Abstract
Mammals that hibernate (hibernators) exhibit a circannual rhythm of food intake and body mass. In the laboratory during the winter hibernation period, many hibernators enter a series of multi-day torpor bouts, dropping their body temperature to near ambient, and cease to feed even if food is present in their cage. The mechanism(s) that regulates food intake in hibernators is unclear. Recently, AMP-activated protein kinase (AMPK) has been shown to play a key role in the central regulation of food intake in mammals. We hypothesized that infusing an AMPK activator, 5-aminoimidazole-4-carboxamide 1 B-D-ribofuranoside (AICAR), intracerebroventricularly (ICV) into the third ventricle of the hypothalamus would stimulate yellow-bellied marmots (Marmota flaviventris) to feed during their hibernation season. Infusion of AICAR ICV into marmots at an ambient temperature of 22°C caused a significant (P<0.05) increase in food intake. In addition, animals stimulated to feed did not enter torpor during the infusion period. Marmots ICV infused with saline did not increase food intake and these animals continued to undergo torpor at an ambient temperature of 22°C. Our results suggest that AICAR stimulated the food intake pathway, presumably by activating AMPK. These results support the hypothesis that AMPK may be involved in regulating food intake in hibernators and that there may be common neural pathways involved in regulating feeding and eliciting torpor.
Keywords: AMPK, appetite, torpor, hibernators, food intake
INTRODUCTION
Before hibernation, many mammals that hibernate (hibernators) dramatically increase their food intake and decrease their energetic costs throughout summer and autumn, resulting in an increase in body mass. Hibernators, such as the yellow-bellied marmot (Marmota flaviventris), nearly double their body mass in the form of white adipose tissue (WAT) over summer, then reduce their food intake to zero prior to hibernation and continue to fast for nearly 7 months (Dark, 2005; Davis, 1976; Ward and Armitage, 1981). Marmots do not store food in their burrow but resume eating in early spring when food is once again available. Similar behavior is shown in the laboratory setting where they do not feed from October to late March even if food is in their cage. Thus, marmots switch from a high food intake state in the summer to a non-feeding state in late autumn and winter. During winter hibernation they undergo torpor bouts which are illustrated by periodic drops in body temperature (Tb) to near ambient temperature (Ta). After several days at low tissue temperature, the animal's Tb rises to 35°C against Ta using endogenously produced heat. During winter hibernation, WAT stored over the summer and autumn is used for endogenous energy. Marmots lose as much as 30% of their body mass during the winter while going through bouts of torpor (Davis, 1976). In spring, marmots cease to hibernate, become reproductively active, and begin the food intake cycle once again. The physiological mechanisms that turn off food intake in these hibernators throughout winter, forcing them to fuel most cellular processes from the energy stored in WAT, are unclear.
AMP-activated protein kinase (AMPK) is a heterotrimeric enzyme that is evolutionarily conserved from yeast to humans (Kahn et al., 2005). It has recently been identified as an intracellular energy sensor and plays a significant role in the central regulation of food intake and energy metabolism (Hardie et al., 1998; Kohno et al., 2008; Minokoshi et al., 2004). AMPK is an important molecular sensor of cellular AMP/ATP ratios within the hypothalamic arcuate nucleus (ARC) and provides information about cellular energy status within the animal. When activated, hypothalamic AMPK decreases the activities of anabolic pathways (ATP utilization) and increases the activity of catabolic pathways, thus acting to maintain normal cellular energy balance. In the brain, AMPK is expressed in hypothalamic ARC neurons [e.g. neurons that release proopiomelanocortin (POMC), agouti-related protein (AgRP) and neuropeptide Y (NPY)] that play a central role in modulating food intake and sensing cellular energy levels (Claret et al., 2007; Kim and Lee, 2005). A decrease in circulating metabolic energy fuels [e.g. free fatty acids (FFA), glucose] initiates hypothalamic AMPK activation, which in turn activates counter-regulatory endocrine responses involving leptin, insulin and adiponectin (Cao et al., 2007; Horman et al., 2005; Kubota et al., 2007). Further, it has been proposed that leptin acts within the hypothalamus to inhibit the action of AMPK by inhibiting NPY and AgRP neurons, leading to a decrease in food intake, while adiponectin stimulates AMPK activity in the ARC, leading to an increase in food intake (Badman and Flier, 2007; Kubota et al., 2007; Mountjoy et al., 2007; Xue and Kahn, 2006). Furthermore, AMPK might play a significant role in regulating metabolic fuel use during torpor, since hibernating animals switch from using carbohydrate to lipid during a torpor bout (Buck and Barnes, 2000; Melvin and Andrews, 2009).
