Abstract
Stress has well-known effects on adrenal glucocorticoid secretion, and chronic elevation of glucocorticoids can have detrimental effects on the brain. Dehydroepiandrosterone (DHEA), an androgen precursor synthesized in the adrenal glands or the brain itself, has anti-glucocorticoid properties, but little is known about the role of DHEA in the stress response, particularly in the brain. Here, we measured the effects of acute restraint on circulating corticosterone (CORT) and DHEA levels in wild song sparrows. Blood was collected from either the brachial or jugular vein. In songbirds, jugular plasma is enriched with neurally synthesized steroids, and therefore, jugular plasma is an indirect index of the neural steroidal milieu. Subjects were sampled during four times of year: breeding, molt, early nonbreeding, and mid-nonbreeding. Baseline CORT and DHEA levels showed similar seasonal changes; both steroids were elevated during the breeding season. Baseline CORT and DHEA levels were similar in jugular and brachial plasma. Acute stress had robust effects on CORT and DHEA that were season specific and vein specific. For CORT, during the molt, stress increased jugular CORT more than brachial CORT. For DHEA, during the breeding season, stress decreased jugular DHEA but not brachial DHEA. During the molt, stress increased jugular DHEA but not brachial DHEA. Acute stress did not affect brachial DHEA. These data suggest that acute stress specifically affects the balance between DHEA synthesis and metabolism in the brain. Furthermore, these results suggest that CORT and DHEA are locally synthesized in the brain during molt, when systemic levels of CORT and DHEA are low.
It is well known that acute stress stimulates the hypothalamic-pituitary-adrenal (HPA) axis and results in glucocorticoid release from the adrenal glands in vertebrates. Interestingly, the HPA axis is plastic in adulthood and varies dramatically across the seasons (1–3). Seasonal variation in HPA axis function may enable animals to adjust behavior and physiology to match seasonal changes in food, weather, predators, or social organization (4).
Like glucocorticoids, dehydroepiandrosterone (DHEA) is regulated by stress. DHEA is a sex steroid precursor with no known classical intracellular steroid receptor (5, 6). In humans, DHEA and its sulfated ester, DHEA-sulfate, are the most abundant steroids secreted by the adrenal cortex. Acute stress and ACTH increase circulating DHEA levels in humans (7–9), and DHEA is more sensitive than cortisol to low doses of ACTH (10). Nonetheless, the function of DHEA in the stress response is not well understood.
Importantly, DHEA is not only produced by the adrenals but can also be synthesized by the gonads and the brain itself (11, 12). In rats and mice, plasma DHEA levels are low, and the adrenals produce little or no DHEA (5). Concentrations of DHEA are higher in the rat brain than plasma, even after adrenalectomy (13), and the brain expresses the necessary steroidogenic enzymes (14). Acute stress and ACTH treatment increase DHEA levels in the rat brain (13, 15, 16). These data suggest that DHEA, as a neurosteroid, is regulated by stress.
Recent evidence suggests a role for DHEA as an anti-glucocorticoid in the nervous system (17). For example, in humans, the ratio of cortisol to DHEA is increased in some studies of patients with stress-related psychiatric diseases, such as major depression and schizophrenia (18, 19). Furthermore, DHEA treatment to depressed patients improves mood (20, 21). In rats, DHEA is a potent anti-glucocorticoid in neuronal cultures (22) and protects against glucocorticoid-induced neuronal death in vivo (23). The mechanisms of action remain unclear because DHEA has low affinity for mineralocorticoid receptors and glucocorticoid receptors (24), but neural metabolism of DHEA to active sex steroids might be important (25).
Here, we examined the effects of acute restraint on plasma DHEA and corticosterone (CORT) levels in a songbird, the song sparrow (Melospiza melodia). We measured DHEA and CORT in different seasons, because there are large seasonal changes in both steroids (2, 26). Song sparrows, unlike rats and mice, have relatively high levels of DHEA in the plasma (26). In the nonbreeding season, DHEA levels are elevated in song sparrow adrenal glands (27), and recent evidence indicates high DHEA levels in the brain (28). A physiological dose of DHEA has robust effects on behavior and neuroanatomy in song sparrows (29). Last, DHEA metabolism to sex steroids in the songbird brain is very high (30–32). Neural metabolism of DHEA by 3β-hydroxysteroid dehydrogenase/isomerase (3β-HSD) is rapidly increased by restraint in breeding male song sparrows (33).
We measured DHEA and CORT in plasma from the brachial vein and jugular vein. Brachial plasma serves as a measure of systemic steroid levels, and jugular plasma serves as an indirect measure of the neural steroidal milieu. Importantly, in songbirds, jugular plasma is enriched with neurally synthesized steroids, such as estradiol (34). When radiolabeled androgen is administered peripherally, radiolabeled estrogens are higher in jugular plasma than carotid plasma (34–36). Recent studies suggest that the songbird brain can synthesize DHEA (37). If the brain is the main source of circulating DHEA, then jugular DHEA will be higher than brachial DHEA. During the nonbreeding season (when the gonads are regressed), the brain might be the main source of circulating DHEA (26). Thus, a difference between jugular and brachial DHEA levels could be season specific. Last, if DHEA or its metabolites have anti-glucocorticoid properties in the brain, acute restraint stress might specifically alter the balance between neural DHEA synthesis and metabolism, thereby affecting jugular DHEA levels.
Materials and Methods
Subjects
Subjects were wild adult male song sparrows (n = 107 total). Song sparrows are a useful model system, because their behavior and physiology in the wild are well characterized (38, 39). Subjects were captured near Vancouver, British Columbia (49° 12′N, 123° 01′W). This population is sedentary, and males maintain territories year-round (39, 40). In 2005, individuals were captured during four seasons: 1) breeding (May 1–25, n = 34); 2) molt (August 15 to September 8, n = 19); 3) early nonbreeding (October 10–27, n = 38); and 4) mid-nonbreeding (December 19–23, n = 16). During breeding, plasma testosterone levels are elevated, and males aggressively defend territories. During the molt, song sparrows replace their feathers, have nondetectable plasma testosterone levels, and show little territorial aggression. During the non-breeding season, the testes are regressed, plasma testosterone levels are nondetectable, but territorial aggression is expressed at high levels (41). Protocols were approved by the University of British Columbia Committee on Animal Care and complied with the guidelines of the Canadian Council of Animal Care.
