Abstract
Background
The objective was to determine the effect of diet induced obesity (DIO) on microvascular and neural function.
Methods
Rats were fed a standard or high fat diet for up to 32 weeks. Measurements were performed of vasodilation in epineurial arterioles by videomicroscopy, endoneurial blood flow by hydrogen clearance, nerve conduction velocity by electrical stimulation, size-frequency distribution of myelinated fibers of the sciatic nerve, intraepidermal nerve fiber density using confocal microscopy and thermal nociception using the Hargreaves method.
Results
Rats fed a high fat diet for 32 weeks developed sensory neuropathy as indicated by slowing of sensory nerve conduction velocity and thermal hypoalgesia. Motor nerve conduction velocity and endoneurial blood flow were not impaired. Mean axonal diameter of myelinated fibers of the sciatic nerve was unchanged in high fat fed rats compared to control. Intraepidermal nerve fiber density was significantly reduced in high fat fed rats. Vascular relaxation to acetylcholine and calcitonin gene-related peptide was decreased and expression of neutral endopeptidase (NEP) increased in epineurial arterioles of rats fed a high fat diet. In contrast, insulin-mediated vascular relaxation was increased in epineurial arterioles. NEP activity was significantly increased in the skin of the hindpaw. Markers of oxidative stress were increased in the aorta and serum of high fat fed rats but not in epineurial arterioles.
Conclusion
Chronic obesity causes microvascular and neural dysfunction. This is associated with increased expression of NEP but not oxidative stress in epineurial arterioles. NEP degrades vasoactive peptides which may explain the decrease in microvascular function.
Keywords: Obesity, vascular function, neutral endopeptidase, neuropathy
Introduction
The metabolic syndrome is a worldwide epidemic, setting the stage for type 2 diabetes and its microvascular complications. Insulin resistance, hyperglycemia, dyslipidemia, hypertension, thrombotic disorders and adiposity define the metabolic syndrome and contribute to microvascular disease [1]. Patients with type 2 diabetes and metabolic syndrome are more prone to microvascular disease than diabetic patients without metabolic syndrome and obesity alone has been suggested to be a primary cause of microvascular dysfunction [2,3]. In our studies with obese Zucker rats, an animal model of the metabolic syndrome, we demonstrated that vascular relaxation in response to acetylcholine and calcitonin gene-related peptide was decreased in epineurial arterioles [4]. In addition, nerve conduction velocity, endoneurial blood flow and thermal nociception were impaired in obese Zucker rats [4]. It is known that hyperglycemia contributes significantly to vascular dysfunction in diabetes [5,6]. However, obese Zucker rats are not hyperglycemic. Therefore, it is likely that conditions, other than hyperglycemia, which are associated with metabolic syndrome, such as insulin resistance and hyperlipidemia, contribute to microvascular dysfunction. In support of this statement, it has been shown that circulating free fatty acids are elevated in obesity and type 2 diabetes and contribute to insulin resistance [7–9]. Increased free fatty acids also exert negative effects on the vessel wall by triggering endothelial cell apoptosis and impairing endothelium-dependent vasodilation [10,11]. Lipid lowering therapy reduces the progression of vascular disease [12,13]. We have also shown that treating obese Zucker rats with Enalapril or Rosuvastatin improved microvascular and neural complications [14]. Since hyperlipidemia may contribute to microvascular dysfunction there is a need to know more about the effect of diet induced obesity on microvascular and neural complications associated with metabolic syndrome. To address this issue in a more representative model we examined microvascular and neural function in Sprague Dawley rats fed a high fat diet for up to 32 weeks.
Materials and Methods
Unless stated otherwise all chemicals used in these studies were obtained from Sigma Chemical Co. (St. Louis, MO).
Animals
Male Sprague-Dawley (Harlan Sprague Dawley, Indianapolis, IN) rats 10–11 weeks of age were housed in a certified animal care facility and food (Harlan Teklad, #7001, Madison, WI) and water were provided ad libitum. All institutional (ACURF #0691101) and NIH guidelines for use of animals were followed. At 12 weeks of age some of the rats were placed on a high fat diet (D12451; Research Diets, New Brunswick, NJ). The high fat diet contained 24 gm% fat, 24 gm% protein and 41 gm% carbohydrate. The primary source of the increased fat content in the diet was soybean oil and lard. The average fat content of the control diet (Harlan Teklad, #7001, Madison, WI) was 4.25 gm%. Rats were maintained on the standard diet or high fat diet for up to 32 weeks. Consumption was monitored weekly by weighing the amount of food eaten and the total weight of the two rats in each cage and recorded as grams of food consumed per kilogram rat.
Glucose tolerance
Glucose tolerance was determined by injecting rats with a saline solution containing 2 g/kg glucose, i.p., after an overnight fast. Immediately prior to the glucose injection and at 10, 20, 30, 45, 60 and 120 min blood samples were taken to measure circulating glucose levels using glucose oxidase reagent strips (Lifescan Inc., Milpitas, CA). Fasting basal levels of insulin was also determined using Luminex technology. Serum leptin levels were also determined using Luminex technology.
Thermal nociceptive response
The day before terminal studies thermal nociceptive response in the hindpaw was measured using the Hargreaves method as previously described [14].
Motor and sensory nerve conduction velocity, endoneurial blood flow, nerve morphometry and biological and oxidative stress markers
On the day of terminal studies rats were anesthetized with Nembutal i.p. (50 mg/kg, i.p., Abbott Laboratories, North Chicago, IL). Serum samples were collected for determination of free fatty acid, triglyceride, free cholesterol, adiponectin and 8-hydroxy deoxyguanosine, using commercial kits from Roche Diagnostics, Mannheim, Germany; Sigma Chemical Co., St. Louis, MO; Bio Vision, Mountain View, CA; ALPCO diagnostics, Windham, NH and Cell Biolabs, Inc., San Diego, CA, respectively. Serum thiobarbituric acid reactive substances (TBARS) levels was determined as an additional marker of oxidative stress by the method of Mihara et al. [15] as modified by Siman and Eriksson [16]. Briefly, 200 μl of serum was boiled in 0.75 ml of phosphoric acid (0.19 M), 0.25 ml thiobarbituric acid (0.42 mM) and 0.3 ml water for 60 min. Afterwards, the samples were precipitated with methanol/NaOH and centrifuged for 5 min. The supernatant was measured fluorometrically at excitation wavelength of 532 nm and emission wavelength of 553 nm. Standards were prepared by the acid hydrolysis of 1,1,3,3-tetraethoxypropane. The data was reported as μg/ml serum.
Motor and sensory nerve conduction velocity and endoneurial blood flow in the sciatic nerve were determined as previously described and afterwards sciatic nerve and tissue containing the epineurial arterioles were collected [14,17–19].
The axonal size-frequency distribution of myelinated fibers in the sciatic nerve was measured as described previously [20]. Briefly, sciatic nerve samples were taken midway between the sciatic notch and the popliteal fossa, fixed in 2.5% glutaraldehyde and dehydrated before processing to araldite blocks. Thick sections (1 μm) were cut, stained with p-phenylenediamine and examined using a light microscope connected via a video camera to a computer running Scion Image software. Axons of myelinated fibers were manually selected for morphometric analysis using a serpentine progression across the nerve fascicle and subsequently sorted into bins of 1 μm axonal diameter increments by an automated process. All slides were coded and 300–700 axons were measured per slide.
Hydroethidine (Molecular Probes Inc., Eugene, OR), an oxidative fluorescent dye, was used to evaluate in situ levels of superoxide (O2−) in epineurial vessels [17]. Hydroethidine is permeable to cell membranes, and in the presence of O2− it is oxidized to fluorescent ethidium bromide, where it is trapped by intercalating with DNA. Unfixed frozen vessel segments were cut in 10 μm sections and placed on glass slides. Hydroethidine (2 μM) was topically applied to each tissue section and cover slipped. Slides were incubated in a light-protected humidified chamber at 37° C for 30 min. Images were obtained with a Zeiss LSM710 confocal microscope. Vessels from control and high fat fed rats were processed and imaged in parallel. Laser settings were identical for acquisition of all images from control and high fat fed rats.
