Abstract
Objective
In pregnancy, a normal result on the oral glucose tolerance test (OGTT) that follows an abnormal screening glucose challenge test (GCT) is considered a reassuring finding, requiring no further intervention. The obstetrical and metabolic implications of this presentation, however, have not been well-studied. Thus, we sought to characterize the obstetrical and postpartum metabolic significance of an abnormal GCT in women with normal glucose tolerance (NGT) on antepartum OGTT.
Design/Patients/Measurements
259 women with NGT on antepartum OGTT (166 with an abnormal GCT and 93 with normal GCT) underwent (i) metabolic evaluation in pregnancy, (ii) assessment of obstetrical outcome at delivery and (iii) repeat metabolic characterization by OGTT at 3-months postpartum.
Results
Neither infant birthweight nor Caesarian-section rate differed between the abnormal GCT and normal GCT groups. At 3-months postpartum, however, compared to the normal GCT group, the abnormal GCT group exhibited greater glycemia (mean area-under-the-glucose-curve (AUCgluc) 19.6 vs 18.3, p=0.0021), lower insulin sensitivity (median ISOGTT 9.5 vs 11.3, p=0.0243) and poorer beta-cell function (median insulinogenic index/HOMA-IR 9.8 vs 14.1, p=0.0013). On multiple linear regression analyses, an abnormal GCT emerged as (i) the strongest independent predictor of postpartum AUCgluc (t=2.77, p=0.006) and (ii) the strongest independent negative predictor of log insulinogenic index/HOMA-IR (t=−2.36, p=0.0191). Furthermore, the GCT was the antepartum parameter that best predicted postpartum pre-diabetes (area-under-receiver-operating-characteristic-curve=0.754).
Conclusions
An abnormal antepartum GCT, even when followed by a normal OGTT, is associated with postpartum glycemia and beta-cell dysfunction, factors that may portend an increased future risk of diabetes in this patient population.
Keywords: glucose challenge test, pregnancy, glucose intolerance, postpartum
INTRODUCTION
Gestational diabetes mellitus (GDM) is associated with (i) adverse obstetrical outcomes, particularly related to fetal macrosomia, and (ii) an increased risk of developing type 2 diabetes (T2DM) in the years following the index pregnancy (1,2). As such, standard obstetrical practice involves screening pregnant women for the presence of GDM. Although universal consensus regarding the appropriate screening protocol is lacking, one common approach is the performance of a 50g glucose challenge test (GCT) in all women in late 2nd trimester, followed by a diagnostic oral glucose tolerance test (OGTT) in those women in whom the GCT is abnormal. Women identified with GDM are then (i) treated with dietary or insulin therapy in order to reduce glucose levels and decrease the risk of infant macrosomia and (ii) advised to undergo postpartum glucose tolerance testing (3,4).
Recently, it has emerged that even women with mildly elevated glucose levels on antepartum GDM screening are still at risk of fetal macrosomia (5). Similarly, we have reported that women with mild glucose intolerance in pregnancy (ie. less severe than GDM) are also at increased risk of developing pre-diabetes or diabetes at 3-months postpartum (6). Thus, taken together, these data suggest that the obstetrical and postpartum metabolic risks of GDM extend to much lower levels of maternal glycemia than currently recognized, a possibility that may have important implications for a large number of women with mild abnormalities on GDM screening.
The mildest possible abnormal category on antepartum GDM screening is represented by those women with normal glucose tolerance (NGT) on the OGTT but an isolated abnormal GCT result. Although NGT on the OGTT following an abnormal GCT is generally considered a reassuring result (one that does not require any clinical intervention), it should be noted that there has been limited study to date of the obstetrical and postpartum metabolic implications of this presentation. Thus, in light of the aforementioned observations regarding the significance of mild dysglycemia in pregnancy, our objective in the current analysis was to systematically evaluate obstetrical outcomes and postpartum metabolic function in women with an abnormal GCT followed by NGT on antepartum OGTT, as compared to a control group of women with both a normal GCT and NGT.