Field protocol
Subjects were caught using mist-nets and less than 5 min of conspecific song playback (mean ± SEM, 2.78 ± 0.02 min). A baseline blood sample (~150 μl) was collected within 3 min of capture (mean ± SEM, 2.2 ± 0.05 min) from the brachial or jugular vein (see below). Then, subjects were restrained in an opaque cloth bag for 30 or 60 min. After restraint, another blood sample (~150 μl) was collected (from the same vein as at baseline). Importantly, separate individuals were bled after 30 min restraint and 60 min in two of four seasons (breeding and early nonbreeding seasons).
From the brachial vein, blood was collected with heparinized microhematocrit tubes after venipuncture with a sterile 26-gauge needle. From the jugular vein, blood was collected with a sterile heparinized 1-ml syringe with a fixed 28-gauge needle, as described previously (42–44). We used cotton and gentle pressure to stop the blood flow. Blood was kept on ice until returned to the laboratory (2–8 h) and centrifuged. Plasma was stored at −20 C.
We also collected the following body measurements: length (to nearest 0.1 mm) of the tarsus, wing, and cloacal protuberance (androgen-dependent secondary sex characteristic); abdominal and furcular fat scores (five-level visual fat index) (45); and body mass (to the nearest 0.1 g). Last, subjects were given a unique combination of three plastic color bands and a numbered aluminum band. Subjects were then released back onto their territory.
CORT RIA
To measure CORT, we used a double-antibody 125I RIA (ImmuChem 07-120103; MP Biomedicals, Orangeburg, NY), which was modified for songbird plasma (46). Each sample was measured in duplicate. Briefly, the manufacturer’s directions were followed, except that the volumes of all reagents were halved and plasma was diluted 1:50 (5 μl of plasma plus 245 μl of assay buffer). The CORT antibody has a low cross-reactivity with deoxycorticosterone (0.35%), testosterone (0.10%), cortisol (0.05%), androstenedione (0.03%), and DHEA (<0.01%). The intraassay variation was 3.8% (n = 12 replicates in one assay), and the interassay variation was 2.4% for the low standard and 2.1% for the high standard (n = 4 assays). The lowest point on the standard curve was 3.1 pg CORT/tube, and the detection limit for our assays with 1 μl plasma/tube was 3.1 ng/ml.
We validated this assay for song sparrow plasma. Using a plasma pool, we compared the CORT concentrations in samples assayed without a steroid extraction step and in samples assayed after a steroid extraction step. For steroid extraction, we used HPLC-grade dichloromethane (DCM) (3 ml × 2). Extracts were dried under N2 at 40 C, resuspended in 250 μl assay buffer, and stored overnight at 4 C before RIA. We also examined parallelism between the standard curve and serially diluted unextracted plasma. Parallelism between the two curves indicates that substances in the plasma do not interfere with the assay (47).
DHEA RIA
To measure DHEA, we used a double-antibody 125I RIA (DSL 8900; Diagnostic Systems Laboratories, Webster, TX). The DHEA RIA was modified to increase sensitivity (48) and has been previously used for songbird plasma (49). Each sample was measured in duplicate. The DHEA antibody has a low cross-reactivity with DHEA-sulfate (0.02%), 16β-OH DHEA (0.041%), androstenedione (0.46%), testosterone (0.028%), and CORT (<0.01%). The intraassay variation was 1.6% (n = 6 replicates in one assay), and the interassay variation was 2.0% for the low standard (25 pg/tube) and 7.0% for the high standard (100 pg/tube) (n = 4 assays). The lowest point on the standard curve was 2 pg DHEA/tube, and the detection limit for our assays with 15 μl plasma/tube was 0.13 ng/ml.
Previous data indicate that DHEA must be extracted from song sparrow plasma before RIA (50), and here we used DCM to extract DHEA (3 ml × 2), as described previously (26). To confirm that DCM extraction was effective, we examined recovery of 50 pg exogenous DHEA that was added to a pool of song sparrow plasma before extraction. DHEA was extracted from 33 μl plasma with DCM, and extracts were dried under nitrogen at 40 C. Dried extracts were resuspended in 220 μl assay buffer and stored at 4 C overnight. Furthermore, plasma was extracted with DCM, and the extracts were serially diluted and measured via RIA. Parallelism between the standard curve and serially diluted extracted plasma indicates that DCM extraction removes substances in the plasma that interfere with the assay (47). The serial dilution was also used to determine optimal plasma volume for the RIA.
Statistical analysis
To determine parallelism between an RIA standard curve and a serial dilution of plasma, we tested equality of slopes using an analysis of covariance (ANCOVA). A lack of significant interaction between the standard curve and serial dilution of plasma indicates that the lines are parallel. To calculate an index of body condition, we used the residuals from a linear regression of body mass on tarsus length (51).
To test for the effects of season and vein on baseline steroid levels, we used a two-factor ANOVA. To examine the effects of season, vein, and stress on hormone levels, we used a three-factor mixed-design ANOVA where season and vein were between-subject factors and stress was a within-subject factor (52). Where applicable, significant interactions with season were broken down using two-factor ANOVA within each season to assess the effects of vein and stress. For post hoc tests, we used Tukey’s honestly significant difference (HSD) tests. We used JMP IN 5.1 (SAS, Cary, NC). We considered test results significant for P < 0.05. Data are presented as mean ± SEM.
Results
Validation of RIAs
CORT RIA
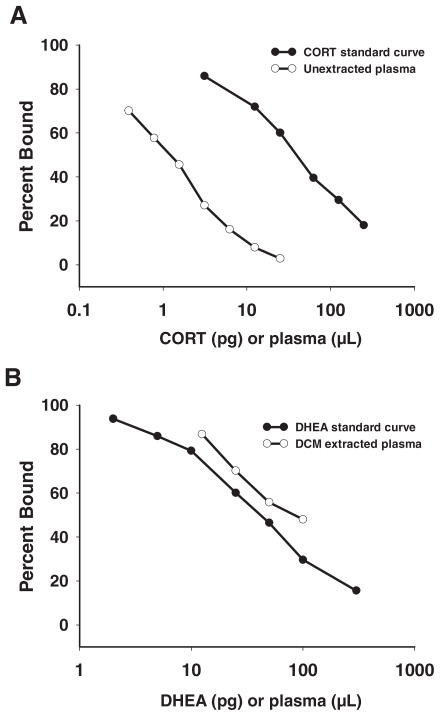
The CORT concentration measured in plasma extracted with DCM (31.34 ± 0.94 ng/ml; n = 6) was not different from the CORT concentration measured in unextracted plasma (31.56 ± 1.16 ng/ml; n = 6) (t test; t = 0.15; df = 10; P = 0.88). Furthermore, a serial dilution of unextracted plasma showed parallelism with the CORT standard curve (ANCOVA, r = 0.99, no significant interaction, F(1,12) = 1.11; P = 0.32) (Fig. 1A). Taken together, these data indicate that CORT can be measured directly in song sparrow plasma, without an extraction step, as described previously (46).