An activator of AMPK is 5-aminoimidazole-4-carboxamide 1 B-D-ribonucleoside (AICAR), which acts by mimicking the effects of a high AMP/ATP ratio and activating AMPK (Hardie et al., 1998; Hardie et al., 1997). AICAR is taken up into cells and phosphorylated to form 5-amino-4-imidazolecarboxamide ribotide (ZMP), which mimics the effects of AMP on AMPK activation (Fryer and Carling, 2005; Sabina et al., 1985). Once activated by AICAR, AMPK stimulates feeding in all rodents studied thus far (Hardie, 2008; Kohno et al., 2008). AMPK activation has effects on many physiological systems including muscles, fat, liver and possibly all cells (for a review, see Towler and Hardie, 2007). To explore the role of AMPK in the regulation of food intake in hibernators we infused AICAR intracerebroventricularly (ICV) into yellow-bellied marmots during the middle of their hibernation and aphagic season to test whether they could be induced to feed. We hypothesized that if AMPK is a fuel sensor within the hypothalamus of hibernators, the infusion of AMPK-activating AICAR should stimulate marmots to feed.
MATERIALS AND METHODS
Nine adult yellow-bellied marmots (Marmota flaviventris, Audubon and Bachman 1841) of both sexes were captured in Colorado over a 3 year period and housed under an approved Institutional Animal Care and Use Committee protocol. In summer, all animals were maintained under natural photoperiod (Paragon Sun Tracker EC72ST; Invensys Controls, Carol Stream, IL, USA) and at room temperature (22±2°C) with food provided ad libitum (Harlan Teklad 8640; Madison, WI, USA). Animals were moved to a cold room in August (15±1°C). The animals became cold acclimated as the temperature of the cold room was steadily decreased from 15°C in August to 5°C in September. Food intake was measured on a daily basis for the duration of the experiment.
In November of 2006 and 2008 marmots were anesthetized using a ketamine–acepromazine–xylazine cocktail (50 mg kg−1, 1.0–1.5 mg kg−1 and 5 mg kg−1, respectively) and then maintained under anesthesia using isoflurane (0.8%). A stereotaxic apparatus (David Kopf Instr., Tujunga, CA, USA) was used to aseptically implant sterile brain cannulae (Plastics One, brain infusion kit no. 3; Roanoke, VA, USA) into the third ventricle of nine animals using a marmot brain atlas (Dellman, 1973). The coordinates were A 12.1 from frontal zero plane and midline. Confirmation of cannula placement was immediately determined by slight suction on the cannula tube and observing cerebrospinal fluid within the tube. The volume of the cannula was approximately 0.58 μl and approximately 3 in (76 mm) of cannula tubing was attached to the cannula; the cannula and tubing were filled with sterile saline and the tubing was sealed at the end. In addition, sterile body temperature data loggers (I-Buttons, Maxim Integrated Products, Sunnyvale, CA, USA) set to record Tb every 2 h, were implanted into the abdominal cavity of all animals at the same time to monitor Tb during all phases of a torpor bout [e.g. the drop in Tb (entrance), low Tb, and inter-bout arousal at high Tb between bouts]. All animals were allowed to recover at room temperature for 1 week and then were returned to the cold room.
In early December (2006) or January (2009), animals were removed from the cold room and anesthetized as described above. The patency of the tubing connected to the brain cannulae was tested to make sure it was clear, and the 7 day ALZET mini-pumps (product no. 1007; ALZET Inc., Cupertino, CA, USA) were connected to the indwelling brain cannula. Three experimental and one control animal, and two experimental and three control animals were used in 2006 and 2009, respectively. Thus, five experimental animals received AICAR (Sigma-Aldrich cat. no. A9978; St Louis, MO, USA) dissolved in 0.9% sterile saline and four control animals received 0.9% sterile saline. The concentration of AICAR used in the 7 day ALZET mini-pumps (volume 100 μl) was 50 μg μl−1 with an infusion rate of 25 μg h−1 based on doses previously used in rodents (Kim et al., 2004; Perrin et al., 2004) and the published pumping rate of 0.5 μl h−1 (ALZET Inc.) at ~37°C. Flow rate for the ALZET mini-pumps was calculated a posteriori via the ALZET mini-pump interactive calculator (see www.alzet.com) using the average Tb each animal maintained during infusion. Sterile 0.9% saline was used for the mini-pumps of the control animals. The infusion rate of the mini-pumps is temperature dependent; therefore, to ensure that the pumps worked properly, animals were removed from the cold room for the infusion period and maintained at a high Ta (22±2°C). Given the flow rate and volume of the tubing, we calculated that AICAR or saline would reach the ventricle after a delay of roughly 46 h (downward pointing arrow, Fig. 1). The mini-pumps were removed 8 days after the AICAR reached the ventricle (Fig. 1, upward pointing arrow) and the animals were returned to the cold room. We did not immediately kill animals to determine whether AMPK was activated in the ARC because we wanted to determine whether animals would progress through the hibernation season normally (e.g. no food intake, torpor exhibited). We defined a torpor bout as a drop in Tb below 30°C for more than 6 h. At the end of each study (March), marmots were killed, Methylene Blue dye was injected through the cannula to confirm its position within the third ventricle, and I-buttons were removed and analyzed. All animals had cannulae within the 3rd ventricle.