Superoxide levels in the aorta were measured by lucigenin-enhanced chemiluminescence [17]. Vessel segments from control and high fat fed rats were incubated in 0.5 ml phosphate-buffered saline containing lucigenin (5 μM); afterward, relative light units (RLUs) were measured using a Zylux FB12 luminometer. For these studies, chemiluminescence was measured for 5 min. Background activity was determined and subtracted, and RLUs were normalized to surface area.
Superoxide anion can interact with nitric oxide to form peroxynitrite [21]. This reaction reduces the efficacy of nitric oxide to act as a signal transduction agent. Peroxynitrite is a highly reactive intermediate known to nitrate protein tyrosine residues and cause cellular oxidative damage [22,23]. To determine whether diet induced obesity promotes the formation of peroxynitrite we measured 3-nitrotyrosine (a stable biomarker of tissue peroxynitrite formation) [24]. Briefly, frozen tissue segments of arterioles were cut into 10 μm sections and then incubated in phosphate buffered saline solution containing 1% Triton X-100 and 0.1% bovine serum albumin for 30 min at room temperature. Afterwards, the samples were incubated in this buffer solution containing mouse anti-nitrotyrosine antibody (Upstate, Lake Placid, NY) overnight at 4° C. After washing, the sections were incubated for 2h with Alexa Fluor 546 goat anti-mouse IgG (Molecular Probes, Eugene, OR). Sections were then rinsed and mounted with VectorShield. The labeled vessels derived from these studies were visualized with a Zeiss LSM710 confocal microscope.
Images for superoxide and nitrotyrosine were quantified using the ZEN image analysis software. The amount of immunostaining was determined by dividing the total intensity of the stained regions by their area. This analysis excludes the area of the unstained lumen.
Intraepidermal nerve fiber density and measurement of neutral endopeptidase activity in the hindpaw
Immunoreactive intraepidermal nerve fiber profiles were visualized using confocal microscopy. Biopsies of skin of the right hindpaw were fixed, dehydrated and embedded in paraffin. Sections (7 μm) were collected and immuno stained with anti-PGP9.5 antibody (rabbit anti human, AbD Serotic, Morpho Sys US Inc., Raleigh, NC) over night followed by treatment with secondary antibody Alexa Fluor 546 goat anti rabbit (Invitrogen, Eugene, OR). Profiles were counted by two individual investigators that were blinded to the sample identity. All immunoreactive profiles within the epidermis were counted and normalized to epidermal length [25]. Length of the epidermis was determined by drawing a polyline along the contour of the epidermis and recording its length in mm. The number of intraepidermal nerve fiber profiles was reported per mm length.
A skin biopsy of the left hindpaw was used to determine neutral endopeptidase activity using a modified method described by Ayoub and Melzig [26]. Briefly, skin sample was homogenized in HEPES buffer and cleared by centrifugation (500g). A 50 μg protein aliquot of the supernatant was used to determine neutral endopeptidase activity. The protein sample was incubated in 0.4 ml 50 mm HEPES buffer solution containing 400 μM succinyl-L-Ala-L-Ala-L-Phe-7-amido-3-methylcoumarin (SAAP-AMC) for 1 h at 37° C. The reaction was stopped by adding 50 μl phosphoramidon (50 μM). A 0.4 ml aliquot of the incubation mixture was transferred into a tube containing 20 μL of aminopeptidase N (1:235 dilution in water) and incubated for 1 h at 56° C. Afterwards, 0.8 ml of acetone was added and the fluorescence of the released AMC was determined using fluorescence spectrophotometer ex 367 nm and em 440 nm. Activity was determined by comparing the results to a standard curve of AMC ran simultaneously. Neutral endopeptidase activity was reported as nmol AMC released/mg protein.
Vascular reactivity
Videomicroscopy was used to investigate in vitro vasodilatory responsiveness of arterioles vascularizing the region of the sciatic nerve as previously described [14,17,18]. Cumulative concentration-response relationships were evaluated for acetylcholine (10−8 – 10−4 M), calcitonin gene related peptide (10−11 – 10−8 M) (CGRP) and insulin (0.5 – 1000 ng/ml) using vessels from each group of rats. At the end of the acetylcholine concentrationresponse curve a maximal dose of sodium nitroprusside (10−4 M) was addedto determine endothelium-independent vascular relaxation response. At the end of each dose response curve for either acetylcholine, CGRP or insulinpapaverine (10−5 M) was added to determine maximal vasodilation. Papaverine is a synthetic opium alkaloid with prominent spasmolytic and anticholinergic action. The mechanism of its action is related to inhibition of phosphodiesterase activity and elevation of the intracellular concentration of cAMP.
Immunohistochemistry and Western blot analysis of neutral endopeptidase in epineurial arterioles
We generally followed the methods described previously [21]. Epineurial arterioles were collected with minimal preparation, embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA) and sectioned. Sections (10 μm) wereincubated with the primary antibody 40 μg/ml (anti-CD-10 rabbit polyclonal IgG (Santa Cruz Biotechnology, CA)) for 16h in 0.01 M phosphate-buffered saline containing 0.1% bovine serum albumin and 0.1% triton-X 100. The sections were then incubated with the secondary antibody Alexa Fluor-546-conjugated IgG in buffer for 2h (Molecular Probes, Eugene, OR). Afterwards, the vessels were washed with 0.01 M PBS, water, mounted with VectorShield and visualized using aZeiss LSM710 confocal microscope. Optimal settings for the microscope and exposure was determined and remained constant for recording of all the samples. Image analysis was performed as described above.
Immunohistochemistry was also used to determine the localization of neutral endopeptidase in the hindpaw of the rat. In this study we used dual labeling. Neutral endopeptidase (as described above) along with VWF antibody (Santa Cruz Biotechnology, CA) and Alexa Fluor-488-conjugated IgG (Molecular Probes, Eugene, OR) was used.
Western blot analysis was used to confirm changes in neutral endopeptidase expression in epineurial arterioles. Briefly, epineurial arterioles were isolated and placed in 100 μl of RIPA buffer containing protease inhibitor cocktail. The samples were homogenized and sonicated at 4°C, centrifuged at 14,000g and supernatant analyzed for protein concentration. 10 μg of protein was separated by SDS-PAGE and transferred to nitrocellulose paper. The blot was blocked for 1 h in 5% non fat dry milk and exposed to CD10 antibody (F-4) (sc-46656 mouse monoclonal (Santa Cruz)) overnight in T-TBS at 4°C. The blots were washed with TBS-T then incubated with secondary antibody anti mouse HRP for 1 hour at room temperature. The blots were washed and develop with ECL. Afterwards, the blots were stripped and reanalyzed for β-actin to standardize for equal loading.
Data Analysis
Results are presented as mean ± SEM. Comparisons between the groups were conducted using unpaired students t-test (Prism software; GraphPad, San Diego, CA). Concentration response curves were compared using a two-way repeated measures analysis of variance with autoregressive covariance structure using proc mixed program of SAS [17,18]. A p value of less than 0.05 was considered significant.
Results
Weight and Metabolic Changes
Rats fed a high fat diet for 32 weeks weighed significantly more than age-matched control rats fed a standard diet (Table 1). The difference in weight gain was noticed after 4 weeks on the high fat diet and this difference was maintained throughout 32 weeks on the high fat diet (data not shown). With the high fat fed rats weighing approximately 14% more then the control rats this model was not extremely obese as some of the genetic obese rodent models. The weight difference between control and high fat fed rats is more consistent with subjects that are overweight. Food consumption was analyzed for 1 week at weeks 20, 24 and 28. Control and high fat fed rats consumed 50.3 ± 6.6 and 32.2 ± 4.4 g/day/kg rat, respectively.
Table 1.
