatic intolerance, as previously described by Stewart et al,66 is accompanied by LRV occlusion in 70% of cases. Idiopathic renal bleeding and massive orthostatic proteinuria are seen in 18% and 14% of patients, respectively,5 and are caused by lysis of red blood cells in the urine.67 Degrees of proteinuria vary depending on postural changes.43
Chronic fatigue syndrome and fatigue symptoms have been associated with NCS with high LRV-IVC pressure gradients.19,66,68 Fatigue symptoms correlated positively with high peak velocity (PV) ratios by DUS and improved in some patients after surgery, balloon angioplasty, or aspirin therapy.3,68,69
In venous reflux with formation of collaterals, NCS may become a cause of pelvic congestion.3,33 In these cases, chronic pelvic pain is associated with dyspareunia, dysuria, dysmenorrhea, increased frequency of polycystic changes of the ovaries, and variable venous duplex waveform during Valsalva maneuver.33,37,58,70-75 All patients have demonstrable pelvic varicoceles.37 In a study by Scultetus et al,58 9 of 51 patients with pelvic congestion were diagnosed as having NCS. In some cases, interruption of the gonadal vein inflow improves symptoms. However, gonadal veins may be outflow conduits, and their interruption may worsen NCS symptoms.33
Controversy exists as to whether benign findings, such as otherwise asymptomatic microhematuria or varicocele, are sufficient to render NCP as NCS or at which point such a distinction should be made. It is also unclear why some patients with radiographically demonstrable NCP, and occasionally even with transected LRV, remain asymptomatic, whereas others have clinical sypmtoms. Lower body mass index (BMI) correlates positively with NCP,20,30 and NCP may manifest after weight loss,76 although many individuals with relatively low BMI and aortomesenteric angles have no signs of NCP.65 On the basis of analogy with the SMA syndrome, a decrease in retroperitoneal fat is believed to reduce the AMA and cause NCP. Increased BMI is associated with decreased prevalence of varicoceles.77,78 However, although correlations with body mass hold for groups of people, they are not necessarily applicable to an individual patient.
Possible coexistence of NCS and other morbid conditions makes the diagnosis challenging. Symptoms of NCS may be triggered or aggravated by pregnancy and multiparity.28,33,79-82 Cases of Henoch-Schonlein purpura,83 IgA nephropathy,84,85 membranous nephropathy,67 and idiopathic hypercalciuria with urolithiasis86 concurrent with NCS have been described. Combined NCS and Dumbar syndrome (also known as median arcuate ligament syndrome) has been reported.87 Hartung et al72 had a patient with previously stented May-Thurner syndrome, and we have described a case of combined May-Thurner and NCS.88
NATURAL HISTORY
The natural history of NCP is not well known. Spontaneous resolution of NCP has been described in children, sometimes after several years of persistence.34,83,89-91 A notable study by Takebayashi et al61 correlated ultrasonographic data, renocaval pressure gradients, and presence of collaterals on phlebography with the respective stage of the pathophysiologic process. It appears that the renocaval pressure gradient cannot be predicted by Doppler flow velocities because it depends on the degree of compensatory collateral vein formation.30,61 We encountered a case in which symptoms improved and renocaval pressure gradient resolved in a woman in whom collaterals formed over time.88
Considering the number of minimally symptomatic NCS diagnoses resulting from mass urinalysis screenings for hematuria and cases of NCS resolution in children, some cases of mild NCS may be due to changes in anatomic proportions associated with growth. Why NCP occurs or becomes symptomatic in adults is less clear. The exceptions may be cases of prior trauma, rapid weight loss, or multiparous women who later present with progressive symptoms of pelvic congestion.
DIAGNOSIS
Normal Anatomic and Physiologic Findings
Variations of normal anatomy should be carefully considered before making the diagnosis or predicting the prognosis of NCP/NCS. The left renal vein is 6 to 10 cm in length and, unlike the right renal vein, receives tributaries of the left adrenal, left gonadal, ureteral, and communicating second (and occasionally third) lumbar veins before joining the IVC.1,17 These tributaries have valves preventing reflux of the blood. In NCP, when the valves are absent or incompetent, the tributaries may decompress the LRV. In contrast, the presence of competent valves in NCP may contribute to an increase in renal pressure and resultant formation of varices and collaterals. The average normal LRV diameter is 4 to 5 mm.23 However, its width is not uniform, and its distal diameter is greater than its proximal diameter by 50% in more than half of all individuals.92 Buschi et al10 analyzed computed tomographic (CT), ultrasonographic, and pathologic data in a series of 72 random patients, showing that the ratio of LRV diameters of the distended to the narrowed portions (LRVD/N) may reach 4:1, which is consistent with ultrasonographic data from other authors.44 The normal pressure gradient between the LRV and IVC is 1 mm Hg or lower (mean ± SD, 1.1±0.9 mm Hg; range, 0-2.5 mm Hg), whereas patients with bleeding of unknown origin visualized from the left ureter have significantly higher values (mean ± SD, 5.0±2.0 mm Hg). On the basis of these data, LRV hypertension occurs when the gradient is 3.0 mm Hg.53,54
