Abstract
The etiology and treatment of hypertrophic scar remain puzzles even after decades of research. A significant reason is the lack of an accepted animal model of the process. The female, red Duroc pig model was described long ago. Since the skin of the pig is similar to that of humans, we are attempting to validate this model and found it to be encouraging. In this project we quantified myofibroblasts, mast cells and collagen nodules in the thick scar of the Duroc pig and compared these to the values for human hypertrophic scar. We found the results to be quite similar and so further validated the model. In addition, we observed that soon after wounding an inflammatory cell layer forms. The thickness of the inflammatory layer approaches the thickness of the skin removed as if the remaining dermis “knows” how much dermis is gone. In deep wounds this inflammatory layer thickens and this thickness is predictive of the thickness of the ultimate scar.
Keywords: Collagen nodules, Mast cells, Hypertrophic scar, Porcine, Swine, Pig, Duroc, Myofibroblasts
1. Introduction
Hypertrophic scar is an undesirable result of deep partial thickness wounds and burns and may have devastating physical, psychosocial and vocational consequences [1-10]. The process has been studied for decades, but the pathophysiology of hypertrophic scarring is still unknown[11-20].
One major reason that the etiology of human hypertrophic scar is unknown is the absence of a useful animal model [19,21-23]. Various animal models have been described [19,22-30] but none of these have risen to being considered a “gold standard”.
Nearly 30 years ago, Silverstein, Goodwin, Raulston, and Pruitt [31] reported that deep donor sites in 12 of 12 female red Duroc pigs healed with “hypertrophic” scarring. Since there is considerable evidence that porcine skin is similar to human skin [32-44] we are attempting to validate this model. To date, we found the model quite encouraging in various respects [45-48], but mast cells, collagen nodules, and myofibroblasts in the Duroc scar have yet to be compared to human hypertrophic scar.
Various authors have suggested that mast cells may be involved in fibrosis [49-54] and that human hypertrophic scar contains more mast cells than uninjured skin and normotrophic scar [55-58] If mast cells can be demonstrated in the Duroc wounds, the model could also be used to define timing and roles of mast cell-mediated immune responses.
Human hypertrophic scar classically is thought to be histologically defined by collagen nodules [12,59-69]. Since these nodules are not found in normotrophic scar they are thought to be characteristic of hypertrophic scars. Identification of this histological organization in deep wounds would further validate this Duroc model of scar formation.
Finally, Gabbiani et al. described modified fibroblasts in 1971 [70]. Majno et al. later termed them myofibroblasts [71]. Most investigators have determined that myofibroblast number is increased in hypertrophic scars. Nedelec et al. reported a greater number of myofibroblasts in hypertrophic scar than in normotrophic scar [72]. Santucci et al. [69] determined that early scars have large numbers of myofibroblasts and late hypertrophic scars have fewer. Ehrlich et al. [68] localized the myofibroblasts in hypertrophic scars to the collagen nodules, however, Santucci et al. and Nedelec et al. could not confirm these findings. Given these contradictory observations about localization and timing of myofibroblast infiltration into human hypertrophic scar, the Duroc model could provide a way to quantify this cellular response.
For this project, we hypothesized that the occurrence of mast cells, collagen nodules and myofibroblasts in the thick scar of the female, red Duroc pig is similar to human hypertrophic scar.
2. Materials and methods
2.1. Duroc skin and scar samples
All animal studies were performed as previously described [45-48]. In accord with Animal Care Committee permission female Duroc pigs (Toth Farm, Bellingham, WA), 6 weeks old, approximately 16–18 kg, were purchased and housed in the Harborview Medical Center Research and Training Vivarium with 12-h light/dark cycles. The animals were observed for 1 week and fed lab porcine grower diet and water ad lib. At 7 weeks, anesthesia was established with Telazol® reconstituted with 5 ml xylazine (100 mg/ml), dosage 1 ml/18 kg body weight (Phoenix Pharmaceuticals Inc., St. Joseph, MO). The hair on the back was clipped and skin cleansed with Betadine® solution and rinsed with 70% alcohol. Tangential wounds were created with a standard electric Padgett dermatome (Padgett Instruments, Kansas City, MO). Wound size was approximately 7 cm × 7 cm and eight wounds were created on the back of each pig. The wounds were allowed to granulate and re-epithelialize without application of topical agents or dressings and were photographed at the times of biopsy.
From our preliminary studies we determined that the settings on the Padgett dermatomes are very precise. However, this does not translate into precise wound depths for multiple reason none of which are truly controllable [73]. Moreover, the thickest setting is 0.040 in. so to achieve greater depths; multiple excisions must be done, which introduces even more error. Therefore, we do not refer to wound depth but rather to total dermatome setting. The total dermatome settings used in this study were 0.020, 0.040 and 0.060 in. creating partial thickness wounds, shallow, intermediate and deep. The three total dermatome settings were rotated on the animals to avoid repeatedly placing one wound depth in the same anatomic location.
Under general anesthesia as described above, 5 × 10 mm surgical biopsies were collected on 7, 14, 21 and 28 days and 2, 3 and 5 months post-wounding. Each wound was biopsied only at one time point. Biopsies were taken from the center of each wound and uninjured skin at each time point. Biopsies were immediately fixed in neutral buffered formalin for 24 h.
2.2. Human skin and scar samples
Human skin and scar samples were acquired at the time of surgical burn reconstruction at Harborview Medical Center. All procedures were carried out in accordance of the University of Washington’s Human Subjects Division. Patient demographics are included in Tables 1 and 2. Human sampling times are different from Duroc times since it is not possible to obtain human samples at specified times.
Table 1.