Effect of 32 Week High Fat Diet on Whole Body, Epididymal and Interscapular Fat and Gastrocnemius Muscle Weight Change, and Blood Pressure
| Determination | Control (23) | High Fat Fed (20) |
|---|---|---|
| Start Body Weight (g) | 269 ± 4 | 272 ± 5 |
| End Body Weight (g) | 509 ± 8 | 579 ± 13a |
| Epididymal (white) fat pad (g) | 4.4 ± 0.3 | 10.5 ± 0.3a |
| Interscapular (brown) fat pad (g) | 0.35 ± 0.03 | 0.71 ± 0.06a |
| Left gastrocnemius muscle (g) | 3.20 ± 0.09 | 3.17 ± 0.10 |
| Blood pressure (mmHg) | 131.6 ± 4.3 | 151.6 ± 6.1a |
Data are presented as the mean ± SEM.
p < 0.05 compared to control.
Parentheses indicate the number of experimental animals.
After 32 weeks the epididymal and interscapular fat pads from high fat fed rats weighed significantly more compared to control rats. In contrast weight of the left gastrocnemius muscle was not different between the two groups of rats (Table 1). Mean arteriole blood pressure was significantly increased in rats fed a high fat diet (Table 1).
Fasting serum insulin levels and non-fasting serum leptin levels were significantly increased in high fat fed rats compared to rats fed a standard diet (Table 2). Data in Table 2 also demonstrate that there was a trend for serum free cholesterol levels to be higher in high fat fed rats but this did not reach significance compared to control rats. Serum free fatty acid levels were significantly higher in high fat fed rats compared to control rats and there was a trend for serum triglyceride levels to be higher in high fat fed rats but this was not significantly different from control rats. Serum adiponectin levels were significantly increased in high fat fed rats compared to control rats. Measurements of markers for oxidative stress demonstrated that serum 8-hydroxy deoxyguanosine (8-OH DG) and thio barbituric acid reactive substances (TBARS) levels were significantly increased in high fat fed rats compared to control rats. Superoxide levels in the aorta were also significantly higher in high fat fed rats compared to control rats.
Table 2.
Effect of 32 Week High Fat Diet on Serum Insulin, Leptin, Cholesterol, Triglycerides, Free Fatty Acids, Adiponectin, 8- hydroxy deoxyguanosine, Thio Barbituric Acid Reactive Substances, and Aorta Superoxide Levels
| Determination | Control (23) | High Fat Fed (20) |
|---|---|---|
| Insulin (ng/ml) | 1.34 ± 0.11 | 3.19 ± 0.47a |
| Leptin (pM) | 217 ± 30 | 2087 ± 499a |
| Cholesterol (mg/dl) | 413.1 ± 32.0 | 541.3 ± 81.6 |
| Triglycerides (mg/dl) | 49.5 ± 5.6 | 95.6 ± 21.8 |
| Free fatty acids (mmol/L) | 0.11 ± 0.02 | 0.26 ± 0.06a |
| Adiponectin (μg/ml) | 7.4 ± 0.4 | 12.3 ± 0.8a |
| 8-OH DG (ng/ml) | 1.85 ± 0.19 | 2.58 ± 0.36a |
| TBARS (μg/ml) | 0.13 ± 0.03 | 0.23 ± 0.02a |
| Aorta superoxide (RLU/mm2) | 1.45 ± 0.15 | 2.55 ± 0.24a |
Data are presented as the mean ± SEM.
p < 0.05 compared to control.
Parentheses indicate the number of experimental animals.
Glucose tolerance
Glucose tolerance was significantly impaired in rats after 32 weeks on the high fat diet (Figure 1). Fasting blood glucose at the beginning of the study was minimally but significantly increased in high fat fed rats compared to control rats. Blood glucose levels peaked in 10 min in rats fed a standard diet and in 20 min in rats fed a high fat diet. For the next 100 min blood glucose levels remained higher in the rats fed a high fat diet.
Figure 1. Effect of a high fat diet on glucose tolerance.
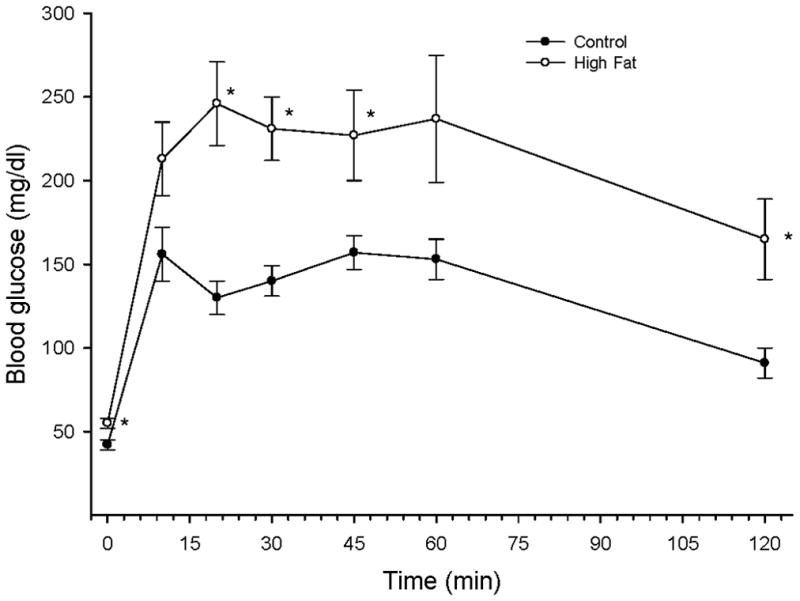
Rats were fed a standard or high fat diet for 32 weeks. Afterwards glucose tolerance was determined as described in the Methods section. Data are presented as the mean ± SEM in mg/dl. * p < 0.05, for individual data points compared to rats fed a standard diet (control). The area under the curve (AUC) was significantly different p < 0.01 for high fat fed rats vs. control. The number of rats in each group was the same as shown in Table 1.
Effect of High Fat Diet on Neural and Vascular Function
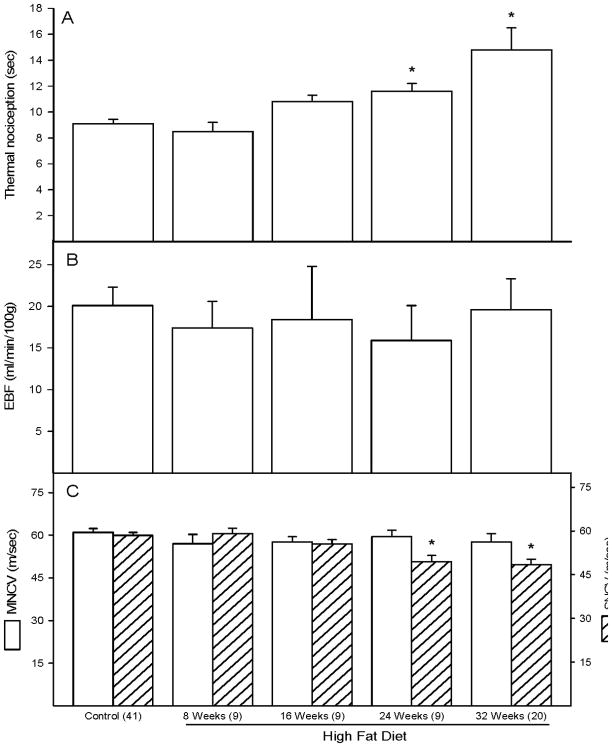
Data in Figure 2A demonstrate that rats fed a high fat diet for 24 and 32 weeks are thermal hypoalgesic. Feeding rats a high fat diet for up to 32 weeks did not affect endoneurial blood flow (Figure 2B). Sensory nerve conduction velocity (SNCV) was significantly decreased in rats fed a high fat diet for 24 and 32 weeks (Figure 2C hatched bars). In contrast, motor nerve conduction velocity (MNCV) was not impaired in high fat fed rats (Figure 2C open bars).