Normal left gonadal vein diameter approximates 3 mm.37,58,93 Ovarian veins (left more commonly than right) can show reflux in healthy asymptomatic parous women.94 Whether the gonadal vein valves are more commonly absent on the left than on the right is unclear.93,95,96 Ovarian veins may have numerous trunks but normally do not communicate with other venous systems.93
Earlier studies reported that the mean AMA is 38° to 56°.97,98 Later studies reported that it was close to 90° in healthy individuals, with angles smaller than half that in patients with NCP.21,23,24 Other studies suggested half smaller numbers for normal and NCP variants, consistent with earlier reports.65,99 Despite this reported variability, smaller AMAs have been associated with NCP compared with healthy controls; however, when the third portion of the duodenum courses between the aorta and the SMA (duodenal interposition), the LRV compression occurs even with a larger AMA.10 The AMA is highly variable depending on the patient position during examination, with greater compression of the LRV by the SMA in an erect position.99,100 Ozkurt et al101 found a moderate positive correlation between BMI and AMA on CT. Ultimately, positional changes are probably unreliable.5,31,58,102
Diagnostic Approach
It appears that NCS is underdiagnosed, and the path to the correct diagnosis is often long and circuitous because many cases have been identified only through mass urinalysis screenings.30,33,34 Considering the association with a lower BMI, at least some cases of “running” hematuria in athletes may be accounted for by NCS.76 The primary diagnostic test should be a careful physical examination and elicitation of history,3 which alert the clinician to the most common symptoms, their evolution, degree of functional impairment, and possible compensation over time. To rule out more common renal conditions, diagnostic methods include blood examinations, urinalysis, urine culture, cytology, urethrocystoscopy, CT urography, and renal biopsy. In some cases, renal biopsy precedes the final diagnosis of NCS.35,84 In cases of hematuria, the number of isomorphic erythrocytes, suggesting nonglomerular origin, may be variable, and this finding is unreliable.67,102-104
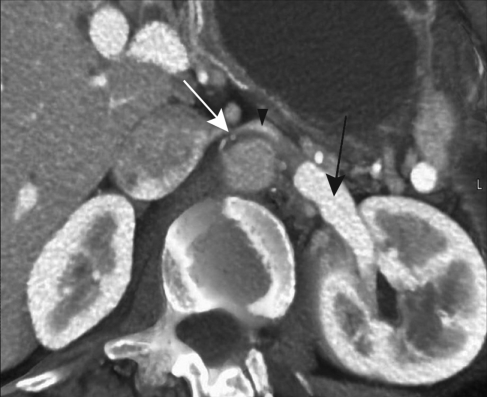
Historically, a number of imaging methods have been used: renal angiography, angiographic CT,17,21,24 digital subtraction angiography,35 standard magnetic resonance (MR) imaging,23 and MR angiography (Figure 2 and Figure 3).18,23,100,105,106 Renal angiography shows delayed venous washout from the left kidney. Intravenous urograms and retrograde pyelograms often reveal normal findings, even in the presence of unilateral hematuria on cystoscopy21,24,107; however, notching from varicosities of the renal pelvis and ureters may be seen.47,56,108
FIGURE 2.
Computed tomographic venogram showing nutcracker phenomenon. Left renal vein is compressed between the aorta and the superior mesenteric artery (white arrow). Left renal vein (black arrowhead) with distended hilar portion (black arrow).
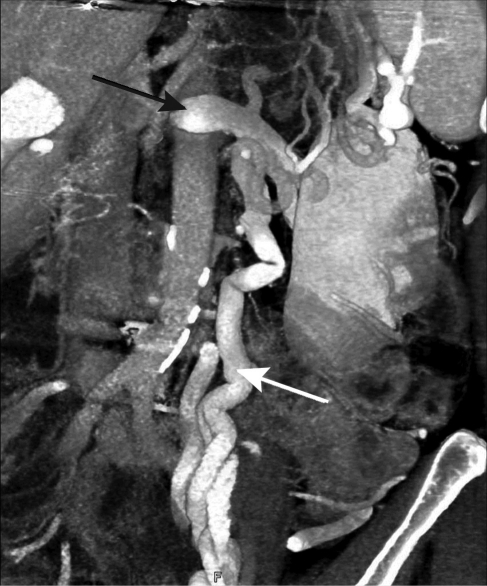
FIGURE 3.
Computed tomographic venogram (digital reconstruction) showing grossly distended gonadal vein (white arrow) in the same patient as in Figure 2. Compression point marked by black arrow.
Attempts to validate LRV diameter ratios have been unsuccessful given that LRV dilatation may be a normal variant.10,92 Standard CT is insufficient to diagnose NCP. However, multiphase CT urography has the ability to reveal other causes of hematuria, such as renal tumors, arteriovenous malformations, and urothelial tumors. It can demonstrate delayed nephrograms in patients with NCP/NCS, can clarify spatial relations between vessels, and is almost always required before surgical interventions to exclude other causes of pathologic findings.
Although regarded as the most informative method, venography (retrograde phlebography) is not commonly performed in patients who do not have severe symptoms, especially in children.108 Venography is not perfectly reliable and occasionally can be misleading.102 Although knowledge of renocaval pressure gradient is extremely helpful, there is some overlap between pressures seen in healthy individuals and those seen in patients with NCS.109
Ultrasound Assessment
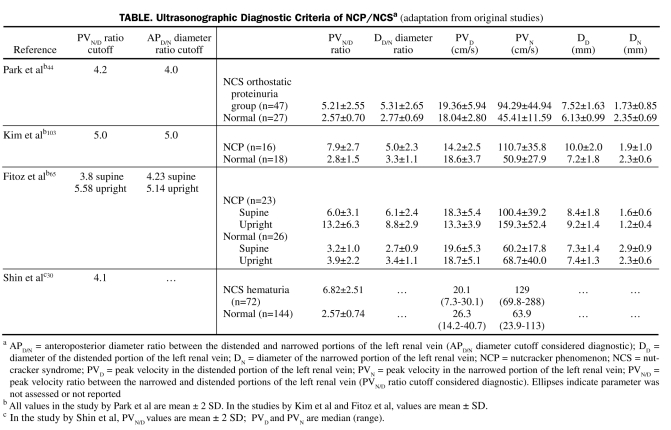
Doppler ultrasonography is a helpful, noninvasive modality and should be the first assessment after NCS is suspected clinically or when a large LRV diameter ratio is noted between its distended and narrowed portions (DD/N) on CT or MR imaging. Whether pediatric and adult DUS criteria should be the same is unclear. A mirror image of the SMA in the aorta (extreme proximal parallel orientation) has 50% sensitivity and 99% specificity for classical NCP.5 Wolfish et al102 proposed diagnostic criteria including standard ultrasonographic findings, positional changes, and urinalysis. Multiple attempts have been made to perfect DUS diagnostic criteria. Takebayashi et al61 found DUS sensitivity and specificity to be 78% and 100%, respectively, and concluded that NCS may exist in either nondistended or distended LRVs; because flow can be normal in a distended LRV, ultrasound assessment is incomplete without assessment of collateral venous flow. Several studies have attempted to validate DUS criteria on the basis of comparing peak ultrasound velocity ratios of the aortomesenteric (narrowed) (PVN) and hilar (distended) (PVD) LRV portions with or without respective diameter ratios (PVN/D and DD/N accordingly). Sensitivity of DUS ranges from 69% to 90%, and specificity from 89% to 100% (summarized findings of these studies are listed in the Table).30,44,65,103 Another study correlated DUS findings with degrees of hematuria in patients with NCP.31
TABLE.