RESEARCH DESIGN AND METHODS
This analysis was conducted in the setting of an ongoing observational study of early events in the natural history of T2DM, in which a cohort of women recruited at the time of GDM screening is undergoing longitudinal metabolic characterization in pregnancy and the postpartum period (6,7). Standard obstetrical practice at our institution involves universal screening for GDM in all pregnant women at 24–28 weeks’ gestation by 50g GCT followed by, if the GCT is abnormal (1-hour post-challenge plasma glucose ≥7.8 mmol/L), referral for a diagnostic OGTT. In the study, healthy pregnant women are recruited either prior to or just after their GCT. Regardless of the GCT result, all study participants undergo a 3-hour 100g OGTT for determination of glucose tolerance status in pregnancy. At 3-months postpartum, participants undergo re-assessment by 2-hour 75g OGTT. The study protocol has been approved by the Mount Sinai Hospital Research Ethics Board and all participants have provided written informed consent. The current analysis was restricted to those women with NGT on the antepartum OGTT (n=259), as defined by National Diabetes Data Group criteria (8). For this analysis, these women were stratified into 2 groups: (i) those with an abnormal GCT (n=166) and (ii) those with a normal GCT (n=93).
Participant Assessments
On the morning of the 3-hour 100g OGTT in pregnancy, data pertaining to personal history of previous GDM and family history of diabetes were collected by interviewer-administered questionnaire. Anthropometric measurements of height and weight were also obtained using a medical scale.
At delivery, data on obstetrical outcome was entered into a database that tracks labour and delivery data at Mount Sinai Hospital. Large-for-gestational-age (LGA) was defined as sex-specific birthweight for gestational age above the 90th percentile of Canadian population fetal growth curves (9). Macrosomia was defined as birthweight ≥4,000 grams.
At 3-months postpartum, participants returned to the clinical investigation unit for a 2-hour 75g OGTT. Physical examination was also performed, including measurement of weight and waist circumference.
Laboratory Measurements and Physiologic Indices
For all OGTTs, participants arrived in the morning after overnight fast. Venous blood samples were drawn for measurement of glucose and insulin at fasting and at 30-, 60- and 120-minutes (and 180-minutes in pregnancy). Specific insulin was measured using the Roche Modular system and the electrochemiluminescence immunoassay kit (Roche Diagnostics catalogue number 12017547122). This assay shows 0.05% cross-reactivity to intact human proinsulin and the primary circulating split form (Des 31,32).
At both baseline and follow-up, glycemia was assessed by the total area-under-the-glucose-curve (AUCgluc) during the OGTT, calculated using the trapezoidal rule. Glucose tolerance status on OGTT at 3-months postpartum was defined according to Canadian Diabetes Association guidelines, as previously described (6,10). Pre-diabetes refers to (i) impaired glucose tolerance (defined as 2-hour glucose between 7.8–11.0 mmol/L inclusive), (ii) impaired fasting glucose (defined as fasting glucose between 6.1–6.9 mmol/L inclusive) or (iii) both (10). Insulin sensitivity was measured using the insulin sensitivity index (ISOGTT) of Matsuda and DeFronzo (11). In pregnant women, ISOGTT exhibits better correlation with insulin sensitivity measured by euglycemic-hyperinsulinemic clamp than either Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) or Quantitative Insulin Sensitivity Check Index (12) Beta-cell function was assessed by the insulinogenic index divided by HOMA-IR (13,14). The insulinogenic index was calculated as the incremental change in insulin concentration during the first 30 minutes of the OGTT divided by the incremental change in glucose during the same time period (15).
Statistical Analyses
All analyses were conducted using the Statistical Analysis System (SAS 9.1, SAS Institute, Cary NC). Continuous variables were tested for normality of distribution (by descriptive histogram and theory-driven Q-Q plot) and natural log transformations of skewed variables were used, where necessary, in subsequent analyses. In Table 1, baseline characteristics at the time of the OGTT in pregnancy (Panel A), obstetrical outcomes (Panel B), and metabolic characteristics at 3-months postpartum (Panel C) are presented for the 2 study groups (normal GCT and abnormal GCT). Continuous variables are presented as median followed by interquartile range if skewed, or mean followed by standard error if normally distributed, while categorical variables are presented as proportions. Univariate differences between the groups were assessed using Analysis of Variance for continuous variables and χ2 test for categorical variables. Conservative Bonferroni adjustment for multiple comparisons was performed. In Table 2, multiple linear regression analysis was used to identify the antepartum factors that independently predicted the following metabolic parameters at 3-months postpartum: (i) log ISOGTT (model A), and (ii) log insulinogenic index/HOMA-IR (model B). Covariates included age, pre-pregnancy BMI, family history of T2DM, previous GDM, ethnicity (with Caucasian as reference group) and abnormal GCT (with normal GCT as reference group). Receiver-operating-characteristic (ROC) analysis was performed to assess the discriminative capacity of antepartum metabolic measures in predicting postpartum pre-diabetes (Figure 1). The following antepartum measures were evaluated: GCT, AUCgluc, insulinogenic index/HOMA-IR, ISOGTT and each glucose value from the OGTT in pregnancy.
