Abstract
Objective
Pre-gravid physical activity has been associated with a reduced risk of gestational diabetes mellitus (GDM), although neither the types of exercise nor the physiologic mechanisms underlying this protective effect have been well studied. Thus, we sought to study the relationships between types of pre-gravid physical activity and metabolic parameters in pregnancy, including glucose tolerance, insulin sensitivity and beta-cell function.
Design/Patients/Measurements
851 women underwent a glucose challenge test (GCT) and a 3-hr oral glucose tolerance test (OGTT) in late pregnancy, yielding 4 glucose tolerance groups: (i) GDM; (ii) gestational impaired glucose tolerance (GIGT); (iii) abnormal GCT with normal glucose tolerance on OGTT (abnormal GCT NGT); and (iv) normal GCT with NGT on OGTT (normal GCT NGT). Pre-gravid physical activity was assessed using the Baecke questionnaire, which measures (i) total physical activity and (ii) its 3 component domains: work, non-sport leisure-time, and vigorous/sports activity.
Results
Glucose tolerance status improved across increasing quartiles of pre-gravid total physical activity (p=0.0244). Whereas neither work nor non-sport leisure-time activity differed between glucose tolerance groups, pre-gravid vigorous/sports activity was significantly higher in women with normal GCT NGT compared to women with (i) abnormal GCT NGT (p=0.0018), (ii) GIGT (p=0.0025), and (iii) GDM (p=0.0044). In particular, vigorous/sports activity correlated with insulin sensitivity (measured by ISOGTT) (r=0.21,p<0.0001). Furthermore, on multiple linear regression analysis, pre-gravid vigorous/sports activity emerged as a significant independent predictor of ISOGTT in pregnancy (t=4.97,p<0.0001).
Conclusions
Pre-gravid vigorous/sports activity is associated with a reduced risk of glucose intolerance in pregnancy, an effect likely mediated by enhanced insulin sensitivity.
Keywords: Physical Activity, Pregnancy, Gestational Diabetes, Insulin Sensitivity
INTRODUCTION
The diagnosis of gestational diabetes mellitus (GDM) frequently foreshadows the future development of type 2 diabetes (T2DM) in the years following the index pregnancy (1–3). This relationship likely reflects the pathophysiologic similarity between GDM and T2DM, insofar as both conditions are characterized by 2 main metabolic defects: (i) insulin resistance and (ii) pancreatic beta-cell dysfunction. Although the mechanistic basis of these defects has not been fully elucidated, it is now recognized that both GDM and T2DM may result from the pathologic interaction between underlying genetic susceptibility and acquired lifestyle factors, including dietary habits and physical inactivity (4–7). As such, there is currently considerable interest in the role of modification of diet and patterns of living as a means of attenuating inherited metabolic risk and thereby preventing the development of GDM and/or T2DM. Furthermore, in light of a recent meta-analysis that suggested that up to one third of parous women with T2DM may have a history of previous GDM (8), it follows that the prevention of GDM (through lifestyle modification) may be a particularly effective approach to combating the diabetes epidemic.
The role of diet in preventing GDM remains unclear, as studies to date have found little consistent association between nutritional factors and gestational glucose intolerance (6,9–12). In contrast, however, a series of recent reports have linked pre-gravid physical activity with a reduced risk of GDM (6,13–17). Although these reports have established this important association, it should be noted that neither the types of exercise nor the physiologic mechanisms underlying this protective effect have been well studied. An enhanced understanding of these issues potentially could inform the development of effective prevention strategies. In this context, we hypothesized that pre-gravid vigorous/sport activity may be particularly beneficial in relation to antepartum glucose homeostasis. Thus, in the current study, our objectives were to elucidate the relationships between specific domains of pre-gravid physical activity and metabolic parameters in pregnancy, including glucose tolerance, insulin sensitivity and beta-cell function.
RESEARCH DESIGN AND METHODS
This analysis was conducted in the setting of an ongoing observational study of early events in the natural history of T2DM, in which participating women recruited at the time of antepartum GDM screening undergo longitudinal metabolic characterization in pregnancy and the postpartum period (18,19). Standard obstetrical practice at our institution involves universal screening for GDM in all pregnant women at 24–28 weeks’ gestation by 50g glucose challenge test (GCT) followed by, if the GCT is abnormal, referral for a diagnostic OGTT. In the study, healthy pregnant women are recruited either prior to or just after their GCT. Regardless of the GCT result, all study participants then undergo a 3-hour 100g OGTT for determination of glucose tolerance status. All participants have provided written informed consent and the study protocol has been approved by the Mount Sinai Hospital Research Ethics Board. The current analysis is a cross-sectional evaluation of the study population at the time of the OGTT in pregnancy.