Patient demographics for mast cells comparison
| Group | Age | Race | Sex | Body part |
|---|---|---|---|---|
| Hypertrophic scar | 33 | Asian | F | Face |
| Hypertrophic scar | 9 | Caucasian | F | Chest |
| Hypertrophic scar | 15 | Hispanic | F | Face |
| Hypertrophic scar | 17 | Multi-racial | M | Arm |
| Hypertrophic scar | 15 | Hispanic | M | Right leg |
| Uninjured skin | 48 | African American | Unknown | Sternum |
| Uninjured skin | 59 | Caucasian | F | Back |
| Uninjured skin | 45 | Caucasian | F | Abdomen |
| Uninjured skin | 57 | Unknown | M | Back |
| Uninjured skin | 18 | Caucasian | M | Arm |
Table 2.
Patient demographics for collagen nodule comparison
| Group (months) | Age | Race | Sex | Body part | Age of scar (months) |
|---|---|---|---|---|---|
| 0–12 | 27 | Caucasian | M | Abdomen | 3.9 |
| 0–12 | 11 | Caucasian | F | Chest | 4.9 |
| 0–12 | 60 | African American | M | Dorsum hand | 5.6 |
| 0–12 | 51 | Caucasian | M | Chest | 6.2 |
| 0–12 | 8 | Caucasian | M | Face | 6.9 |
| 0–12 | 19 | Caucasian | M | Arm | 8 |
| 0–12 | 5 | Caucasian | M | Cheek | 9.6 |
| 0–12 | 48 | Caucasian | F | Face | 10.1 |
| 0–12 | 6 | African American | F | Neck | 10.3 |
| 24–67 | 17 | Multi-racial | M | Arm | 24.1 |
| 24–67 | 15 | Hispanic | F | Face | 25.3 |
| 24–67 | 16 | Hispanic | M | Leg | 25.7 |
| 24–67 | 4 | Caucasian | M | Sternum | 26 |
| 24–67 | 4 | Caucasian | M | Face | 27.3 |
| 24–67 | 38 | African American | M | Arm | 27.5 |
| 24–67 | 7 | Asian | F | Arm | 27.8 |
| 24–67 | 9 | Caucasian | F | Chest | 27.9 |
| 24–67 | Unknown | Unknown | F | Leg | 28.8 |
| 24–67 | 33 | Asian | F | Face | 29.6 |
| 24–67 | 3 | Caucasian | M | Cheek | 29.8 |
| 24–67 | 9 | Caucasian | M | Back | 36.5 |
| 24–67 | 46 | Caucasian | M | Shoulder | 37.2 |
| 24–67 | 45 | Caucasian | M | Leg | 62.2 |
| 24–67 | 12 | Hispanic | M | Chest | 64.1 |
| 24–67 | 16 | Caucasian | F | Arm | 67 |
2.3. Histology
Biopsies from eight wounds on each of eight Duroc pigs were examined with H&E staining. Number of samples at each time point are listed in Table 3.
Table 3.
Number of samples at each time point and each type of wound
| 1 week | 2 weeks | 3 weeks | 4 weeks | 2 months | 3 months | 5 months | |
|---|---|---|---|---|---|---|---|
| Uninjured | 4 | 4 | 3 | 2 | 3 | 5 | 5 |
| Shallow | 4 | 4 | 4 | 3 | 3 | 5 | 7 |
| Intermediate | 2 | 2 | 2 | 2 | 1 | 3 | 4 |
| Deep | 4 | 2 | 2 | 4 | 3 | 2 | 6 |
Wounds were grouped according to total dermatome settings including shallow (0.020 in.), intermediate (0.040 in.) and deep wounds (0.060 in.) at 1, 2, 3 and 4 weeks, and 2, 3 and 5 months. The thickness of the dermis left in the wound bed at the time of wounding, the inflammatory response, the new tissue at the time of epithelialization (1 week for shallow wounds and 4 weeks for deep wounds), and of the late healed skin/scar was measured with an eyepiece micrometer. We used the Wilcoxon Mann–Whitney test to determine statistical differences.
2.4. Processing for mast cell counts
Samples of uninjured human tissue (five patients) and samples of human hypertrophic scars (five patients) taken 24–30 months after injury were analyzed (Table 1). These were compared to samples of Duroc scar (five pigs) from deep wounds and samples of uninjured Duroc tissue (five pigs) taken 5 months from injury. Sections containing all the skin layers were embedded in paraffin, cut into 5 μm sections and mounted onto slides. Histological staining was performed with Giemsa Stain Solution (Crescent Chemical Company Inc., Islandia, NY) [74-78]. Slides were baked at 60 °C for 20 min to deparaffinize and then hydrated to distilled water. Samples were incubated at room temperature for 8 min in non-filtered diluted 2% Giemsa solution, differentiated in 90% ethyl alcohol (three washes), dehydrated through absolute ethyl alcohol (three washes), cleared in xylene and cover-slipped.
An eyepiece with a standard rectangular reticule was used with a Nikon Labophot-2 standard light microscope. For mast cells quantification 20 consecutive non-overlapping rectangular fields of the upper papillary dermis were examined at 40×. Mast cells were identified by the characteristic morphology of the granules (Fig. 1); only intact mast cells with distinct blue staining were counted. Measurement was repeated five times for each field with the researcher blind to the age of the scar. The average number of mast cells was calculated for each sample and the Wilcoxon Mann–Whitney test used to determine statistical significance.
Fig. 1.
Intact mast cells stained with Giemsa in human (a) and Duroc (b) skin, 40×.
2.5. Processing for collagen nodules
Biopsies of human hypertrophic scar (25 patients, 27 scars, 9 from 3.9–12 months postinjury, 18 from 24–67 months postinjury, Table 2) were compared to deep Duroc scars (6 animals, 13 scars, 6 from less than 5 months postinjury, 7 from 5 months postinjury). Tissue was processed as for mast cell quantification but was stained with H&E. The presence or absence of nodules was confirmed by histological evaluation with light microscopy (Fig. 2). Fisher’s exact test was used to determine the statistical difference between groups.