Table 1.
Comparison of women with normal GCT NGT and women with abnormal GCT NGT with respect to (Panel A) antepartum characteristics, (Panel B) obstetrical outcomes, and (Panel C) metabolic characteristics at 3-months postpartum
| Panel A: Antepartum Characteristics | |||
|---|---|---|---|
| Normal GCT NGT n=93 |
Abnormal GCT NGT n=166 |
p |
|
| Age (yrs) | 34.0 [4.4] | 33.8 [4.2] | 0.7632 |
| Weeks gestation | 32.2 [2.6] | 29.1 [2.4] | <0.0001 |
| Pre-pregnancy BMI (kg/m2) | 23.0 [21.5 – 26.1] | 23.5 [21.1 – 27.5] | 0.5501 |
| Family history of DM (%) | 41.9 | 50.6 | 0.1811 |
| Previous GDM/macrosomia (%) | 0.0 | 3.6 | 0.0633 |
| Ethnicity: | 0.8444 | ||
| Caucasian (%) | 79.6 | 79.5 | |
| Asian (%) | 7.5 | 9.0 | |
| Other (%) | 12.9 | 11.5 | |
| ISOGTT | 5.4 [3.6 – 7.3] | 5.4 [3.6 – 7.3] | 0.9248 |
| Insulinogenic Index/HOMA-IR | 13.9 [9.7 – 19.6] | 12.8 [8.6 – 18.1] | 0.3535 |
| Glucose metabolism: | |||
| Glucose challenge test (mmol/L) | 5.8 [1.0] | 8.6 [0.8] | <0.0001 |
| OGTT: | |||
| Fasting glucose (mmol/L) | 4.2 [0.5] | 4.4 [0.4] | 0.0033 |
| 1-hr glucose (mmol/L) | 8.0 [7.0 – 8.5] | 8.6 [7.9 – 9.3] | <0.0001 |
| 2-hr glucose (mmol/L) | 6.6 [5.9 – 7.5] | 7.6 [6.7 – 8.3] | <0.0001 |
| 3-hr glucose (mmol/L) | 6.1 [5.2 – 6.8] | 6.0 [4.8 – 6.9] | 0.6351 |
| AUCgluc | 19.6 [18.3 – 21.1] | 21.3 [19.9 – 22.7] | <0.0001 |
| Panel B: Obstetrical Outcomes | |||
|
Normal GCT NGT n=93 |
Abnormal GCT NGT n=166 |
p |
|
| Length of gestation (wks) | 39 [38 – 40] | 39 [38 – 40] | 0.4001 |
| Infant gender (% M/F) | 44/56 | 50/50 | 0.3757 |
| 1 minute Apgar < 7 (%) | 8.9 | 5.2 | 0.2617 |
| 5 minute Apgar < 7 (%) | 0 | 0 | ------ |
| Caesarian-section (%) | 28.6 | 36.1 | 0.1221 |
| Infant birthweight (g) | 3360 [526] | 3395 [574] | 0.6355 |
| Macrosomia (%) | 7.5 | 12.1 | 0.2542 |
| LGA (%) | 5.6 | 12.2 | 0.0943 |
| Panel C: Metabolic Characteristics at 3-months Postpartum | |||
|
Normal GCT NGT n=93 |
Abnormal GCT NGT n=166 |
p |
|
| Months postpartum | 3.2 [2.9 – 3.6] | 3.2 [2.9 – 4.1] | 0.2198 |
| BMI (kg/m2) | 24.6 [22.6 – 27.9] | 25.8 [23.4 – 29.8] | 0.3272 |
| Waist circumference (cm) | 86.6 [10.9] | 87.1 [11.8] | 0.7149 |
| ISOGTT | 11.3 [8.2 – 15.7] | 9.5 [6.6 – 14.2] | 0.0243 |
| Insulinogenic Index/HOMA-IR | 14.1 [9.6 – 20.0] | 9.8 [7.1 – 14.9] | 0.0013 |
| Glucose metabolism: | |||
| OGTT: | |||
| Fasting glucose (mmol/L) | 4.4 [0.4] | 4.5 [0.4] | 0.1395 |
| 2-hr glucose (mmol/L) | 5.6 [1.1] | 6.0 [1.3] | 0.0074 |
| AUCgluc | 18.3 [3.1] | 19.6 [3.4] | 0.0021 |
| Glucose tolerance status: | 0.0429 | ||
| NGT (%) | 96.8 | 89.8 | |
| Pre-diabetes (%) | 3.2 | 10.2 | |
Continuous data are presented as median followed by interquartile range, if skewed, or mean followed by standard deviation, if normally-distributed. Categorical data are presented as percentages. p-values refer to overall differences across groups as derived from ANOVA analysis for continuous variables (parametric test for normally-distributed variables and nonparametric test for skewed variables) or χ2 test for categorical variables.
