Abstract
Pericardial diseases can present clinically as acute pericarditis, pericardial effusion, cardiac tamponade, and constrictive pericarditis. Patients can subsequently develop chronic or recurrent pericarditis. Structural abnormalities including congenitally absent pericardium and pericardial cysts are usually asymptomatic and are uncommon. Clinicians are often faced with several diagnostic and management questions relating to the various pericardial syndromes: What are the diagnostic criteria for the vast array of pericardial diseases? Which diagnostic tools should be used? Who requires hospitalization and who can be treated as an outpatient? Which medical management strategies have the best evidence base? When should corticosteroids be used? When should surgical pericardiectomy be considered? To identify relevant literature, we searched PubMed and MEDLINE using the keywords diagnosis, treatment, management, acute pericarditis, relapsing or recurrent pericarditis, pericardial effusion, cardiac tamponade, constrictive pericarditis, and restrictive cardiomyopathy. Studies were selected on the basis of clinical relevance and the impact on clinical practice. This review represents the currently available evidence and the experiences from the pericardial clinic at our institution to help guide the clinician in answering difficult diagnostic and management questions on pericardial diseases.
CMR = cardiac magnetic resonance imaging; CT = computed tomography; CYP = cytochrome P450; ECG = electrocardiographic; ESC = European Society of Cardiology; IVC = inferior vena cava; LV = left ventricular; NSAID = nonsteroidal anti-inflammatory drug; RA = right atrium; RV = right ventricle
The pericardium is a thin covering that separates the heart from the remaining mediastinal structures and provides structural support while also having a substantial hemodynamic impact on the heart. The pericardium is not essential—normal cardiac function can be maintained in its absence—however, diseased pericardium presenting clinically as acute or chronic recurrent pericarditis, pericardial effusion, cardiac tamponade, and pericardial constriction can be challenging to manage and life-threatening in some cases. The etiology of pericardial disease is often difficult to determine or remains idiopathic. However, microorganisms, including viruses and bacteria; systemic illnesses, including neoplasia, autoimmune disease, and connective tissue disease; renal failure; previous cardiac surgery; previous myocardial infarction; trauma; aortic dissection; radiation; and, rarely, drugs have been associated with pericardial diseases.
The diagnosis and management of pericardial diseases remain challenging because of the vast spectrum of manifestations and the lack of clinical data on which to base guidelines by the American College of Cardiology and the American Heart Association. However, the European Society of Cardiology (ESC) published guidelines on pericardial disease in 2004.1 This review aims to describe the methods of diagnosing and managing major pericardial syndromes on the basis of the literature and the clinical experience of our pericardial clinic. Searches were performed on PubMed and MEDLINE using the keywords diagnosis, treatment, management, acute pericarditis, relapsing or recurrent pericarditis, pericardial effusion, cardiac tamponade, constrictive pericarditis, and restrictive cardiomyopathy. No date limitations were set. Studies were selected on the basis of clinical relevance and the impact on clinical practice.
NORMAL PERICARDIUM
Normal pericardium consists of an outer sac or fibrous pericardium and an inner double-layered sac called the serous pericardium. The 2 layers of serous pericardium include the visceral layer, or epicardium, which covers the heart and proximal great vessels. The visceral layer is reflected to form the parietal pericardium, which lines the fibrous pericardium. The visceral and parietal layers are separated by the pericardial cavity, which in healthy people contains 15 to 50 mL of a plasma ultrafiltrate. The pericardium provides mechanical protection for the heart and lubrication to reduce friction between the heart and surrounding structures. The pericardium also has a considerable hemodynamic effect on the atria and ventricles.
ACUTE PERICARDITIS
Acute pericarditis is a common disorder caused by inflammation of the pericardium and can occur as an isolated entity or as a manifestation of an underlying systemic disease. It is diagnosed in approximately 0.1% of hospitalized patients and in 5% of patients admitted to the emergency department with noncardiac chest pain.2 In most patients, the cause of acute pericarditis is thought to be idiopathic because the yield of diagnostic tests to confirm etiology has been relatively low.3,4 Major known causes include viral and bacterial (eg, tuberculous) microorganisms; systemic diseases, such as autoreactive or immune-mediated diseases; neoplastic invasion of the pericardium; uremia; previous acute myocardial infarction; aortic dissection and chest wall trauma; and previous cardiotomy or thoracic surgery (Table).
TABLE.
Etiology and Estimated Incidence of Acute Pericarditis
Diagnosis
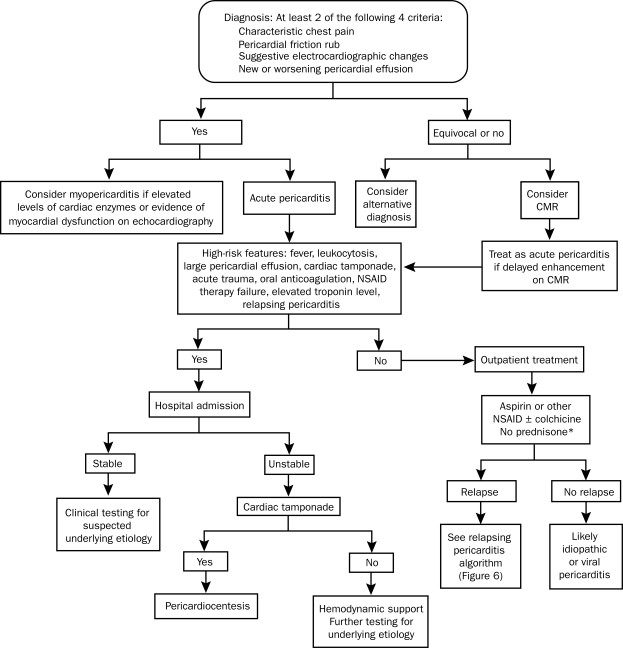
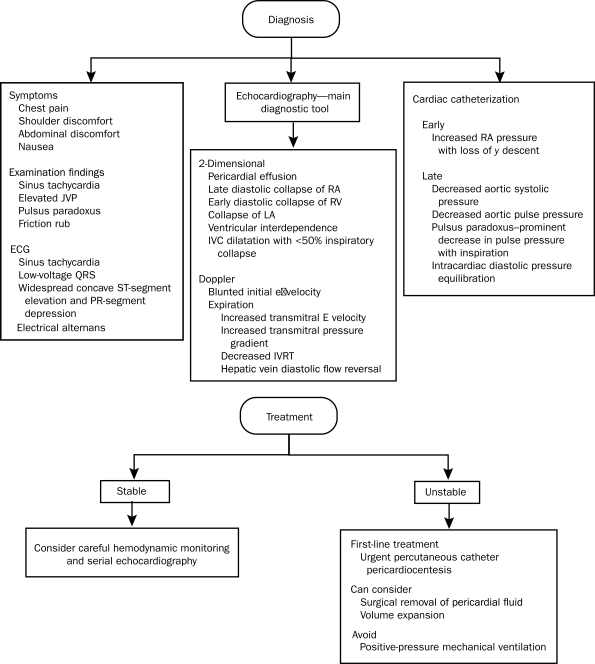
Although no criteria for the diagnosis of acute pericarditis have been established, prior studies6-8 have suggested that at least 2 of the following 4 criteria should be present: (1) characteristic chest pain, (2) pericardial friction rub, (3) suggestive electrocardiographic (ECG) changes, and (4) new or worsening pericardial effusion (Figure 1).
FIGURE 1.
Overview of the diagnosis and management of acute pericarditis. CMR = cardiac magnetic resonance imaging; NSAID = nonsteroidal anti-inflammatory drug.
*Corticosteroids should not be routinely used initially unless there is a rheumatologic etiology or NSAIDs and colchicine are contraindicated.
In some patients, acute pericarditis is accompanied by some degree of myocardial involvement. The diagnosis of myopericarditis implies a predominantly pericardial involvement with associated myocardial inflammation. Clinically, the diagnosis of myopericarditis can be made once pericarditis has been definitively diagnosed and there is evidence of either increased levels of cardiac enzymes, suggesting inflammatory myocardial damage,9 or new onset of focal or diffuse depressed left ventricular (LV) function on imaging in the absence of any other cause.10 An endomyocardial biopsy is not needed for the diagnosis of myopericarditis. A recent observational study by Imazio et al10 suggested the following clues to myocardial involvement in acute pericarditis: male sex, younger age at presentation, presence of cardiac arrhythmias, ST-segment elevation, recent febrile syndrome with gastrointestinal symptoms and/or skeletal muscle myalgias, raised serum cardiac enzyme levels, and evidence of myocardial dysfunction on echocardiography.
Chest Pain
The chest pain of acute pericarditis is usually sudden in onset, retrosternal, and pleuritic in that it is exacerbated by inspiration. The chest pain can be affected by position as patients may note lessening of the pain when they lean forward or are in the upright position. As in myocardial infarction, chest pain can radiate to the neck, arms, or left shoulder; however, radiation of the pain to 1 or both trapezius muscle ridges is probably due to pericarditis because the phrenic nerve that innervates these muscles traverses the pericardium. Dull, oppressive pain can also occur in pericarditis, making it difficult to distinguish from myocardial ischemia.
Pericardial Friction Rub
A pericardial friction rub, which is thought to be generated by friction of the 2 inflamed layers of the pericardium, corresponds to the movement of the heart within the pericardial sac. The rub tends to vary in intensity over time, and thus patients should be examined repeatedly. An audible friction rub, which has been shown to be present in 85% of patients at some time during the course of their disease,3 is highly specific for pericarditis. A pericardial friction rub is usually a high-pitched, scratchy or squeaky sound heard best at the left sternal border. The classic friction rub consists of 3 phases that correspond to the movement of the heart during 3 phases of the cardiac cycle: (1) atrial systole, (2) ventricular systole, and (3) rapid ventricular filling during early diastole.5 However, some rubs are present in only 1 (monophasic) or 2 (biphasic) components of the cardiac cycle,11 and it is difficult to know whether these rubs are truly due to pericardial disease.
Electrocardiography
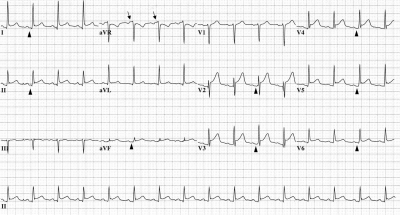
Typical ECG changes in acute pericarditis include wide-spread upward concave ST-segment elevation and PR-segment depression12-15 (Figure 2). However, 4 stages of ECG abnormalities have been described previously.7,8 In stage 1, diffuse ST-segment elevation secondary to epicardial inflammation and PR-segment depression can be seen within the first hours to days with reciprocal ST-segment depression in the aVR and V1 leads. There can also be PR-segment elevation in the aVR lead suggestive of an atrial current of injury. Subsequently, the ST and PR segments normalize (stage 2), followed by the development of widespread T-wave inversions (stage 3). In stage 4, findings on ECG may become normal or the T-wave inversions may persist indefinitely. Typical ECG evolution in acute pericarditis has been shown in up to 60% of patients in a clinical series,16 and stage 1 changes have been observed in 80% of patients with pericarditis.17 However, clinicians must distinguish between ECG changes in acute pericarditis and those in myocardial infarction. A recent retrospective study performed at Mayo Clinic revealed that approximately 17% of all patients with acute pericarditis underwent urgent coronary angiography,18 especially patients with anginal pain, ST-segment elevation, and elevated troponin T values. Although patients with ST-segment elevation myocardial infarction must receive prompt reperfusion, clinicians must also consider the diagnosis of pericarditis to avoid unnecessary coronary angiography. If available, prompt echocardiography can be helpful in this regard by demonstrating normal LV regional contractility.