Ultrasonographic Diagnostic Criteria of NCP/NCSa (adaptation from original studies)
Digital subtraction angiography, DUS data, and venography pressures were compared in 93 pediatric cases to develop NCP ultrasound criteria, and 7 ultrasound grades with criteria for definite, probable, and borderline NCP were defined.5 Kim et al103 showed that renocaval pressure gradients did not correlate well with LRV diameter and peak velocity because of high pressure variability. However, only 4 patients with definite NCP morphologic features (LRV stenosis and dilatation) had pressure gradients of 4 mm Hg or lower (suggesting a lack of compensation through collaterals and reflux in early stages), a finding consistent with other studies.58 The gradients decreased in the most extreme forms of NCP (extreme LRV dilatation, stenosis, or occlusion) associated with increased formation of collaterals and reflux.5
Peak velocities are highly variable depending on the position of the patient,5 and thus PV ratios may be more predictive. Transducer compression in a supine position may produce artifacts.5,31 Because DUS findings vary with positional changes, careful assessment will document the variations in supine, Fowler semisitting, upright, and prone positions.5 Despite convenience and affordability, DUS methods are limited by technical difficulties, eg, very small sampling area.5,19,31,44
TREATMENT
Pastershank15 reported the first case of treatment of NCS in 1974. Management options range from observation to nephrectomy, depending on the severity of symptoms. Conservative treatment is recommended for mild hematuria.47 For patients younger than 18 years, the best option is a conservative approach with observation for at least 2 years because as many as 75% of patients will have complete resolution of hematuria.34,110-112 Angiotensin inhibitors may be helpful in improving orthostatic proteinuria in patients with NCS.41,42,45
The correlation between imaging evidence of LRV compression and clinical symptoms remains challenging, and therefore interventions should be considered only when symptoms are severe or persistent, including severe unrelenting pain, severe hematuria, renal insufficiency, and failure to respond to conservative treatment after 24 months.23,24,50,113,114 Most interventions aim to decrease LRV hypertension, but others are directed against pelvic venous reflux. A variety of surgical approaches have been used, including medial nephropexy with excision of renal varicosities,22 LRV bypass,36 LRV transposition with or without Dacron wedge insertion between SMA and aorta,3,20,23,27,64,113,114 SMA transposition,64,108 renal-to-IVC shunt,20 renal autotransplant,18,24,115 gonadocaval bypass,58 and even nephrectomy for persistent hematuria.23
External stenting with ringed polytetrafluoroethylene graft interposition around the LRV2 and intravascular stenting have been applied relatively recently. Both balloon-expandable and self-expanding stents have been used in adult and pediatric patients.2,26,55,64,72,109,111,112,116-119 Intravascular stenting approaches in NCS were extrapolated from the stenting experience in May-Thurner and superior vena cava syndromes.58 Long-term NCS follow-up data are lacking. Reported complications of stenting include stent migration64,72 and, rarely, thrombosis.118 Until stent endothelialization occurs (within 2-3 months), anticoagulation is recommended.116,118 Some patients are successfully managed with aspirin or clopidogrel without long-term anticoagulation.72,117,118 Other potential concerns with stents include stent restenosis, deformities, and erosions at the placement site.
Treatment should be based on severity of symptoms and their expected reversibility with regard to the patient's age and stage of the syndrome.90 Both stenting and open surgical interventions may relieve symptoms64,114; however, selection criteria are not well-defined. Surgical outcomes may be less impressive in patients with lower pressure gradients. Hematuria gradually resolves or decreases substantially postoperatively, but lower pressure gradients may persist after surgical interventions.20,58,114
Ligation of the collateral veins may increase renocaval pressure gradients,36 and ablation of pelvic venous collaterals should be combined with a procedure to relieve renocaval pressure gradients.3 Coil embolization of ovarian veins in patients with pelvic congestion syndrome and demonstrable pelvic varicoceles may provide symptomatic improvement in 56% to 98% of patients. In rare cases, complications include dislodgement of the coils to the lung. Pelvic vein ruptures are substantially more common but benign.37,59,74,114
CONCLUSION
Although NCP and NCS have been recognized for a long time, may be encountered by physicians in a variety of disciplines, and cause substantial morbidity, understanding of these entities is limited. Patients at any age can develop NCP or NCS. Hematuria, pain, pelvic varicosities, and varicoceles are the most common clinical signs that should raise suspicion for the diagnosis. The severity of clinical symptoms varies depending on the stages of the pathologic process, and many findings are nonspecific, creating an overlap with other clinical entities. Although many physicians are aware of NCS, the clinically important distinction from NCP is less commonly appreciated. Insufficient knowledge of the natural history of these conditions results in uncertainty in treatment selection and diagnostic criteria. Although some cases are dramatic and can be treated effectively, recognition of others is delayed, and therapy is uncertain. Many young patients are asymptomatic or have a benign clinical course and may outgrow their symptoms. Those with serious impairment or severe symptoms may benefit from a surgical or intravascular intervention.