Table 2.
Multiple linear regression analyses of the following dependent variables at 3-months postpartum: (Model A) log ISOGTT and (Model B) log insulinogenic index/HOMA-IR
| Model A: Dependent variable log ISOGTT at 3-months postpartum | ||||
|---|---|---|---|---|
| Variable |
Parameter Estimate |
Standard Error |
t value |
p |
| Age | 0.00198 | 0.00755 | 0.26 | 0.7934 |
| Pre-pregnancy BMI | −0.04212 | 0.00623 | −6.76 | <0.0001 |
| Family history of diabetes | −0.05934 | 0.06575 | −0.90 | 0.3677 |
| Previous GDM | −0.82694 | 0.21090 | −3.92 | 0.0001 |
| Ethnicity: | ||||
| Asian ethnicity | −0.40628 | 0.12000 | −3.39 | 0.0008 |
| Other non-white ethnicity | −0.12875 | 0.10446 | −1.23 | 0.2190 |
| Abnormal GCT | −0.08882 | 0.06760 | −1.31 | 0.1902 |
| Model B: Dependent variable log insulinogenic index/HOMA-IR at 3-months postpartum | ||||
|
Variable |
Parameter Estimate |
Standard Error |
t value |
p |
| Age | −0.01887 | 0.01199 | −1.57 | 0.1168 |
| Pre-pregnancy BMI | −0.01754 | 0.00972 | −1.80 | 0.0725 |
| Family history of diabetes | 0.08028 | 0.10348 | 0.78 | 0.4386 |
| Previous GDM | −0.12335 | 0.32976 | −0.37 | 0.7087 |
| Ethnicity: | ||||
| Asian ethnicity | 0.17288 | 0.18744 | 0.92 | 0.3573 |
| Other non-white ethnicity | 0.16976 | 0.16889 | 1.01 | 0.3159 |
| Abnormal GCT | −0.25147 | 0.10656 | −2.36 | 0.0191 |
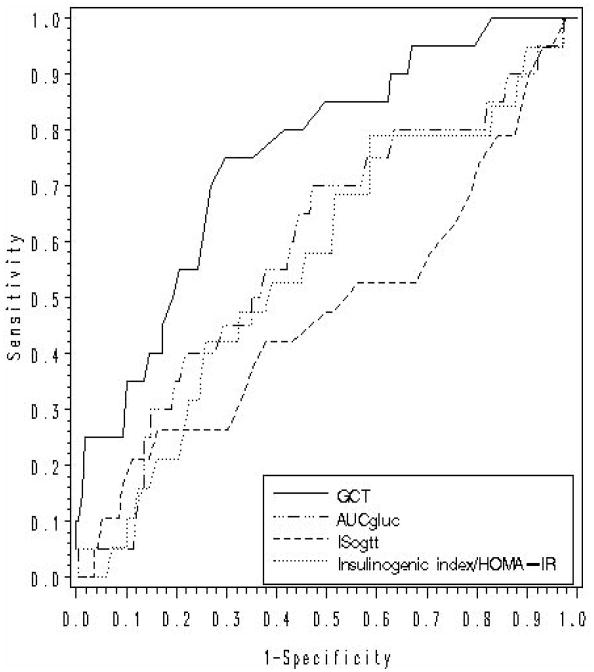
Figure 1.