Fig. 1.
Validation of the CORT (A) and DHEA (B) RIAs. A, Serially diluted, unextracted plasma was parallel to the CORT standard curve; B, serially diluted, DCM-extracted plasma was parallel to the DHEA standard curve.
DHEA RIA
Recovery of 50 pg DHEA added to song sparrow plasma before extraction was 104.12 ± 8.6% (n = 4 pairs). Recoveries of 25- and 100-pg DHEA standards were 89.6 ± 4.5 and 91.3 ± 8.3%, respectively (n = 4 assays each). Furthermore, a serial dilution of DCM-extracted plasma was parallel to the DHEA standard curve (ANCOVA, r = 0.99, no significant interaction; F(1,10) = 2.44; P = 0.17) (Fig. 1B). Lastly, all water blanks were nondetectable (<2 pg DHEA) (n = 2 per assay, 8 total). Taken together, these data indicate that extraction of DHEA from song sparrow plasma with DCM was effective.
Baseline steroid levels
Baseline CORT
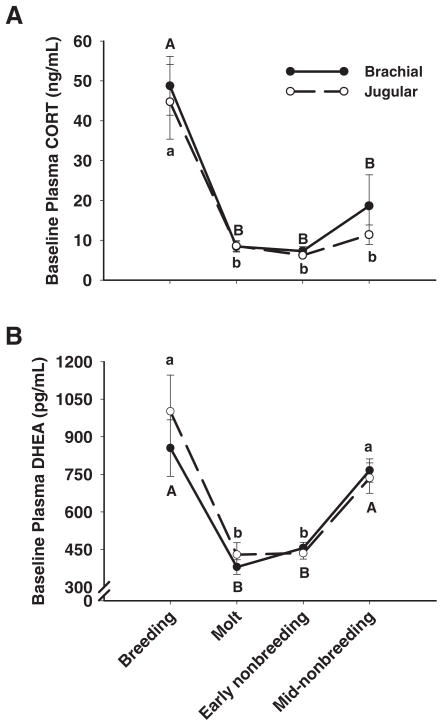
At baseline, there was a significant effect of season on plasma CORT (F(3,105) = 55.20; P < 0.0001); however, there was no main effect of vein (F(1,105) = 0.12; P = 0.73) and no interaction between season and vein (F(3,105) = 1.31; P = 0.27). Baseline plasma CORT levels were highest during the breeding season and similarly low during the other three seasons (Tukey’s HSD, P < 0.05) (Fig. 2A).
Fig. 2.
Baseline levels of plasma CORT and DHEA. Uppercase letters refer to brachial plasma and lowercase letters refer to jugular plasma. Data points that share the same letter are not significantly different. A, Baseline CORT was highest during the breeding season and lower during the molt and early and mid-nonbreeding seasons. Baseline CORT did not differ between veins in any season. B, Baseline DHEA was highest during the breeding and mid-nonbreeding seasons and lower during the molt and early nonbreeding season. Baseline DHEA did not differ between veins in any season. Data are means ± SEM.
Baseline DHEA
Similar to CORT, there was a significant main effect of season on baseline plasma DHEA (F(3,106) = 29.84; P < 0.0001) and no main effect of vein (F(1,106) = 0.08; P = 0.78) and no interaction between season and vein (F(3,106) = 0.33; P = 0.80). Baseline plasma DHEA levels were highest during the breeding season and mid-nonbreeding season, compared with the molt and early nonbreeding season (Tukey’s HSD, P < 0.05) (Fig. 2B).
Stressed steroid levels
Within a vein and season, there were no significant differences in CORT or DHEA concentrations in plasma samples collected after 30 or 60 min of restraint (Table 1). Previous studies on sparrows have also shown that CORT levels do not differ between 30 and 60 min of restraint (53, 54). Therefore, 30- and 60-min data were pooled within a vein and within a season. Moreover, separate analyses using 1) stressed steroid levels at 30 min or 2) pooled stressed steroid levels (30 and 60 min) were not different; thus, pooling the data did not alter the results.
TABLE 1.
No significant differences between 30-min restraint and 60-min restraint
| Breeding season |
Early nonbreeding season |
|||||||
|---|---|---|---|---|---|---|---|---|
| 30 min | 60 min | t | P | 30 min | 60 min | t | P | |
| Brachial | ||||||||
| Plasma CORT (ng/ml) | 199.71 ± 21.96 (8) | 146.6 ± 23.05 (8) | 1.74 | 0.11 | 39.50 ± 6.80 (9) | 52.14 ± 6.64 (9) | −1.30 | 0.21 |
| Plasma DHEA (pg/ml) | 961.82 ± 177.17 | 1138.83 ± 204.58 | −0.65 | 0.53 | 420.57 ± 48.72 | 385.89 ± 40.69 | 0.55 | 0.59 |
| Jugular | ||||||||
| Plasma CORT (ng/ml) | 161.96 ± 22.71 (8) | 160.62 ± 22.71 (10) | 0.04 | 0.97 | 38.52 ± 3.83 (10) | 35.87 ± 3.83 (10) | 0.49 | 0.63 |
| Plasma DHEA (pg/ml) | 762.26 ± 212.48 | 619.9 ± 190.05 | 0.50 | 0.62 | 394.31 ± 41.15 | 395.91 ± 36.69 | −0.03 | 0.98 |
Sample sizes for brachial and jugular plasma samples are indicated in parentheses. The 60-min restraint was conducted only during the breeding season and early nonbreeding season.
Stressed CORT
To examine the effects of season, vein, and stress on plasma CORT, we used a three-factor mixed-design ANOVA. The main effects of season, vein, and stress were significant (Table 2). Also, the interaction between season and stress was significant, and thus, this interaction was broken down using a two-factor ANOVA within each season to examine the main effects of vein and stress on plasma CORT.
TABLE 2.