REFERENCES
- 1.Cope C, Isard HJ. Left renal vein entrapment: a new diagnostic finding in retroperitoneal disease. Radiology 1969;92(4):867-872 [DOI] [PubMed] [Google Scholar]
- 2.Barnes RW, Fleisher HL, III, Redman JF, Smith JW, Harshfield DL, Ferris EJ. Mesoaortic compression of the left renal vein (the so-called nutcracker syndrome): repair by a new stenting procedure. J Vasc Surg. 1988;8(4):415-421 [DOI] [PubMed] [Google Scholar]
- 3.Rudloff U, Holmes RJ, Prem JT, Faust GR, Moldwin R, Siegel D. Mesoaortic compression of the left renal vein (nutcracker syndrome): case reports and review of the literature. Ann Vasc Surg. 2006;20(1):120-129 [DOI] [PubMed] [Google Scholar]
- 4.Shin JI, Lee JS. Nutcracker phenomenon or nutcracker syndrome [letter]? Nephrol Dial Transplant. 2005;20(9):2015 [DOI] [PubMed] [Google Scholar]
- 5.Takahashi Y, Sano A, Matsuo M. An ultrasonographic classification for diverse clinical symptoms of pediatric nutcracker phenomenon. Clin Nephrol. 2005;64(1):47-54 [DOI] [PubMed] [Google Scholar]
- 6.El-Sadr AR, Mina E. Anatomical and surgical aspects in the operative management of varicocele. Urol Cutaneous Rev. 1950;54(5):257-262 [PubMed] [Google Scholar]
- 7.de Schepper A. “Nutcracker” phenomenon of the renal vein and venous pathology of the left kidney [in Dutch]. J Belge Radiol. 1972;55(5):507-511 [PubMed] [Google Scholar]
- 8.Chait A, Matasar KW, Fabian CE, Mellins HZ. Vascular impressions on the ureters. Am J Roentgenol Radium Ther Nucl Med. 1971;111(4):729-749 [DOI] [PubMed] [Google Scholar]
- 9.Grant JCB. Method of Anatomy Baltimore, MD: Williams & Wilkins; 1937:158 [Google Scholar]
- 10.Buschi AJ, Harrison RB, Norman A, et al. Distended left renal vein: CT/sonographic normal variant. AJR Am J Roentgenol. 1980;135(2):339-342 [DOI] [PubMed] [Google Scholar]
- 11.Hearin JB. Duodenal ileus with special reference to superior mesenteric artery compression. Radiology 1966;86(2):305-310 [DOI] [PubMed] [Google Scholar]
- 12.Wilson-Storey D, MacKinlay GA. The superior mesenteric artery syndrome. J R Coll Surg Edinb. 1986;31(3):175-178 [PubMed] [Google Scholar]
- 13.Bedoya R, Lagman SM, Pennington GP, Kirdnual A. Clinical and radiological aspects of the superior mesenteric artery syndrome. J Fla Med Assoc. 1986;73(9):686-689 [PubMed] [Google Scholar]
- 14.Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome: the disease that isn't, or is it? J Clin Gastroenterol. 1985;7(2):113-116 [DOI] [PubMed] [Google Scholar]
- 15.Pastershank SP. Left renal vein obstruction by a superior mesenteric artery. J Can Assoc Radiol. 1974;25(1):52-54 [PubMed] [Google Scholar]
- 16.Barsoum MK, Shepherd RF, Welch TJ. Patient with both Wilkie syndrome and nutcracker syndrome. Vasc Med. 2008;13(3):247-250 [DOI] [PubMed] [Google Scholar]
- 17.Urban BA, Ratner LE, Fishman EK. Three-dimensional volume-rendered CT angiography of the renal arteries and veins: normal anatomy, variants, and clinical applications. Radiographics 2001;21(2):373-386 [DOI] [PubMed] [Google Scholar]
- 18.Ali-El-Dein B, Osman Y, Shehab El-Din AB, El-Diasty T, Mansour O, Ghoneim MA. Anterior and posterior nutcracker syndrome: a report on 11 cases. Transplant Proc. 2003;35(2):851-853 [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, Ohta S, Sano A, et al. Does severe nutcracker phenomenon cause pediatric chronic fatigue? Clin Nephrol. 2000;53(3):174-181 [PubMed] [Google Scholar]
- 20.Shaper KR, Jackson JE, Williams G. The nutcracker syndrome: an uncommon cause of haematuria. Br J Urol. 1994;74(2):144-146 [DOI] [PubMed] [Google Scholar]
- 21.Fu WJ, Hong BF, Xiao YY, et al. Diagnosis of the nutcracker phenomenon by multislice helical computed tomography angiography. Chin Med J (Engl) 2004;117(12):1873-1875 [PubMed] [Google Scholar]
- 22.Wendel RG, Crawford ED, Hehman KN. The “nutcracker” phenomenon: an unusual cause for renal varicosities with hematuria. J Urol. 1980;123(5):761-763 [DOI] [PubMed] [Google Scholar]
- 23.Hohenfellner M, Steinbach F, Schultz-Lampel D, et al. The nutcracker syndrome: new aspects of pathophysiology, diagnosis and treatment. J Urol. 1991;146(3):685-688 [DOI] [PubMed] [Google Scholar]
- 24.Shokeir AA, el-Diasty TA, Ghoneim MA. The nutcracker syndrome: new methods of diagnosis and treatment. Br J Urol. 1994;74(2):139-143 [DOI] [PubMed] [Google Scholar]
- 25.Zerhouni EA, Siegelman SS, Walsh PC, White RI. Elevated pressure in the left renal vein in patients with varicocele: preliminary observations. J Urol. 1980;123(4):512-513 [DOI] [PubMed] [Google Scholar]