Fig. 2.
Collagen nodules in human (a) and Duroc (b) scar, 4×. Human scar is 10 months since injury and Duroc scar 5 months.
2.6. Processing for myofibroblasts
Three shallow and three deep wound samples at 1–4 weeks, and 3 and 5 months were analyzed. The immunohistochemical study was performed on 4 μm paraffin sections. The sections were microwaved in 0.1 M pH6.0 citrate buffer for 5 min, and allowed to cool to room temperature. Anti-α-smooth muscle actin monoclonal antibody (obtained from Giulio Gabbiani, Geneva, Switzerland) was diluted with PBS at 1:300 and incubated with the sections at room temperature for 60 min. Endogenous peroxidase was inactivated by incubation with 0.3% H2O2 in methanol for 10 min at room temperature. The secondary antibody was biotinylated goat anti-mouse antibody (Vector Laboratories, Burlingame, CA) at 1:250 dilutions for 30 min, followed by Streptavidin AH–biotin complex (SABC Kit, Zymed Laboratories, San Francisco, CA) at 1:250 dilutions for 30 min. All antibodies were diluted in the serum/TBS blocking solution and all incubations occurred at room temperature. Sections were visualized for immunoreactivity using (0.12%) 3,3′-diaminobenzidine (Sigma, St. Louis, MO) as a chromogen for 20 min and counterstained in hematoxylin (Fig. 3). An eyepiece with a standard rectangular reticule was used with a Nikon Labophot-2 light microscope. To count myofibroblasts, the designated rectangular area was started at one edge of the tissue in the upper papillary layer. Eight consecutive non-overlapping rectangular fields were examined at 20×. The Wilcoxon Mann–Whitney test was used to test for statistical differences.
Fig. 3.
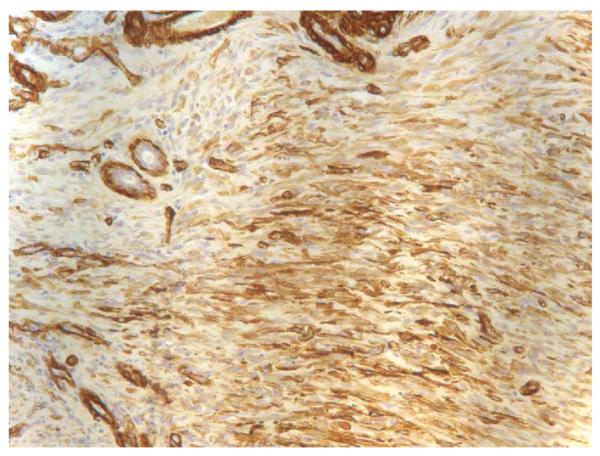
Myofibroblasts in deep wound at 2 weeks, 20×.
3. Results
3.1. Wound healing and histology
As in previous publications [45-48], shallow wounds epithelialized at about 1 week after-wounding whereas deeper wounds required approximately 4 weeks. For shallow wounds, the healed wound consisted of normal epidermis, well-organized collagen fibers and hair follicles, and minor contraction. The healed wound was difficult to discern from uninjured skin. Deep wounds developed thick scar, disorganized collagen, no skin appendages and major contraction.
3.2. Wound inflammatory layer
By “inflammatory layer” we refer to a layer of maturing granulation tissue or cellular fibrosis (early scar) with significant inflammation. This discrete layer was identified by intense hematoxylin staining of the inflammatory cells. At 1 week after-wounding, the excised tissue was replaced by an inflammatory layer of almost equal thickness (Fig. 4). In shallow wounds the inflammatory layer was gone at 4 weeks. In deep wounds, at 4 weeks when the wounds were finally epithelialized, the inflammatory layer was still very thick (Fig. 5). Also in these deep wounds, during the subsequent weeks the inflammatory tissue layer was replaced with a thicker layer of disorganized fibrous tissue.
Fig. 4.

Thickness of residual dermis, removed tissue, inflammatory layer, healed skin/scar, and uninjured skin in shallow, intermediate and deep Duroc wounds.
Fig. 5.
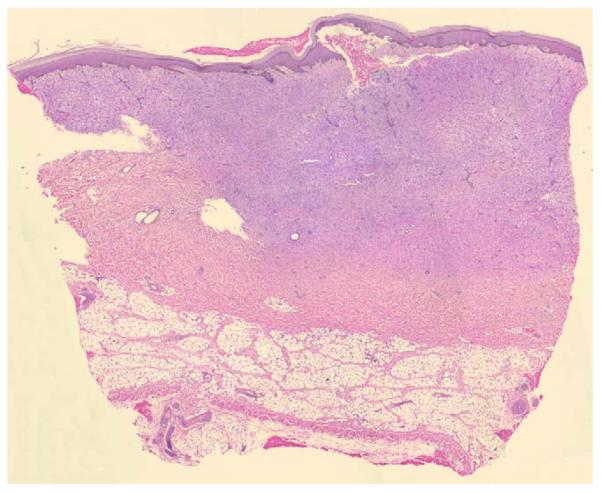
Wound (0.060 in.) at 1 month, 4×. Note the thick, blue inflammatory layer, i.e. the layer of maturing granulation tissue or cellular fibrosis (early scar) with inflammation.
3.3. Wound thickness
The thickness of the wounds at the various time points is shown in Fig. 6a–c. For shallow and intermediate wounds (Fig. 6a and b) the thickness of the wounds resembles uninjured skin. For the deep wounds, the thickness of the wound was significantly greater than that of uninjured skin at 8, 12 and 21 weeks (p < 0.05). The thickness of the inflammatory layer increases with wound depth and the difference is significant for the deep wounds at 2, 3 and 4 weeks (p < .05).