FIGURE 2.
Electrocardiographic abnormalities in acute pericarditis. Diffuse up-sloping ST-segment elevation is seen in leads I, II, aVF, and V1 to V6. The PR segment is elevated in the aVR lead (arrows) and subtly depressed in leads I, II, aVF, and V2 to V6 (arrowheads). Reciprocal ST-segment depression is seen in the aVR lead.
Serologic Testing
Markers of inflammation, such as white blood cell count, erythrocyte sedimentation rate, and serum C-reactive protein concentration, are usually elevated; however, these tests provide little information about the underlying cause of pericarditis and so are of limited use in devising a strategy for further management. It has been suggested that many patients with idiopathic acute pericarditis have an underlying viral infection; however, viral cultures and antibody titers have not proven to be clinically useful,3,4,19 and the diagnosis of viral acute pericarditis does not alter management. Given the frequency of autoimmune disease as the underlying cause of acute pericarditis, routine serologic testing for antinuclear antibody and rheumatoid factor is of low yield and should be ordered only if the clinical presentation is suggestive of these diseases. Serum cardiac troponin I levels have been shown to be minimally elevated,9,20 presumably as a result of the involvement of the epicardium in the inflammatory process; however, most patients with an elevated troponin level and acute pericarditis have normal findings on coronary angiography.20 An elevated troponin level is not associated with a worse prognosis, and troponin levels usually return to normal within 1 to 2 weeks.5
Chest Radiography
Cardiomegaly with clear lung fields suggests a significant pericardial effusion and indicates at least 200 mL of pericardial fluid.2 However, cardiomegaly is an uncommon finding, and most findings on chest radiography are normal in patients with acute pericarditis.
Echocardiography
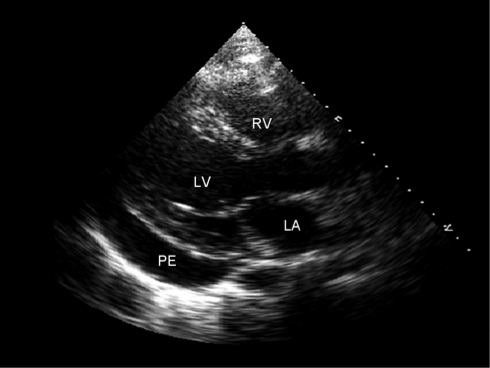
Transthoracic echocardiography is recommended in patients with suspected acute pericarditis who have evidence of hemodynamic compromise.21 The finding of a significant pericardial effusion (Figure 3) supports the diagnosis and guides further management, especially if there is evidence of cardiac tamponade and a need for emergent pericardiocentesis.
FIGURE 3.
Two-dimensional transthoracic echocardiogram showing the parasternal long-axis view in a patient diagnosed as having acute pericarditis. A moderate-sized pericardial effusion (PE) is present posteriorly. LA = left atrium; LV = left ventricle; RV = right ventricle.
Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging
Cardiac computed tomography (CT) and cardiac magnetic resonance imaging (CMR) are increasingly being used in the diagnosis of pericarditis. Both imaging modalities are very sensitive in the detection of generalized or loculated effusions and can also be used to measure pericardial thickness. Normal pericardial thickness is less than 4 mm and is usually 1 to 2 mm.1,22 Cardiac CT can show increased pericardial thickness in a patient with acute pericarditis, but such findings are not diagnostic for pericarditis. The most sensitive method for the diagnosis of acute pericarditis is delayed enhancement of the pericardium on CMR. On CMR, the pericardium normally appears black because of its low water content; however, in patients with pericarditis, enhanced gadolinium uptake in the inflamed pericardium is delayed (Figure 4). Findings on CMR are also helpful in demonstrating myocardial involvement in patients with myopericarditis.
FIGURE 4.
Short-axis cardiac magnetic resonance imaging with delayed gadolinium enhancement in a patient with acute pericarditis. The brightly enhanced pericardium (arrowheads) is suggestive of inflammation in a patient with acute pericarditis.
Initial Evaluation
Because most cases of acute pericarditis follow a benign course, a full diagnostic evaluation is not needed for all patients with acute pericarditis. However, the initial evaluation should identify patients with high-risk features of acute pericarditis, including significant effusion and cardiac tamponade. In addition, tuberculous, purulent, uremic, or neoplastic causes of acute pericarditis should be adequately investigated.
An initial history and physical examination should be performed with particular attention to physical signs of cardiac tamponade, such as pulsus paradoxus and Kussmaul sign with elevated jugular venous pressure. Transthoracic echocardiography would be reasonable in all patients with suspected acute pericarditis who have evidence of hemodynamic compromise.21
Testing should include ECG, chest radiography, complete blood cell count, and sedimentation rate and/or measurement of C-reactive protein concentration for most patients; for patients in whom an autoimmune or infectious disease is suspected, an antinuclear antibody titer, tuberculin skin test or QuantiFERON-TB assay, human immunodeficiency virus serology, or blood cultures should be added. If the history and physical examination findings suggest a specific cause such as malignancy, then appropriate additional tests should be performed. The yield of routine viral studies is low, and management is not usually altered.4 In cases in which the diagnosis of acute pericarditis remains uncertain, CMR may be useful.
Hospital admission is not necessary for all patients with acute pericarditis; however, patients with high-risk features should be hospitalized.16,23 High-risk features include fever (temperature >38°C), leukocytosis, a large pericardial effusion (echo-free space >20 mm), cardiac tamponade, acute trauma, immunosuppressed state, concurrent oral anticoagulation, failure of nonsteroidal anti-inflammatory drug (NSAID) therapy, elevated troponin levels, and recurrent or incessant pericarditis. In a study evaluating patients who had no poor prognostic indicators and who were treated for acute pericarditis without admission to the hospital, no serious complications were reported in 39 months of follow-up.16
Treatment
When the identified etiology of acute pericarditis is not viral or idiopathic, management should be directed toward treating the underlying cause. Patients with no high-risk features can be managed as outpatients. Medical management for viral or idiopathic acute pericarditis has been centered on 3 major agents—NSAIDs, colchicine, and corticosteroids. The management and prognosis of patients with myopericarditis are similar to those of patients with acute pericarditis.10
Nonsteroidal Anti-inflammatory Drugs. The 2004 ESC guidelines recommended the use of an NSAID for the treatment of idiopathic or viral acute pericarditis,1 with the goal of therapy being the relief of pain and the resolution of inflammation. Ibuprofen or aspirin has been most commonly used and provides prompt relief of pain in most patients6,8,16,24 but does not alter the natural history of the disease. High-dose aspirin (800 mg orally every 6 to 8 hours for 7 to 10 days followed by gradual tapering of the dose by 800 mg per week for 3 additional weeks) in combination with gastroprotection with misoprostol (600 to 800 μg/d) or omeprazole (20 mg/d) has been used in multiple studies6,16 and has shown excellent efficacy in patients with mainly idiopathic or presumed viral pericarditis. Indomethacin25 and ketorolac26 have also shown efficacy in treating acute pericarditis. In patients who do not respond to NSAID therapy in 1 week, an etiology other than idiopathic or viral pericarditis is likely.16 Aspirin should be used preferentially in patients with acute pericarditis in the setting of myocardial infarction because of the requirement for antiplatelet therapy; the anti-inflammatory actions of an NSAID other than aspirin may interfere with myocardial healing and scar formation.27-30 Indomethacin should be avoided in patients with coronary artery disease because it decreases coronary blood flow.27 A proton pump inhibitor or another form of gastric protection should be provided in all patients treated with an NSAID.
Colchicine. Colchicine has been shown in observational studies to be effective in relieving pain in patients with acute pericarditis and in preventing recurrences.31,32 Routine use of colchicine in the treatment of acute pericarditis has been supported by the COPE (Colchicine for Acute Pericarditis) trial.6 In this prospective study, patients with a first episode of acute pericarditis were randomized to colchicine plus aspirin or aspirin alone. Colchicine significantly reduced symptoms at 72 hours and recurrence at 18 months, and no patients in the colchicine group developed cardiac tamponade or pericardial constriction.
The major adverse effect prompting discontinuation of colchicine in approximately 8% of patients was diarrhea.6 However, less common published adverse effects of colchicine include bone marrow suppression, hepatotoxicity, and myotoxicity.33-35 Chronic renal insufficiency can lead to increased colchicine levels and appears to be the major risk factor for adverse effects.36,37 In addition, colchicine undergoes extensive hepatic metabolism by cytochrome P450 (CYP) 3A4,38 and thus drugs that interact with CYP can increase the levels of colchicine. Colchicine is also a substrate for P-glycoprotein, a transporter involved in drug elimination. Macrolides inhibit P-glycoprotein and CYP and may impair colchicine elimination, resulting in drug toxicity.35-37,39
We recommend that 4 to 6 weeks of colchicine therapy be considered in all patients with acute pericarditis, especially in patients who have not benefitted from NSAID therapy after 1 week. However, colchicine should be avoided or used with caution in patients with severe renal insufficiency, hepatobiliary dysfunction, blood dyscrasias, and gastrointestinal motility disorders.
Corticosteroids. Although acute pericarditis appears to respond dramatically to corticosteroids, early use of corticosteroids has been associated with an increased risk of relapsing pericarditis in multiple studies.5,6,40 In the COPE trial,6 corticosteroid use was an independent risk factor for recurrence (odds ratio, 4.3). Thus, corticosteroids should only be considered if the patient has clearly received no benefit from NSAID and colchicine therapy and a specific cause for pericarditis has been excluded. The 2004 ESC guidelines1 recommend the use of systemic corticosteroids as an initial treatment of acute pericarditis when the underlying cause is an immune-mediated disease, a connective tissue disorder, or uremic pericarditis. We recommend slow tapering of high-dose prednisone (1 mg/kg per day) when corticosteroid treatment is indicated in acute pericarditis. Tapering should begin after approximately 2 to 4 weeks and only when the patient is asymptomatic and the serum C-reactive protein concentration has normalized. Slow tapering is often the key in preventing recurrence. Intrapericardial administration of corticosteroids has been reported to be effective in acute pericarditis, but its invasive nature limits its clinical utility.41,42
Pericardiocentesis
The 2004 ESC guidelines1 support the use of pericardiocentesis if purulent, tuberculous, or neoplastic pericarditis is suspected. Pericardiocentesis can also be performed for patients with persistent symptomatic pericardial effusions.