- 26.Neste MG, Narasimham DL, Belcher KK. Endovascular stent placement as a treatment for renal venous hypertension. J Vasc Interv Radiol. 1996;7(6):859-861 [DOI] [PubMed] [Google Scholar]
- 27.Ariyoshi A, Nagase K. Renal hematuria caused by “nutcracker” phenomenon: a more logical surgical management. Urology 1990;35(2):168-170 [DOI] [PubMed] [Google Scholar]
- 28.Radisic MV, Feldman D, Diaz C, Froment RO. Unexplained hematuria during pregnancy: right-sided nutcracker phenomenon. Int Urol Nephrol. 2007;39(3):709-711 [DOI] [PubMed] [Google Scholar]
- 29.Shin JI, Lee JS, Kim MJ. The prevalence, physical characteristics and diagnosis of nutcracker syndrome [letter]. Eur J Vasc Endovasc Surg. 2006;32(3):335-336 [DOI] [PubMed] [Google Scholar]
- 30.Shin JI, Park JM, Lee JS, Kim MJ. Effect of renal Doppler ultrasound on the detection of nutcracker syndrome in children with hematuria. Eur J Pediatr. 2007;166(5):399-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada M, Tsuzuki K, Ito S. Diagnosis of the nutcracker phenomenon using two-dimensional ultrasonography. Clin Nephrol. 1998;49(1):35-40 [PubMed] [Google Scholar]
- 32.Matsukura H, Arai M, Miyawaki T. Nutcracker phenomenon in two siblings of a Japanese family [letter]. Pediatr Nephrol. 2005;20(2):237-238 [DOI] [PubMed] [Google Scholar]
- 33.Rogers A, Beech A, Braithwaite B. Transperitoneal laparoscopic left gonadal vein ligation can be the right treatment option for pelvic congestion symptoms secondary to nutcracker syndrome. Vascular 2007;15(4):238-240 [DOI] [PubMed] [Google Scholar]
- 34.Shin JI, Park JM, Lee SM, et al. Factors affecting spontaneous resolution of hematuria in childhood nutcracker syndrome. Pediatr Nephrol. 2005;20(5):609-613 [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y, Akaishi K, Sano A, Kuroda Y. Intra-arterial digital subtraction angiography for children with idiopathic renal bleeding: a diagnosis of nutcracker phenomenon. [published correction appears in Clin Nephrol. 1989;31(5):280] Clin Nephrol. 1988;30(3):134-140 [PubMed] [Google Scholar]
- 36.Coolsaet BL. Ureteric pathology in relation to right and left gonadal veins. Urology 1978;12(1):40-49 [DOI] [PubMed] [Google Scholar]
- 37.Park SJ, Lim JW, Ko YT, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182(3):683-688 [DOI] [PubMed] [Google Scholar]
- 38.Devarajan P. Mechanisms of orthostatic proteinuria: lessons from a transplant donor. J Am Soc Nephrol. 1993;4(1):36-39 [DOI] [PubMed] [Google Scholar]
- 39.Ekim M, Bakkaloglu SA, Tümer N, Sanlidilek U, Salih M. Orthostatic proteinuria as a result of venous compression (nutcracker phenomenon): a hypothesis testable with modern imaging techniques. Nephrol Dial Transplant. 1999;14(4):826-827 [DOI] [PubMed] [Google Scholar]
- 40.Ekim M, Ozçakar ZB, Fitoz S, et al. The “nutcracker phenomenon” with orthostatic proteinuria: case reports. Clin Nephrol. 2006;65(4):280-283 [DOI] [PubMed] [Google Scholar]
- 41.Ha T-S, Lee E-J. ACE inhibition can improve orthostatic proteinuria associated with nutcracker syndrome. Pediatr Nephrol. 2006;21(11):1765-1768 [DOI] [PubMed] [Google Scholar]
- 42.Ha T-S, Lee E-J. ACE inhibition in orthostatic proteinuria associated with nutcracker syndrome would be individualized [letter reply]. Pediatr Nephrol. 2007;22(5):759-760 [Google Scholar]
- 43.Lee SJ, You ES, Lee JE, Chung EC. Left renal vein entrapment syndrome in two girls with orthostatic proteinuria. Pediatr Nephrol. 1997;11(2):218-220 [DOI] [PubMed] [Google Scholar]
- 44.Park SJ, Lim JW, Cho BS, Yoon TY, Oh JH. Nutcracker syndrome in children with orthostatic proteinuria: diagnosis on the basis of Doppler sonography. J Ultrasound Med. 2002;21(1):39-45 [DOI] [PubMed] [Google Scholar]
- 45.Shin JI, Lee JS. ACE inhibition in nutcracker syndrome with orthostatic proteinuria: how about a hemodynamic effect [letter]? Pediatr Nephrol. 2007;22(5):758 [DOI] [PubMed] [Google Scholar]
- 46.Shintaku N, Takahashi Y, Akaishi K, Sano A, Kuroda Y. Entrapment of left renal vein in children with orthostatic proteinuria. Pediatr Nephrol. 1990;4(4):324-327 [DOI] [PubMed] [Google Scholar]
- 47.Dever DP, Ginsburg ME, Millet DJ, Feinstein MJ, Cockett AT. Nutcracker phenomenon. Urology 1986;27(6):540-542 [DOI] [PubMed] [Google Scholar]
- 48.Beinart C, Sniderman KW, Saddekni S, Weiner M, Vaughan ED, Jr, Sos TA. Left renal vein hypertension: a cause of occult hematuria. Radiology 1982;145(3):647-650 [DOI] [PubMed] [Google Scholar]