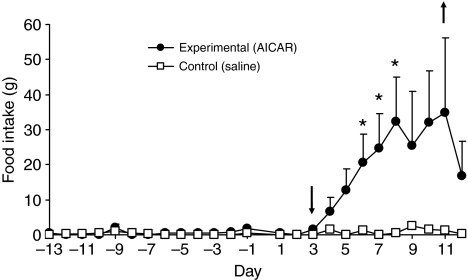
Fig. 1.
Mean food intake (±s.e.m.) in five marmots infused with AICAR and four marmots infused with saline in winter. The downward pointing arrow indicates the approximate time that infused AICAR reached the ventricle (~46 h). The upward pointing arrow indicates when the pumps were removed. The cannulae were implanted several weeks before the 7 day ALZET pumps were attached. All marmots had not eaten for at least 2 months prior to infusion. The increase in food intake of the AICAR-infused animals was significantly different from saline-infused animals beginning on day 6 through to day 8 (*P<0.02). The saline-infused animals' food intake was essentially zero.
All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA). Statistical significance was determined by Student's t-test and ANCOVA was used for repeated measures: differences were considered significant at the P<0.05 level.
RESULTS
Food intake
Before the attachment of the ALZET mini-pump to the cannula, all marmots had not eaten for at least 2 months, although food was present in their cage. The five animals that received AICAR began to eat within 48 h of the pump being connected to the cannula (Fig. 1), which is approximately when AICAR was calculated to reach the 3rd ventricle. All five marmots increased their food intake significantly from the previous month (P<0.01) and several increased their food intake by ~50 g day−1 over the infusion period, while their prior food intake had been zero. During the infusion period, none of the four saline-infused animals significantly increased their food intake compared with the month before infusion. Food intake was significantly higher for AICAR-infused animals (P<0.02) during days 6–8 of the infusion period compared with the saline-infused animals. Food intake dropped as the osmotic pumps became depleted (i.e. Fig. 1, day 11, upward pointing arrow). Animals were returned to the cold room following infusion and food intake had decreased by 50% by the third day post-infusion (day 14). Once returned to the cold room, 8 of the 9 animals resumed hibernation within days (Figs 2 and 3) and food intake returned to zero.
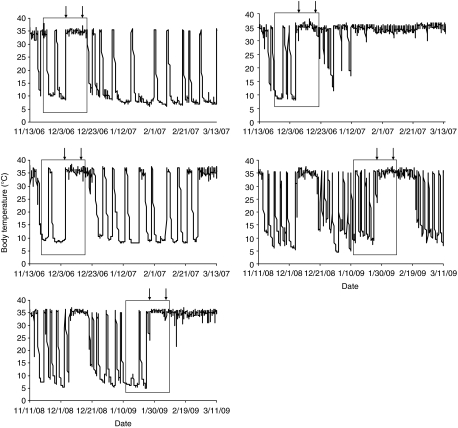
Fig. 2.
The Tb records of all five AICAR-infused animals. Animals were in the cold room which was 5±2°C except during the infusion period when it was 22±2°C (the time between the arrows on the boxes). The arrows denote the period of infusion of AICAR. Food was available to the animals throughout the entire experiment; however, the box represents the period of time that food intake was reported in Fig. 1 and I-buttons recorded Tb every 2 h from November to March.
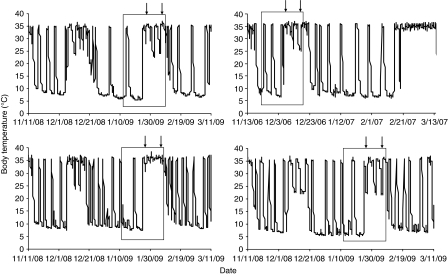
Fig. 3.
The Tb records for all four saline-infused animals. Ambient temperature is as described in Fig. 2. The arrows denote the period of saline infusion. Food availability, intake and I-button recordings were the same as in Fig. 2.
Body temperature
Before infusion, both AICAR-infused and saline-infused animals exhibited deep torpor bouts, with Tb near Ta (5±1°C) (Figs 2 and 3, respectively). The infusion rate of the ALZET mini-pumps is dependent on temperature and works optimally at 37°C. Therefore, we were required to perform the experiment at room temperature (>20°C) in order to keep marmots from dropping Tb too low for optimal infusion and to be able to have an infusion rate comparable to that in other rodent studies (Fryer and Carling, 2005; Hardie, 2008; Xue and Kahn, 2006). As shown in Fig. 2, none of the AICAR-infused animals underwent torpor bouts during the period of infusion. Three of the four animals infused with saline, however, did enter torpor within 48 h and their Tb decreased to near Ta (22±1°C) (Fig. 3). These animals continued to enter torpor throughout the infusion period. The saline-infused animal that did not enter torpor remained euthermic during the infusion, but did not eat. All saline-infused animals exhibited periodic and deep torpor bouts at 5°C within 4 days of returning to the cold room following infusion. Four of the five AICAR-infused animals also exhibited periodic and deep torpor bouts within days of being returned to the cold room.