Evaluation of Study Participants in Pregnancy
On the morning of the OGTT, anthropometric measurements of height (to nearest 0.5 cm) and weight (to nearest 0.1 kg) were obtained using a medical scale. Data pertaining to medical, obstetrical, and family history were collected by interviewer-administered questionnaire. Pre-gravid physical activity in the year prior to pregnancy was assessed using the Baecke questionnaire, an established instrument that has been extensively validated in several populations, including women of childbearing age (20,21). The questionnaire was completed during the OGTT (ie. prior to knowledge of glucose tolerance status), with participants asked to report on their physical activity in the year prior to the pregnancy. The Baecke questionnaire measures (i) total physical activity and (ii) its 3 component domains: occupation-associated activity (work index); leisure-time activity not including sports (non-sport leisure-time activity index); and sport-related physical activity (vigorous/sports index). The work index quantifies the exertion related to occupational activities, such as sitting, standing, lifting, and walking, as well as associated effects on the individual (eg. fatigue, perspiration) The leisure-time index quantifies the exertion associated with non-sport recreational activities (such as walking and television viewing). The vigorous/sports index characterizes vigorous/sports activity with respect to intensity (using the updated Compendium of Physical Activities (22)), duration and frequency.
In conjunction with the GCT, the OGTT determined glucose tolerance status in pregnancy, as represented by the following 4 groups:
GDM, as defined by National Diabetes Data Group (NDDG) criteria (23) (requires at least 2 of the following on the OGTT: fasting glucose ≥5.8 mmol/L, 1-hr blood glucose ≥10.6 mmol/L, 2-hr blood glucose ≥9.2 mmol/L, or 3-hr blood glucose ≥8.1 mmol/L);
Gestational impaired glucose tolerance (GIGT), as defined by meeting only 1 of the above NDDG criteria;
Abnormal GCT Normal Glucose Tolerance (NGT), as defined by having an abnormal 50g GCT (1-hour post-challenge plasma glucose ≥ 7.8 mmol/L) followed by NGT on the OGTT (defined by meeting none of the NDDG criteria);
Normal GCT NGT, defined as having a normal GCT followed by NGT on the OGTT
Laboratory Measurements and Physiologic Indices
On the day of the OGTT, participants arrived in the morning after overnight fast. Venous blood samples were drawn for measurement of plasma glucose and insulin at fasting and at 30-, 60- and 120-minutes and 180-minutes. Specific insulin was measured using the Roche Modular system and the electrochemiluminescence immunoassay kit (Roche Diagnostics catalogue number 12017547122). This assay shows 0.05% cross-reactivity to intact human proinsulin and the primary circulating split form created by processing of the insulin pro-hormone (Des 31,32).
Glycemic measures included glucose tolerance status (as described above) and the total area-under-the-glucose-curve (AUCgluc) during the OGTT (calculated using the trapezoidal rule applied to the 0-, 60-, 120- and 180-minute plasma glucose values). Insulin sensitivity was measured using the insulin sensitivity index (ISOGTT) of Matsuda and DeFronzo (24). In pregnant women, ISOGTT has been shown to exhibit stronger correlation with insulin sensitivity measured by euglycemic-hyperinsulinemic clamp than either Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) or Quantitative Insulin Sensitivity Check Index (25). Beta-cell function was assessed by the insulinogenic index divided by HOMA-IR (26,27). The insulinogenic index was calculated as the incremental change in insulin concentration during the first 30 minutes of the OGTT divided by the incremental change in glucose during the same time period (28). HOMA-IR was calculated as described by Matthews et al (29).