- 49.Beckmann CF, Abrams HL. Idiopathic renal vein varices: incidence and significance. Radiology 1982;143(3):649-652 [DOI] [PubMed] [Google Scholar]
- 50.Stewart BH, Reiman G. Left renal venous hypertension “nutcracker” syndrome: managed by direct renocaval reimplantation. Urology 1982;20(4):365-369 [DOI] [PubMed] [Google Scholar]
- 51.Hayashi M, Kume T, Nihira H. Abnormalities of renal venous system and unexplained renal hematuria. J Urol. 1980;124(1):12-16 [DOI] [PubMed] [Google Scholar]
- 52.Sayfan J, Halevy A, Oland J, Nathan H. Varicocele and left renal vein compression. Fertil Steril. 1984;41(3):411-417 [DOI] [PubMed] [Google Scholar]
- 53.Nishimura Y, Fushiki M, Yoshida M, et al. Left renal vein hypertension in patients with left renal bleeding of unknown origin. Radiology 1986;160(3):663-667 [DOI] [PubMed] [Google Scholar]
- 54.Beinart C, Sniderman KW, Tamura S, Vaughan ED, Jr, Sos TA. Left renal vein to inferior vena cava pressure relationship in humans. J Urol. 1982;127(6):1070-1071 [DOI] [PubMed] [Google Scholar]
- 55.Segawa N, Azuma H, Iwamoto Y, et al. Expandable metallic stent placement for nutcracker phenomenon. Urology 1999;53(3):631-633 [DOI] [PubMed] [Google Scholar]
- 56.Weiner SN, Bernstein RG, Morehouse H, Golden RA. Hematuria secondary to left peripelvic and gonadal vein varices. Urology 1983;22(1):81-84 [DOI] [PubMed] [Google Scholar]
- 57.Trambert JJ, Rabin AM, Weiss KL, Tein AB. Pericaliceal varices due to the nutcracker phenomenon. AJR Am J Roentgenol. 1990;154(2):305-306 [DOI] [PubMed] [Google Scholar]
- 58.Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg. 2001;34(5):812-819 [DOI] [PubMed] [Google Scholar]
- 59.Maleux G, Stockx L, Wilms G, Marchal G. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical results. J Vasc Interv Radiol. 2000;11(7):859-864 [DOI] [PubMed] [Google Scholar]
- 60.Unlu M, Orguc S, Serter S, Pekindil G, Pabuscu Y. Anatomic and hemodynamic evaluation of renal venous flow in varicocele formation using color Doppler sonography with emphasis on renal vein entrapment syndrome. Scand J Urol Nephrol. 2007;41(1):42-46 [DOI] [PubMed] [Google Scholar]
- 61.Takebayashi S, Ueki T, Ikeda N, Fujikawa A. Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. AJR Am J Roentgenol. 1999;172(1):39-43 [DOI] [PubMed] [Google Scholar]
- 62.Braedel HU, Schindler E, Polsky MS. Selective renal phlebography in the diagnosis of renal pelvic and ureteric varices. Br J Urol. 1977;49(5):365-370 [DOI] [PubMed] [Google Scholar]
- 63.Stavros AT, Sickler KJ, Menter RR. Color duplex sonography of the nutcracker syndrome (aortomesenteric left renal vein compression). J Ultrasound Med. 1994;13(7):569-574 [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Li M, Jin W, San P, Xu P, Pan S. The left renal entrapment syndrome: diagnosis and treatment. Ann Vasc Surg. 2007;21(2):198-203 [DOI] [PubMed] [Google Scholar]
- 65.Fitoz S, Ekim M, Ozcakar ZB, Elhan AH, Yalcinkaya F. Nutcracker syndrome in children: the role of upright position examination and superior mesenteric artery angle measurement in the diagnosis. J Ultrasound Med. 2007;26(5):573-580 [DOI] [PubMed] [Google Scholar]
- 66.Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr. 1999;135(2, pt 1):218-225 [DOI] [PubMed] [Google Scholar]
- 67.Russo D, Minutolo R, Iaccarino V, Andreucci M, Capuano A, Savino FA. Gross hematuria of uncommon origin: the nutcracker syndrome. Am J Kidney Dis. 1998;32(3):E3 [DOI] [PubMed] [Google Scholar]
- 68.Takahashi Y, Sano A, Matsuo M. An effective “transluminal balloon angioplasty” therapy for pediatric chronic fatigue syndrome with nutcracker phenomenon [letter]. Clin Nephrol. 2000;53(1):77-78 [PubMed] [Google Scholar]
- 69.Shin JI, Lee JS. Can chronic fatigue symptoms associated with nutcracker phenomenon be treated with aspirin [letter]? Med Hypotheses 2007;69(3):704-705 [DOI] [PubMed] [Google Scholar]
- 70.Scholbach T. From the nutcracker-phenomenon of the left renal vein to the midline congestion syndrome as a cause of migraine, headache, back and abdominal pain and functional disorders of pelvic organs. Med Hypotheses 2007;68(6):1318-1327 [DOI] [PubMed] [Google Scholar]
- 71.Gunter J. Chronic pelvic pain: an integrated approach to diagnosis and treatment. Obstet Gynecol Surv. 2003;58(9):615-623 [DOI] [PubMed] [Google Scholar]