RELAPSING PERICARDITIS
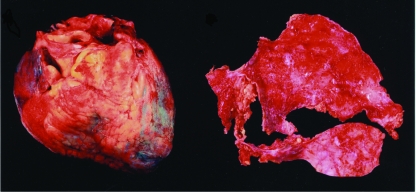
Relapsing pericarditis is one of the most challenging complications of acute pericarditis, and no optimal treatment for this disorder of the pericardium has been definitively established. Relapsing or recurrent pericarditis can be classified as either incessant or intermittent. In the incessant type, discontinuation of or attempts to wean patients from anti-inflammatory treatment (eg, aspirin, indomethacin, ibuprofen) nearly always ensure a relapse in less than 6 weeks.43 This type of relapsing or recurrent pericarditis appears to be particularly frequent in patients receiving corticosteroid therapy; several studies have shown that the mean number of relapses was much higher in those receiving corticosteroid therapy than in those who were not.5,6,40 In the intermittent type, patients have symptom-free intervals of greater than 6 weeks without treatment. Gross anatomic features of recurrent pericarditis are illustrated in Figure 5.
FIGURE 5.
Gross anatomic features of relapsing pericarditis. Left, Anterior view of fibrinous pericardium in a patient with recurrent pericarditis. Right, Thickened fibrinous pericardium after surgical pericardiectomy. Photograph courtesy of William D. Edwards, MD.
The frequency of occurrences of relapsing pericarditis has not been well established because previous studies have used relatively small patient populations. However, the frequency in clinical series including more than 40 patients varies between 8% and 80%,43-45 with an average of 24%, strongly suggesting that recurrent pericarditis is a serious clinical dilemma.
Because recurrent pericarditis often responds to corticosteroid and other immunosuppressive therapies and is characterized by the presence of autoantibodies, its primary etiology is thought to be autoimmune.1,46 Other causes of recurrent pericarditis include viruses or infections and postpericardial and postmyocardial injury syndromes; it may also have an idiopathic etiology.42,47,48 However, tuberculous, purulent, and neoplastic pericarditis are not recognized causes of relapsing pericarditis.49,50
Diagnosis
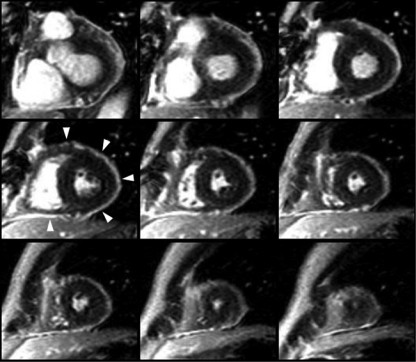
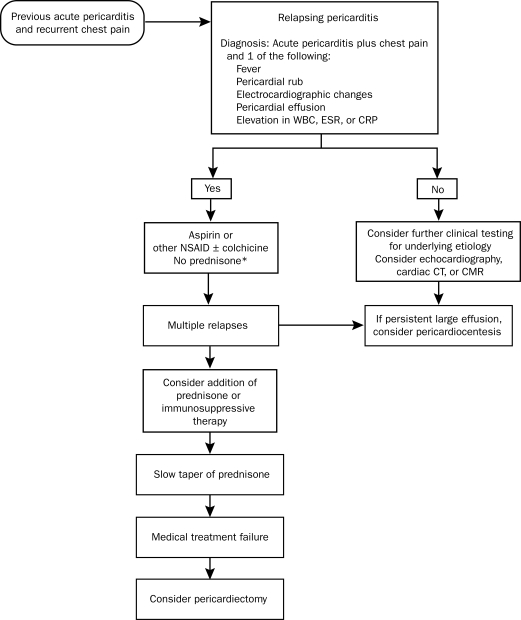
The diagnosis of recurrent pericarditis requires a documented first episode of acute pericarditis and evidence of recurrent pericardial chest pain plus one of the following51: fever, pericardial friction rub, ECG changes typical of acute pericarditis (Figure 2), pericardial effusion on echocardiography, or an elevation in white blood cell count, erythrocyte sedimentation rate, or C-reactive protein concentration (Figure 6). Cardiac CT can be used to evaluate pericardial thickness and pericardial effusions, which can be helpful in the diagnosis of recurrent pericarditis (Figure 7, left). Delayed gadolinium enhancement of the pericardium by CMR (Figure 7, right) is a reliable and objective method to detect inflammation of the pericardium.52
FIGURE 6.
Overview of the management of relapsing pericarditis. CMR = cardiac magnetic resonance imaging; CRP = C-reactive protein; CT = computed tomography; ESR = erythrocyte sedimentation rate; NSAID = nonsteroidal anti-inflammatory drug; WBC = white blood cell count.
*Corticosteroids should not be routinely used initially unless there is a rheumatologic etiology or NSAIDs and colchicine are contraindicated.
FIGURE 7.
Cardiac computed tomography (CT) and cardiac magnetic resonance imaging in chronic relapsing pericarditis. Left, Cardiac CT showing thickened areas of pericardium (arrows) and areas of loculated pericardial effusion (*). Pericardium of normal thickness (arrowheads) is also present in this patient with chronic recurrent pericarditis. Right, Cardiac magnetic resonance imaging from the same patient shows a delayed gadolinium enhancement short-axis image. The brightly enhanced pericardium (arrows) is suggestive of inflammation. A loculated pericardial effusion (*) is also shown.
The first symptoms of recurrent pericarditis usually occur within 18 to 20 months after the initial attack.6,53 The initial episode of acute pericarditis is usually more severe than subsequent episodes. Chest pain is usually sharp and progressive, worsens with recumbency, and is relieved by leaning forward. Most patients are well between attacks; however, some patients have a more persistent or chronic course.41,42
Treatment
Although constrictive pericarditis, myocardial disease, and tamponade are rare in patients with relapsing pericarditis, incessant recurrences can severely impair quality of life in some patients.43 The goals of management should be symptomatic relief, prevention of recurrences, and restriction of physical activity. The underlying disease should be treated in recurrent pericarditis. Idiopathic and viral relapsing pericarditis can be treated with aspirin (650 mg orally every 4-6 hours), other NSAIDs (eg, ibuprofen, 200-400 mg every 4-6 hours, and indomethacin, 25-50 mg every 6-8 hours), or acetaminophen (500-750 mg every 4-6 hours), alone or in combination.43 Several studies have shown that colchicine is effective in preventing recurrences.6,31,51 The COPE trial6 demonstrated that colchicine was effective in preventing recurrent pericarditis if given after the first episode of acute pericarditis, and the Colchicine for Recurrent Pericarditis (CORE) trial51 demonstrated that colchicine was effective in preventing relapses when given after the first episode of recurrent pericarditis. Thus, colchicine can be administered to patients after the first episode of acute pericarditis but should be avoided or used with caution in patients with severe renal insufficiency, hepatobiliary dysfunction, blood dyscrasias, and gastrointestinal motility disorders. Starting dosages of 2 to 3 mg/d can be followed by maintenance dosages of 0.5 to 1 mg/d, which should be given for at least 1 year after the last episode of pericarditis.
Although current guidelines recommend limiting the use of corticosteroids in the treatment of relapsing pericarditis, corticosteroids are administered in 60% to 90% of patients in most clinical series.54 Corticosteroids should be avoided when possible because studies have suggested that administration may favor relapses; however, some authors have advocated that the administration of a short course of high-dose prednisolone may reduce the frequency of relapse.55,56 We recommend limiting corticosteroid treatment to patients in whom NSAIDs and colchicine are contraindicated or in whom recurrent pericarditis has an autoimmune or rheumatologic etiology. In our clinical experience with patients already receiving corticosteroid therapy for relapsing pericarditis, high-dose NSAIDs or aspirin for several months with a very slow taper of prednisone by 1-mg decrements weekly or biweekly has been effective in preventing recurrences. Other clinicians have also reported the usefulness of low-dose corticosteroids with slow tapers in the treatment of recurrent pericarditis.57,58
Although pericardiectomy has been proposed for the relief of refractory relapsing pericarditis, it does not always result in cessation of recurrences. However, pericardiectomy should be considered in patients with severe relapsing pericarditis that has not responded to adequate treatment.1 Our preliminary experience indicates that pericardiectomy in this setting may be beneficial. Of 41 patients who had a pericardiectomy at Mayo Clinic between 1975 and 2000 for chronic relapsing pericarditis and whose records were retrospectively reviewed, 25 (61%) had follow-up at 1 year; at follow-up, all patients reported symptomatic improvement, and 23 (92%) were no longer taking corticosteroids.59
CARDIAC TAMPONADE
Cardiac tamponade is characterized by the accumulation of pericardial fluid under pressure. As the pericardial effusion increases, the movement of the parietal pericardium decreases. Tamponade occurs when all cardiac chambers are compressed as a result of increased intrapericardial pressure to the point of compromising systemic venous return to the right atrium (RA).2,7 Increased intrapericardial pressure reduces the myocardial transmural pressure, and the cardiac chambers become smaller, with reduced chamber diastolic compliance and a decrease in cardiac output and blood pressure. Despite tamponade, much of the inspiratory decline in thoracic pressure is transmitted through the pericardium to the right side of the heart, resulting in an increase in systemic venous return with inspiration and distention of the right ventricle (RV). However, once the intrapericardial pressure is high enough, the pericardium prevents the free wall of the RV from expanding. Expansion is limited to the interventricular septum, resulting in bulging of the RV into the LV. This bulging results in reduced LV compliance and decreased filling of the LV during inspiration.2,7
Occurrences of tamponade can be acute, subacute, regional, or characterized by low pressure. Acute tamponade is sudden, life-threatening if not treated promptly, and often associated with hypotension as well as chest pain and dyspnea. Cardiac tamponade should be considered in patients in cardiogenic shock, especially if they have increased jugular venous pressure or pulseless electrical activity. In the current era, invasive cardiac procedures have become one of the most common causes of acute tamponade.60 Many of these procedures require intraprocedural anticoagulation and left atrial access using transseptal puncture and thus carry the risk of pericardial effusion and tamponade.
Diagnosis
Physical Examination. Depending on the type and severity of tamponade, a variety of physical findings may be present (Figure 8).1 Chest pain is the result of fluid accumulation within the pericardium and acute pericardial irritation. Typically, substernal chest discomfort radiates up to the neck and jaw. Atypical symptoms include shoulder discomfort, abdominal discomfort, or even nausea. In patients with subacute tamponade, a prominent presenting symptom can be right upper quadrant pain due to hepatic venous congestion and peripheral edema. Sinus tachycardia occurs in most patients as a physiologic response to maintain cardiac output. The jugular venous pressure is almost always elevated, with preservation of the x descent but absence or attenuation of the y descent due to a blunting of diastolic filling of the ventricle. Pulsus paradoxus, which is an exaggeration of the normal variation in the pulse during the inspiratory phase of respiration, is commonly found in tamponade and can be quantified by an inspiratory reduction in systolic blood pressure of greater than 10 mm Hg. Pulsus paradoxus has been shown to be predictive of the severity of cardiac tamponade.61 A pericardial friction rub, while not common, can also be heard in cardiac tamponade if the underlying cause is an inflammatory pericarditis.7 Kussmaul sign is an elevation in the jugular venous pressure during inspiration and can be seen in tamponade, but not usually in the absence of pericardial constriction.
FIGURE 8.