Figure 2. Effect of a high fat diet on thermal nociception, endoneurial blood flow and motor and sensory nerve conduction velocity.
Data are presented as the mean ± SEM for thermal nociception in sec (A), nutritive blood flow in ml/min/100g (B), and motor and sensory nerve conduction velocity in m/sec (C). The number of experimental determinations is presented in parentheses. For these studies control rats were age matched for each time point. Data from each of these time points for the control rats were not affected by age so these data were combined. * p < 0.05, compared to rats fed the standard diet (control).
The axonal size-frequency distribution of myelinated nerve fibers in the sciatic nerve from rats fed a standard or high fat diet for 32 weeks was not different (data not shown). The mean axonal diameter in the sciatic nerve from control and 32 week high fat fed rats was 4.7 ± 0.3 and 4.6 ± 0.2, micrometers, respectively. A qualitative assessment of sciatic nerves by light microscopy did not identify any overt pathologic damage to axons or any changes in myelin structure such as splitting ballooning or thinning.
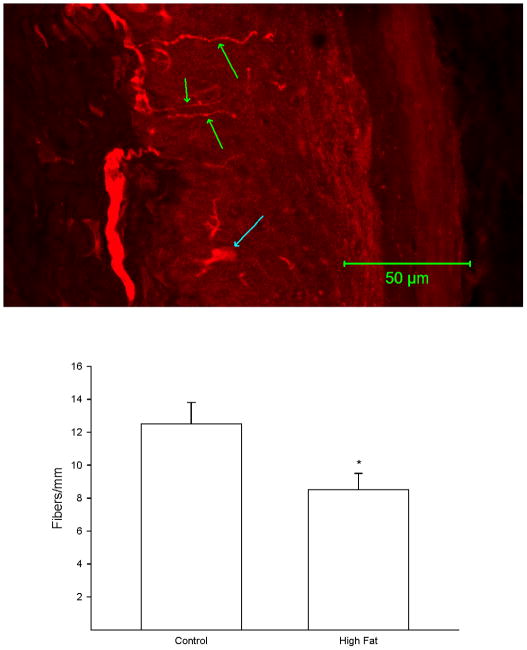
A representative image of a footpad from a control rat following immunostaining for PGP9.5 is shown in Figure 3 (top). The number of intraepidermal nerve fiber profiles in a skin biopsy from the right hindpaw was significantly decreased in rats fed a high fat diet for 32 weeks compared to control rats (Figure 3 bottom).
Figure 3. Effect of a high fat diet on intraepidermal nerve fiber profiles.
Rats were fed a standard or high fat diet for 32 weeks. Afterwards, the number of intraepidermal nerve fiber profiles were determined as described in the Methods section. A representative image for intraepidermal nerve fiber profiles is provided (top). The three green arrows point at individual IENF profiles. The blue arrow near the bottom of the image points at a Langerhans cell. The number of rats in each group was the same as shown in Table 1. * p < 0.05, compared to rats fed the standard diet (control).
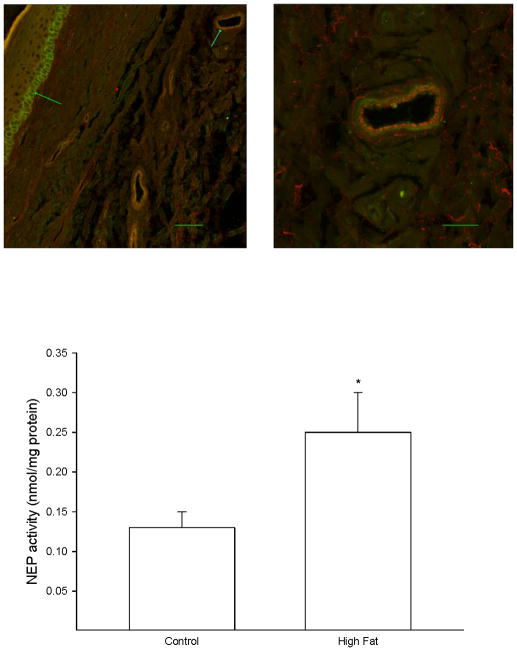
A skin biopsy of the left hindpaw was analyzed by immunohistochemistry for neutral endopeptidase localization and activity. Figure 4 (top) demonstrates that neutral endopeptidase is found in basal keratinocytes in the stratum basale (left image) and in the smooth muscle layer of blood vessels that occur in the dermis (right image). Data in Figure 4 (bottom) demonstrate that the activity of neutral endopeptidase was significantly increased in the skin from high fat fed rats compared to control rats.
Figure 4. Effect of a high fat diet on neutral endopeptidase activity in the skin of the hindpaw.
Rats were fed a standard or high fat diet for 32 weeks. Afterwards, activity of neutral endopeptidase in a skin biopsy of the hindpaw was determined as described in the Methods section. A representative image for expression of neutral endopeptidase in the hindpaw is provided (top). The left image (inserted bar 50 μm) demonstrates the staining for neutral endopeptidase (green) that occurs in basal keratinocytes in the stratum basale (green arrow on left). The blue arrow pointing at the vessel in the upper right portion of the image on the left side is enlarged and shown on the right side. The right image (inserted bar 20 μm) demonstrates that staining for VWF occurring in the endothelium (red staining) does not overlap with the staining for neutral endopeptidase (green staining) suggesting that neutral endopeptidase in the vasculature is primarily located in the smooth muscle layer. The number of rats in each group was the same as shown in Table 1. * p < 0.05, compared to rats fed the standard diet (control).
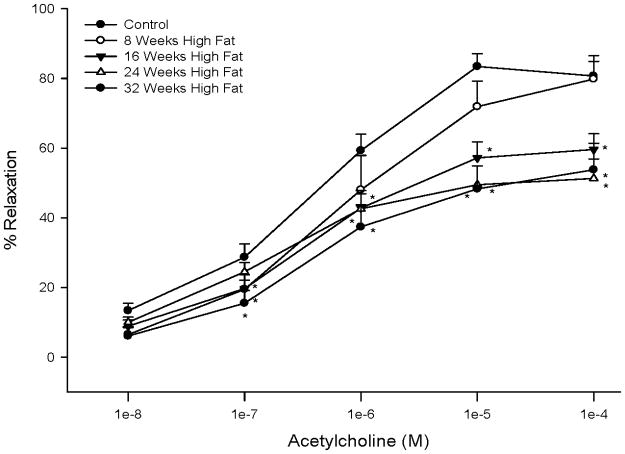
Feeding rats a high fat diet caused a significant impairment in acetylcholine-mediated vascular relaxation by epineurial arterioles that were dependent on duration on the high fat diet (Figure 5). Maximal acetylcholine-mediated vasodilation by epineurial arterioles from control rats was about 80%, which is consistent with our previous report [27]. Acetylcholine-mediated vasodilation by epineurial arterioles from rats fed a high fat diet for 8 weeks was similar to control rats. However, after 16 weeks on a high fat diet vascular relaxation to acetylcholine was significantly impaired and a maximal affect was observed after 24 weeks on the high fat diet. Relaxation to 10−4 M sodium nitroprusside (a maximal dose), a marker for non-endothelium dependent vasodilation, was not significantly impaired in epineurial arterioles from high fat fed rats (data not shown).
Figure 5. Effect of a high fat diet on acetylcholine-mediated vascular relaxation of epineurial arterioles.
Pressurized arterioles (40 mm Hg and ranging from 60–100 μm luminal diameter) were constricted with U46619 (30–50%) and incremental doses of acetylcholine were added to the bathing solution while recording steady state vessel diameter. Data are presented as the mean of % relaxation ± SEM. For these studies two vessels were collected from each rat, studied and the data combined. * p < 0.05, compared to rats fed the standard diet (control). The minimum number of rats in each group is 9.
We had previously demonstrated that epineurial arterioles are innervated by sensory nerves that contain calcitonin gene related peptide (CGRP) and that CGRP is a potent vasodilator in epineurial arterioles [28]. Data in Figure 6 demonstrate that vascular relaxation to CGRP was significantly impaired at lower doses of CGRP in rats fed a high fat diet for 32 weeks compared to rats fed a standard diet.