Effect of season, vein, and stress on plasma CORT and DHEA
| Variable | Three-way mixed-design ANOVA |
|||||
|---|---|---|---|---|---|---|
| CORT |
DHEA |
|||||
| (degrees of freedom) | F-ratio | P | (degrees of freedom) | F-ratio | P | |
| Season | 3, 105 | 131.72 | <0.0001 | 3, 106 | 41.32 | <0.0001 |
| Vein | 1, 105 | 4.58 | 0.03 | 1, 106 | 0.07 | 0.67 |
| Season × vein | 3, 105 | 0.78 | 0.51 | 3, 106 | 4.93 | 0.003 |
| Stress | 1, 105 | 420.94 | <0.0001 | 1, 106 | 1.22 | 0.27 |
| Season × stress | 3, 105 | 5.06 | 0.0002 | 3, 106 | 1.58 | 0.20 |
| Vein × stress | 1, 105 | 0.01 | 0.91 | 1, 106 | 1.59 | 0.21 |
| Season × vein × stress | 3, 105 | 1.55 | 0.20 | 3, 106 | 14.01 | <0.0001 |
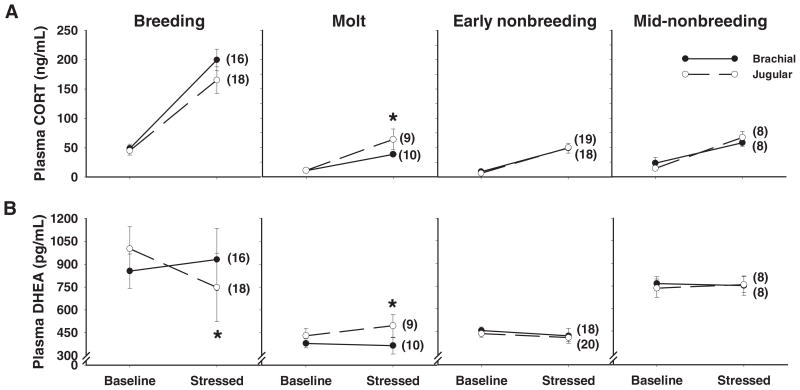
Within all seasons, there was a significant main effect of stress (Fig. 3A and Table 3). Interestingly, only during the molt was there a significant main effect of vein and a significant interaction between vein and stress (Fig. 3A and Table 3). During molt, jugular CORT levels were significantly higher than brachial CORT levels after stress (Tukey’s HSD, P < 0.05) (Fig. 3A).
Fig. 3.
Effects of acute restraint stress on plasma CORT and DHEA levels. A, In all four seasons, stress increased plasma CORT in the brachial and jugular veins. Importantly, during the molt, stress increased CORT significantly more in the jugular vein. B, Stress decreased jugular DHEA during the breeding season and increased jugular DHEA during the molt. Stress did not affect brachial DHEA in any season. Asterisks indicate a significant vein × stress interaction in a two-factor ANOVA (Table 3). Data are means ± SEM, and numbers in parentheses indicate sample sizes.
TABLE 3.
Effects of vein and stress on plasma CORT and DHEA within each season
| Season and variable | Two-way mixed-design ANOVA |
|||||
|---|---|---|---|---|---|---|
| CORT |
DHEA |
|||||
| df | F-ratio | P | df | F-ratio | P | |
| Breeding | ||||||
| Vein | 1, 33 | 0.58 | 0.45 | 1, 34 | 4.46 | 0.04 |
| Stress | 1, 33 | 100.42 | <0.0001 | 1, 34 | 3.59 | 0.07 |
| Vein × stress | 1, 33 | 0.39 | 0.54 | 1, 34 | 22.10 | <0.0001 |
| Molt | ||||||
| Vein | 1, 18 | 7.95 | 0.01 | 1, 18 | 0.70 | 0.41 |
| Stress | 1, 18 | 207.81 | <0.0001 | 1, 18 | 0.33 | 0.57 |
| Vein × stress | 1, 18 | 4.81 | 0.04 | 1, 18 | 21.38 | 0.0002 |
| Early nonbreeding | ||||||
| Vein | 1, 37 | 0.14 | 0.71 | 1, 37 | 0.99 | 0.33 |
| Stress | 1, 37 | 391.04 | <0.0001 | 1, 37 | 2.65 | 0.11 |
| Vein × stress | 1, 37 | 1.09 | 0.40 | 1, 37 | 0.03 | 0.87 |
| Mid-nonbreeding | ||||||
| Vein | 1, 15 | 1.21 | 0.29 | 1, 15 | 0.28 | 0.60 |
| Stress | 1, 15 | 83.72 | <0.0001 | 1, 15 | 0.03 | 0.87 |
| Vein × stress | 1, 15 | 0.11 | 0.74 | 1, 15 | 0.02 | 0.88 |
Stressed DHEA
To examine the effects of season, vein, and stress on plasma DHEA, we used a three-factor mixed-design ANOVA. There was a significant main effect of season; however, the main effects of vein and stress were not significant (Table 2). The interaction between season and vein was significant, as was the three-way interaction of season × vein × stress (Table 2). Because the three-way interaction was significant, we used a two-factor ANOVA within each season to examine the effects of vein and stress on plasma DHEA.
During the breeding season, the main effect of vein was significant, the main effect of stress approached significance, and the interaction between vein and stress was significant (Table 3). Stressed jugular DHEA was significantly lower than baseline jugular DHEA (Tukey’s HSD, P < 0.05), but stressed brachial DHEA was not different from baseline brachial DHEA (Tukey’s HSD, P > 0.05). These results indicate that stress decreased jugular DHEA but did not affect brachial DHEA during the breeding season (Fig. 3B and Table 3).
During the molt, the main effects of vein and stress were not significant, but the interaction between vein and stress was significant (Table 3). Stressed jugular DHEA was significantly higher than baseline jugular DHEA (Tukey’s HSD, P < 0.05). Stressed jugular DHEA was also significantly higher than baseline and stressed brachial DHEA (Tukey’s HSD, P < 0.05). Stressed brachial DHEA was not different from baseline brachial DHEA (Tukey’s HSD, P > 0.05). These results indicate that stress increased jugular DHEA but did not affect brachial DHEA during the molt (Fig. 3B and Table 3).
During the early and mid-nonbreeding seasons, neither the main effects of vein and stress nor the interaction between vein and stress were significant (Fig. 3B and Table 3).