Fig. 6.
(a) Thickness of shallow wound compared to uninjured skin. There is very little difference. (b) Thickness of intermediate wound compared to uninjured skin. The difference is not significant.
3.4. Mast cells
We counted mast cells in four groups (Duroc scar, uninjured Duroc skin, human hypertrophic scar and uninjured human skin). For each wound type, the mean number of mast cells was calculated. The Kruskal–Wallis test was used to determine statistical differences.
Duroc scars at 5 months contained more mast cells than uninjured Duroc skin (p < .05) and human hypertrophic scar contained more mast cells than uninjured human skin (p < 0.01) (Table 4).
Table 4.
Mast cell counts
| Tissue type | Age of scar (months) |
N | Mean ± S.D. | p-Value |
|---|---|---|---|---|
| Duroc scar from deep wounds |
5 | 5 | 55 ± 34 | <0.05 |
| Uninjured Duroc skin |
5 | 5 | 20 ± 9 | |
| Human hypertrophic scar |
24–30 | 5 | 272 ± 109 | <0.01 |
| Uninjured human skin |
24–30 | 5 | 53 ± 13 |
3.5. Collagen nodules
We grouped the sample data into two categories according to age of the scar, <5 and 5 months for Duroc scar and 0–12 and 24–67 months for human hypertrophic scar and calculated the percent of samples in each group containing collagen nodules.
Human hypertrophic scars (33%) less than 12 months old contained nodules; 81% of human hypertrophic scar greater than 24 months old (p < .05). Whereas no Duroc scars less than 5 months old demonstrated nodules, 50% of Duroc scars at 5 months demonstrated distinct nodular structures (p < .05) (Table 5).
Table 5.
Presence of collagen nodules
| Type of scar | Group (months) |
Nodules |
p-Value | |
|---|---|---|---|---|
| Absent | Present | |||
| Duroc scar from deep wounds |
<5 | 6 | 0 | <0.05 |
| 5 | 3 | 4 | ||
| Human hypertrophic scar |
5–12 | 6 | 3 | <0.05 |
| 24–67 | 4 | 14 | ||
3.6. Myofibroblasts
The counts of α-SMA positive spindle shaped cells are shown in Table 6. In the deep wounds the counts were higher than in shallow wounds at 1, 2 and 3 weeks and then declined. There was no difference at 3 and 5 months.
Table 6.
Counts of α-SMA positive cells
| Time | Shallow wounds (mean ± S.D.) |
Deep wounds (mean ± S.D.) |
p-Value |
|---|---|---|---|
| 1 week | 59 ± 92 | 259 ± 85 | <0.05 |
| 2 weeks | 6 ± 5 | 276 ± 92 | <0.05 |
| 3 weeks | 8 ± 3 | 179 ± 162 | <0.05 |
| 3 months | 2 ± 1 | 2 ± 2 | |
| 5 months | 1 ± 1 | 1 ± 1 |
4. Discussion
4.1. Histology, the inflammatory layer and thickness
The thickness of the deep wounds at 5 months is greater than the thickness of uninjured skin and the thickness of shallow wounds.
It is interesting in our Duroc model that the thickness of the inflammatory layer at 1 week after-wounding is essentially the same as the thickness of the tissue removed. It suggests a delicate monitoring system that communicates to the local cells how much tissue is missing and how much tissue is needed to mend the defect. At 1 week in shallow and deep wounds, the amount of skin removed was replaced with almost equal amount of inflammatory tissue (Fig. 4).
The events in shallow and deep wounds are very different. In shallow wounds, the inflammatory tissue layer rapidly disappears and the skin is essentially the same as uninjured skin. In deep wounds, however, the inflammatory tissue layer continues to increase in thickness and, in time, becomes a thick layer of disorganized scar. This suggests that if the inflammatory tissue layer could be affected perhaps the thick scar could also be minimized.
The concept that cellular communication between different segments of the skin regulates response to injury is intriguing. It has been hypothesized that epidermal–dermal interactions are involved in skin morphogenetic responses including repair [79-84]. Disruption of epidermal–mesenchymal communication due to a delay in epithelialization increases the frequency of developing fibrotic conditions in skin [85]. By using a tissue-engineered model of reconstructed human skin [86], Bellemare demonstrated that hypertrophic scar keratinocytes plays a role in the development of pathological fibrosis by influencing the proliferation of dermal cells and matrix accumulation, reflected in the balance of synthesis and degradation of collagen.
4.2. Mast cell counts
Mast cell proliferation and infiltration is common in various pathologic skin conditions including wounds, however, their interactions with other cell types such as fibroblasts, endothelial cells and neurons is still unclear. Their presence may prove to be a valuable indicator of fibroblast cell activation and extracellular fibrosis [49-54]. This along with their hematopoietic derivation and strategic location between vessels and neurons suggests a key role in the formation of scar. Human hypertrophic scar has been reported to exhibit as many as four times more mast cells than normal skin [55-58].
We found that Duroc scars at 5 months contained 2.4 times more mast cells than uninjured Duroc tissue (p < 0.05) and that human hypertrophic scar contained 4.2 times more mast cells than uninjured human tissue (p < 0.01). These results are similar to that reported in the literature for human hypertrophic scar. This further validates this model of scarring and may also validate the Duroc model for the study of mast cell role in responses to injury.
4.3. Collagen nodules
Formation of nodules with whorl-like patterns, hyalinized collagen, and spheroid delineation has been described as characteristic of human hypertrophic scar [12,59-69]. We noted that in the early papers nodules were thought to be present in “all” human hypertrophic scar whereas the recent paper by Santucci et al. [69] clarified that early hypertrophic scars do not always contain nodules.