- 72.Hartung O, Grisoli D, Boufi M, et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg. 2005;42(2):275-280 [DOI] [PubMed] [Google Scholar]
- 73.Kim HS, Malhotra AD, Rowe PC, Lee JM, Venbrux AC. Embolotherapy for pelvic congestion syndrome: long-term results. J Vasc Interv Radiol. 2006;17(2, pt 1):289-297 [DOI] [PubMed] [Google Scholar]
- 74.Nicholson T, Basile A. Pelvic congestion syndrome, who should we treat and how? Tech Vasc Interv Radiol. 2006;9(1):19-23 [DOI] [PubMed] [Google Scholar]
- 75.Reiter RC. A profile of women with chronic pelvic pain. Clin Obstet Gynecol. 1990;33(1):130-136 [PubMed] [Google Scholar]
- 76.Hanna HE, Santella RN, Zawada ET, Jr, Masterson TE. Nutcracker syndrome: an underdiagnosed cause for hematuria? S D J Med. 1997;50(12):429-436 [PubMed] [Google Scholar]
- 77.Handel LN, Shetty R, Sigman M. The relationship between varicoceles and obesity. J Urol. 2006;176(5):2138-2140 [DOI] [PubMed] [Google Scholar]
- 78.Shin JI, Lee JS. Re: The relationship between varicoceles and obesity [letter]. J Urol. 2007;178(5):2223-2224 [DOI] [PubMed] [Google Scholar]
- 79.Itoh S, Yoshida K, Nakamura Y, Mitsuhashi N. Aggravation of the nutcracker syndrome during pregnancy. Obstet Gynecol. 1997;90(4, pt 2):661-663 [DOI] [PubMed] [Google Scholar]
- 80.Shin JI, Lee JS. Comment on: nutcracker syndrome in pregnancy: a tough nut to crack? (Aust N Z J Obstet Gynaecol 2008 February; 48(1): 119-20) [letter] Aust N Z J Obstet Gynaecol. 2008;48(5):521 [DOI] [PubMed] [Google Scholar]
- 81.Singh R, Griffiths A, Trivedi A. Nutcracker syndrome in pregnancy: a tough nut to crack? Aust N Z J Obstet Gynaecol. 2008;48(1):119-120 [DOI] [PubMed] [Google Scholar]
- 82.Hiromura T, Nishioka T, Nishioka S, Ikeda H, Tomita K. Reflux in the left ovarian vein: analysis of MDCT findings in asymptomatic women. AJR Am J Roentgenol. 2004;183(5):1411-1415 [DOI] [PubMed] [Google Scholar]
- 83.Shin JI, Park JM, Shin YH, Lee JS, Kim MJ. Superimposition of nutcracker syndrome in a haematuric child with Henoch-Schönlein purpura. Int J Clin Pract. 2005;59(12):1472-1475 [DOI] [PubMed] [Google Scholar]
- 84.Ozono Y, Harada T, Namie S, et al. The “nutcracker” phenomenon in combination with IgA nephropathy. J Int Med Res. 1995;23(2):126-131 [DOI] [PubMed] [Google Scholar]
- 85.Shin JI, Park JM, Shin YH, Lee JS, Kim MJ, Jeong HJ. Nutcracker syndrome combined with IgA nephropathy in a child with recurrent hematuria. Pediatr Int. 2006;48(3):324-326 [DOI] [PubMed] [Google Scholar]
- 86.Shin JI, Park JM, Lee JS, Han SW, Kim MJ. Superimposition of nutcracker syndrome in a hematuric child with idiopathic hypercalciuria and urolithiasis. Int J Urol. 2006;13(6):814-816 [DOI] [PubMed] [Google Scholar]
- 87.Linares P, Vivas S, Dominguez A, et al. An uncommon association of abdominal vascular compression syndromes: Dumbar and Nutcracker. Eur J Gastroenterol Hepatol. 2002;14(10):1151-1153 [DOI] [PubMed] [Google Scholar]
- 88.Kurklinsky A, Shepherd R. A case of combined nutcracker and May-Thurner Syndromes in an adult with resolution of hemodynamic findings Presented at: XVI World Meeting of the International Union of Phlebology; August 31-September 4, 2009; Principality of Monaco, France GE3.7-10 [Google Scholar]
- 89.Tanaka H, Waga S. Spontaneous remission of persistent severe hematuria in an adolescent with nutcracker syndrome: seven years' observation. Clin Exp Nephrol. 2004;8(1):68-70 [DOI] [PubMed] [Google Scholar]
- 90.Shin JI, Baek SY, Lee JS, Kim MJ. Follow-up and treatment of nutcracker syndrome [letter]. Ann Vasc Surg. 2007;21(3):402 [DOI] [PubMed] [Google Scholar]
- 91.Shin JI, Park JM, Lee JS, Kim MJ. Morphologically improved nutcracker syndrome in an 11-year-old girl with hematuria. Pediatr Int. 2007;49(5):677-679 [DOI] [PubMed] [Google Scholar]
- 92.Zerin JM, Hernandez RJ, Sedman AB, Kelsch RC. “Dilatation” of the left renal vein on computed tomography in children: a normal variant. Pediatr Radiol. 1991;21(4):267-269 [DOI] [PubMed] [Google Scholar]
- 93.Lechter A, Lopez G, Martinez C, Camacho J. Anatomy of the gonadal veins: a reappraisal. Surgery 1991;109(6):735-739 [PubMed] [Google Scholar]
- 94.Rozenblit AM, Ricci ZJ, Tuvia J, Amis ES., Jr Incompetent and dilated ovarian veins: a common CT finding in asymptomatic parous women. AJR Am J Roentgenol. 2001;176(1):119-122 [DOI] [PubMed] [Google Scholar]
- 95.Ahlberg NE, Bartley O, Chidekel N. Right and left gonadal veins: an anatomical and statistical study. Acta Radiol Diagn (Stockh) 1966;4(6):593-601 [DOI] [PubMed] [Google Scholar]