Relationship between CORT and DHEA
Baseline levels of CORT and DHEA were positively correlated across seasons [r = 0.47; n = 100, P < 0.0001]. However, within each season, there was not a significant correlation between baseline levels of CORT and DHEA.
Stressed levels of CORT and DHEA were also positively correlated across seasons [(r = 0.36; n = 101, P = 0.0003]. However, within each season, there was not a significant correlation between stressed levels of CORT and DHEA.
The CORT/DHEA ratios at baseline and after acute restraint were significantly higher during the breeding season in brachial and jugular plasma (Table 4).
TABLE 4.
CORT/DHEA ratio at baseline and after acute restraint stress
| Breeding (n = 16, 18) | Molt (n = 10, 8) | Early nonbreeding (n = 18, 18) | Mid-nonbreeding (n = 8, 8) | F-ratio | P | |
|---|---|---|---|---|---|---|
| Brachial | ||||||
| Baseline | 1.58 ± 0.04a | 1.45 ± 0.04b | 1.40 ± 0.02b | 1.46 ± 0.03b | 13.52 | <0.0001 |
| Stressed | 1.87 ± 0.03a | 1.73 ± 0.02b | 1.78 ± 0.03b | 1.73 ± 0.02b | 10.87 | <0.0002 |
| Jugular | ||||||
| Baseline | 1.62 ± 0.02a | 1.45 ± 0.4b | 1.45 ± 0.02b | 1.49 ± 0.04b | 14.43 | <0.0001 |
| Stressed | 1.95 ± 0.03a | 1.77 ± 0.04b | 1.76 ± 0.01b | 1.75 ± 0.03b | 12.63 | <0.0001 |
Numbers in parentheses indicate sample sizes for brachial and jugular plasma, respectively.
Within a row, values that share the same letter are not significantly different.
Body condition
Body condition was calculated using the residuals from a regression of body mass on tarsus length. Body condition changed significantly with season, and subjects in the mid-nonbreeding season had the highest body condition (Table 5). Baseline CORT levels were not correlated with body condition in any season; however, stressed CORT levels were negatively correlated with body condition in the breeding season [r = −0.39; n = 32, P = 0.05]. In contrast, baseline DHEA levels were positively correlated with body condition in the breeding season [r = 0.45; n = 32, P = 0.02].
TABLE 5.
Seasonal changes in body condition, fat and cloacal protuberance length.
| Breeding (n = 34) | Molt (n = 19) | Early nonbreeding (n = 38) | Mid-nonbreeding (n = 16) | F-ratio | P | |
|---|---|---|---|---|---|---|
| Body condition | −0.33 ± 0.23a,b | 0.44 ± 0.23a | −0.73 ± 0.18b | 2.26 ± 0.36c | 24.70 | <0.0001 |
| Fat score | 0.42 ± 0.06a | 0.69 ± 0.07b | 0.55 ± 0.06a,b | 1.97 ± 0.46c | 38.48 | <0.0001 |
| Cloacal protuberance (mm) | 9.50 ± 0.15a | 5.23 ± 0.18b | 4.46 ± 0.08c | 3.32 ± 0.16d | 353.55 | <0.0001 |
Within a row, values that share the same letter are not significantly different.
Fat score also changed significantly with season, with fat stores being much greater during the mid-nonbreeding season (Table 5).
The androgen-dependent cloacal protuberance length changed significantly with season (Table 5). The cloacal protuberance was largest during the breeding season and decreased progressively in the molt, early nonbreeding season, and mid-nonbreeding season (Table 5). A small cloacal protuberance indicates low circulating testosterone levels (55, 56).
Discussion
Plasma CORT and DHEA changed dramatically over the seasons. Both steroids were regulated by acute restraint stress; however, the effect of stress was dependent on season and vein. Stress decreased jugular DHEA during breeding, whereas stress increased jugular DHEA during molt. Stress did not affect brachial DHEA in any season. Across seasons, stress increased CORT in both veins. However, during molt, stress increased jugular CORT significantly more than brachial CORT. Previous songbird studies have shown differences between jugular and systemic plasma levels of neurally synthesized radiolabeled estrogens (34–36), but this study is the first to suggest differences between jugular and systemic levels of endogenous steroids in adults. Moreover, this is the first study to examine acute regulation of DHEA in multiple seasons.
Baseline steroid levels
At baseline, brachial CORT did not differ from jugular CORT in any season. Baseline CORT levels were significantly higher during the breeding season than during the rest of the year, and this robust seasonal pattern is consistent with previous studies in songbirds (2). Seasonal modulation of plasma CORT levels can occur due to changes at the level of the hypothalamus, the pituitary, or the adrenals (1). Elevated baseline CORT in breeding males may facilitate energy mobilization to meet the demands of feeding and raising young, which is energetically demanding in this biparental species (2, 57).
Baseline levels of brachial DHEA were significantly higher during the breeding and mid-nonbreeding seasons, consistent with previous data in song sparrows (26). During the breeding season, the testes are recrudesced and secrete high levels of androgens. During the nonbreeding season, the testes are fully regressed and circulating testosterone is non-detectable (41). Importantly, at baseline, jugular DHEA did not differ from brachial DHEA at any time of year. Thus, these data do not support the hypothesis that the brain is the main source of circulating DHEA in the nonbreeding season. Nonetheless, it remains possible that DHEA is synthesized in the brain (58) and acts locally, without being secreted into the jugular. Future studies will measure DHEA directly in specific brain regions. Baseline CORT and DHEA in both veins were positively correlated across all seasons. The striking similarity in the pattern of seasonal change suggests that both CORT and DHEA may be of adrenal origin.
Chronic elevations in CORT have detrimental effects on brain and immune function (59). DHEA is a potent anti-glucocorticoid, and thus, an elevation in DHEA during the breeding season could mitigate the detrimental effects of CORT. For example, DHEA is a potent anti-glucocorticoid in the brain and diminishes the neurodegenerative effects of CORT in the rodent brain (17, 23). In mice, DHEA also ameliorates the immunosuppressive effects of CORT (60). During the breeding season (when CORT is high), elevated DHEA may play a neuroprotective or immune-enhancing role. Interestingly, baseline DHEA was positively correlated with body condition during the breeding season, further suggesting a beneficial effect of high DHEA.