Overview of the diagnosis and management of cardiac tamponade. E = early diastolic filling; ECG = electrocardiography; IVC = inferior vena cava; IVRT = isovolumic relaxation time; JVP = jugular venous pressure; LA = left atrium; RA = right atrium; RV = right ventricle.
Electrocardiography. Several ECG findings are associated with tamponade. Sinus tachycardia, low-voltage QRS complex, and typical ECG findings in acute pericarditis, including widespread upward concave ST-segment elevation and PR-segment depression, can all be found in cardiac tamponade. Low-voltage QRS complex, defined as a maximum QRS amplitude of less than 0.5 mV in the limb leads, has been shown to resolve within 1 week after treatment of tamponade by pericardiocentesis or anti-inflammatory medications.62 Electrical alternans, defined as the alteration of the QRS complex amplitude or axis between beats, is specific but not very sensitive for cardiac tamponade.2 Electrical alternans reflects the swinging motion of the heart in pericardial fluid.
Chest Radiography. In acute tamponade, findings on chest radiography are usually normal, and the cardiac silhouette does not enlarge until at least 200 mL of pericardial fluid has accumulated.2 However, as the pericardial effusion becomes larger, the cardiac shadow becomes globular on chest radiography.
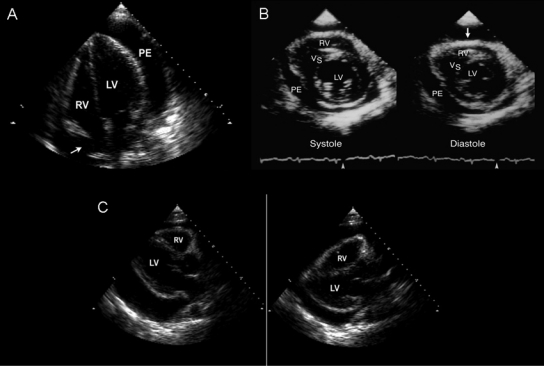
Imaging Studies. The use of echocardiography in cardiac tamponade is a class I indication.21 Typical echocardiographic findings in tamponade (Figure 963) include late diastolic collapse of the RA and early diastolic collapse of the RV when the intrapericardial pressure exceeds intracavitary pressure.1,64,65 Maximal pericardial pressure in tamponade occurs during end-diastole, when RA volume is minimal, causing buckling of the RA.66 Persistence of RA collapse for more than one-third of the cardiac cycle is highly sensitive and specific for tamponade.66 The collapse of right heart chambers does not occur even with tamponade if right heart pressure is elevated. Left atrial collapse, which can also occur in tamponade, is very specific1,64,67 but is not sensitive for tamponade. Left ventricular collapse is rare because of the muscular nature of the LV wall.1,64,68 When cardiac tamponade is due to a life-threatening condition, such as aortic dissection or cardiac free wall rupture, 2-dimensional echocardiography may show a coagulated mass within the pericardium.
FIGURE 9.
Two-dimensional echocardiographic features of cardiac tamponade. A, Still-frame image of an apical 4-chamber view showing late diastolic collapse of the right atrium (RA, arrow). Persistence of RA collapse for more than one-third of the cardiac cycle is highly sensitive and specific for tamponade. B, Early diastolic collapse (arrow) of the right ventricle (RV) is specific for tamponade. C, Parasternal long-axis views showing the swinging motion of the heart within the pericardial cavity of a large pericardial effusion; the swinging motion is responsible for the electrocardiographic manifestation termed electrical alternans. LV = left ventricle; PE = pericardial effusion; VS = ventricular septum.
Adapted from reference 63.
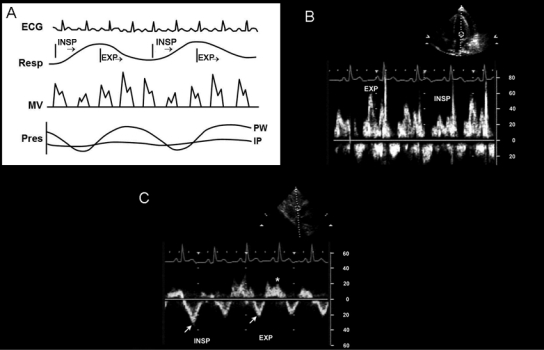
During respiration, reciprocal changes occur in the RV and LV and are indicative of ventricular interdependence. During inspiration, the interventricular septum bulges into the LV due to increased systemic venous return to the RV and limited expansion of the RV free wall due to the increase in intrapericardial pressure. With expiration, the transmitral pressure gradient increases and systemic venous return decreases with reversal of diastolic flow in the hepatic veins (Figure 1063). Additional echocardiographic findings in tamponade include inferior vena cava (IVC) dilatation, with less than a 50% reduction in the diameter of the IVC during inspiration.69
FIGURE 10.
Doppler echocardiographic features in cardiac tamponade. A, Schematic diagram of simultaneous electrocardiographic (ECG), respirometer (Resp), mitral valve (MV) Doppler, and pressure (Pres) changes in intrapericardial (IP) and pulmonary capillary wedge (PW) pressure. Intrapericardial pressure does not change much with respiration, whereas PW pressure decreases with inspiration (INSP), which results in respiratory variation in left ventricular filling and MV inflow Doppler velocity recording. B, Doppler mitral inflow velocities with respiratory variation; mitral E velocity is higher with expiration (EXP) than with INSP. C, Pulsed-wave Doppler recording of the hepatic vein velocities; forward flow (arrows) decreases with EXP and diastolic flow reversal increases (*) with EXP.
Adapted from reference 63.
Cardiac CT and CMR are not usually needed if 2-dimensional and Doppler echocardiography are available. However, cardiac CT may be able to detect early signs of tamponade. Findings such as pericardial effusion, ventricular interdependence, RA and RV collapse, and IVC and hepatic vein distention can be detected by CT.70,71 A loculated pericardial effusion or hematoma soon after cardiac surgery often escapes detection by transthoracic echocardiography. Cardiac CT or transesophageal echocardiography can be useful to diagnose such conditions in hemodynamically unstable patients during the postoperative period.
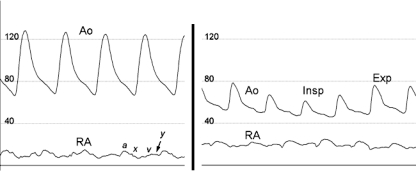
Cardiac Catheterization. Cardiac catheterization is not usually performed for the diagnosis of acute tamponade, but as percutaneous procedures become more complex, procedure-related iatrogenic acute cardiac tamponade is becoming more common, especially in the setting of intraprocedural anticoagulation. Prompt recognition is essential so that hemodynamic collapse can be averted. Various hemodynamic findings in the catheterization laboratory can provide indirect evidence of cardiac perforation and subsequent tamponade.60 Although hypotension is a hallmark of tamponade, systemic aortic pressure and heart rate can increase in the early stages of acute tamponade as a result of the sympathetic response to pericardial irritation. In early tamponade, RA pressure will begin to increase, with a loss of y descent and a more pronounced a wave (Figure 11) at the time of atrial contraction in patients who are in sinus rhythm. As cardiac tamponade progresses, aortic systolic pressure and pulse pressure decrease and pulsus paradoxus develops, as evidenced by a more pronounced decrease in pulse pressure during inspiration. In contrast to aortic pressure, RA pressure continues to increase significantly (Figure 11). Other important hemodynamic changes include intracardiac diastolic pressure equilibration.
FIGURE 11.
Hemodynamics of acute cardiac tamponade by cardiac catheterization. Left, In the very early stages of cardiac tamponade, the right atrial (RA) pressure is mildly elevated, with a more pronounced a wave and a diminution of rapid y descent (arrow) suggesting left ventricular filling abnormalities. The aortic pressure (Ao) has not yet decreased because of increased vasoconstriction, and the pulse pressure remains normal. Right, As tamponade progresses, the Ao and pulse pressure significantly decrease. The further drop in Ao during inspiration (Insp) compared with expiration (Exp) is evidence of pulsus paradoxus. a = a wave; x = x descent; v = v wave.
Treatment
Acute cardiac tamponade with hemodynamic compromise requires urgent pericardiocentesis or surgical removal of pericardial fluid.72 However, in hemodynamically stable patients, careful hemodynamic monitoring with serial echocardiography and treatment of the underlying cause of tamponade may be sufficient. Pericardiocentesis, however, is the most direct means to relieve cardiac tamponade. In hypotensive patients, volume expansion with saline, blood, plasma, and dextran can be used as a temporary measure.73 Volume expansion has been associated with increased intrapericardial pressure, RA pressure, and LV end-diastolic pressure.74 Inotropic therapy remains controversial in cardiac tamponade because endogenous inotropic stimulation is usually maximal in tamponade.21 Positive-pressure mechanical ventilation should be avoided in acute tamponade because it further reduces cardiac filling.75
Evacuation of pericardial fluid in cardiac tamponade can be achieved by percutaneous catheter pericardiocentesis or surgical pericardiectomy. In patients with malignant effusions, percutaneous balloon pericardiotomy is an alternative approach.76,77 Percutaneous catheter pericardiocentesis is the treatment of choice in most patients1 and is less expensive and less invasive than surgery, while allowing for accurate hemodynamic measurement. At Mayo Clinic, pericardiocentesis is almost always performed under echocardiographic guidance. Echocardiography identifies the optimal site for pericardiocentesis by visualizing the location and distribution of pericardial effusion. Evaluation of the Mayo Clinic Echocardiography-Guided Pericardiocentesis Registry from 1979 to 2000 revealed that 1127 therapeutic echocardiography-guided pericardiocenteses were performed in 977 patients.78 The procedural success rate was 97% overall, with a total complication rate of 4.7% and only 1 procedural death. The para-apical site was the most common entry site for pericardiocentesis and, in 89% of procedures, only 1 attempt at needle passage was necessary to gain access to the pericardial space. The following tests should be performed on the pericardial fluid, as clinically indicated: Gram stain, bacterial cultures, acid-fast bacilli and culture, polymerase chain reaction, and cytology. In the setting of aortic dissection, pericardiocentesis is relatively contraindicated, and an urgent surgical evaluation should be obtained.
PERICARDIAL EFFUSION WITHOUT TAMPONADE
Pericardial effusions can occur in the absence of cardiac tamponade.16 However, nearly one-third of patients with large idiopathic pericardial effusions developed cardiac tamponade unexpectedly,79 and some experts recommend that large pericardial effusions (>20 mm) be drained if the effusion persists for more than a month or if there is right-sided collapse.75 If smaller pericardial effusions recur and the patient remains asymptomatic without hemodynamic compromise, regular follow-up with clinical examination and/or echocardiography is recommended. When recurrent pericardial effusions are related to chylopericardium, the underlying etiology may be thoracic duct obstruction, requiring surgical treatment.80
CONSTRICTIVE PERICARDITIS
Constrictive pericarditis is a potentially curable entity in patients with heart failure and normal ejection fraction; however, it is not well appreciated and is underdiagnosed because of the difficulty in differentiating it from restrictive cardiomyopathy or other entities responsible for predominantly right-sided heart failure. Even after extensive testing with 2-dimensional and Doppler echocardiography, cardiac CT and CMR, and conventional cardiac catheterization, the diagnosis may remain equivocal.81-83 However, with new insights and advances in noninvasive imaging, a definitive diagnosis of pericardial constriction is becoming easier to establish.