Receiver-operating-characteristic (ROC) curves of antepartum metabolic parameters (GCT, AUCgluc, insulinogenic index/HOMA-IR, ISOGTT) for the prediction of pre-diabetes at 3-months postpartum
RESULTS
Baseline Characteristics of Study Groups
Table 1 Panel A shows the baseline characteristics of the study subjects stratified into two groups: (i) normal GCT (n=93) and (ii) abnormal GCT (n=166). The antepartum OGTT was performed slightly later in pregnancy in the normal GCT group than in the abnormal GCT group (mean 32.2 vs 29.1 weeks, p<0.0001). Otherwise, the two groups were not significantly different with respect to age, pre-pregnancy BMI, family history of T2DM, previous GDM, ethnicity, insulin sensitivity (ISOGTT) and beta-cell function (insulinogenic index/HOMA-IR) in pregnancy. As would be expected, mean GCT value was significantly higher in the abnormal GCT group (8.6 vs 5.8 mmol/L, p<0.0001). More importantly, although all of the women had NGT on the OGTT, almost all glycemic measures from the OGTT (including fasting glucose, 1-hr glucose, 2-hour glucose, and AUCgluc) were significantly higher in the abnormal GCT group compared to the normal GCT group (all p<0.005).
Obstetrical Outcomes of Study Groups
Having demonstrated greater antepartum glycemia in the women with an abnormal GCT, we next compared the study groups with respect to obstetrical outcomes (Table 1 Panel B). This comparison revealed that infant birthweight was not significantly different between the abnormal GCT (3395 +/−574g) and normal GCT group (3360 +/−526g) (p=0.6355). There were also no significant differences with respect to length of gestation, infant gender, Apgar scores, and Caesarian-section rates. Although rates of macrosomia and LGA were both higher in the abnormal GCT group, neither difference reached statistical significance (p=0.2542 and p=0.0943, respectively).
Postpartum Metabolic Characteristics of Study Groups
At 3-months postpartum (Table 1 Panel C), neither BMI nor waist circumference differed between the two groups. Nevertheless, clear metabolic differences between the groups were apparent. Specifically, at 3-months postpartum, women with an abnormal GCT in pregnancy had lower insulin sensitivity (ISOGTT: p=0.0243) and poorer beta-cell function (insulinogenic index/HOMA-IR: p=0.0013) than their peers who had had a normal GCT. These features translated into greater glycemia in the abnormal GCT group, as reflected by 2-hr blood glucose (p=0.0074) and AUCgluc (p=0.0021). Most importantly, the prevalence of pre-diabetes was significantly higher in the abnormal GCT group than in the normal GCT group (10.2% vs 3.2%, p=0.0429).
Antepartum Predictors of Postpartum Metabolic Dysfunction
Having demonstrated that an abnormal GCT was associated with postpartum metabolic defects, we next sought to determine if these associations were independent of other antepartum factors. Indeed, on multiple linear regression analysis, an abnormal GCT was the strongest independent predictor of postpartum AUCgluc (t=2.77, p=0.006) (data not shown). The mechanism linking the abnormal GCT group to postpartum dysglycemia did not appear to involve decreased insulin sensitivity, as abnormal GCT was not significantly associated with log ISOGTT at 3-months postpartum, after adjustment for age, pre-pregnancy BMI, family history of T2DM, previous GDM and ethnicity (Table 2 model A). In contrast, however, abnormal GCT was the sole independent negative predictor of log insulinogenic index/HOMA-IR (t=−2.36, p=0.0191) (Table 2 model B), thereby implicating beta-cell dysfunction as a likely factor contributing to postpartum dysglycemia in the abnormal GCT group.
Finally, we compared the discriminative capacity of the GCT with that of other antepartum metabolic measures for the prediction of postpartum pre-diabetes. As shown in Figure 1, the area-under-the-ROC curve (AROC) for the GCT (0.754) exceeded that of other metabolic measures in pregnancy, including AUCgluc (0.592), insulinogenic index/HOMA-IR (0.564) and ISOGTT (0.483), reflecting the superior discriminative capacity of the GCT in this context. The GCT was also superior to each glucose measure on the OGTT in pregnancy, including fasting glucose (AROC=0.541) (data not shown).
DISCUSSION
In this report, we demonstrate that, in women with normal glucose tolerance on antepartum OGTT, an abnormal GCT in pregnancy is associated with postpartum metabolic dysfunction. Specifically, the abnormal GCT is an independent predictor of both glycemia and beta-cell dysfunction at 3-months postpartum. Furthermore, when compared to other antepartum metabolic measures on ROC analysis, the GCT emerged as the best indicator of those women at risk of postpartum pre-diabetes. Overall, these data suggest that the finding of an isolated abnormal GCT in pregnancy may hold significance by potentially identifying a population of women at increased risk of developing T2DM in the future.