The CORT/DHEA ratio was highest during the breeding season. An elevated CORT/DHEA ratio supports the idea that the breeding season is a particularly demanding period. The CORT/DHEA ratio is also increased in some studies of patients with major depression and schizophrenia (18, 19). Further studies are needed to clarify the significance of the CORT/DHEA ratio in songbirds.
Effects of acute restraint on plasma CORT
Restraint stress increased brachial and jugular CORT levels in every season, but the magnitude of the increase showed large seasonal variation, consistent with previous studies (2). This is the first study to examine seasonal changes in jugular CORT levels. In both veins, the CORT response to stress was significantly higher during the breeding season and similar in the other seasons. Increased CORT responsiveness during the breeding season may prepare an individual for a subsequent stressor (4). Thus, stressed CORT should be maximal when the probability of encountering a stressor (e.g. predator, parasite, or competitor for mates) is greatest, and for many species, this period is the breeding season (2).
Specifically during the molt, the increase in jugular CORT was significantly greater than the increase in brachial CORT, even though baseline levels were similar in the two veins. The molt is the annual replacement of feathers after the breeding season (61). Previous studies have shown that baseline CORT and stressed CORT levels are dramatically reduced during molt (62). Furthermore, CORT treatment slows feather growth in molting birds (61). During molt, the decrease in systemic CORT levels may facilitate protein deposition during feather growth and avoid the catabolic actions of CORT (61).
The present data raise the intriguing hypothesis that during molt, when adrenal synthesis of CORT is down-regulated, neural synthesis of CORT is up-regulated. There is some evidence that the brain has the capacity to synthesize CORT in mammals (63–66), and future studies will measure CORT directly in brain tissue. Increased local synthesis of CORT in the brain during molt might allow for behavioral responses to stressors (e.g. predators) while avoiding the costs of high circulating CORT on feather growth. Similar mechanisms may operate during the nonbreeding season to avoid the costs of high circulating testosterone (67). An alternative hypothesis is that circulating CORT is sequestered in the brain during molt and released in response to stress, but at present, there is little evidence for this hypothesis.
Effects of acute restraint on plasma DHEA
Acute restraint stress for 30 or 60 min did not affect brachial DHEA levels in any season. These data are consistent with a previous study that used a 30-min restraint (26). Thus, even though CORT and DHEA levels are positively correlated across the seasons, suggesting similar long-term regulation, short-term regulation by stress is quite different for these two steroids. In humans, both cortisol and DHEA are synthesized in the adrenal cortex and are acutely regulated by stress and ACTH (7–9, 68). In song sparrows, during the nonbreeding season, DHEA concentrations in the adrenals are nearly 10 times higher than in plasma (26, 28). Nonetheless, acute stress has no effect on systemic DHEA levels. Similarly, the bovine adrenal synthesizes DHEA (69), but short-term ACTH treatment has no effect on systemic DHEA levels (70). In contrast, long-term ACTH treatment increases circulating DHEA levels in cows (70). If ACTH has chronic, but not acute, effects on adrenal DHEA synthesis in song sparrows, that might explain why seasonal changes in systemic CORT and DHEA are positively correlated but the effects of acute stress differ.
Unlike brachial DHEA, jugular DHEA was significantly affected by acute stress in a season-specific manner. During the breeding season, stress significantly decreased jugular DHEA levels. A decrease in jugular DHEA suggests a decrease in neural DHEA synthesis or an increase in neural DHEA metabolism. In captive breeding male song sparrows, acute restraint increases the activity of brain 3β-HSD, the enzyme that metabolizes DHEA to androstenedione (33). These data are consistent with stress increasing neural DHEA metabolism in the breeding season.
During the breeding season, there was greater individual variation in both brachial and jugular DHEA. This individual variability could be the result of differences in breeding sub-stage. Within the breeding season, hormone levels fluctuate according to substage (e.g. nest building, egg laying, incubation, feeding chicks, and re-nesting) and also decline from the first brood to the second brood (41). This variability may have affected our ability to assess the effect of vein between individuals. However, the effect of stress was assessed within individuals, which controlled for individual differences.
In contrast to the breeding season, stress increased jugular DHEA levels during the molt. An increase in jugular DHEA suggests either an increase in neural DHEA synthesis or a decrease in neural DHEA metabolism. Molt is the only season when jugular DHEA increased in response to stress, and this coincides with the data on jugular CORT during molt. During molt, when systemic levels of CORT and DHEA are low, it is possible that the down-regulation of systemic steroid signals is accompanied by an up-regulation of local steroid production. The balance between systemic and local steroid signaling mechanisms remains enigmatic (71) but has important implications for patients with adrenal insufficiency and Addison’s disease (72) and also for the stress-hyporesponsive period during development (73).
Conclusions
The present results indicate pronounced season-dependent effects of acute restraint stress on CORT and DHEA levels. Furthermore, the effects depend on whether the steroids are measured in brachial or jugular plasma. The positive correlation between baseline CORT and DHEA levels across seasons suggests that CORT and DHEA are regulated similarly in the long term. However, systemic CORT but not systemic DHEA is regulated by stress in the short term. Importantly, the effects of acute stress on jugular DHEA suggest a role for DHEA in the brain. Future studies shall focus on CORT and DHEA levels in specific brain regions and the effects of natural chronic stress (74). Lastly, these data highlight the importance of the blood sampling site. In endocrine studies, blood is collected from a variety of sites, including the brachial vein, jugular vein, tail vein, saphenous vein, and retroorbital sinus. It is possible that blood samples from these different sites have different steroid profiles.
Acknowledgments
We thank David Hope for help in the field, Kim Schmidt and Dr. Scott MacDougall-Shackleton for useful comments on the manuscript, and Dr. Joanne Weinberg and Wayne Yu for equipment. We are especially grateful to Dr. Ryan Norris for his insight during manuscript development and to Drs. Beren Robinson and Andreas Klein for statistical guidance.
This research was supported by grants from the Natural Sciences and Engineering Council of Canada (NSERC), Canadian Institutes of Health Research, Canada Foundation for Innovation, and the Michael Smith Foundation for Health Research (MSFHR) to K.K.S. and by NSERC and MSFHR to A.E.M.N.
Abbreviations
- ANCOVA
Analysis of covariance
- CORT
corticosterone
- DCM
dichloromethane
- DHEA
dehydroepiandrosterone
- HPA
hypothalamic-pituitary-adrenal
- HSD
honestly significant difference
- 3β-HSD
3β-hydroxysteroid dehydrogenase/isomerase
Footnotes
Disclosure Statement: The authors have nothing to declare.