Figure 6. Effect of a high fat diet on calcitonin gene-related peptide (CGRP) mediated vascular relaxation of epineurial arterioles.
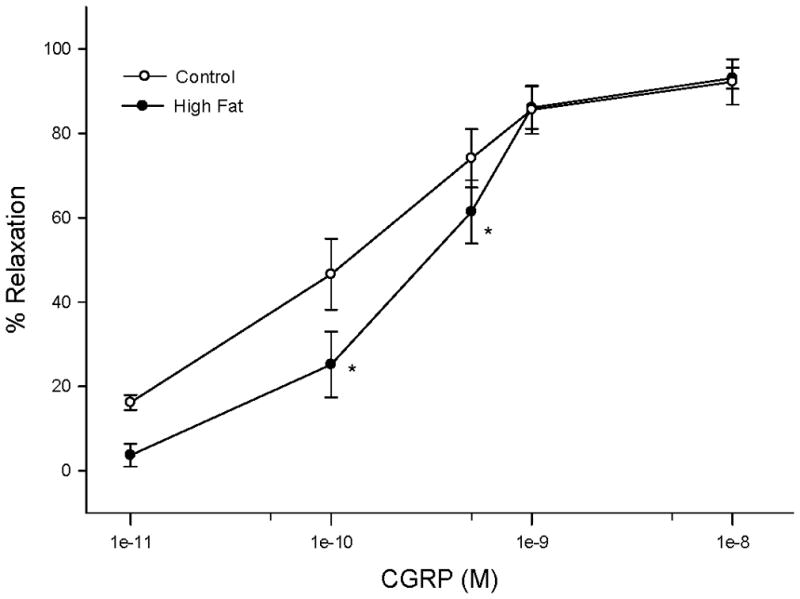
Arterioles were derived from control 32 week high fat fed rats as described in Figure 5. Incremental doses of CGRP were added to the bathing solution while recording steady state vessel diameter. Data are presented as the mean of % relaxation ± SEM. The number of rats in each group was the same as shown in Table 1. * p < 0.05, compared to rats fed the standard diet (control).
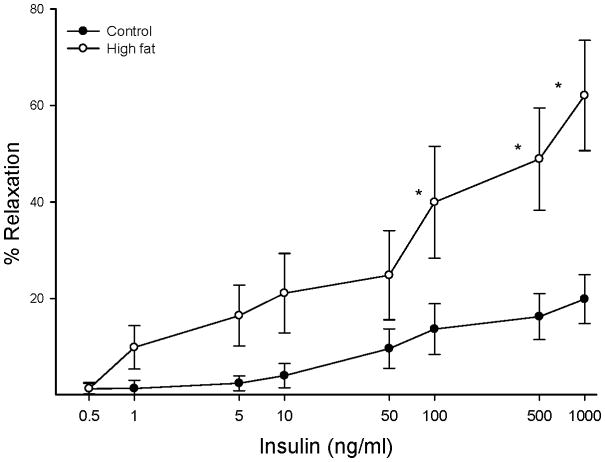
Data in Figure 7 demonstrate that insulin causes a dose dependent relaxation in epineurial arterioles and that feeding rats a high fat diet for 32 weeks results in a significant increase in vascular relaxation in response to insulin compared to rats fed a standard diet.
Figure 7. Effect of a high fat diet on insulin-mediated vascular relaxation by epineurial arterioles.
Arterioles were derived from control 32 week high fat fed rats as described in Figure 5. Incremental doses of insulin were added to the bathing solution while recording steady state vessel diameter. The number of rats in each group was the same as shown in Table 1. Data are presented as the mean of % relaxation ± SEM. * p < 0.05, compared to rats fed the standard diet (control).
We have previously demonstrated that superoxide and nitrotyrosine staining is increased in epineurial arterioles of the sciatic nerve from diabetic rats [24,29]. However, we found that superoxide and nitrotyrosine staining in epineurial arterioles from high fat fed rats was not increased. Analysis of RLU from 18 individual vessels from control and 24 – 32 week high fat fed rats was 98.5 ± 11.6 and 78.3 ± 5.6 for superoxide and 131.9 ± 7.1 and 115.9 ± 5.9 for nitrotyrosine, respectively.
Effect of a High Fat Diet on Expression of Neutral Endopeptidase in Epineurial Arterioles
Our previous studies with diabetic rats have linked increased expression of neutral endopeptidase in epineurial arterioles with impaired vascular function [24,29]. Therefore, we examined whether expression of neutral endopeptidase is changed in epineurial arterioles from high fat fed rats. Data in Figure 8 demonstrate that expression of neutral endopeptidase was increased about 2.5 fold in epineurial arterioles from rats fed a high fat diet for 32 weeks as indicated by immunohistochemistry and Western blot analysis.
Figure 8. Effect of a high fat diet on expression of neutral endopeptidase in epineurial arterioles.
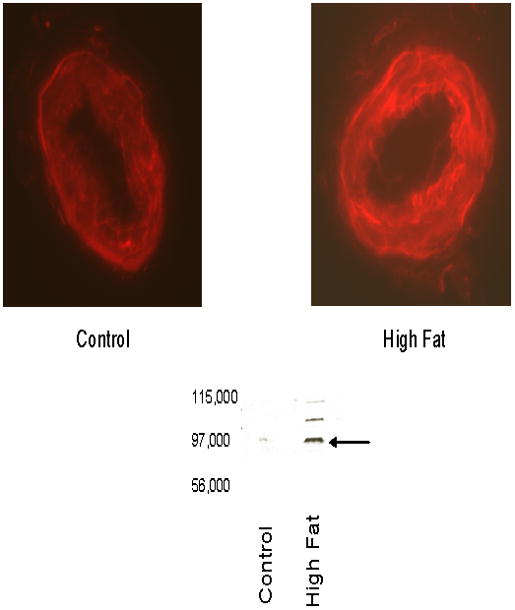
Presented are representative fluorescent photomicrographs of confocal microscopic sections of epineurial arterioles of the sciatic nerve for neutral endopeptidase immunostaining (top) and Western blot analysis (bottom). The arrow points to the NEP band in the Western blot. The identity of the two other bands in the Western blot from the high fat fed sample is unknown but may be higher molecular weight isoforms. Each of the two vessels was examined on the same day using identical laser and photomultiplier settings. The experiment was repeated three times and the fold increase in expression of NEP in epineurial arterioles from rats fed a high fat diet was 2.6 ± 0.6 as determined by analysis of the immunostaining.
Discussion
We previously reported that obese Zucker rats develop vascular and neural impairment independent of hyperglycemia [4]. To extend this line of study we investigated the development of vascular and neural complications in rats fed a high fat diet. The physiologic characteristics of the diet induced obesity rat model are insulin resistance, impaired glucose tolerance, dyslipidemia and increased fat deposition [30]. In the present study our rats also developed a concurrent range of nerve disorders, including SNCV slowing, thermal hypoalgesia and a decrease in intraepidermal nerve fiber profiles. In contrast, sciatic nerve MNCV mean axonal diameter of myelinated fibers and endoneurial blood flow, were not changed. Sensory neuropathy was accompanied by vascular dysfunction, as indicated by decreased relaxation of epineurial arterioles to acetylcholine and CGRP, and increased relaxation to insulin. Finally, expression and activity of neutral endopeptidase was increased in epineurial arterioles and skin from the hindpaw of high fat fed rats, whereas markers of oxidative stress were not increased.