Marmots infused with AICAR remained euthermic during the infusion period but only exhibited an average Tb of 34.9°C, less than the 37°C expected for a euthermic mammal. This resulted in an adjusted flow rate of 0.45 μl h−1, compared with the expected rate of 0.5 μl h−1 at a Tb of 37°C. This result could explain why some animals started to eat a few days after the pump was attached. In addition, three of the four animals infused with saline entered torpor at the high Ta resulting in an average Tb of 32.6°C for these animals. This resulted in a flow rate of 0.39 μl h−1.
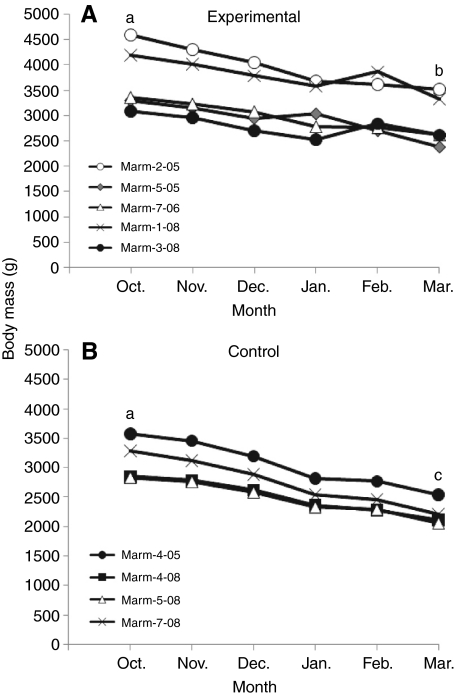
Body mass was recorded monthly for all marmots for the duration of the experiment. In addition, in 2009 body mass was measured just before and directly following infusion. The body mass for all marmots was significantly (P<0.05) lower in March than it was in October. During the experimental period (October to March) all of the control animals lost body mass. In contrast, some of the experimental animals (marm-1-08, 3-08 and 7-06) ate enough food to increase their body mass during the time of infusion (Fig. 4), and lost significantly less body mass over the course of the hibernation season as a result of eating during the infusion period, than controls (b vs c). Once the animals were returned to the cold room, all animals continued to lose body mass.
Fig. 4.
Changes in body mass for five AICAR-infused (A) and four saline-infused animals (B) over the hibernation period (October to March). Each line shows the body mass trajectory for an individual animal. The body mass for each group was not significantly different at the start of the experiment (denoted by the same letter). The males were heavier than the females. The AICAR-infused and saline-infused animals did differ significantly (P<0.05) in March. Both groups lost a significant amount of body mass (denoted by different letters) from October to March (P<0.05).
DISCUSSION
The purpose of this experiment was to induce a hibernator that normally does not feed for several months during the winter to feed in the middle of the winter hibernation period. To accomplish this, we infused an AMPK agonist, AICAR, into the 3rd ventricle of a hibernator and demonstrated that feeding resumed and continued throughout the infusion of the agonist. We found that infusion of AICAR, which activates AMPK within the ARC, stimulated a significant increase in food intake over the entire infusion period and animals did not undergo torpor. For three of the AICAR-infused animals there was an increase or no change in body mass during the infusion period (Fig. 4A); however, saline-infused animals decreased body mass as expected because animals were in the weight-loss phase of their body mass cycle (Fig. 4B).
Our data are consistent with previous studies examining the actions of AICAR on food intake. AICAR acts by mimicking the effects of a high AMP/ATP ratio and activating AMPK (Hardie et al., 1998; Hardie et al., 1997). Once activated by AICAR, AMPK stimulates feeding in all rodents studied thus far (Hardie, 2008; Kohno et al., 2008). Previous rodent studies have injected AICAR into the 3rd ventricle and recorded an increase in food intake over, at most, a 24 h period (Horman et al., 2005; Minokoshi et al., 2004). However, to our knowledge, this is the first demonstration of AICAR-induced feeding when delivered chronically to the 3rd ventricle. AICAR can have other actions within isolated cells (for review, see Towler and Hardie, 2007) including altering the metabolic syndrome related to glucose metabolism, fatty acid oxidation and protein synthesis (Wong et al., 2009). AICAR was also used to demonstrate astrocyte stellation due to AMPK and ACC activation (Favero and Mandell, 2007). We studied the intact animal and therefore were only able to record certain physiological responses such as food intake and Tb. Thus, it is possible that AICAR effected other physiological responses, such as hypoglycaemia (Wong et al., 2009), but this seems unlikely because glucose levels are already low during hibernation (Galster and Morrison, 1970).