Etiology
Constrictive pericarditis can occur after any pericardial disease process. In developed nations, idiopathic causes and cardiac surgery are the 2 most predominant underlying etiologies, followed by pericarditis and mediastinal radiation therapy.83,84 In developing and underdeveloped nations, as well as in immunosuppressed patients, tuberculosis is a major cause of constrictive pericarditis.85 Miscellaneous causes of constrictive pericarditis include connective tissue disorder, malignancy, trauma, medications, asbestosis, sarcoidosis, and uremic pericarditis.
Restrictive cardiomyopathy, a rarer disorder, is often seen in patients with a systemic disorder, such as amyloidosis, sarcoidosis, hypereosinophilic syndromes, endomyocardial fibrosis, and disorders caused by chemotherapy or radiation. Restrictive hemodynamic features without restrictive cardiomyopathy can also arise in any myocardial disease, including hypertrophic, dilated, hypertensive, and ischemic heart disease.
Pathophysiology
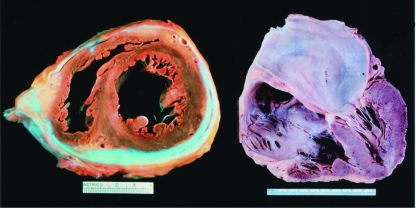
Both constrictive pericarditis and restrictive cardiomyopathy limit diastolic filling and result in diastolic heart failure, with relatively preserved global systolic function. In constrictive pericarditis, diastolic filling is restricted by an inelastic pericardium after an initial expansion of the myocardium. The upper limit of ventricular volume is constrained by an inflamed, scarred, or calcified pericardium (Figure 12, left) that is usually, but not always, thicker than normal.1,86,87 Restrictive cardiomyopathy (Figure 12, right) is defined by a nondilated ventricle with a rigid myocardium that causes a major decrease in the effective operative compliance of the heart muscle itself.88 This decrease distinguishes it from constrictive pericarditis, in which no such decrease in myocardial compliance is usually seen.
FIGURE 12.
Gross pathological features of constrictive pericarditis and restrictive cardiomyopathy. Left, Heart specimen of a patient who died with constrictive pericarditis. The pericardium is thickened and calcified; fibrosis and adhesion of the pericardial layers can be observed. Diastolic filling of the right and left ventricles was markedly reduced. Right, Heart specimen of a patient who died with restrictive cardiomyopathy. Diastolic filling is limited by an abnormal myocardium, resulting in biatrial enlargement. The ventricles are not dilated in restrictive cardiomyopathy. Photograph courtesy of William D. Edwards, MD.
Diagnosis
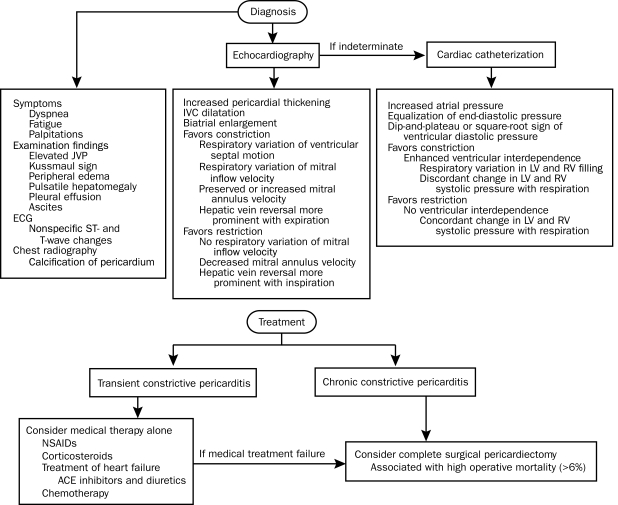
History. A history of cardiac surgery or a systemic disease that affects the pericardium makes the diagnosis of constrictive pericarditis more likely, whereas a history of an infiltrative disease that may involve the heart muscle favors the diagnosis of restrictive cardiomyopathy. In both conditions, the most common symptoms are related to either fluid overload (eg, peripheral edema, elevated central venous pressure, hepatomegaly, pleural effusion, ascites, and anasarca) or decreased cardiac output (eg, dyspnea on exertion, fatigue, palpitations, weakness, and exercise intolerance) (Figure 13).
FIGURE 13.
Overview of the diagnosis and management of constrictive pericarditis. ACE = angiotensin-converting enzyme; ECG = electrocardiography; IVC = inferior vena cava; JVP = jugular venous pressure; LV = left ventricle; NSAID = nonsteroidal anti-inflammatory drug; RV = right ventricle.
Physical Examination. The cardiovascular examination may not differentiate constrictive pericarditis from restrictive cardiomyopathy. The jugular venous pressure is always elevated in constrictive pericarditis with a deep, steep y descent. Evidence of Kussmaul sign and a pericardial knock favor constrictive pericarditis. However, a pericardial knock may be difficult to distinguish from an S3 because the origin of a pericardial knock and S3 is similar in that the ventricular wall vibrates with rapid early diastolic filling. Both coincide with the nadir of the y descent. Pulsus paradoxus, a finding in tamponade, is uncommon in constrictive pericarditis.
Electrocardiography. Electrocardiography may assist in distinguishing constrictive pericarditis from restrictive cardiomyopathy but is never diagnostic. Nonspecific ST-segment and T-wave changes are common features of constrictive pericarditis, whereas low QRS voltage and isolated repolarization abnormalities are more typical in constrictive pericarditis. Depolarization abnormalities, such as bundle branch block, ventricular hypertrophy, pathologic Q waves, and impaired atrioventricular conduction, strongly favor restrictive cardiomyopathy. Late-stage atrial fibrillation can occur in both conditions.
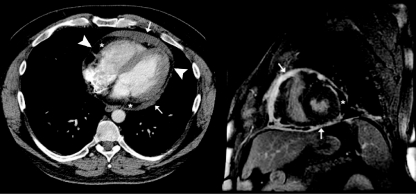
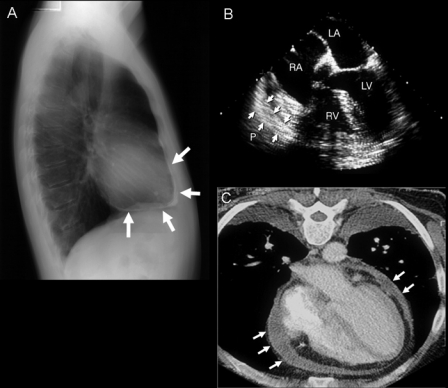
Chest Radiography. Calcification of the pericardium (Figure 14, A63) strongly suggests constrictive pericarditis in patients with heart failure. It is best seen from a lateral view and located over the RV and diaphragmatic surfaces of the heart. In our most recent review,22 only 25% of patients with constrictive pericarditis were found to have pericardial calcification on chest radiography. Pericardial calcification is associated with a longer duration of the constrictive process but not with a specific etiology. Therefore, absence of calcification does not exclude constrictive pericarditis.
FIGURE 14.
Chest radiograph, transesophageal echocardiogram (TEE), and cardiac computed tomogram typical of constrictive pericarditis. A, Pericardial calcification (arrows) on chest radiography is best seen from the lateral view over the right ventricle (RV) and across the diaphragmatic surface of the heart. Pericardial calcification reflects chronicity of constrictive pericarditis and is associated with a higher surgical mortality. B, Thickness of the pericardium is often difficult to determine by transthoracic echocardiography, but TEE is usually reliable in measuring the pericardial thickness (arrows). C, Increased pericardial thickness (arrows) can be visualized on computed tomogram of the same patient. LA = left atrium; LV = left ventricle; RA = right atrium.
Adapted from reference 63.
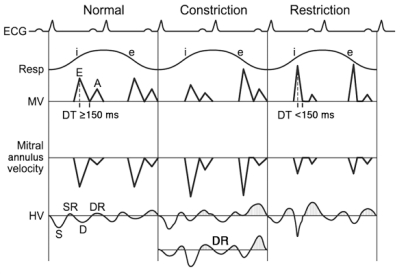
Echocardiography. Echocardiography is usually the initial diagnostic imaging and hemodynamic study in patients with suspected constrictive pericarditis. Its diagnostic accuracy for constrictive pericarditis has increased since characteristic hemodynamic changes and mitral annulus motion were identified. Left ventricular systolic function as judged by the ejection fraction is typically normal but may be impaired in mixed constrictive-restrictive disease. The following findings may be present with echocardiography in constrictive pericarditis1,7,89-94: (1) increased pericardial thickness (measured with greater sensitivity by transesophageal echocardiography and cardiac CT than transthoracic echocardiography [Figure 1463]); however, constrictive pericarditis can occur without increased pericardial thickness; (2) abnormal ventricular septal motion; (3) dilatation and absent or diminished collapse of the IVC and hepatic veins; (4) restrictive mitral and tricuspid inflow velocities, typically (but not always) with respiratory variation (Figure 1563); (5) preserved or increased medial mitral annulus early diastolic (e′) velocity (Figure 15), which is an important distinction from restrictive cardiomyopathy in which the e′ is diminished with a cutoff value of 7 cm/s95; and (6) increased hepatic vein flow reversal with expiration, reflecting the ventricular interaction and the dissociation of the intracardiac and intrathoracic pressures (Figure 1563).
FIGURE 15.
Schematic diagram of Doppler echocardiographic features in constrictive pericarditis vs restrictive cardiomyopathy. Schematic illustration of Doppler velocities from mitral inflow (MV), mitral annulus velocity, and hepatic vein (HV). Electrocardiographic (ECG) and respirometer (Resp) recordings indicating inspiration (i) and expiration (e) are also shown. A = atrial filling; D = diastolic flow; DR = diastolic flow reversal; DT = deceleration time; E = early diastolic filling; S = systolic flow; SR = systolic flow reversal. Stippled areas under the curve represent flow reversal.
From reference 63.
In restrictive cardiomyopathy, the mitral inflow velocity rarely shows respiratory variation, and hepatic vein systolic flow reversals are more prominent with inspiration. Therefore, invasively or noninvasively, the differentiation of restriction from constriction should be based on the respiratory patterns of ventricular filling. Measurement of septal mitral annular velocity by Doppler tissue imaging also helps distinguish constriction from restriction. Mitral annular velocity is markedly decreased in restrictive cardiomyopathy because myocardial relaxation is reduced in myocardial diseases. In contrast, it is well preserved or even augmented in constrictive pericarditis because the longitudinal motion of the heart is the main mechanism of diastolic filling in this condition (Figure 1563).
Cardiac CT and CMR
Computed tomography is useful in diagnosing constrictive pericarditis and can demonstrate increased pericardial thickness (>4 mm) and calcification1,22 (Figure 14, C63). Inferior vena cava dilatation, deformed ventricular contours, and angulation of the ventricular septum can be seen. In addition, failure of the immediately adjacent pulmonary structures to pulsate during the cardiac cycle in the presence of a thickened pericardium is highly suggestive of constrictive physiology. However, almost 20% of patients with surgically proven constrictive pericarditis did not have increased pericardial thickness on cardiac CT.22 Therefore, the lack of increased pericardial thickness does not exclude the diagnosis of constriction. Cardiac magnetic resonance imaging has been shown to be useful in detecting increased pericardial thickness and dilatation of the IVC.