The finding that rats fed a high fat diet develop indices of neuropathy is consistent with our studies of obese Zucker rats [4,14] and also with clinical studies in which pre-diabetes and impaired glucose tolerance have been associated with an early-onset, length-dependent neuropathy that is typically sensory predominant [31–33]. It is unknown to what extent impaired glucose tolerance directly causes nerve injury or is simply a covariant with other factors such as obesity [34]. The selectively sensory neuropathy in our rats fed a high fat diet is distinct from the polyneuropathy of most rat models of diabetes. However, there is precedence in models of toxic neuropathies, such as pyridoxine intoxication, in which sensory nerves are more prone to dysfunction [35]. The mechanisms inducing a selective sensory neuropathy in our high fat fed rats are not yet clear but it may be pertinent to note that the cell bodies of all sensory neurons lie outside the blood brain barrier and are more exposed to circulating factors than the motor neuron cell bodies located in the spinal cord.
The sensory neuropathy phenotype of rats fed a high fat diet extended to both large and small fiber dysfunction. SNCV slowing in large fibers of high fat fed rats was not accompanied by MNCV slowing or a discernable decrease in either axonal caliber or size:frequency distribution. We have seen a similar lack of effect on axonal caliber in both the Zucker and ZDF rat models of obesity and insulin resistance (unpublished observations), whereas mean axonal caliber is decreased in the sciatic nerve of rats after 2 months of insulin deficient diabetes [36]. This suggests that SNCV slowing in rats fed a high fat diet may have a metabolic origin, although we cannot yet discount the possibility that a selective effect on sensory axonal caliber is obscured by normal motor axons in the mixed sciatic nerve. In contrast to the lack of structure:function association in large fibers, small fiber dysfunction, as implied by paw thermal hypoalgesia, was accompanied by loss of small fiber terminal projections in the epidermis. This suggests a possible structural basis to the loss of sensation in high fat fed rats that parallel observations in diabetic rats [37–40]. However, this association should be interpreted with caution as thermal hypoalgesia also occurs in mice fed a high fat fed diet in the absence of intraepidermal nerve fiber loss [41] and thermal hypoalgesia precedes intraepidermal nerve fiber loss in diabetic mice [42]. It is possible that our finding of increased neutral endopeptidase activity in hindpaw skin of high fat fed rats also contributes to thermal hypoalgesia, as this enzyme degrades neuropeptides such as calcitonin gene-related peptide and substance P that contribute to small fiber sensory function [43] and that are reduced in nerve of thermally hypoalgesic diabetic rats [44]. Indeed, we have recently shown that thermal hypoalgesia induced by insulin deficient diabetes or a high fat diet is absent in mice lacking neutral endopeptidase [45]. Interestingly, neutral endopeptidase activity is also increased in skin of patients with diabetic ulcers [46].
One manifestation of neuropathy that appears to differ between high fat fed rats and humans with impaired glucose tolerance is in the clinical presentation of painful sensory neuropathy [31,47,48] versus sensory loss in the rats. There is a recent report showing rapid progression from hyperalgesia to hypoalgesia in rat models of impaired glucose tolerance [49] and it is possible that we may have missed indices of hyperalgesia by not measuring earlier than the 8 week time point. Moreover, it should be acknowledged that thermal hyperalgesia in rats is a poor surrogate for many of the pain sensations experienced by patients.
We found that endoneurial blood flow in the sciatic nerve was not impaired in high fat fed rats even though vascular relaxation in epineurial arterioles to acetylcholine and CGRP was decreased. Previously, in type 1 and type 2 diabetic rats we found that impaired vasodilation to acetylcholine occurred early and preceded slowing of endoneurial blood flow and MNCV [4,50,51]. Impairment in vascular relaxation of epineurial arterioles from high fat fed rats to acetylcholine and CGRP was not as severe as it is in vessels isolated from diabetic rats [4,28,50,51]. Furthermore, we found that vascular relaxation to insulin was significantly increased in epineurial arterioles from high fat fed rats. If our in vitro vascular response data to insulin can be translated to in vivo conditions it would suggest that at levels of insulin seen in control rats vascular relaxation due to insulin would be negligible. However, in high fat fed rats fasting insulin levels are increased as is vascular relaxation of epineurial arterioles to insulin, which could mean in vivo insulin is causing about a 20% relaxation of epineurial arterioles. It is possible that in vivo the reduced vascular relaxation in response to acetylcholine and CGRP is compensated by the increased response to insulin and thus endoneurial blood flow is maintained. This could also help explain why MNCV was not impaired in high fat fed rats. In studies by my laboratory and others reduced endoneurial blood flow has been shown to precede slowing of MNCV [17,18,27,50–52]. Since endoneurial blood flow in high fat fed rats is normal, ischemia and other damaging processes that lead to slowing of MNCV may not occur.
The mechanism responsible for insulin mediated vascular relaxation in epineurial arterioles is unknown. Furthermore, additional studies are needed to determine how diet induced obesity leads to increased vascular relaxation to insulin in epineurial arterioles even though insulin resistance exists by evidence of impaired glucose tolerance. In large vessels such as the aorta from animal models of obesity, diabetes and/or hypertension impaired insulin signaling in the vasculature has been linked to increased reactive oxygen species and impairment in insulin signaling pathways [53,54]. In high fat fed rats we observed an increase in superoxide in the aorta as determined by a lucigenin based assay and markers for oxidative stress were also increased in the serum. However, we did not detect an increase in superoxide or peroxynitrite in epineurial arterioles of the sciatic nerve using hydroethidine and nitrotyrosine staining. Due to the lack of increased oxidative stress in epineurial arterioles from high fat fed rats insulin signaling may be unaffected. However, this does not explain the up regulation in insulin-mediated vascular relaxation in epineurial arterioles in high fat fed rats. Unlike the aorta little is known about the effect of obesity on insulin signaling in the vasculature of resistance size vessels. In the mesentery insulin-induced vascular relaxation is thought to be mediated by calcium activated potassium channels [55]. It is possible that in the vasculature of resistence vessels pathways linked to insulin action are up-regulated in obesity. Future studies will focus on the mechanism for insulin mediated vascular relaxation in epineurial arterioles and how this is changed in obesity.
Previously we have demonstrated increased reactive oxygen species in epineurial arterioles from type 1 and type 2 diabetic rats and that reducing oxidative stress in these vessels improved vascular relaxation to acetylcholine as well as MNCV [4,17,18,24,56]. The increase in superoxide in the aorta of high fat fed rats is likely due to increased NAD(P)H oxidase activity and/or expression, which has been linked to increased activity of angiotensin in obesity [57]. However, in epineurial arterioles from diabetic rats increased superoxide levels have been linked to the mitochondria and NAD(P)H oxidase may not play a significant role [58]. The lack of superoxide/peroxynitrite production by epineurial arterioles from high fat fed rats could explain why vascular relaxation to acetylcholine is not impaired as severely as it is in diabetic rats. Nitric oxide and endothelium-derived hyperpolarizing factor provide two signals responsible for acetylcholine-mediated vascular relaxation in epineurial arterioles [27]. Impairment of nitric oxide mediated vascular relaxation occurs when superoxide binds with nitric oxide forming peroxynitrite. In epineurial arterioles from high fat fed rats we have found no evidence for increased nitrotyrosine staining a marker for peroxynitrite formation. This would suggest that acetylcholine-induced relaxation via nitric oxide formation is not impaired in high fat fed rats.
The decrease in acetylcholine-mediated vascular relaxation could be due to the increased expression of neutral endopeptidase in epineurial arterioles from high fat fed rats. In cultured human microvascular cells high fat causes an increase in expression and activity of neutral endopeptidase [59]. Neutral endopeptidase regulates the biological activity of vasoactive peptides including natriuretic peptides and CGRP via degradation prior to binding at active sites [60,61]. We have demonstrated that C-type natriuretic peptide has characteristics that qualify it as a candidate for endothelium-derived hyperpolarizing factor in epineurial arterioles and others have suggested the same for C-type natriuretic peptide in the mesentery [24,62]. Furthermore, epineurial arterioles express C-type natriuretic peptide [24]. It is possible that the increased expression of neutral endopeptidase seen in epineurial arterioles from high fat fed rats reduces the biological activity of C-type natriuretic peptide, and thus acetylcholine, by increasing degradation [24]. This would also explain why the vasodilation activity of CGRP is impaired only at lower doses of CGRP. Alternatively, it has been reported that hyperlipidemia directly can impair endothelial function and cause a decrease in acetylcholine-mediated vasodilation in conductance and resistance vessels [63,64]. Since circulating lipids are increased in high fat fed rats we cannot rule out a possible lipid effect on acetylcholine-mediated vascular relaxation in epineurial arterioles.