We found that in both Duroc scar and human hypertrophic scar demonstrate increasing numbers of nodules with age confirming the literature findings and further validating the model. Since we have demonstrated nodule formation in these thick Duroc wounds, these histological findings may provide one way to measure effects of scar modulation by mechanical perturbations of scarring such as pressure garments.
4.4. Myofibroblasts
Santucci et al. [69] demonstrated that myofibroblasts are common in human hypertrophic scars less than 12 months old and then decline. Kamath et al. [87] also reported on the presence of myofibroblasts over time and found counts to be highest at approximately 2 months and declining thereafter. The time course in the thick Duroc scar was similar although greatly compressed compared to the Santucci et al. report but similar to the Kamath et al. report. These findings further validate the model and suggest that the model may be useful in studying the role of myofibroblasts in scarring.
5. Conclusions
The purpose of this study was to quantify the number of mast cells, collagen nodules and myofibroblasts in Duroc scar and human hypertrophic scar to further validate the female, red Duroc model of hypertrophic scarring. We found that increased numbers of myofibroblasts, mast cells and collagen nodules are present in Duroc scar similar to human hypertrophic scar.
We also found that the thickness of the inflammatory tissue layer correlates with the thickness of the ultimate scar.
Acknowledgements
This work was funded in part by Washington State Council of Firefighters Burn Foundation; National Institute on Disability and Rehabilitation Research/Office of Special Education and Rehabilitation Services/US Department of Education; National Institutes of Health.
References
- [1].Ehrlich HP, Kelley SF. Hypertrophic scar: an interruption in the remodeling of repair — a laser Doppler blood flow study. Plast Reconstr Surg. 1992;90(6):993–8. [PubMed] [Google Scholar]
- [2].Rudolph R. Wide spread scars, hypertrophic scars, and keloids. Clin Plast Surg. 1987;14(2):253–60. [PubMed] [Google Scholar]
- [3].Engrav LH, Heimbach DM, Reus JL, Harnar TJ, Marvin JA. Early excision and grafting versus nonoperative treatment of burns of indeterminant depth: a randomized prospective study. J Trauma. 1983;23(11):1001–4. doi: 10.1097/00005373-198311000-00007. [DOI] [PubMed] [Google Scholar]
- [4].Abdullah A, Blakeney P, Hunt R, Broemeling L, Phillips L, Herndon DN, et al. Visible scars and self-esteem in pediatric patients with burns. J Burn Care Rehabil. 1994;15(2):164–8. doi: 10.1097/00004630-199403000-00011. [DOI] [PubMed] [Google Scholar]
- [5].Fauerbach JA, Heinberg LJ, Lawrence JW, Munster AM, Palombo DA, Richter D, et al. Effect of early body image dissatisfaction on subsequent psychological and physical adjustment after disfiguring injury. Psychosom Med. 2000;62(4):576–82. doi: 10.1097/00006842-200007000-00017. [DOI] [PubMed] [Google Scholar]
- [6].Brych SB, Engrav LH, Rivara FP, Ptacek JT, Lezotte DC, Esselman PC, et al. Time off work and return to work rates after burns: systematic review of the literature and a large two-center series. J Burn Care Rehabil. 2001;22(6):401–5. doi: 10.1097/00004630-200111000-00010. [DOI] [PubMed] [Google Scholar]
- [7].Fauerbach JA, Heinberg LJ, Lawrence JW, Bryant AG, Richter L, Spence RJ. Coping with body image changes following a disfiguring burn injury. Health Psychol. 2002;21(2):115–21. [PubMed] [Google Scholar]
- [8].Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4(4):245–72. doi: 10.2165/00128071-200304040-00004. [DOI] [PubMed] [Google Scholar]
- [9].Herndon DN, LeMaster J, Beard S, Bernstein N, Lewis SR, Rutan TC, et al. The quality of life after major thermal injury in children: an analysis of 12 survivors with greater than or equal to 80% total body 70% third-degree burns. J Trauma. 1986;26(7):609–19. [PubMed] [Google Scholar]
- [10].Engrav LH, Covey MH, Dutcher KD, Heimbach DM, Walkinshaw MD, Marvin JA. Impairment, time out of school, and time off from work after burns. Plast Reconstr Surg. 1987;79(6):927–34. doi: 10.1097/00006534-198706000-00012. [DOI] [PubMed] [Google Scholar]
- [11].Linares HA. From wound to scar. Burns. 1996;22(5):339–52. doi: 10.1016/0305-4179(95)00164-6. [DOI] [PubMed] [Google Scholar]
- [12].Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989;84(5):827–37. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]
- [13].Murray JC. Keloids and hypertrophic scars. Clin Dermatol. 1994;12(1):27–37. doi: 10.1016/0738-081x(94)90254-2. [DOI] [PubMed] [Google Scholar]
- [14].Su CW, Alizadeh K, Boddie A, Lee RC. The problem scar. Clin Plast Surg. 1998;25(3):451–65. [PubMed] [Google Scholar]
- [15].Dasu MR, Hawkins HK, Barrow RE, Xue H, Herndon DN. Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J Pathol. 2004;202(4):476–85. doi: 10.1002/path.1539. [DOI] [PubMed] [Google Scholar]