Invasive Hemodynamic Evaluation of Constrictive Pericarditis vs Restrictive Cardiomyopathy
Despite the difference in the pathophysiologic mechanisms of restriction and constriction, considerable overlap is seen in the invasive hemodynamic parameters of these 2 entities. Increased atrial pressures, equalization of end-diastolic pressures, and dip-and-plateau or square-root sign of the ventricular diastolic pressure recording have traditionally been considered hemodynamic features typical of constrictive pericarditis. However, almost identical hemodynamic pressure tracings can be obtained in patients with restrictive cardiomyopathy. Therefore, in addition to these hemodynamic features, respiratory variation in ventricular filling and increased ventricular interdependence should be demonstrated to diagnose constriction, either invasively or noninvasively.96,97
In both conditions, a high driving pressure across the valves at the time of atrioventricular valve opening results in early rapid diastolic filling and an abrupt increase in ventricular pressure. In constrictive pericarditis, once the volume of ventricular filling approximates what the diseased pericardium allows in mid to late diastole, the filling of the heart chambers stops abruptly because the fixed and stiffened pericardial sac cannot stretch any further. As a result, the predominantly ventricular filling occurs rapidly in the first third of diastole, and neither ventricular chamber fills to any substantial amount in mid to end diastole. This leads to the hemodynamic signs of dip (the rapid y descent in the jugular venous pressure) and plateau during right heart catheterization, a phenomenon known as the square-root sign. As a consequence of these limitations, there is an elevation and near equalization of diastolic pressures in the RA, RV, and pulmonary wedge pressure, which corresponds to the left heart diastolic pressure.84 However, the 2 pressures are not precisely equal throughout the respiratory cycle; the pulmonary wedge pressure decreases during inspiration, whereas the systemic venous pressure remains constant. In restrictive cardiomyopathy, stiff ventricular walls result in severe diastolic dysfunction and restrictive filling, with elevated filling pressures similar to those in constrictive pericarditis. Restrictive cardiomyopathy may have similar findings of elevation with end-equalization of diastolic pressures in all 4 cardiac chambers.
Respiratory Changes in Ventricular Filling
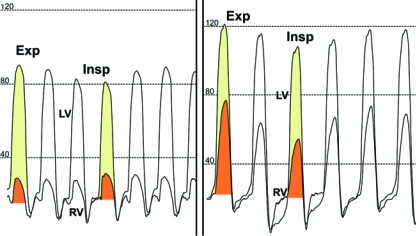
The most specific hemodynamic feature of constrictive pericarditis is enhanced ventricular interdependence, manifested as a discordant change in the total area of the LV and RV systolic pressure curve with respiration97 (Figure 16). Respiratory variation in the filling of the LV and RV occurs in patients with constrictive pericarditis but not in those with restrictive cardiomyopathy.96,97 In the heart with a normal pericardium, inspiration causes a decrease in intrathoracic pressure, with some increase in filling of the RV due to enhanced venous return. Filling of the LV is unaffected throughout the cardiac cycle because the decrease in intrathoracic pressure with inspiration is transmitted to intracardiac chambers to maintain relatively the same driving pressure gradient from the pulmonary circulation to the left-sided chamber throughout different respiratory phases. However, in a patient with constrictive pericarditis, the rigid pericardium does not allow the decrease in intrathoracic pressure to be transmitted to the heart chambers, causing a decrease in the driving pressure gradient from the pulmonary circulation to the left-sided chambers with inspiration. With a constant combined cardiac volume, the reduction in LV filling allows increased RV filling with inspiration. During inspiration, the RV filling cannot expand into the free wall because of the rigid pericardium. As a consequence, the interventricular septum shifts toward the LV, but the total cardiac volume does not change. During expiration, conversely, LV filling is increased. Hence, the left septum shifts toward the RV, and RV filling decreases. In patients with restrictive cardiomyopathy or other myocardial diseases with biventricular failure, these findings are absent, and the area of the LV and RV systolic pressures changes concordantly during the respiratory cycle (Figure 16).
FIGURE 16.
Ventricular interdependence and respiratory variation in ventricular filling in constrictive pericarditis and restrictive cardiomyopathy. Ventricular interdependence is observed in simultaneous recordings of left ventricular (LV) and right ventricular (RV) pressures. Left, In constrictive pericarditis, inspiration (Insp) induces less filling of the LV, and the area of the LV pressure curve decreases (yellow shaded area) as compared with expiration (Exp). The opposite changes occur in the RV so that area of the RV pressure curve increases with Insp (orange shaded area). Ejection time also varies with respiration in opposite directions in LV and RV. This discordant pressure change between the LV and RV occurs in constrictive pericarditis. Right, In restrictive cardiomyopathy, the changes in LV and RV systolic pressures with respiration are concordant. During Insp, the area of the RV pressure curve decreases (orange shaded area) as compared with Exp. The area of the LV pressure curve (yellow shaded area) is unchanged during Insp as compared with Exp. Note that both patients have early rapid filling and elevation and end-equalization of the LV and RV pressures at end Exp.
Treatment
In patients with transient constrictive pericarditis caused by pericardial inflammation, symptoms and constrictive features may resolve with medical therapy alone.98,99 In chronic constrictive pericarditis, surgery is the accepted standard.83,100-102 However, pericardiectomy is associated with a significant operative mortality of greater than 6% at most experienced centers.83,100 The largest surgical series indicate that independent adverse predictors of long-term outcome include older age, worse New York Heart Association class, renal dysfunction, pulmonary hypertension, LV dysfunction, hyponatremia, and prior ionizing radiation.83,100 Because the symptoms of constrictive pericarditis may persist after partial pericardiectomy, it is important that the pericardiectomy is complete, with as much of the pericardium removed as possible.
EFFUSIVE-CONSTRICTIVE PERICARDITIS
Effusive-constrictive pericarditis is a unique clinical condition consisting of both pericardial effusion and constrictive pericarditis. The patient presents with a pericardial effusion and evidence of increased filling pressure with cardiac tamponade or constriction.103,104 Evacuation of the pericardial fluid can result in resolution of constrictive physiology due to the decrease in intrapericardial pressure; however, in some cases, constrictive hemodynamics can still persist even after the pericardial effusion is removed. Effusive-constrictive pericarditis can be treated by catheter pericardiocentesis or surgical drainage and pericardiectomy. However, in a subset of patients in whom constrictive pericarditis is due to reversible inflammation of the pericardium, the pericardial effusion may resolve after treatment with anti-inflammatory medications; this condition has been termed transient constrictive pericarditis.99 This transient constrictive phase may last 2 to 3 months before it gradually resolves, either spontaneously or with treatment with anti-inflammatory agents. When hemodynamics and findings typical of constriction develop in patients with acute pericarditis, the initial treatment should be NSAIDs for 2 to 3 weeks. If there is no response, corticosteroid treatment can be considered in patients in whom infection is not the underlying etiology of pericarditis. Corticosteroids can be given for 1 to 2 months with gradual tapering during 6 to 8 weeks.
CONGENITALLY ABSENT PERICARDIUM
Complete absence of the pericardium is very rare and usually asymptomatic. More commonly, a portion of the pericardium, usually on the left, is absent. The defect is more common in male patients. With extreme cardiac shift to the left, on rare occasion the patient may experience left-sided nonexertional chest pain or prominent cardiac pulsation. Because the heart shifts to the left, cardiac motion is exaggerated, especially that of the posterior wall of the LV. Traditional echocardiography shows prominence of the right-sided cardiac chambers and abnormal septal motion.105,106 These findings may lead to an erroneous diagnosis of RV volume overload or atrial septal defect. Congenital absence of the pericardium is associated with atrial septal defect, bicuspid aortic valve, and bronchogenic cysts. Rarely, herniation of cardiac chambers through a partial defect of the pericardium may cause sudden death, presumably because of marked ischemia from compression of the coronary artery. Closure of the pericardial defect is necessary in symptomatic patients. The diagnosis can be confirmed with cardiac CT or CMR.
PERICARDIAL CYST
A pericardial cyst is a benign structural abnormality of the pericardium that is usually detected as an incidental mass lesion on chest radiography in an asymptomatic person. Most frequently, it is located at the right costophrenic angle, but it may also be found at the left costophrenic angle, hilum, or superior mediastinum. The differential diagnosis of such a chest radiographic finding includes malignant tumor, cardiac chamber enlargement, and diaphragmatic hernia. Pericardial cyst appears as a cystic structure attached to the heart on cardiac imaging. Two-dimensional echocardiography, cardiac CT, or CMR may be used to differentiate pericardial cysts from other solid tumors. In asymptomatic patients, no treatment is necessary.
CONCLUSION
Because of the wide spectrum of pericardial diseases, diagnosis and management remain difficult. This review attempts to describe useful diagnostic criteria and tools and to outline evidence-based medical and surgical treatments of major pericardial diseases. Summary algorithms for the diagnosis and management of acute pericarditis, relapsing pericarditis, cardiac tamponade, and constrictive pericarditis are provided.
In acute and relapsing pericarditis, history and findings on examination, ECG, and laboratory studies can be used to make a diagnosis. Additional imaging tools, including echocardiography, cardiac CT, and CMR, can be used when the diagnosis is equivocal or there is evidence of hemodynamic compromise. Management should be directed toward treating the underlying cause. Nonsteroidal anti-inflammatory drugs and colchicine are first-line treatment for idiopathic or viral acute or relapsing pericarditis, and corticosteroids should be limited to patients in whom NSAID and colchicine therapy has failed or is contraindicated or those whose relapsing pericarditis has an autoimmune or rheumatologic etiology.
In cardiac tamponade, echocardiography is the main diagnostic tool, and first-line treatment of unstable patients is urgent percutaneous catheter pericardiocentesis. The diagnosis of constrictive pericarditis and restrictive cardiomyopathy is challenging because of the considerable overlap in hemodynamic parameters. Increased ventricular dependence and discordant changes in LV and RV systolic pressure during respiration are features of pericardial constriction; in restrictive cardiomyopathy, these findings are not present, and LV and RV systolic pressures change concordantly during the respiratory cycle. Although constrictive pericarditis can be transient in a subset of patients and managed medically, most require surgical pericardiectomy, which can be performed with a very acceptable risk at experienced centers.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr Sharmi Shafi for her significant help with the preparation of the submitted manuscript.