In summary, feeding Sprague-Dawley rats a high fat diet causes sensory nerve dysfunction, a decrease in intraepidermal nerve fiber profiles and impaired vascular relaxation to acetylcholine and CGRP in epineurial arterioles. In contrast, relaxation to insulin was increased. These changes were associated with increased expression of NEP but not oxidative stress in epineurial arterioles and increased NEP activity in the skin of the hindpaw. NEP degrades vasoactive peptides which may explain the decrease in microvascular function.
Acknowledgments
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development and by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK073990 (MAY) and DK057629 (NAC) from NIH. The authors express their thanks to Ms. Veronica Lopez for excellent technical support. The content of this manuscript are new and solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies. The authors have no conflicts of interest to report.
References
- 1.Caglayan E, Blaschke F, Takata Y, Hsueh WA. Metabolic syndrome-interdependence of the cardiovascular and metabolic pathways. Curr Opin Pharmacol. 2005;5:135–142. doi: 10.1016/j.coph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Costa LA, Canani LH, Lisboa HR, Tres GS, Gross JL. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabetic Med. 2004;21:252–255. doi: 10.1111/j.1464-5491.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- 3.de Jongh RT, Serne EH, IJzerman RG, de Vries G, Stehouwer SD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- 4.Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA. Progression of vascular and neural dysfunction in sciatic nerves of Zucker Diabetic Fatty (ZDF) and Zucker rats. Am J Physiol. 2005;289:E113–122. doi: 10.1152/ajpendo.00594.2004. [DOI] [PubMed] [Google Scholar]
- 5.LeRoith D, Rayfield EJ. The benefits of tight glycemic control in type 2 diabetic mellitus. Clinical Cornerstone. 2007;8:S19–S29. doi: 10.1016/s1098-3597(07)80018-4. [DOI] [PubMed] [Google Scholar]
- 6.Jellinger PS. Metabolic consequences of hyperglycemia and insulin resistance. Clinical Cornerstone. 2007;8:S30–S42. doi: 10.1016/s1098-3597(07)80019-6. [DOI] [PubMed] [Google Scholar]
- 7.Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wellnitz B, Boehm BO, Schaefer JR, Marz W. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab. 2006;91:2542–2547. doi: 10.1210/jc.2006-0195. [DOI] [PubMed] [Google Scholar]
- 8.Bays H, Mandarino L, DeFronzo RA. Role of the adipocytes, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 9.Poynten AM, Gan SK, Kriketos AD, Campbell LV, Chisholm DJ. Circulating fatty acids, non-high density lipoprotein cholesterol, and insulin-infused fat oxidation acutely influence whole body insulin sensitivity in non-diabetic men. J Clin Endocrinol Metab. 2005;90:1035–1040. doi: 10.1210/jc.2004-0943. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer JR, Maisch B, Klumpp S, Krieglstein J. Why does atherosclerosis occur where it occurs? Atherosclerosis. 2005;180:417–418. doi: 10.1016/j.atherosclerosis.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Hufnagel B, Dworak M, Soufi M, Mester Z, Zhu Y, Schaefer JR, Klumpp S, Krieglstein J. Unsaturated fatty acids isolated from human lipoproteins activate protein phosphatase type 2Cβ and induce apoptosis in endothelial cells. Atherosclerosis. 2005;180:245–254. doi: 10.1016/j.atherosclerosis.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimi R, Saleh JR, Toggart EJ, Hayatdavoudi B, Wolf CJ, Wadhani NN, Shah AP. Lipid lowering therapy in patients with peripheral arterial disease. J Cardiovascular Pharmacology Therapeutics. 2004;9:271–277. doi: 10.1177/107424840400900407. [DOI] [PubMed] [Google Scholar]
- 13.Kim CK, Schmalfuss CM, Schofield RS, Sheps DS. Pharmacological treatment of patients with peripheral arterial disease. Drugs. 2003;63:637–647. doi: 10.2165/00003495-200363070-00002. [DOI] [PubMed] [Google Scholar]
- 14.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Attenuation of vascular/neural dysfunction in Zucker rats treated with Enalapril or Rosuvastatin. Obesity. 2008;16:82–89. doi: 10.1038/oby.2007.19. [DOI] [PubMed] [Google Scholar]
- 15.Mihara M, Uchiyama M, Fukuzama K. Thiobarbituric acid value of fresh homogenate of rat as a parameter of lipid peroxidation in aging, CC14 intoxication, and vitamin E deficiency. Biochem Med. 1980;23:302–311. doi: 10.1016/0006-2944(80)90040-x. [DOI] [PubMed] [Google Scholar]
- 16.Siman CM, Eriksson UJ. Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia. 1997;40:1416–1424. doi: 10.1007/s001250050844. [DOI] [PubMed] [Google Scholar]
- 17.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50:1927–1937. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- 18.Coppey LJ, Davidson EP, Rinehart TW, Gellett JS, Oltman CL, Lund DD, Yorek MA. ACE inhibitor or angiotensin II receptor antagonist attenuates diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2006;55:341–348. doi: 10.2337/diabetes.55.02.06.db05-0885. [DOI] [PubMed] [Google Scholar]
- 19.Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 20.Forcier NJ, Mizisin AP, Rimmer MA, Powell HC. Cellular pathology of the nerve microenvironment in galactose intoxication. J Neuropath Exp Neurology. 1991;50:235–255. doi: 10.1097/00005072-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Wattanapitayakul SK, Weinstein DM, Holycross BJ, Bauer JA. Endothelial dysfunction and peroxynitrite formation are early events in angiotensin-induced cardiovascular disorders. FASEB J. 2000;14:271–278. doi: 10.1096/fasebj.14.2.271. [DOI] [PubMed] [Google Scholar]
- 22.Pryor WA, Squadrito GI. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 23.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 24.Davidson EP, Kleinschmidt TL, Oltman CL, Lund DD, Yorek MA. Treatment of streptozotocin-induced diabetic rats with AVE7688, a vasopeptidase inhibitor, on vascular and neural disease. Diabetes. 2007;56:355–362. doi: 10.2337/db06-1180. [DOI] [PubMed] [Google Scholar]
- 25.Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 2008;110:351–362. doi: 10.1016/j.acthis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayoub S, Melzig MF. Induction of neutral endopeptidase (NEP) activity of SK-N-SH cells by natural compounds from green tea. J Pharmacy Pharmacology. 2006;58:495–501. doi: 10.1211/jpp.58.4.0009. [DOI] [PubMed] [Google Scholar]
- 27.Terata K, Coppey LJ, Davidson EP, Dunlap JA, Gutterman DD, Yorek MA. Acetylcholine-induced arteriolar dilation is reduced in streptozotocin-induced diabetic rats with motor nerve dysfunction. British J Pharm. 1999;128:837–843. doi: 10.1038/sj.bjp.0702856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yorek MA, Coppey LJ, Gellett JS, Davidson EP. Sensory nerve innervation of epineurial arterioles of the sciatic nerve containing calcitonin-gene related peptide (CGRP): effect of streptozotocin-induced diabetes. Exp Diabetes Res. 2004;5:187–193. doi: 10.1080/15438600490486732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Yorek MA. Treatment of Zucker Diabetic Fatty rats with AVE7688 improves vascular and neural dysfunction. Diabetes, Obesity and Metabolism. 2009;11:223–233. doi: 10.1111/j.1463-1326.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tschop M, Heiman ML. Rodent obesity models: an overview. Expt Clin Endocrinology Diabetes. 2001;109:307–319. doi: 10.1055/s-2001-17297. [DOI] [PubMed] [Google Scholar]