Presumably, the mechanism for AICAR activation of feeding behavior involves neurons within the ARC. By knocking out AMPK within AgRP neurons of the ARC, Claret and colleagues demonstrated in a mouse model that animals developed a lean phenotype, whereas knockout of AMPK within POMC neurons resulted in an obese phenotype (Claret et al., 2007). Although it is likely that, in the present study, infusion of AICAR into the 3rd ventricle activated AMPK-containing neurons within the ARC, we have not yet identified the exact neuronal phenotypes of cells involved in this phenomenon. Nonetheless, it is clear that AMPK activation in the hypothalamus can induce feeding and prevent torpor bouts in hibernating mammals.
Alternatively, an argument could be made that transfer to a higher Ta used for these studies may have initiated food intake independently of the AICAR infusion, because marmots do not normally experience high Ta in winter months. The saline-infused animals did not eat, however, even though food was present in their cage. Moreover, they continued to enter torpor, indicating that Ta did not stimulate food intake.
Although the marmots infused with AICAR remained euthermic during the infusion, their Tb was slightly, but not significantly, higher than that of saline-infused animals. This is likely due to the absence of torpor bouts in the AICAR-infused animals. As a result, their pumps operated at a slightly higher rate (0.45 μl h−1 vs 0.39 μl h−1, respectively) than those of the saline-infused animals. It might be argued that the animals receiving AICAR simply ate as a result of a higher flow rate of fluid into their brains. We find this possibility highly unlikely, however, as there is no evidence in the literature to suggest that such low flow rate differences in the brain can alter any behavior.
Hibernators cease eating prior to entering torpor and it is obvious that animals in torpor do not eat. However, it is unclear whether the neuronal pathways involved in food intake must be inhibited first in order to allow animals to undergo torpor by depressing metabolism and switching to fat metabolism. We found that if we activate AMPK in winter, animals will refrain from entering torpor and will eat. One possible mechanism by which AMPK activation may exert this effect is through the acetyl CoA carboxylase (ACC)-malonyl CoA-creatine phosphate transferase 1c (CPT-1c) pathway, which regulates fuel utilization and food intake (Horman et al., 2005; Lane et al., 2005). Since malonyl CoA has been implicated in the control of food intake and is involved in fat metabolism through CPT-1c, it is likely that malonyl CoA and AMPK are involved in the switch to fat metabolism (i.e. respiratory quotient, RQ=0.7) during autumn and winter in hibernators (Buck and Barnes, 2000). A low level of AMPK activation within the hypothalamus may be needed for lipid oxidation and torpor to continue, but high levels of AMPK activation will stimulate food intake and prevent torpor. For example, it appears that AMPK may be involved in this switch to fat utilization in heart muscle (Fryer and Carling, 2005; Houten et al., 2009). In the heart of hibernating thirteen-lined ground squirrels (Spermophilus tridecemlineatus), it has been shown that FFAs and insulin shift the fuel utilization within heart tissue by activating pyruvate dehydrogenase kinase isoenzyme 4 and this switch from carbohydrate to lipid metabolism is necessary for successful torpor and may require activated AMPK (Carey et al., 2003). In addition, since some mammalian hibernators do eat during the hibernation period [e.g. chipmunks (Humphries et al., 2001)], it is possible that varying the activation of AMPK allows some animals to eat during an inter-bout arousal. Even so, all hibernators do show a progressive decrease in food intake prior to the winter hibernation season suggesting that there is a mechanism that promotes a decrease in food intake regardless of whether the animal exhibits long or short torpor bouts.
Similar to what we saw following AICAR infusion, previous experiments have demonstrated that NPY injection into the brain of hibernators and animals that exhibit daily torpor stimulates feeding (Boswell et al., 1993; Paul et al., 2005; Pelz and Dark, 2007). The mechanism by which NPY stimulates feeding in hibernators could potentially be AMPK activation within NPY-secreting neurons. It is known that when AMPK is activated within NPY/AgRP neurons it stimulates the release of NPY (Fryer and Carling, 2005; Mountjoy et al., 2007). This is consistent with the results of Claret and colleagues showing lean phenotype when AMPK is knocked out of AgRP neurons (Claret et al., 2007). Although the results of our study support the hypothesis that activated AMPK stimulates feeding in hibernators, the role of NPY neurons in this behavior is unclear as we have not measured NPY secretion or neuronal activity in these animals. Nonetheless, from previous work on non-hibernators, we could hypothesize that upon activation of AMPK, NPY-secreting neurons would be stimulated (Kohno et al., 2008), thus leading to increases in feeding.