On completion of this article, you should be able to: (1) identify the diagnostic criteria for acute and relapsing pericarditis, cardiac tamponade, and constrictive pericarditis; (2) integrate the information obtained from a history, physical examination, electrocardiography, and laboratory studies to determine if further imaging modalities or invasive cardiac catheterization is necessary for the diagnosis of the various pericardial syndromes; and (3) apply management strategies on the basis of evidence and clinical experience to decrease morbidity and improve survival in patients with pericardial disease.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary: The task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25(7):587-610 [DOI] [PubMed] [Google Scholar]
- 2.Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349(7):684-690 [DOI] [PubMed] [Google Scholar]
- 3.Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995;75(5):378-382 [DOI] [PubMed] [Google Scholar]
- 4.Permanyer-Miralda G, Sagrista-Sauleda J, Soler-Soler J. Primary acute pericardial disease: a prospective series of 231 consecutive patients. Am J Cardiol. 1985;56(10):623-630 [DOI] [PubMed] [Google Scholar]
- 5.Lange RA, Hillis LD. Clinical practice: acute pericarditis [published correction appears in N Engl J Med. 2005;352(11):1163] N Engl J Med. 2004;351(21):2195-2202 [DOI] [PubMed] [Google Scholar]
- 6.Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005;112(13):2012-2016 [DOI] [PubMed] [Google Scholar]
- 7.Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004;363(9410):717-727 [DOI] [PubMed] [Google Scholar]
- 8.Spodick DH. Acute pericarditis: current concepts and practice. JAMA 2003;289(9):1150-1153 [DOI] [PubMed] [Google Scholar]
- 9.Bonnefoy E, Godon P, Kirkorian G, Fatemi M, Chevalier P, Touboul P. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21(10):832-836 [DOI] [PubMed] [Google Scholar]
- 10.Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008;94(4):498-501 [DOI] [PubMed] [Google Scholar]
- 11.Spodick DH. Pericardial rub: prospective, multiple observer investigation of pericardial friction in 100 patients. Am J Cardiol. 1975;35(3):357-362 [DOI] [PubMed] [Google Scholar]
- 12.Ginzton LE, Laks MM. The differential diagnosis of acute pericarditis from the normal variant: new electrocardiographic criteria. Circulation 1982;65(5):1004-1009 [DOI] [PubMed] [Google Scholar]
- 13.Spodick DH. Diagnostic electrocardiographic sequences in acute pericarditis: significance of PR segment and PR vector changes. Circulation 1973;48(3):575-580 [DOI] [PubMed] [Google Scholar]
- 14.Surawicz B, Lasseter KC. Electrocardiogram in pericarditis. Am J Cardiol. 1970;26(5):471-474 [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349(22):2128-2135 [DOI] [PubMed] [Google Scholar]
- 16.Imazio M, Demichelis B, Parrini I, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43(6):1042-1046 [DOI] [PubMed] [Google Scholar]
- 17.Bruce MA, Spodick DH. Atypical electrocardiogram in acute pericarditis: characteristics and prevalence. J ElectroCardiol. 1980;13(1):61-66 [DOI] [PubMed] [Google Scholar]
- 18.Salisbury AC, Olalla-Gomez C, Rihal CS, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84(1):11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Permanyer-Miralda G. Acute pericardial disease: approach to the aetiologic diagnosis. Heart 2004;90(3):252-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imazio M, Demichelis B, Cecchi E, et al. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42(12):2144-2148 [DOI] [PubMed] [Google Scholar]
- 21.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Coll Cardiol. 2003;42(5):954-970 [DOI] [PubMed] [Google Scholar]
- 22.Ling LH, Oh JK, Breen JF, et al. Calcific constrictive pericarditis: is it still with us? Ann Intern Med. 2000;132(6):444-450 [DOI] [PubMed] [Google Scholar]
- 23.Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007;115(21):2739-2744 [DOI] [PubMed] [Google Scholar]
- 24.Berman J, Haffajee CI, Alpert JS. Therapy of symptomatic pericarditis after myocardial infarction: retrospective and prospective studies of aspirin, indomethacin, prednisone, and spontaneous resolution. Am Heart J. 1981;101(6):750-753 [DOI] [PubMed] [Google Scholar]
- 25.McGinn JT, Rosati M, McGinn TG. Indomethacin in treatment of pericarditis. N Y State J Med. 1970;70(13):1783-1788 [PubMed] [Google Scholar]
- 26.Arunasalam S, Siegel RJ. Rapid resolution of symptomatic acute pericarditis with ketorolac tromethamine: a parenteral nonsteroidal antiinflammatory agent. Am Heart J. 1993;125(5, pt 1):1455-1458 [DOI] [PubMed] [Google Scholar]
- 27.Schifferdecker B, Spodick DH. Nonsteroidal anti-inflammatory drugs in the treatment of pericarditis. Cardiol Rev. 2003;11(4):211-217 [DOI] [PubMed] [Google Scholar]
- 28.Brown EJ, Jr, Kloner RA, Schoen FJ, Hammerman H, Hale S, Braunwald E. Scar thinning due to ibuprofen administration after experimental myocardial infarction. Am J Cardiol. 1983;51(5):877-883 [DOI] [PubMed] [Google Scholar]
- 29.Hammerman H, Kloner RA, Schoen FJ, Brown EJ, Jr, Hale S, Braunwald E. Indomethacin-induced scar thinning after experimental myocardial infarction. Circulation 1983;67(6):1290-1295 [DOI] [PubMed] [Google Scholar]
- 30.Jugdutt BI, Basualdo CA. Myocardial infarct expansion during indomethacin or ibuprofen therapy for symptomatic post infarction pericarditis: influence of other pharmacologic agents during early remodelling. Can J Cardiol. 1989;5(4):211-221 [PubMed] [Google Scholar]
- 31.Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: a decade of experience. Circulation 1998;97(21):2183-2185 [DOI] [PubMed] [Google Scholar]
- 32.Adler Y, Zandman-Goddard G, Ravid M, et al. Usefulness of colchicine in preventing recurrences of pericarditis. Am J Cardiol. 1994;73(12):916-917 [DOI] [PubMed] [Google Scholar]
- 33.Lange U, Schumann C, Schmidt KL. Current aspects of colchicine therapy—classical indications and new therapeutic uses. Eur J Med Res. 2001;6(4):150-160 [PubMed] [Google Scholar]
- 34.Wilbur K, Makowsky M. Colchicine myotoxicity: case reports and literature review. Pharmacotherapy 2004;24(12):1784-1792 [DOI] [PubMed] [Google Scholar]
- 35.Brucato A, Adler Y, Spodick DH. Letter regarding article by Imazio et al, “colchicine in addition to conventional therapy for acute pericarditis. Circulation 2006;113(14):e693 [DOI] [PubMed] [Google Scholar]
- 36.Rollot F, Pajot O, Chauvelot-Moachon L, Nazal EM, Kelaidi C, Blanche P. Acute colchicine intoxication during clarithromycin administration. Ann Pharmacother. 2004;38(12):2074-2077 [DOI] [PubMed] [Google Scholar]
- 37.Hung IF, Wu AK, Cheng VC, et al. Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: a retrospective study. Clin Infect Dis. 2005;41(3):291-300 [DOI] [PubMed] [Google Scholar]
- 38.Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum. 2009;38(6):411-419 [DOI] [PubMed] [Google Scholar]
- 39.Adler Y, Spodick DH, Shabetai R, Brucato A. Can colchicine prevent recurrence of new-onset acute pericarditis? Nat Clin Pract Cardiovasc Med. 2006;3(2):78-79 [DOI] [PubMed] [Google Scholar]
- 40.Imazio M, Demichelis B, Parrini I, et al. Recurrent pain without objective evidence of disease in patients with previous idiopathic or viral acute pericarditis. Am J Cardiol. 2004;94(7):973-975 [DOI] [PubMed] [Google Scholar]
- 41.Spodick DH. Intrapericardial treatment of persistent autoreactive pericarditis/myopericarditis and pericardial effusion. Eur Heart J. 2002;23(19):1481-1482 [DOI] [PubMed] [Google Scholar]
- 42.Maisch B, Ristic AD, Pankuweit S. Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone: the way to avoid side effects of systemic corticosteroid therapy. Eur Heart J. 2002;23(19):1503-1508 [DOI] [PubMed] [Google Scholar]
- 43.Soler-Soler J, Sagrista-Sauleda J, Permanyer-Miralda G. Relapsing pericarditis. Heart 2004;90(11):1364-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carmichael DB, Sprague HB, Wyman SM, Bland EF. Acute nonspecific pericarditis: clinical, laboratory, and follow-up considerations. Circulation 1951;3(3):321-331 [DOI] [PubMed] [Google Scholar]
- 45.Admetlla M, Cequier A, Amer R, Javaloyas M, Sabate X, Gausi C. Acute idiopathic pericarditis: clinical features, development and complications: prospective study of 101 cases [in Spanish]. Med Clin (Barc) 1985;85(14):563-567 [PubMed] [Google Scholar]
- 46.Brucato A, Brambilla G, Moreo A, et al. Long-term outcomes in difficult-to-treat patients with recurrent pericarditis. Am J Cardiol. 2006;98(2):267-271 [DOI] [PubMed] [Google Scholar]
- 47.Maisch B. Recurrent pericarditis: mysterious or not so mysterious? Eur Heart J. 2005;26(7):631-633 [DOI] [PubMed] [Google Scholar]
- 48.Fowler NO, Harbin AD., III Recurrent acute pericarditis: follow-up study of 31 patients. J Am Coll Cardiol. 1986;7(2):300-305 [DOI] [PubMed] [Google Scholar]
- 49.Sagrista-Sauleda J, Permanyer-Miralda G, Soler-Soler J. Tuberculous pericarditis: ten year experience with a prospective protocol for diagnosis and treatment. J Am Coll Cardiol. 1988;11(4):724-728 [DOI] [PubMed] [Google Scholar]
- 50.Galve E, Permanyer-Miralda G, Tornos MP, Oller G, Roma F, Soler-Soler J. Self-limited acute pericarditis as initial manifestation of primary cardiac tumor. Am Heart J. 1992;123(6):1690-1692 [DOI] [PubMed] [Google Scholar]
- 51.Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med. 2005;165(17):1987-1991 [DOI] [PubMed] [Google Scholar]
- 52.Misselt AJ, Harris SR, Glockner J, Feng D, Syed IS, Araoz PA. MR imaging of the pericardium. Magn Reson Imaging Clin N Am. 2008;16(2):185-199, vii [DOI] [PubMed] [Google Scholar]
- 53.Imazio M, Demichelis B, Parrini I, et al. Management, risk factors, and outcomes in recurrent pericarditis. Am J Cardiol. 2005;96(5):736-739 [DOI] [PubMed] [Google Scholar]
- 54.Imazio M, Brucato A, Adler Y, et al. Prognosis of idiopathic recurrent pericarditis as determined from previously published reports. Am J Cardiol. 2007;100(6):1026-1028 [DOI] [PubMed] [Google Scholar]