- 96.Nascimento AB, Mitchell D, Holland G. Gonadal veins: MR imaging findings in an unselected population. Proc Intl Soc Mag Reson Med. 2001;9:2086 http://cds.ismrm.org/ismrm-2001/PDF7/2086.pdf Accessed December 9, 2009 [Google Scholar]
- 97.Derrick JR, Fadhli HA. Surgical anatomy of the superior mesenteric artery. Am Surg. 1965August;31:545-547 [PubMed] [Google Scholar]
- 98.Mansberger AR, Jr, Hearn JB, Byers RM, Fleisig N, Buxton RW. Vascular compression of the duodenum: emphasis on accurate diagnosis. Am J Surg. 1968;115(1):89-96 [DOI] [PubMed] [Google Scholar]
- 99.Arima M, Hosokawa S, Ogino T, Ihara H, Terakawa T, Ikoma F. Ultrasonographically demonstrated nutcracker phenomenon: alternative to angiography. Int Urol Nephrol. 1990;22(1):3-6 [DOI] [PubMed] [Google Scholar]
- 100.Kaneko K, Ohtomo Y, Yamashiro Y, Obinata K, Kurokawa S, Aizawa S. Magnetic resonance angiography in nutcracker phenomenon [letter]. Clin Nephrol. 1999;51(4):259-260 [PubMed] [Google Scholar]
- 101.Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat. 2007;29(7):595-599 [DOI] [PubMed] [Google Scholar]
- 102.Wolfish NM, McLaine PN, Martin D. Renal vein entrapment syndrome: frequency and diagnosis: a lesson in conservatism. Clin Nephrol. 1986;26(2):96-100 [PubMed] [Google Scholar]
- 103.Kim SH, Cho SW, Kim HD, Chung JW, Park JH, Han MC. Nutcracker syndrome: diagnosis with Doppler US. Radiology 1996;198(1):93-97 [DOI] [PubMed] [Google Scholar]
- 104.Felip A, Caralps A, Donoso L, Montserrat E. Diagnosis of left renal vein entrapment syndrome [letter]. Clin Nephrol. 1988;30(1):56 [PubMed] [Google Scholar]
- 105.Chen YM, Wang IK, Ng KK, Huang CC. Nutcracker syndrome: an overlooked cause of hematuria. Chang Gung Med J. 2002;25(10):700-705 [PubMed] [Google Scholar]
- 106.Takemura T, Iwasa H, Yamamoto S, et al. Clinical and radiological features in four adolescents with nutcracker syndrome. Pediatr Nephrol. 2000;14(10-11):1002-1005 [DOI] [PubMed] [Google Scholar]
- 107.Chang CT, Hung CC, Ng KK, Yen TH. Nutcracker syndrome and left unilateral haematuria. Nephrol Dial Transplant. 2005;20(2):460-461 [DOI] [PubMed] [Google Scholar]
- 108.Thompson PN, Darling RC, III, Chang BB, Shah DM, Leather RP. A case of nutcracker syndrome: treatment by mesoaortic transposition. J Vasc Surg. 1992;16(4):663-665 [DOI] [PubMed] [Google Scholar]
- 109.Park YB, Lim SH, Ahn JH, et al. Nutcracker syndrome: intravascular stenting approach. Nephrol Dial Transplant. 2000;15(1):99-101 [DOI] [PubMed] [Google Scholar]
- 110.Kim JY, Joh JH, Choi HY, Do YS, Shin SW, Kim DI. Transposition of the left renal vein in nutcracker syndrome. Eur J Vasc Endovasc Surg. 2006;31(1):80-82 [DOI] [PubMed] [Google Scholar]
- 111.Chiesa R, Anzuini A, Marone EM, et al. Endovascular stenting for the nutcracker phenomenon. J Endovasc Ther. 2001;8(6):652-655 [DOI] [PubMed] [Google Scholar]
- 112.Shin JI, Lee JS, Kim MJ. Re: endovascular stent placement for the treatment of nutcracker phenomenon in three pediatric patients [letter]. J Vasc Interv Radiol. 2006;17(6):1063 [DOI] [PubMed] [Google Scholar]
- 113.Hohenfellner M, D'Elia G, Hampel C, Dahms S, Thüroff JW. Transposition of the left renal vein for treatment of the nutcracker phenomenon: long-term follow-up. Urology 2002;59(3):354-357 [DOI] [PubMed] [Google Scholar]
- 114.Reed NR, Kalra M, Bower TC, Vrtiska TJ, Ricotta JJ, II, Gloviczki P. Left renal vein transposition for nutcracker syndrome. J Vasc Surg. 2009;49(2):386-394 [DOI] [PubMed] [Google Scholar]
- 115.Chuang CK, Chu SH, Lai PC. The nutcracker syndrome managed by autotransplantation. J Urol. 1997;157(5):1833-1834 [PubMed] [Google Scholar]
- 116.Chen W, Chu J, Yang JY, et al. Endovascular stent placement for the treatment of nutcracker phenomenon in three pediatric patients. J Vasc Interv Radiol. 2005;16(11):1529-1533 [DOI] [PubMed] [Google Scholar]
- 117.Basile A, Tsetis D, Calcara G, et al. Percutaneous nitinol stent implantation in the treatment of nutcracker syndrome in young adults. J Vasc Interv Radiol. 2007;18(8):1042-1046 [DOI] [PubMed] [Google Scholar]
- 118.Kim SJ, Kim CW, Kim S, et al. Long-term follow-up after endovascular stent placement for treatment of nutcracker syndrome. J Vasc Interv Radiol. 2005;16(3):428-431 [DOI] [PubMed] [Google Scholar]
- 119.Wei SM, Chen ZD, Zhou M. Intravenous stent placement for treatment of the nutcracker syndrome. J Urol. 2003;170(5):1934-1935 [DOI] [PubMed] [Google Scholar]