It is well known that hibernators deposit large amounts of WAT prior to hibernation and in marmots and woodchucks there is a significant increase in plasma leptin and insulin associated with the increase in fat mass (Concannon et al., 2001; Florant et al., 1985; Florant et al., 2004). Both insulin and leptin levels provide feedback to the ARC by activating POMC/cocaine- and amphetamine-regulated neurons and inhibiting NPY/AgRP neurons (Könner et al., 2009; Mountjoy et al., 2007). Furthermore, leptin's inhibition of NPY/AgRP neurons decreases activation of Y1 and Y5 receptors by NPY and reduces the inhibition of melanocortin 3 and 4 receptors (Badman and Flier, 2007). Activation of these neurons leads to a decrease in appetite and increased thermogenesis (Gao et al., 2004), as well as other peripheral metabolic effects. In hibernators such as the marmot, plasma leptin levels increase before hibernation and could provide a signal to the ARC neurons to shut down food intake. In addition, the increase in leptin during the hibernation period is likely a part of the mechanism that turns off food intake and promotes lipid oxidation through the activation of ACC during hibernation (Ahima et al., 1996). Interestingly, in the Siberian hamster, which is photoperiodic, leptin sensitivity changes with photoperiod (Klingenspor et al., 2000) and leptin levels must be reduced for the initiation of torpor (Freeman et al., 2004; Paul et al., 2005).
The exact physiological (e.g. neuronal) mechanism(s) that initiates and terminates torpor is unknown. It is known that the regulation of Tb resides in the hypothalamus and that lesions in this area can disrupt and/or prevent torpor (Heller, 1979; Ruby, 1995). Furthermore, there is a general metabolic depression and reduction in sympathetic tone prior to the drop in Tb (Braulke and Heldmaier, 2009; Heldmaier et al., 2004). However, the direct interaction of the food intake pathway and the regulation of Tb has not been investigated, although it has been suggested that the paraventricular nucleus may influence daily torpor indirectly by regulating body mass or the availability of metabolic fuels (Ruby, 1995). We suggest here that when animals reach their peak body mass, prior to entering torpor, endogenous factors such as metabolic fuels (e.g. fatty acids) or hormones (e.g. leptin) may play a role in shutting down food intake and stimulating or releasing the inhibition of thermoregulatory pathways, allowing the animal to lower Tb in a regulated manner. It is well known that metabolic fuels dramatically increase during the autumn when body mass peaks, prior to the onset of torpor (Florant et al., 2004; Melvin and Andrews, 2009). Since food intake and the thermoregulatory pathways coexist within the hypothalamus, it is not inconceivable that the pathways share common nuclei that promote physiological responses. We hypothesize that food intake and the ability of an animal to enter torpor share common neuronal pathways. We suggest that high concentrations of metabolic fuels (e.g. fatty acids) and/or hormones (e.g. leptin, insulin and adiponectin) in autumn may feed back to the neurons involved in producing feeding behavior and inhibit food intake, whereupon pathways leading to metabolic depression are stimulated. Our previous studies support the hypothesis that changes in hormonal factors may lead to decreased food intake (Florant et al., 2004). Our current study suggests that the hibernation phenotype is plastic in that hibernators can change from a feeding state (no torpor) to a non-feeding state (torpor) in a short period of time, which supports a neuronal mechanism. The exact neuronal changes that occur in the brain of hibernators to produce this dramatic alteration in feeding behaviors and Tb are unknown, although manipulation of brain fuels and circulating hormones should provide answers to this question.
The results from hibernators are unique and suggest they may be excellent animal models to study the regulation of food intake in mammals. Many rodent studies have relied solely on genetically engineered animal models. The use of an animal model that stops eating naturally for months at a time, not just hours, days or even weeks, represents a true natural ‘knockout’ which might provide important clues about food intake regulation and energy metabolism.
ACKNOWLEDGEMENTS
We would like to thank Melanie Richter for helping in this research, Dr Tim Bartness for reading an earlier version of this manuscript and two anonymous reviewers for helpful criticisms. This work was supported by NIH grant DK 067017-5 to G.L.F. and NIH NS 039951 to R.J.H. Deposited in PMC for release after 12 months. We also thank the Fox Acres Country Club for allowing us to trap marmots on their property.