Pregnancy provides a natural test of a woman’s gluco-regulatory physiology, wherein the pancreatic beta-cells must compensate for the acquired insulin resistance of late gestation, if normoglycemia is to be maintained (16). In this context, it stands to reason that any abnormal glucose homeostasis in pregnancy should reflect some degree of underlying beta-cell dysfunction and hence should be associated with an increased risk of postpartum glucose intolerance. Indeed, consistent with this concept, we have recently demonstrated that any abnormal finding on antepartum GDM screening predicts an increased risk of postpartum pre-diabetes (6,7). In the current analysis, we extend these data by specifically studying the metabolic features of those women with the mildest such abnormality (an abnormal GCT followed by NGT on OGTT) in comparison to their peers with truly normal glucose tolerance in pregnancy (i.e. a normal GCT and NGT on OGTT). Remarkably, the seemingly mild abnormality of an isolated elevated GCT is found to be associated with postpartum metabolic dysfunction, including dysglycemia/glucose intolerance, lower insulin sensitivity and poorer beta-cell function. As such, these data raise the important possibility that an abnormal GCT with NGT in pregnancy may identify a population of women at increased risk of developing T2DM in the future.
To date, there has been very limited study of the postpartum metabolic implications of an abnormal GCT with NGT on antepartum OGTT. In a single previous report, Carr et al used administrative data to demonstrate that women with GCT results above 5.4 mmol/L have a >1.7-fold higher risk of developing diabetes over median follow-up of 8.8 years when compared to their peers with lower GCT values, after limited adjustment for age, primigravidity and pre-term delivery (17). The current study extends this data by (i) systematic evaluation of all subjects by GCT and OGTT in pregnancy (i.e. thereby confirming that all of the women under study had NGT on OGTT), (ii) postpartum metabolic characterization of all subjects by OGTT, (ii) full adjustment for covariates associated with glucose intolerance (including age, BMI, family history of T2DM, previous GDM and ethnicity), and (iii) assessment of insulin sensitivity and beta-cell function. Specifically, we demonstrate that, in women with NGT in pregnancy, an abnormal GCT independently predicts postpartum glycemia and beta-cell dysfunction. The association with poorer postpartum beta-cell function is particularly important, since chronic beta-cell dysfunction is likely the underlying basis for the enhanced risk of T2DM in women with a history of previous GDM (1). Thus, taken together with the findings of Carr et al (17), the current analysis (i) suggests that an abnormal GCT with NGT in pregnancy indeed identifies a population of women at risk of future diabetes and (ii) implicates beta-cell dysfunction as a potential mechanism underlying this increased risk. Further study will be needed to determine if this group of women warrants enhanced postpartum surveillance for the development of pre-diabetes/diabetes.
As an isolated elevated GCT with NGT on OGTT represents the mildest possible abnormality on antepartum GDM screening, it is to be expected that the increased risk of postpartum glucose intolerance in this group of women will be less than that of their peers with GDM or gestational impaired glucose intolerance (ie. >/=2 or 1 abnormal value(s) on OGTT in pregnancy, respectively). As such, if postpartum surveillance is to even be considered in this patient population (abnormal GCT with NGT), the identification of those women within this group at greatest risk of future glucose intolerance emerges as an important issue. In this regard, our ROC analysis suggests that the discriminative capacity of the GCT for predicting postpartum pre-diabetes amongst women with NGT in pregnancy exceeds that of other antepartum metabolic measures. While further study will be needed to determine the optimal strategy for identifying those women at greatest risk, the current data nevertheless highlights the potential significance of the GCT result in women with NGT in pregnancy.
It should be recognized that the usefulness of the GCT as a predictor of postpartum pre-diabetes may be limited by the variability associated with this test. Specifically, a woman’s glycemic response to the 50g GCT has been shown to be influenced by several factors, including (i) weeks gestation at the time of testing (18), (ii) time of day of the test (19,20), and (iii) the amount of time since her last meal (21,22). As none of these data were available in this study, we cannot determine whether the predictive capacity of the GCT with respect to postpartum pre-diabetes is truly independent of these factors. Thus, further study aimed at elucidating the role of the GCT for predicting postpartum pre-diabetes will need to adjust for each of these factors (weeks gestation, time of day and the amount of time since the last meal) in order to evaluate the potential independent value of the GCT in this context.