- 31.Singleton JR, Smith AG, Russell J, Feldman EL. Polyneuropathy with impaired glucose tolerance: implications for diagnosis and therapy. Curr Treatment Options Neurol. 2005;7:33–42. doi: 10.1007/s11940-005-0004-4. [DOI] [PubMed] [Google Scholar]
- 32.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24:1448–1453. doi: 10.2337/diacare.24.8.1448. [DOI] [PubMed] [Google Scholar]
- 33.Summer CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- 34.Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist. 2008;14 :23–29. doi: 10.1097/NRL.0b013e31815a3956. [DOI] [PubMed] [Google Scholar]
- 35.Perry TA, Weerasuriya A, Mouton PR, Holloway HW, Greig NH. Pyridoxine-induced toxicity in rats: a stereological quantification of the sensory neuropathy. Exp Neurol. 2004;190:133–144. doi: 10.1016/j.expneurol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Calcutt NA, Dines KC, Cesena RM. Effects of the peptide HP228 on nerve disorders in diabetic rats. Metabolism. 1998;47:650–656. doi: 10.1016/s0026-0495(98)90025-7. [DOI] [PubMed] [Google Scholar]
- 37.Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647. doi: 10.1016/j.nurt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. J Peripher Nerv Syst. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- 39.Piriz J, Torres-Aleman I, Nunez A. Independent alterations in the central and peripheral somatosensory pathways in rat diabetic neuropathy. Neuroscience. 2009;160:402–411. doi: 10.1016/j.neuroscience.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 40.Calcutt NA, Freshwater JD, Mizisin AP. Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia. 2004;47:718–724. doi: 10.1007/s00125-004-1354-2. [DOI] [PubMed] [Google Scholar]
- 41.Obrosova IG, Ilnytska O, Lyzogubov VV, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effect of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 42.Beiswenger KK, Calcutt NA, Mizisin AP. Dissociation of thermal hypoalgesia and epidermal denervation in streptozotocin-diabetic mice. Neurosci Lett. 2008;442:267–272. doi: 10.1016/j.neulet.2008.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu LC, Hou JF, Fu FH, Zhang YX. Roles of calcitonin gene-related peptide and its receptor in pain-related behavioral responses in the central nervous system. Neuroscience Biobehavioral Rev. 2009;33:1185–1191. doi: 10.1016/j.neubiorev.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Calcutt NA, Freshwater JD, Hauptmann N, Taylor EM, Mizisin AP. Protection of sensory function in diabetic rats by Neotrofin. Eur J Pharmacol. 2006;534:187–193. doi: 10.1016/j.ejphar.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 45.Davidson EP, Coppey LJ, Lu B, Arballo V, Calcutt NA, Gerard C, Yorek MA. The roles of streptozotocin neurotoxicity and neutral endopeptidase in murine experimental diabetic neuropathy. Exp Diabetes Res. doi: 10.1155/2009/431980. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antezana MA, Sullivan SR, Usui ML, Gibran NS, Spenny ML, Larsen JA, Ansel JC, Bunnett NW, Olerud JE. Neutral endopeptidase activity is increased in the skin of subjects with diabetic ulcers. J Invest Dermatol. 2002;119:1400–1404. doi: 10.1046/j.1523-1747.2002.19618.x. [DOI] [PubMed] [Google Scholar]
- 47.Singleton JR, Smith AG, Bromberg MB. Painful sensory neuropathy associated with impaired glucose tolerance. Muscle & Nerve. 2001;24:1225–1228. doi: 10.1002/mus.1136. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch P. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol. 2006;63:1075–1079. doi: 10.1001/archneur.63.8.noc50336. [DOI] [PubMed] [Google Scholar]
- 49.Sugimoto K, Rashid IB, Kojima K, Shoji M, Tanabe J, Tamasawa N, Suda T, Yasujima M. Time course of pain sensation in rat models of insulin resistance, type 2 diabetes, and exogenous hyperinsulinaemia. Diabetes Metab Res Rev. 2008;24:652–650. doi: 10.1002/dmrr.903. [DOI] [PubMed] [Google Scholar]
- 50.Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that provide circulation to the sciatic nerve. Int J Exp Diabetes Res. 2000;1:131–143. doi: 10.1155/EDR.2000.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Changes in epineurial blood flow, motor nerve conduction velocity and vascular relaxation of epineurial arterioles of the sciatic nerve in ZDF-obese diabetic rats. Diabetes/Metabolism Res and Rev. 2002;18:1–9. doi: 10.1002/dmrr.257. [DOI] [PubMed] [Google Scholar]
- 52.Cameron NE, Cotter MA, Low PA. Nerve blood flow in early experimental diabetes in rats: relation to conduction defects. Am J Physiol. 1991;261:E1–E8. doi: 10.1152/ajpendo.1991.261.1.E1. [DOI] [PubMed] [Google Scholar]
- 53.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension. 2001;37:554–560. doi: 10.1161/01.hyp.37.2.554. [DOI] [PubMed] [Google Scholar]
- 54.Frank GD, Eguchi S, Motley ED. The role of reactive oxygen species in insulin signaling in the vasculature. Antioxidants and redox signaling. 2005;7:1053–1061. doi: 10.1089/ars.2005.7.1053. [DOI] [PubMed] [Google Scholar]
- 55.Iida S, Taguchi H, Watanabe N, Kushiro T, Kanmatsuse K. Insulin-induced relaxation of rat mesenteric artery is mediated by Ca(2+)-activated K(+) channels. Eur J Pharmacol. 2001;411:155–160. doi: 10.1016/s0014-2999(00)00892-x. [DOI] [PubMed] [Google Scholar]
- 56.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Adebara ET, Yorek MA. Vascular and neural dysfunction in Zucker Diabetic Fatty rats: a difficult condition to reverse. Diabetes, Obesity and Metabolism. 2008;10:67–74. doi: 10.1111/j.1463-1326.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 57.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 58.Coppey LJ, Gellett JS, Davidson EP, Yorek MA. Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radical Res. 2003;37:33–40. doi: 10.1080/1071576021000028442. [DOI] [PubMed] [Google Scholar]
- 59.Muangman P, Spenny ML, Tamura RN, Gibran NS. Fatty acids and glucose increase neutral endopeptidase activity in human microvascular endothelial cells. Shock. 2003;19:508–512. doi: 10.1097/01.shk.0000055815.40894.16. [DOI] [PubMed] [Google Scholar]
- 60.Pu Q, Schiffrin EL. Effect of ACE/NEP inhibition on cardiac and vascular collagen in stroke-prone spontaneously hypertensive rats. Am J Hypertension. 2001;14:1067–1072. doi: 10.1016/s0895-7061(01)02157-4. [DOI] [PubMed] [Google Scholar]
- 61.Katayama M, Nadel JA, Bunnett NW, Di Maria GU, Haxhiu M, Borson DB. Catabolism of calcitonin gene-related peptide and substance P by neutral endopeptidase. Peptides. 1991;12:563–567. doi: 10.1016/0196-9781(91)90102-u. [DOI] [PubMed] [Google Scholar]
- 62.Chauhan SD, Hobbs AJ, Ahluwalia A. C-type natriuretic peptide: new candidate for endothelium-derived hyperpolarizing factor. Int J Biochem Cell Biol. 2004;36:1878–1881. doi: 10.1016/j.biocel.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Naderali EK, Williams G. Effects of short-term feeding of a highly palatable diet on vascular reactivity in rats. Euro J Clin Invest. 2001;31:1024–1028. doi: 10.1046/j.1365-2362.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 64.Song GY, Gao Y, Di YW, Pan LL, Zhou Y, Ye JM. High-fat feeding reduces endothelium-dependent vasodilation in rats: differential mechanisms for saturated and unsaturated fatty acids? Clin Exp Pharmacology Physiology. 2006;33:708–713. doi: 10.1111/j.1440-1681.2006.04422.x. [DOI] [PubMed] [Google Scholar]