- 55.Melchior TM, Ringsdal V, Hildebrandt P, Torp-Pedersen C. Recurrent acute idiopathic pericarditis treated with intravenous methylprednisolone given as pulse therapy. Am Heart J. 1992;123(4, pt 1):1086-1088 [DOI] [PubMed] [Google Scholar]
- 56.Marcolongo R, Russo R, Laveder F, Noventa F, Agostini C. Immunosuppressive therapy prevents recurrent pericarditis. J Am Coll Cardiol. 1995;26(5):1276-1279 [DOI] [PubMed] [Google Scholar]
- 57.Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation 2008;118(6):667-671 [DOI] [PubMed] [Google Scholar]
- 58.Imazio M, Brucato A, Trinchero R, Shabetai R, Spodick D, Adler Y. Corticosteroid therapy for pericarditis: a double-edged sword. Nat Clin Pract Cardiovasc Med. 2008;5(3):118-119 [DOI] [PubMed] [Google Scholar]
- 59.Greason K, Danielson GK, Oh JK, Nishimura RA. Pericardiectomy for chronic relapsing pericarditis [abstract 1005-1204]. J Am Coll Cardiol. 2001;37(478A). [Google Scholar]
- 60.Holmes DR, Jr, Nishimura R, Fountain R, Turi ZG. Iatrogenic pericardial effusion and tamponade in the percutaneous intracardiac intervention era. JACC Cardiovasc Interv. 2009;2(8):705-717 [DOI] [PubMed] [Google Scholar]
- 61.Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J. 1988;115(2):391-398 [DOI] [PubMed] [Google Scholar]
- 62.Bruch C, Schmermund A, Dagres N, et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol. 2001;38(1):219-226 [DOI] [PubMed] [Google Scholar]
- 63.Oh JK, Seward JB, Tajik AJ, eds. The Echo Manual 3rd ed.Philadelphia, PA: Lippincott, Williams & Wilkins; 2007. [Google Scholar]
- 64.Reydel B, Spodick DH. Frequency and significance of chamber collapses during cardiac tamponade. Am Heart J. 1990;119(5):1160-1163 [DOI] [PubMed] [Google Scholar]
- 65.Leimgruber PP, Klopfenstein HS, Wann LS, Brooks HL. The hemodynamic derangement associated with right ventricular diastolic collapse in cardiac tamponade: an experimental echocardiographic study. Circulation 1983;68(3):612-620 [DOI] [PubMed] [Google Scholar]
- 66.Gillam LD, Guyer DE, Gibson TC, King ME, Marshall JE, Weyman AE. Hydrodynamic compression of the right atrium: a new echocardiographic sign of cardiac tamponade. Circulation 1983;68(2):294-301 [DOI] [PubMed] [Google Scholar]
- 67.Torelli J, Marwick TH, Salcedo EE. Left atrial tamponade: diagnosis by transesophageal echocardiography. J Am Soc Echocardiogr. 1991;4(4):413-414 [DOI] [PubMed] [Google Scholar]
- 68.Fusman B, Schwinger ME, Charney R, Ausubel K, Cohen MV. Isolated collapse of left-sided heart chambers in cardiac tamponade: demonstration by two-dimensional echocardiography. Am Heart J. 1991;121(2, pt 1):613-616 [DOI] [PubMed] [Google Scholar]
- 69.Himelman RB, Kircher B, Rockey DC, Schiller NB. Inferior vena cava plethora with blunted respiratory response: a sensitive echocardiographic sign of cardiac tamponade. J Am Coll Cardiol. 1988;12(6):1470-1477 [DOI] [PubMed] [Google Scholar]
- 70.Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics 2007;27(6):1595-1610 [DOI] [PubMed] [Google Scholar]
- 71.Gold MM, Spindola-Franco H, Jain VR, Spevack DM, Haramati LB. Coronary sinus compression: an early computed tomographic sign of cardiac tamponade. J Comput Assist Tomogr. 2008;32(1):72-77 [DOI] [PubMed] [Google Scholar]
- 72.Reddy PS, Curtiss EI, O'Toole JD, Shaver JA. Cardiac tamponade: hemodynamic observations in man. Circulation 1978;58(2):265-272 [DOI] [PubMed] [Google Scholar]
- 73.Kerber RE, Gascho JA, Litchfield R, Wolfson P, Ott D, Pandian NG. Hemodynamic effects of volume expansion and nitroprusside compared with pericardiocentesis in patients with acute cardiac tamponade. N Engl J Med. 1982;307(15):929-931 [DOI] [PubMed] [Google Scholar]
- 74.Sagrista-Sauleda J, Angel J, Sambola A, Permanyer-Miralda G. Hemodynamic effects of volume expansion in patients with cardiac tamponade. Circulation 2008;117(12):1545-1549 [DOI] [PubMed] [Google Scholar]
- 75.Little WC, Freeman GL. Pericardial disease. Circulation 2006;113(12):1622-1632 [DOI] [PubMed] [Google Scholar]
- 76.Jackson G, Keane D, Mishra B. Percutaneous balloon pericardiotomy in the management of recurrent malignant pericardial effusions. Br Heart J. 1992;68(6):613-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swanson N, Mirza I, Wijesinghe N, Devlin G. Primary percutaneous balloon pericardiotomy for malignant pericardial effusion. Catheter Cardiovasc Interv. 2008;71(4):504-507 [DOI] [PubMed] [Google Scholar]
- 78.Tsang TS, Enriquez-Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77(5):429-436 [DOI] [PubMed] [Google Scholar]
- 79.Sagrista-Sauleda J, Angel J, Permanyer-Miralda G, Soler-Soler J. Long-term follow-up of idiopathic chronic pericardial effusion. N Engl J Med. 1999;341(27):2054-2059 [DOI] [PubMed] [Google Scholar]
- 80.Melduni RM, Oh JK, Bunch TJ, Sinak LJ, Gloviczki P. Reconstruction of occluded thoracic duct for treatment of chylopericardium: a novel surgical therapy. J Vasc Surg. 2008;48(6):1600-1602 [DOI] [PubMed] [Google Scholar]
- 81.Hatle LK, Appleton CP, Popp RL. Differentiation of constrictive pericarditis and restrictive cardiomyopathy by Doppler echocardiography. Circulation 1989;79(2):357-370 [DOI] [PubMed] [Google Scholar]
- 82.Oh JK, Hatle LK, Seward JB, et al. Diagnostic role of Doppler echocardiography in constrictive pericarditis. J Am Coll Cardiol. 1994;23(1):154-162 [DOI] [PubMed] [Google Scholar]
- 83.Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999;100(13):1380-1386 [DOI] [PubMed] [Google Scholar]
- 84.Schwefer M, Aschenbach R, Heidemann J, Mey C, Lapp H. Constrictive pericarditis, still a diagnostic challenge: comprehensive review of clinical management. Eur J Cardiothorac Surg. 2009;36:502-510 [DOI] [PubMed] [Google Scholar]
- 85.Mayosi BM. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart 2007;93(10):1176-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.White PD. Chronic constrictive pericarditis. Circulation 1951;4(2):288-294 [DOI] [PubMed] [Google Scholar]
- 87.Shabetai R, Fowler NO, Guntheroth WG. The hemodynamics of cardiac tamponade and constrictive pericarditis. Am J Cardiol. 1970;26(5):480-489 [DOI] [PubMed] [Google Scholar]
- 88.Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. N Engl J Med. 1997;336(4):267-276 [DOI] [PubMed] [Google Scholar]
- 89.Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation 2003;108(15):1852-1857 [DOI] [PubMed] [Google Scholar]
- 90.Schnittger I, Bowden RE, Abrams J, Popp RL. Echocardiography: pericardial thickening and constrictive pericarditis. Am J Cardiol. 1978;42(3):388-395 [DOI] [PubMed] [Google Scholar]
- 91.Agatston AS, Rao A, Price RJ, Kinney EL. Diagnosis of constrictive pericarditis by pulsed Doppler echocardiography. Am J Cardiol. 1984;54(7):929-930 [DOI] [PubMed] [Google Scholar]
- 92.Tei C, Child JS, Tanaka H, Shah PM. Atrial systolic notch on the interventricular septal echogram: an echocardiographic sign of constrictive pericarditis. J Am Coll Cardiol. 1983;1(3):907-912 [DOI] [PubMed] [Google Scholar]
- 93.Hoit BD. Imaging the pericardium. Cardiol Clin. 1990;8(4):587-600 [PubMed] [Google Scholar]
- 94.D'Cruz IA, Dick A, Gross CM, Hand CR, Lalmalani GG. Abnormal left ventricular-left atrial posterior wall contour: a new two-dimensional echo cardiographic sign in constrictive pericarditis. Am Heart J. 1989;118(1):128-132 [DOI] [PubMed] [Google Scholar]
- 95.Rajagopalan N, Garcia MJ, Rodriguez L, et al. Comparison of new Doppler echocardiographic methods to differentiate constrictive pericardial heart disease and restrictive cardiomyopathy. Am J Cardiol. 2001;87(1):86-94 [DOI] [PubMed] [Google Scholar]
- 96.Hurrell DG, Nishimura RA, Higano ST, et al. Value of dynamic respiratory changes in left and right ventricular pressures for the diagnosis of constrictive pericarditis. Circulation 1996;93(11):2007-2013 [DOI] [PubMed] [Google Scholar]
- 97.Talreja DR, Nishimura RA, Oh JK, Holmes DR. Constrictive pericarditis in the modern era: novel criteria for diagnosis in the cardiac catheterization laboratory. J Am Coll Cardiol. 2008;51(3):315-319 [DOI] [PubMed] [Google Scholar]
- 98.Sagrista-Sauleda J, Permanyer-Miralda G, Candell-Riera J, Angel J, Soler-Soler J. Transient cardiac constriction: an unrecognized pattern of evolution in effusive acute idiopathic pericarditis. Am J Cardiol. 1987;59(9):961-966 [DOI] [PubMed] [Google Scholar]
- 99.Haley JH, Tajik AJ, Danielson GK, Schaff HV, Mulvagh SL, Oh JK. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004;43(2):271-275 [DOI] [PubMed] [Google Scholar]
- 100.Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43(8):1445-1452 [DOI] [PubMed] [Google Scholar]
- 101.DeValeria PA, Baumgartner WA, Casale AS, et al. Current indications, risks, and outcome after pericardiectomy. Ann Thorac Surg. 1991;52(2):219-224 [DOI] [PubMed] [Google Scholar]
- 102.Chowdhury UK, Subramaniam GK, Kumar AS, et al. Pericardiectomy for constrictive pericarditis: a clinical, echocardiographic, and hemodynamic evaluation of two surgical techniques. Ann Thorac Surg. 2006;81(2):522-529 [DOI] [PubMed] [Google Scholar]
- 103.Sagrista-Sauleda J, Angel J, Sanchez A, Permanyer-Miralda G, Soler-Soler J. Effusive-constrictive pericarditis. N Engl J Med. 2004;350(5):469-475 [DOI] [PubMed] [Google Scholar]
- 104.Hancock EW. A clearer view of effusive-constrictive pericarditis. N Engl J Med. 2004;350(5):435-437 [DOI] [PubMed] [Google Scholar]
- 105.Nasser WK, Helmen C, Tavel ME, Feigenbaum H, Fisch C. Congenital absence of the left pericardium: clinical, electrocardiographic, radiographic, hemodynamic, and angiographic findings in six cases. Circulation 1970;41(3):469-478 [DOI] [PubMed] [Google Scholar]
- 106.Connolly HM, Click RL, Schattenberg TT, Seward JB, Tajik AJ. Congenital absence of the pericardium: echocardiography as a diagnostic tool. J Am Soc Echocardiogr. 1995;8(1):87-92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.