- [16].Rahban SR, Garner WL. Fibroproliferative scars. Clin Plast Surg. 2003;30(1):77–89. doi: 10.1016/s0094-1298(02)00069-x. [DOI] [PubMed] [Google Scholar]
- [17].Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38(2):72–140. doi: 10.1067/msg.2001.111167. [DOI] [PubMed] [Google Scholar]
- 18.Robson MC. Proliferative scarring. Surg Clin N Am. 2003;83(3):557–69. doi: 10.1016/S0039-6109(02)00197-4. [DOI] [PubMed] [Google Scholar]
- [19].Aksoy MH, Vargel I, Canter IH, Erk Y, Sargon M, Pinar A, et al. A new experimental hypertrophic scar model in guinea pigs. Aesthet Plast Surg. 2002;26(5):388–96. doi: 10.1007/s00266-002-1121-z. [DOI] [PubMed] [Google Scholar]
- [20].Gallant CL, Olson ME, Hart DA. Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveals an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen. 2004;12(3):305–19. doi: 10.1111/j.1067-1927.2004.012311.x. [DOI] [PubMed] [Google Scholar]
- [21].Mast BA. The skin. In: Cohen IK, Diegelmann RF, Lindblad WJ, editors. Wound healing: biochemical and clinical aspects. W.B. Saunders Co.; Philadelphia: 1992. p. 353. [Google Scholar]
- [22].Morris DE, Wu L, Zhao LL, Bolton L, Roth SI, Ladin DA, et al. Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg. 1997;100(3):674–81. doi: 10.1097/00006534-199709000-00021. [DOI] [PubMed] [Google Scholar]
- [23].Polo M, Kim YJ, Kucukcelebi A, Hayward PG, Ko F, Robson MC. An in vivo model of human proliferative scar. J Surg Res. 1998;74(2):187–95. doi: 10.1006/jsre.1997.5251. [DOI] [PubMed] [Google Scholar]
- [24].Kischer CW, Pindur J, Shetlar MR, Shetlar CL. Implants of hypertrophic scars and keloids into the nude (athymic) mouse: viability and morphology. J Trauma. 1989;29(5):672–7. doi: 10.1097/00005373-198905000-00023. [DOI] [PubMed] [Google Scholar]
- [25].Kischer CW, Sheridan D, Pindur J. Use of nude (athymic) mice for the study of hypertrophic scars and keloids: vascular continuity between mouse and implants. Anat Rec. 1989;225(3):189–96. doi: 10.1002/ar.1092250303. [DOI] [PubMed] [Google Scholar]
- [26].Wang X, Smith P, Pu LL, Kim YJ, Ko F, Robson MC. Exogenous transforming growth factor beta(2) modulates collagen I and collagen III synthesis in proliferative scar xenografts in nude rats. J Surg Res. 1999;87(2):194–200. doi: 10.1006/jsre.1999.5757. [DOI] [PubMed] [Google Scholar]
- [27].Polo M, Smith PD, Kim YJ, Wang X, Ko F, Robson MC. Effect of TGF-beta2 on proliferative scar fibroblast cell kinetics. Ann Plast Surg. 1999;43(2):185–90. [PubMed] [Google Scholar]
- [28].Shetlar MR, Shetlar CL, Kischer CW, Pindur J. Implants of keloid and hypertrophic scars into the athymic nude mouse: changes in the glycosaminoglycans of the implants. Connect Tissue Res. 1991;26(1–2):23–36. doi: 10.3109/03008209109152161. [DOI] [PubMed] [Google Scholar]
- [29].Shetlar MR, Shetlar CL, Hendricks L, Kischer CW. The use of athymic nude mice for the study of human keloids. Proc Soc Exp Biol Med. 1985;179(4):549–52. doi: 10.3181/00379727-179-rc3. [DOI] [PubMed] [Google Scholar]
- [30].Robb EC, Waymack JP, Warden GD, Nathan P, Alexander JW. A new model for studying the development of human hypertrophic burn scar formation. J Burn Care Rehabil. 1987;8(5):371–5. doi: 10.1097/00004630-198709000-00006. [DOI] [PubMed] [Google Scholar]
- [31].Silverstein P, Goodwin MN, Raulston GL, Pruitt B. Hypertrophic scar in the experimental animal. In: Longacre JJ, editor. The ultrastructure of collagen; its relation to the healing of wounds and to the management of hypertrophic scar. Thomas; Springfield, IL: 1976. pp. 213–36. [Google Scholar]
- [32].Moritz AR, Henriques FC., Jr Studies of thermal injury. Part II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol. 1947;23:695–720. [PMC free article] [PubMed] [Google Scholar]
- [33].Montagna W, Yun J. The skin of the domestic pig. J Invest Dermatol. 1964;43:11–21. [PubMed] [Google Scholar]
- [34].Marcarian HQ, Calhoun ML. Microscopic anatomy of the integument of adult swine. Am J Vet Res. 1966;27(118):765–72. [PubMed] [Google Scholar]
- [35].Nicolaides N, Fu HC, Rice GR. The skin surface lipids of man compared with those of eighteen species of animals. J Invest Dermatol. 1968;51(2):83–9. doi: 10.1038/jid.1968.96. [DOI] [PubMed] [Google Scholar]
- [36].Forbes PD. Vascular supply of the skin and hair in swine. In: Montagna W, Dobson RL, editors. Advances in the biology of skin: hair growth. vol 9. Pergamon; Oxford: 1969. [Google Scholar]
- [37].Douglas WR. Of pigs and men and research. Space Life Sci. 1972;3:226–34. doi: 10.1007/BF00928167. [DOI] [PubMed] [Google Scholar]
- [38].Gray GM, Yardley HJ. Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res. 1975;16(6):434–40. [PubMed] [Google Scholar]
- [39].Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol. 1978;7:39–52. doi: 10.1159/000401274. [DOI] [PubMed] [Google Scholar]
- [40].Morris GM, Hopewell JW. Epidermal cell kinetics of the pig: a review. Cell Tissue Kinet. 1990;23(4):271–82. doi: 10.1111/j.1365-2184.1990.tb01124.x. [DOI] [PubMed] [Google Scholar]