Footnotes
- ACC
- acetyl-CoA carboxylase
- AgRP
- agouti-related protein
- AICAR
- 5-aminoimidazole-4-carboxamide 1 B-D-ribofuranoside
- AMPK
- AMP-activated protein kinase
- ARC
- arcuate nucleus
- FFA
- free fatty acids
- ICV
- intracerebroventricularly
- NPY
- neuropeptide Y
- POMC
- proopiomelanocortin
- Ta
- ambient temperature
- Tb
- body temperature
REFERENCES
- Ahima R. S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., Flier J. S. (1996). Role of leptin in the neuroendocrine response to fasting. Nature 382, 250-252 [DOI] [PubMed] [Google Scholar]
- Badman M. K., Flier J. S. (2007). The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 132, 2103-2115 [DOI] [PubMed] [Google Scholar]
- Boswell T., Richardson R. D., Schwartz M. W., D'Alessio D. A., Woods S. C., Sipols A. J., Baskin D. G., Kenagy G. J. (1993). NPY and galanin in a hibernator: hypothalamic gene expression and effects on feeding. Brain Res. Bull. 32, 379-384 [DOI] [PubMed] [Google Scholar]
- Braulke L. J., Heldmaier G. (2009). Torpor and ultradian rhythms require an intact signalling of the sympathetic nervous system. Cryobiology 60, 198-203 [DOI] [PubMed] [Google Scholar]
- Buck C. L., Barnes B. M. (2000). Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J. Physiol. Regul. Integr. Comp. Physiol. 279, R255-R262 [DOI] [PubMed] [Google Scholar]
- Cao Z. P., Wang F., Xiang X. S., Cao R., Zhang W. B., Gao S. B. (2007). Intracerebroventricular administration of conjugated linoleic acid (CLA) inhibits food intake by decreasing gene expression of NPY and AgRP. Neurosci. Lett. 418, 217-221 [DOI] [PubMed] [Google Scholar]
- Carey H. V., Andrews M. T., Martin S. L. (2003). Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153-1181 [DOI] [PubMed] [Google Scholar]
- Claret M., Smith M. A., Batterham R. L., Selman C., Choudhury A. I., Fryer L. G., Clements M., Al-Qassab H., Heffron H., Xu A. W., et al. (2007). AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest. 117, 2325-2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon P., Levac K., Rawson R., Tennant B., Bensadoun A. (2001). Seasonal changes in serum leptin, food intake, and body weight in photoentrained woodchucks. Am J. Physiol. Regul. Integr. Comp. Physiol. 281, R951-R959 [DOI] [PubMed] [Google Scholar]
- Dark J. (2005). Annual lipid cycles in hibernators: integration of physiology and behavior. Annu. Rev. Nutr. 25, 469-497 [DOI] [PubMed] [Google Scholar]
- Davis D. E. (1976). Hibernation and circannual rhythms of food consumption in marmots and ground squirrels. Q. Rev. Biol. 51, 477-514 [DOI] [PubMed] [Google Scholar]
- Dellman H., Breazile J. E., South F. (1973). A Stereotaxic Guide to the Brain of the Marmot Columbia: Private printing, University of Missouri; [Google Scholar]
- Favero C. B., Mandell J. W. (2007). A pharmacological activator of AMP-activated protein kinase (AMPK) induces astrocyte stellation. Brain Res. 1168, 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florant G. L., Lawrence A. K., Williams K., Bauman W. A. (1985). Seasonal changes in pancreatic B-cell function in euthermic yellow-bellied marmots. Am J. Physiol. 249, R159-R165 [DOI] [PubMed] [Google Scholar]
- Florant G. L., Porst H., Peiffer A., Hudachek S. F., Pittman C., Summers S. A., Rajala M. W., Scherer P. E. (2004). Fat-cell mass, serum leptin and adiponectin changes during weight gain and loss in yellow-bellied marmots (Marmota flaviventris). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 174, 633-639 [DOI] [PubMed] [Google Scholar]
- Freeman D. A., Lewis D. A., Kauffman A. S., Blum R. M., Dark J. (2004). Reduced leptin concentrations are permissive for display of torpor in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R97-R103 [DOI] [PubMed] [Google Scholar]
- Fryer L. G., Carling D. (2005). AMP-activated protein kinase and the metabolic syndrome. Biochem. Soc. Trans. 33, 362-366 [DOI] [PubMed] [Google Scholar]
- Galster W. A., Morrison P. (1970). Cyclic changes in carbohydrate concentrations during hibernation in the arctic ground squirrel. Am J. Physiol. 218, 1228-1232 [DOI] [PubMed] [Google Scholar]
- Gao Z., Zhang X., Zuberi A., Hwang D., Quon M. J., Lefevre M., Ye J. (2004). Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol. Endocrinol. 18, 2024-2034 [DOI] [PubMed] [Google Scholar]
- Hardie D. G. (2008). AMPK: a key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. (Lond) 32, S7-S12 [DOI] [PubMed] [Google Scholar]
- Hardie D. G., Corton J., Ching Y. P., Davies S. P., Hawley S. (1997). Regulation of lipid metabolism by the AMP-activated protein kinase. Biochem. Soc. Trans. 25, 1229-1231 [DOI] [PubMed] [Google Scholar]