In contrast to the metabolic findings, we did not observe evidence of adverse obstetrical outcomes in this patient population. In this regard, previous studies have reported mixed results. In some cases, abnormal GCT with NGT has been associated with adverse perinatal outcomes and LGA (23–25). Moreover, consistent with these findings, Bevier et al showed that dietary counselling with a euglycemic diet (consisting of 40% carbohydrate, 20% protein and 40% fat) and home glucose monitoring of fasting and postprandial glucose levels can reduce infant birthweight and Caesarian-section rates in this patient population (26). In contrast, other investigators have not found evidence of fetal macrosomia or adverse perinatal outcomes in women with an abnormal GCT followed by NGT (27,28), rendering the obstetrical implications of this presentation somewhat uncertain.
A limitation of the current study is that the sample size (n=259) may not have provided sufficient power to detect associations with obstetrical outcomes. Since many other factors may impact fetal growth (29,30), limitations in power could obscure an otherwise modest effect of antepartum glycemia on obstetrical outcomes. Although possibly under-powered for obstetrical associations, this report nevertheless represents the first demonstration of postpartum metabolic dysfunction in this patient population and should lead to further studies.
In summary, an abnormal GCT in pregnancy, even in the presence of normal glucose tolerance on antepartum OGTT, is associated with abnormalities in postpartum metabolic function. As such, these data raise the important possibility that pregnant women presenting in this way may represent a patient population at increased risk of developing T2DM in the future. Further study is thus warranted, as this population of young women may benefit from enhanced postpartum surveillance.
Acknowledgments
We wish to thank the Mount Sinai Hospital Department of Pathology and Laboratory Medicine and Patient Care Services. The study was supported by operating grants (MOP 67063 and 84206) from the Canadian Institutes of Health Research (CIHR). R Retnakaran is supported by a CIHR Clinical Research Initiative New Investigator Award, Canadian Diabetes Association (CDA) Clinician-Scientist incentive funding, and a University of Toronto Banting and Best Diabetes Centre New Investigator Award. AJ Hanley holds a Tier II Canada Research Chair in Diabetes Epidemiology and is supported through a CDA Scholarship. B Zinman holds the Sam and Judy Pencer Family Chair in Diabetes Research at Mount Sinai Hospital and University of Toronto.
References
- 1.Buchanan TA, Xiang AH. Gestational diabetes mellitus. Journal of Clinical Investigation. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 3.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 4.Bentley-Lewis R, Levkoff S, Stuebe A, Seely EW. Gestational diabetes mellitus: postpartum opportunities for the diagnosis and prevention of type 2 diabetes mellitus. Nature Clinical Practice Endocrinology and Metabolism. 2008;4:552–558. doi: 10.1038/ncpendmet0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358:1991–2002. [Google Scholar]
- 6.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31:2026–2031. doi: 10.2337/dc08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1-hour on oral glucose tolerance test in pregnancy resembles gestational diabetes in predicting postpartum metabolic dysfunction. Diabetes Care. 2008;31:1275–1281. doi: 10.2337/dc08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 9.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, Blondel B, Breart G Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System. A new and improved population-based Canadian reference for birthweight for gestational age. Pediatrics. 2001;108:e35–41. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 10.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. 2003 Canadian Diabetes Association Clinical Practice Guidelines. Canadian Journal of Diabetes. 2003;27 (Suppl 2):S7–S9. [Google Scholar]
- 11.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 12.Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24:1602–1607. doi: 10.2337/diacare.24.9.1602. [DOI] [PubMed] [Google Scholar]
- 13.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 14.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R, Murphy MB, Shamoon H. The Diabetes Prevention Program Research Group (2005) Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes. 54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabetic Medicine. 1995;12:931. doi: 10.1111/j.1464-5491.1995.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan TA. Pancreatic β-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2001;86:989–93. doi: 10.1210/jcem.86.3.7339. [DOI] [PubMed] [Google Scholar]