- [41].Wollina U, Berger U, Mahrle G. Immunohistochemistry of porcine skin. Acta Histochem. 1991;90(1):87–91. doi: 10.1016/S0065-1281(11)80166-2. [DOI] [PubMed] [Google Scholar]
- [42].Monteiro-Riviere NA, Stromberg MW. Ultrastructure of the integument of the domestic pig (Sus scrofa) from one through 14 weeks of age. Anat Histol Embryol. 1985;14(2):97–115. doi: 10.1111/j.1439-0264.1985.tb00270.x. [DOI] [PubMed] [Google Scholar]
- [43].Vardaxis NJ, Brans TA, Boon ME, Kreis RW, Marres LM. Confocal laser scanning microscopy of porcine skin: implications for human wound healing studies. J Anat. 1997;190(Pt 4):601–11. doi: 10.1046/j.1469-7580.1997.19040601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9(2):66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- [45].Zhu KQ, Engrav LH, Gibran NS, Cole JK, Matsumura H, Piepkorn M, et al. The female, red Duroc pig as an animal model of hypertrophic scarring and the potential role of the cones of skin. Burns. 2003;29(7):649–64. doi: 10.1016/s0305-4179(03)00205-5. [DOI] [PubMed] [Google Scholar]
- [46].Liang Z, Engrav LH, Muangman P, Muffley LA, Zhu KQ, Carrougher GJ, et al. Nerve quantification in female red Duroc pig (FRDP) scar compared to human hypertrophic scar. Burns. 2004;30(1):57–64. doi: 10.1016/j.burns.2003.09.004. [DOI] [PubMed] [Google Scholar]
- [47].Zhu KQ, Engrav LH, Tamura RN, Cole JK, Muangman P, Carrougher GJ, et al. Further similarities between cutaneous scarring in the female, red Duroc pig and human hypertrophic scarring. Burns. 2004;30(6):518–30. doi: 10.1016/j.burns.2004.02.005. [DOI] [PubMed] [Google Scholar]
- [48].Zhu KQ, Engrav LH, Armendariz RT, Muangman P, Klein MB, Carrougher GJ, et al. Changes in VEGF and nitric oxide after deep dermal injury in the female, red Duroc pig-further similarities between female. Duroc scar and human hypertrophic scar. Burns. 2005;31(1):5–10. doi: 10.1016/j.burns.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [49].Gruber BL. Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2003;5(2):147–53. doi: 10.1007/s11926-003-0043-3. [DOI] [PubMed] [Google Scholar]
- [50].Trautmann A, Toksoy A, Engelhardt E, Brocker EB, Gillitzer R. Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol. 2000;190(1):100–6. doi: 10.1002/(SICI)1096-9896(200001)190:1<100::AID-PATH496>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [51].Choi KL, Claman HN. Mast cells, fibroblasts, and fibrosis. New clues to the riddle of mast cells. Immunol Res. 1987;6(3):145–52. doi: 10.1007/BF02918088. [DOI] [PubMed] [Google Scholar]
- [52].Gruber BL. Mast cells: accessory cells which potentiate fibrosis. Int Rev Immunol. 1995;12(2–4):259–79. doi: 10.3109/08830189509056717. [DOI] [PubMed] [Google Scholar]
- [53].LeRoy EC, Trojanowska M, Smith EA. The pathogenesis of scleroderma (systemic sclerosis SSc) Clin Exp Rheumatol. 1991;9(2):173–7. [PubMed] [Google Scholar]
- [54].Atkins FM, Clark RA. Mast cells and fibrosis. Arch Dermatol. 1987;123(2):191–3. [PubMed] [Google Scholar]
- [55].Mikhail GR, Miller-Milinska A. Mast cell population in human skin. J Invest Dermatol. 1964;43:249–54. [PubMed] [Google Scholar]
- [56].Kischer CW, Bailey JF. The mast cell in hypertrophic scars. Tex Rep Biol Med. 1972;30(4):327–38. [PubMed] [Google Scholar]
- [57].Kischer CW, Bunce H, 3rd, Shetlah MR. Mast cell analyses in hypertrophic scars, hypertrophic scars treated with pressure and mature scars. J Invest Dermatol. 1978;70(6):355–7. doi: 10.1111/1523-1747.ep12543553. [DOI] [PubMed] [Google Scholar]
- [58].Reich JD, Cazzaniga AL, Mertz PM, Kerdel FA, Eaglstein WH. The effect of electrical stimulation on the number of mast cells in healing wounds. J Am Acad Dermatol. 1991;25(1 Pt 1):40–6. doi: 10.1016/0190-9622(91)70171-w. [DOI] [PubMed] [Google Scholar]
- [59].Glücksmann A. Local factors in the histogenesis of hypertrophic scar. Br J Plast Surg. 1951;4:88–103. doi: 10.1016/s0007-1226(51)80014-6. [DOI] [PubMed] [Google Scholar]
- [60].Blackburn WR, Cosman B. Histologic basis of keloid and hypertrophic scar differentiation. Arch Pathol. 1966;82(1):65–71. [PubMed] [Google Scholar]
- [61].Linares HA, Kischer CW, Dobrkovsky M, Larson DL. The histiotypic organization of the hypertrophic scar in humans. J Invest Dermatol. 1972;59(4):323–31. doi: 10.1111/1523-1747.ep12627386. [DOI] [PubMed] [Google Scholar]
- [62].Linares HA, Kischer CW, Dobrkovsky M, Larson DL. On the origin of the hypertrophic scar. J Trauma. 1973;13(1):70–5. doi: 10.1097/00005373-197301000-00013. [DOI] [PubMed] [Google Scholar]
- [63].Linares HA, Larson DL. Early differential diagnosis between hypertrophic and nonhypertrophic healing. J Invest Dermatol. 1974;62(5):514–6. doi: 10.1111/1523-1747.ep12681048. [DOI] [PubMed] [Google Scholar]
- [64].Kischer CW, Brody GS. Structure of the collagen nodule from hypertrophic scars and keloids. Scan Electron Microsc. 1981;(Pt 3):371–6. [PubMed] [Google Scholar]