- Hardie D. G., Carling D., Carlson M. (1998). The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67, 821-855 [DOI] [PubMed] [Google Scholar]
- Heldmaier G., Ortmann S., Elvert R. (2004). Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317-329 [DOI] [PubMed] [Google Scholar]
- Heller H. C. (1979). Hibernation: neural aspects. Annu. Rev. Physiol. 41, 305-321 [DOI] [PubMed] [Google Scholar]
- Horman S., Hussain N., Dilworth S. M., Storey K. B., Rider M. H. (2005). Evaluation of the role of AMP-activated protein kinase and its downstream targets in mammalian hibernation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 142, 374-382 [DOI] [PubMed] [Google Scholar]
- Houten S. M., Chegary M., Te Brinke H., Wijnen W. J., Glatz J. F., Luiken J. J., Wijburg F. A., Wanders R. J. (2009). Pyruvate dehydrogenase kinase 4 expression is synergistically induced by AMP-activated protein kinase and fatty acids. Cell. Mol. Life Sci. 66, 1283-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. M., Thomas D. W., Kramer D. L. (2001). Torpor and digestion in food-storing hibernators. Physiol. Biochem. Zool. 74, 283-292 [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Alquier T., Carling D., Hardie D. G. (2005). AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1, 15-25 [DOI] [PubMed] [Google Scholar]
- Kim E. K., Miller I., Aja S., Landree L. E., Pinn M., McFadden J., Kuhajda F. P., Moran T. H., Ronnett G. V. (2004). C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J. Biol. Chem. 279, 19970-19976 [DOI] [PubMed] [Google Scholar]
- Kim M. S., Lee K. U. (2005). Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J. Mol. Med. 83, 514-520 [DOI] [PubMed] [Google Scholar]
- Klingenspor M., Niggemann H., Heldmaier G. (2000). Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 170, 37-43 [DOI] [PubMed] [Google Scholar]
- Kohno D., Sone H., Minokoshi Y., Yada T. (2008). Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem. Biophys. Res. Commun. 366, 388-392 [DOI] [PubMed] [Google Scholar]
- Könner A. C., Klöckener T., Brüning J. C. (2009). Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Physiol. Behav. 5, 632-638 [DOI] [PubMed] [Google Scholar]
- Kubota N., Yano W., Kubota T., Yamauchi T., Itoh S., Kumagai H., Kozono H., Takamoto I., Okamoto S., Shiuchi T., et al. (2007). Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 6, 55-68 [DOI] [PubMed] [Google Scholar]
- Lane M. D., Hu Z., Cha S. H., Dai Y., Wolfgang M., Sidhaye A. (2005). Role of malonyl-CoA in the hypothalamic control of food intake and energy expenditure. Biochem. Soc. Trans. 33, 1063-1067 [DOI] [PubMed] [Google Scholar]
- Melvin R. G., Andrews M. T. (2009). Torpor induction in mammals: recent discoveries fueling new ideas. Trends Endocrinol. Metab. 20, 490-498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferre P., Birnbaum M. J., et al. (2004). AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569-574 [DOI] [PubMed] [Google Scholar]
- Mountjoy P. D., Bailey S. J., Rutter G. A. (2007). Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia 50, 168-177 [DOI] [PubMed] [Google Scholar]
- Paul M. J., Freeman D. A., Park J. H., Dark J. (2005). Neuropeptide Y induces torpor-like hypothermia in Siberian hamsters. Brain Res. 1055, 83-92 [DOI] [PubMed] [Google Scholar]
- Pelz K. M., Dark J. (2007). ICV NPY Y1 receptor agonist but not Y5 agonist induces torpor-like hypothermia in cold-acclimated Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R2299-R2311 [DOI] [PubMed] [Google Scholar]
- Perrin C., Knauf C., Burcelin R. (2004). Intracerebroventricular infusion of glucose, insulin, and the adenosine monophosphate-activated kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, controls muscle glycogen synthesis. Endocrinology 145, 4025-4033 [DOI] [PubMed] [Google Scholar]
- Ruby N. F. (1995). Paraventricular nucleus ablation disrupts daily torpor in Siberian hamsters. Brain Res. Bull. 37, 193-198 [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Patterson D., Holmes E. W. (1985). 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J. Biol. Chem. 260, 6107-6114 [PubMed] [Google Scholar]
- Towler M. C., Hardie D. G. (2007). AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 100, 328-341 [DOI] [PubMed] [Google Scholar]
- Ward J. M., Jr, Armitage K. B. (1981). Circannual rhythms of food consumption, body mass, and metabolism in Yellow-Bellied Marmots. Comp. Biochem. Physiol. A 69, 621-626 [Google Scholar]
- Wong A. K., Howie J., Petrie J. R., Lang C. C. (2009). AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin. Sci. (Lond) 116, 607-620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Kahn B. B. (2006). AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 574, 73-83 [DOI] [PMC free article] [PubMed] [Google Scholar]