References
- 1.Romero LM, Soma KK, Wingfield JC. Hypothalamic-pituitary-adrenal axis changes allow seasonal modulation of corticosterone in a bird. Am J Physiol. 1998;274:R1338–R1344. doi: 10.1152/ajpregu.1998.274.5.R1338. [DOI] [PubMed] [Google Scholar]
- 2.Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 3.Pyter LM, Adelson JD, Nelson RJ. Short days increase hypothalamic-pituitary-adrenal axis responsiveness. Endocrinology. 2007;148:3402–3409. doi: 10.1210/en.2006-1432. [DOI] [PubMed] [Google Scholar]
- 4.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 5.Labrie F, Luu-The V, Belanger A, Lin S, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 6.Widstrom RL, Dillon JS. Is there a receptor for dehydroepiandrosterone or dehydroepiandrosterone sulfate? Semin Reprod Med. 2004:289–298. doi: 10.1055/s-2004-861546. [DOI] [PubMed] [Google Scholar]
- 7.Oberbeck R, Benschop RJ, Jacobs R, Hosch W, Jetschmann JU, Schurmeyer TH, Schmidt RE, Schedlowski M. Endocrine mechanisms of stress-induced DHEA-secretion. J Endocrinol Invest. 1998;21:148–153. doi: 10.1007/BF03347293. [DOI] [PubMed] [Google Scholar]
- 8.Kroboth P, Salek F, Pittenger A, Fabian T, Frye R. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- 9.Salek F, Bigos K, Kroboth P. The influence of hormones and pharmaceutical agents on DHEA and DHEA-S concentrations: a review of clinical studies. J Clin Pharmacol. 2002;42:247–266. doi: 10.1177/00912700222011274. [DOI] [PubMed] [Google Scholar]
- 10.Arvat E, DiVito L, Lanfranco F, Maccario M, Baffoni C, Rossetto R, Aimaretti G, Camanni F, Ghigo E. Stimulatory effect of adrenocorticotropin on cortisol, aldosterone, and dehydroepiandrosterone secretion in normal humans: dose-response study. J Clin Endocrinol Metab. 2000;85:3141–3146. doi: 10.1210/jcem.85.9.6784. [DOI] [PubMed] [Google Scholar]
- 11.Robel P, Baulier EE. Dehydroepiandrosterone (DHEA) is a neuroactive neurosteroid. Ann NY Acad Sci. 1995;774:82–110. doi: 10.1111/j.1749-6632.1995.tb17374.x. [DOI] [PubMed] [Google Scholar]
- 12.Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 13.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu E. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mensah-Nyagan AG, Do-Rego J, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev. 1999;51:63–82. [PubMed] [Google Scholar]
- 15.Torres JM, Ortega E. DHEA, PREG and their sulphate derivatives on plasma and brain after CRH and ACTH administration. Neurochem Res. 2003;28:1187–1191. doi: 10.1023/a:1024276328127. [DOI] [PubMed] [Google Scholar]
- 16.Torres JM, Ruiz E, Ortega E. Effects of CRH and ACTH administration on plasma and brain neurosteroid levels. Neurochem Res. 2001;26:555–558. doi: 10.1023/a:1010925331768. [DOI] [PubMed] [Google Scholar]
- 17.Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA) Mol Cell Biochem. 1994;131:99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- 18.Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am J Psychiatry. 2002;159:1237–1239. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]
- 19.Ritsner M, Maayan R, Gibel A, Strous RD, Modai I, Weizman A. Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients. Eur Neuropsychopharmacol. 2004;14:267–273. doi: 10.1016/j.euroneuro.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Wolkowitz OM, Reus VI, Roberts E, Manfredi F, Chan T, Raum WJ, Ormiston S, Johnson R, Canick J, Brizendine L, Weingartner H. Dehydroepiandrosterone (DHEA) treatment of depression. Biol Psychiatry. 1997;41:311–318. doi: 10.1016/s0006-3223(96)00043-1. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, Simpson-St Clair L, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- 22.Kimonides VG, Spillantini MG, Sofroniew MV, Fawcett JW, Herbert J. Dehydroepiandrosterone antagonizes the neurotoxic effects of corticosterone and translocation of stress-activated protein kinase 3 in hippocampal primary cultures. Neuroscience. 1999;89:429–436. doi: 10.1016/s0306-4522(98)00347-9. [DOI] [PubMed] [Google Scholar]
- 23.Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, Moreno C, Schmidt A, Harada S, Freedman L, Reszka A. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005;146:4568–4576. doi: 10.1210/en.2005-0368. [DOI] [PubMed] [Google Scholar]
- 25.Hajszan T, MacLusky NJ, Leranth C. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- 26.Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol. 2001;123:144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- 27.Soma KK. Testosterone and aggression: Berthold, birds and beyond. J Neuroendocrinol. 2006;18:543–551. doi: 10.1111/j.1365-2826.2006.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman AE, Soma KK. Effect of aggressive encounters on steroid levels in plasma and brain. Proc Society of Behavioral Neuroendocrinology Meeting; June 21–24, 2007; Pacific Grove, CA. (Poster No. 3.27) [Google Scholar]
- 29.Soma KK, Wissman AM, Brenowitz EA, Wingfield JC. Dehydroepiandrosterone (DHEA) increases territorial song and the size of an associated brain region in a male songbird. Horm Behav. 2002;41:203–212. doi: 10.1006/hbeh.2001.1750. [DOI] [PubMed] [Google Scholar]
- 30.Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- 31.Schlinger BA, Pradhan DS, Soma KK. 3β-HSD activates DHEA in the songbird brain. Neurochem Int. 2008;52:611–620. doi: 10.1016/j.neuint.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of DHEA metabolism in the male and female songbird brain. J Neurochem. 2008;104:244–253. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soma KK, Alday NA, Schlinger BA. Program No. 189 1 2002 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2002. 3β-HSD and aromatase in songbird brain: DHEA metabolism, aggression and song. Online. [Google Scholar]
- 34.Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci USA. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlinger BA, Arnold AP. Estrogen synthesis in vivo in the adult zebra finch: additional evidence that circulating estrogens can originate in brain. Endocrinology. 1993;133:2610–2616. doi: 10.1210/endo.133.6.8243284. [DOI] [PubMed] [Google Scholar]
- 36.Saldanha CJ, Schlinger BA. Estrogen synthesis and secretion in the brown-Headed cowbird (Molothrus ater) Gen Comp Endocrinol. 1997;105:390–401. doi: 10.1006/gcen.1996.6841. [DOI] [PubMed] [Google Scholar]