- [65].Kischer CW, Shetlar MR, Chvapil M. Hypertrophic scars and keloids: a review and new concept concerning their origin. Scan Electron Microsc. 1982;(Pt 4):1699–713. [PubMed] [Google Scholar]
- [66].Kischer CW. Comparative ultrastructure of hypertrophic scars and keloids. Scan Electron Microsc. 1984;(Pt 1):423–31. [PubMed] [Google Scholar]
- [67].Kischer CW. The microvessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol. 1992;24(2):281–96. [PubMed] [Google Scholar]
- [68].Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145(1):105–13. [PMC free article] [PubMed] [Google Scholar]
- [69].Santucci M, Borgognoni L, Reali UM, Gabbiani G. Keloids and hypertrophic scars of Caucasians show distinctive morphologic and immunophenotypic profiles. Virchows Arch. 2001;438(5):457–63. doi: 10.1007/s004280000335. [DOI] [PubMed] [Google Scholar]
- [70].Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- [71].Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971;173(996):548–50. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- [72].Nedelec B, Shankowsky H, Scott PG, Ghahary A, Tredget EE. Myofibroblasts and apoptosis in human hypertrophic scars: the effect of interferon-alpha2b. Surgery. 2001;130(5):798–808. doi: 10.1067/msy.2001.116453. [DOI] [PubMed] [Google Scholar]
- [73].Fang P, Engrav LH, Gibran NS, Honari S, Kiriluk DB, Cole JK, et al. Dermatome setting for autografts to cover INTEGRA. J Burn Care Rehabil. 2002;23(5):327–32. doi: 10.1097/00004630-200209000-00005. [DOI] [PubMed] [Google Scholar]
- [74].Vila AT, Puig L, Fernandez-Figueras MT, Laiz AM, Vidal D, Alomar A. Adverse cutaneous reactions to anakinra in patients with rheumatoid arthritis: clinicopathological study of five patients. Br J Dermatol. 2005;153(2):417–23. doi: 10.1111/j.1365-2133.2005.06635.x. [DOI] [PubMed] [Google Scholar]
- [75].Rios JD, Horikawa Y, Chen LL, Kublin CL, Hodges RR, Dartt DA, et al. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp Eye Res. 2005;80(4):477–91. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hochegger K, Siebenhaar F, Vielhauer V, Heininger D, Mayadas TN, Mayer G, et al. Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur J Immunol. 2005;35(10):3074–82. doi: 10.1002/eji.200526250. [DOI] [PubMed] [Google Scholar]
- [77].Hendrix S, Warnke K, Siebenhaar F, Peters EM, Nitsch R, Maurer M. The majority of brain mast cells in B10.PL mice is present in the hippocampal formation. Neurosci Lett. 2005 doi: 10.1016/j.neulet.2005.09.029. [DOI] [PubMed] [Google Scholar]
- [78].Acikalin MF, Oner U, Topcu I, Yasar B, Kiper H, Colak E. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Liver Dis. 2005;37(3):162–9. doi: 10.1016/j.dld.2004.09.028. [DOI] [PubMed] [Google Scholar]
- [79].Garner WL. Epidermal regulation of dermal fibroblast activity. Plast Reconstr Surg. 1998;102(1):135–9. doi: 10.1097/00006534-199807000-00021. [DOI] [PubMed] [Google Scholar]
- [80].Andriessen MP, Niessen FB, Van de Kerkhof PC, Schalkwijk J. Hypertrophic scarring is associated with epidermal abnormalities: an immunohistochemical study. J Pathol. 1998;186(2):192–200. doi: 10.1002/(SICI)1096-9896(1998100)186:2<192::AID-PATH163>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [81].Niessen FB, Andriessen MP, Schalkwijk J, Visser L, Timens W. Keratinocyte-derived growth factors play a role in the formation of hypertrophic scars. J Pathol. 2001;194(2):207–16. doi: 10.1002/path.853. [DOI] [PubMed] [Google Scholar]
- [82].Niessen FB, Schalkwijk J, Vos H, Timens W. Hypertrophic scar formation is associated with an increased number of epidermal Langerhans cells. J Pathol. 2004;202(1):121–9. doi: 10.1002/path.1502. [DOI] [PubMed] [Google Scholar]
- [83].Machesney M, Tidman N, Waseem A, Kirby L, Leigh I. Activated keratinocytes in the epidermis of hypertrophic scars. Am J Pathol. 1998;152(5):1133–41. [PMC free article] [PubMed] [Google Scholar]
- [84].Hakvoort TE, Altun V, Ramrattan RS, van der Kwast TH, Benner R, van Zuijlen PP, et al. Epidermal participation in post-burn hypertrophic scar development. Virchows Arch. 1999;434(3):221–6. doi: 10.1007/s004280050331. [DOI] [PubMed] [Google Scholar]
- [85].Ghahary A, Marcoux Y, Karimi-Busheri F, Li Y, Tredget EE, Kilani RT, et al. Differentiated keratinocyte-releasable stratifin (14-3-3 sigma) stimulates MMP-1 expression in dermal fibroblasts. J Invest Dermatol. 2005;124(1):170–7. doi: 10.1111/j.0022-202X.2004.23521.x. [DOI] [PubMed] [Google Scholar]
- [86].Bellemare J, Roberge CJ, Bergeron D, Lopez-Valle CA, Roy M, Moulin VJ. Epidermis promotes dermal fibrosis: role in the pathogenesis of hypertrophic scars. J Pathol. 2005;206(1):1–8. doi: 10.1002/path.1737. [DOI] [PubMed] [Google Scholar]
- [87].Kamath NV, Ormsby A, Bergfeld WF, House NS. A light microscopic and immunohistochemical evaluation of scars. J Cutan Pathol. 2002;29(1):27–32. doi: 10.1034/j.1600-0560.2002.290105.x. [DOI] [PubMed] [Google Scholar]