- 17.Carr DB, Newton KM, Utzschneider KM, Tong J, Gerchman F, Kahn SE, Heckbert SR. Modestly elevated glucose levels during pregnancy are associated with a higher risk of future diabetes among women without gestational diabetes mellitus. Diabetes Care. 2008;31:1037–1039. doi: 10.2337/dc07-1957. [DOI] [PubMed] [Google Scholar]
- 18.Watson WJ. Serial changes in the 50-g oral glucose test in pregnancy: implications for screening. Obstetrics and Gynecology. 1989;74:40–43. [PubMed] [Google Scholar]
- 19.McElduff A, Hitchman R. Screening for gestational diabetes: the time of day is important. Medical Journal of Australia. 2002;176:136. doi: 10.5694/j.1326-5377.2002.tb04327.x. [DOI] [PubMed] [Google Scholar]
- 20.Aparicio NJ, Joao MA, Cortelezzi M, Guz M, Sturgeon C, Galimberti DM, Fernandez CA. Pregnant women with impaired tolerance to an oral glucose load in the afternoon: evidence suggesting that they behave metabolically as patients with gestational diabetes. American Journal of Obstetrics and Gynecology. 1998;178:1059–1066. doi: 10.1016/s0002-9378(98)70548-4. [DOI] [PubMed] [Google Scholar]
- 21.Sermer M, Naylor CD, Gare DJ, Kenshole AB, Ritchie JW, Farine D, Cohen HR, McArthur K, Holzapfel S, Biringer A. Impact of time since last meal on the gestational glucose challenge test. The Toronto Tri-Hospital Gestational Diabetes Project. American Journal of Obstetrics and Gynecology. 1994;171:607–616. doi: 10.1016/0002-9378(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 22.Coustan DR, Widness JA, Carpenter MW, Rotondo L, Pratt DC, Oh W. Should the fifty-gram, one-hour plasma glucose screening test for gestational diabetes be administered in the fasting or fed state? American Journal of Obstetrics of Gynecology. 1986;154:1031–1035. doi: 10.1016/0002-9378(86)90744-1. [DOI] [PubMed] [Google Scholar]
- 23.Grotegut CA, Tatineni H, Dandolu V, Whiteman VE, Katari S, Geifman-Holtzman O. Obstetric outcomes with a false-positive one-hour glucose challenge test by the Carpenter-Coustan criteria. Journal of Maternal Fetal and Neonatal Medicine. 2008;21:315–320. doi: 10.1080/14767050801909564. [DOI] [PubMed] [Google Scholar]
- 24.Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstetrics and Gynecology. 2003;102:850–56. doi: 10.1016/s0029-7844(03)00661-6. [DOI] [PubMed] [Google Scholar]
- 25.Okun N, Verma A, Mitchell BF, Flowerdew G. Relative importance of maternal constitutional factors and glucose intolerance of pregnancy in the development of newborn macrosomia. Journal of Maternal Fetal Medicine. 1997;6:285–290. doi: 10.1002/(SICI)1520-6661(199709/10)6:5<285::AID-MFM9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Bevier WC, Fischer R, Jovanovic L. Treatment of women with an abnormal glucose challenge test (but a normal oral glucose tolerance test) decreases the prevalence of macrosomia. American Journal of Perinatology. 1999;16:269–275. doi: 10.1055/s-2007-993871. [DOI] [PubMed] [Google Scholar]
- 27.Stamilio DM, Olsen T, Ratcliffe S, Sehdev HM, Macones GA. False-positive 1-hour glucose challenge test and adverse perinatal outcomes. Obstetrics and Gynecology. 2004;103:148–156. doi: 10.1097/01.AOG.0000109220.24211.BD. [DOI] [PubMed] [Google Scholar]
- 28.Dudhbhai M, Lim L, Bombard A, Juliard K, Meenakshi B, Trachelenberg Y, Weiner Z. Characteristics of patients with abnormal glucose challenge test and normal oral glucose tolerance test results: comparison with normal and gestational diabetic patients. American Journal of Obstetrics and Gynecology. 2006;194:e42–e45. doi: 10.1016/j.ajog.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Ricart W, López J, Mozas J, Pericot A, Sancho MA, González N, Balsells M, Luna R, Cortázar A, Navarro P, Ramírez O, Flández B, Pallardo LF, Hernández-Mijas A, Ampudia J, Fernández-Real JM, Corcoy R Spanish Group for the Study of the Impact of Carpenter and Coustan GDM Thresholds. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycemia. Diabetologia. 2005;48:1736–1742. doi: 10.1007/s00125-005-1877-1. [DOI] [PubMed] [Google Scholar]
- 30.Segal P, Hamilton JK, Sermer M, Connelly PW, Hanley AJ, Zinman B, Retnakaran R. Maternal obesity and familial history of diabetes have opposing effects on infant birth weight in women with mild glucose intolerance in pregnancy. Journal of Maternal Fetal and Neonatal Medicine. 2008;21:73–79. doi: 10.1080/14767050701827148. [DOI] [PubMed] [Google Scholar]