- 37.London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nice MM. Studies in the life history of the song sparrow. I. A population study of the song sparrow. Trans Linn Soc NY. 1937;4:1–247. [Google Scholar]
- 39.Wingfield JC. Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Horm Behav. 1994;28:1–15. doi: 10.1006/hbeh.1994.1001. [DOI] [PubMed] [Google Scholar]
- 40.Arcese P, Sogge MK, Marr AB, Patten MA. Song sparrow (Melospiza melodia) In: Poole A, editor. The birds of North America. Ithaca, NY: Cornell Lab of Ornithology; 2002. retrieved from http://bna.birds.cornell.edu/bna/species/704. [Google Scholar]
- 41.Wingfield JC, Hahn TP. Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim Behav. 1994;47:77–89. [Google Scholar]
- 42.Hoysak DJ, Weatherhead PJ. Sampling blood from birds: A technique and an assessment of its effect. The Condor. 1991;93:746–752. [Google Scholar]
- 43.Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm Behav. 2004;45:225–234. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Sheldon LD, Chin EH, Gill SA, Schmaltz G, Newman AEM, Soma KK. Effects of blood collection on wild birds: an update. J Avian Biol. in press. [Google Scholar]
- 45.Helms CW, Drury WH., Jr Winter and migratory weight and fat. Field studies on some North American buntings. Bird Banding. 1960;31:1–40. [Google Scholar]
- 46.Washburn BE, Morris DL, Millspaugh JJ, Faaborg J, Schulz JH. Using a commercially available radioimmunoassay to quantify corticosterone in avian plasma. Condor. 2002;104:558–563. [Google Scholar]
- 47.Chard T. An introduction to radioimmunoassay and related techniques. New York: Elsevier; 1995. p. 316. [Google Scholar]
- 48.Granger DA, Schwartz EB, Booth A, Curran M, Zakaria D. Assessing dehydroepiandrosterone in saliva: a simple radioimmunoassay for use in studies of children, adolescents and adults. Psychoneuroendocrinology. 1999;24:567–579. doi: 10.1016/s0306-4530(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 49.Goodson JL, Evans AK, Soma KK. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport. 2005;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman AE, Chin EH, Schmidt KL, Bond L, Wynne-Edwards KE, Soma KK. Analysis of steroids in songbird plasma and brain by coupling solid-phase extraction to radioimmunoassay. Gen Comp Endocrinol. 2008;155:503–510. doi: 10.1016/j.ygcen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. Restitution of mass-size residuals: validating body condition indices. Ecology. 2005;86:155–163. [Google Scholar]
- 52.Zar JH. Biostatistical analysis. Englewood Cliffs, NJ: Prentice Hall; 1999. p. 663. [Google Scholar]
- 53.Breuner CW, Orchinik M. Seasonal regulation of membrane and intracellular corticosteroid receptors in the house sparrow brain. J Neuroendocrinol. 2001;13:412–420. doi: 10.1046/j.1365-2826.2001.00646.x. [DOI] [PubMed] [Google Scholar]
- 54.Romero LM, Romero RC. Corticosterone responses in wild birds: the importance of rapid initial sampling. Condor. 2002;104:129–135. [Google Scholar]
- 55.Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5α-reductase, and 5β-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- 56.Soma KK, Tramontin AD, Featherstone J, Brenowitz EA. Estrogen contributes to seasonal plasticity of the adult avian song control system. J Neurobiol. 2004;58:413–422. doi: 10.1002/neu.10288. [DOI] [PubMed] [Google Scholar]
- 57.Hambly C, Markman S, Roxburgh L, Pinshow B. Seasonal sex-specific energy expenditure in breeding and non-breeding Palestine sunbirds Nectarinia osea. J Avian Biol. 2007;38:190–197. [Google Scholar]
- 58.Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neuroscience Res. 2000;36:261–273. doi: 10.1016/s0168-0102(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 59.Vanitallie TB. Stress: a risk factor for serious illness. Metabolism. 2002;51:S40–S45. doi: 10.1053/meta.2002.33191. [DOI] [PubMed] [Google Scholar]
- 60.Blauer K, Poth M, Rogers W, Bernton E. Dehydroepiandrosterone antagonizes the suppressive effects of dexamethasone on lymphocyte proliferation. Endocrinology. 1991;129:3174–3179. doi: 10.1210/endo-129-6-3174. [DOI] [PubMed] [Google Scholar]
- 61.Romero LM, Strochlic D, Wingfield JC. Corticosterone inhibits feather growth: potential mechanism explaining seasonal down regulation of corticosterone during molt. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:65–73. doi: 10.1016/j.cbpa.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Romero LM. Seasonal changes in hypothalamic-pituitary-adrenal axis sensitivity in free-living house sparrows (Passer domesticus) Gen Comp Endocrinol. 2006;149:66–71. doi: 10.1016/j.ygcen.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 63.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 64.Davies E, MacKenzie SM. Extra-adrenal production of corticosteroids. Clin Exp Pharmacol Physiol. 2003;30:437–445. doi: 10.1046/j.1440-1681.2003.03867.x. [DOI] [PubMed] [Google Scholar]
- 65.Stoeffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann NY Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288:E342–E346. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- 67.Wingfield JC, Lynn SE, Soma KK. Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evol. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- 68.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Ogo A, Haji M, Ohashi M, Nawata H. Decreased expression of cytochrome P450 17α-hydroxylase mRNA in senescent bovine adrenal gland. Gerontology. 1991;37:262–271. doi: 10.1159/000213270. [DOI] [PubMed] [Google Scholar]
- 70.Marinelli L, Trevisi E, DaDalt L, Merlo M, Bertoni G, Gabai G. Dehydroepiandrosterone secretion in dairy cattle is episodic and unaffected by ACTH stimulation. J Endocrinol. 2007;194:627–635. doi: 10.1677/JOE-07-0226. [DOI] [PubMed] [Google Scholar]
- 71.Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3β-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003;361:1881–1893. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 73.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 74.Clinchy M, Zanette L, Boonstra R, Wingfield JC, Smith JNM. Balancing food and predator pressure induces chronic stress in songbirds. Proc Biol Sci. 2004;271:2473–2479. doi: 10.1098/rspb.2004.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]