Statistical Analyses
All analyses were conducted using the Statistical Analysis System (SAS 9.1, SAS Institute, Cary NC). Continuous variables were tested for normality of distribution and natural log transformations of skewed variables were used, where necessary, in subsequent analyses. For each quartile of total physical activity score, continuous variables are presented as mean followed by standard deviation, if normally-distributed, or as median followed by interquartile range, if skewed, while categorical variables are presented as proportions. Univariate differences across the groups were assessed using Analysis of Variance (ANOVA) for continuous variables and χ2 for categorical variables. Mean values of total physical activity score, work index, leisure-time index, and vigorous/sport index were compared across the 4 glucose tolerance groups by ANOVA. The odds ratio for abnormal glucose tolerance in pregnancy (GDM, GIGT, or abnormal GCT NGT) was determined for women above the 50th percentile for vigorous/sports index, compared to those below the 50th percentile. Univariate correlations between the domains of physical activity and metabolic parameters were assessed by Spearman correlation analysis. Multiple linear regression analysis was used to identify factors independently associated with the dependent variable ISOGTT. Covariates of interest included age, weeks’ gestation, pre-pregnancy BMI, weight gain in pregnancy preceding the OGTT, previous GDM, family history of T2DM, ethnicity (with Caucasian as reference group), and all 3 domains of pre-gravid physical activity (work index, non-sport leisure-time index, sport index). Covariates were considered to be retained in the model at a significance level of 0.05. Since the residual plot for the model with logarithmically-transformed ISOGTT showed no difference compared to that of the model with ISOGTT in original scale, we have presented the latter.
RESULTS
Characteristics of Study Population by Quartiles of Total Physical Activity
Table 1 shows the demographic, clinical and metabolic characteristics of the 851 study participants stratified by quartile of pre-gravid total physical activity score. There were no significant differences between the 4 groups with respect to age, pre-pregnancy BMI, weight gain per week of gestation, smoking exposure, parity, previous GDM, or family history of T2DM. Women in the 4th quartile underwent the OGTT slightly later (median 30 weeks’ gestation) than women in the other three quartiles (each median 29 weeks) (p=0.0034). As total physical activity score increased, the proportion of Caucasian women rose while that of Asian women decreased (p=0.0046). Finally, as would be expected, total physical activity score and each of its 3 domains (work index, non-sport leisure-time index, vigorous/sport index) progressively increased across the 4 quartiles (each p<0.0001, respectively). The sport activities most frequently reported by women in the highest quartile of physical activity were the vigorous activities of running (24%), gym training (12%) and swimming (8%).
Table 1.
Clinical and metabolic characteristics of study population stratified by quartiles of pre-gravid total physical activity score
| Lowest Quartile n=242 |
Second Quartile n=195 |
Third Quartile n=221 |
Fourth Quartile n=193 |
p |
|
|---|---|---|---|---|---|
| Age (yrs) | 33.2 [4.1] | 33.9 [4.0] | 33.4 [4.6] | 33.8 [5.5] | 0.3567 |
| Weeks’ gestation at OGTT (wks) | 29 [28–30] | 29 [28–31] | 29 [28–31] | 30 [28–32] | 0.0034 |
| Pre-p regnancy BMI (kg/m2) | 24.1 [21.4–28.6] | 24.2 [21.3–28.8] | 23.0 [21.2–27.4] | 23.1 [21.0–27.0] | 0.0537 |
| Weight gain per week (kg/wk) | 0.3 [0.2–0.4] | 0.3 [0.3–0.4] | 0.3 [0.3–0.5] | 0.4 [0.3–0.5] | 0.0647 |
| Smoking exposure: | 0.3530 | ||||
| Never (%) | 70 | 70 | 71 | 63 | |
| Remote (%) | 27 | 28 | 28 | 35 | |
| Current (%) | 3 | 2 | 1 | 2 | |
| Parity: | 0.2916 | ||||
| Nulliparous (%) | 55 | 51 | 55 | 61 | |
| One (%) | 33 | 44 | 33 | 29 | |
| Greater than one (%) | 12 | 6 | 12 | 10 | |
| Previous GDM/macrosomia (%) | 5.0 | 7.7 | 4.1 | 4.7 | 0.5616 |
| Family history of DM (%) | 51.7 | 54.9 | 48.0 | 51.8 | 0.6761 |
| Ethnicity: | 0.0046 | ||||
| Caucasian (%) | 68 | 75 | 74 | 79 | |
| Asian (%) | 18 | 12 | 13 | 8 | |
| Other (%) | 14 | 13 | 13 | 13 | |
| Pre-g ravid physical actitivity score | 6.2 [0.6] | 7.3 [0.2] | 8.1 [0.3] | 9.4 [0.7] | <0.0001 |
| Work index | 2.0 [0.4] | 2.4 [0.5] | 2.6 [0.6] | 2.9 [0.6] | <0.0001 |
| Leisure-time index | 2.5 [0.4] | 2.9 [0.5] | 3.2 [0.5] | 3.5 [0.5] | <0.0001 |
| Vigorous/Sport index | 1.6 [0.4] | 2.1 [0.5] | 2.4 [0.5] | 3.0 [0.7] | <0.0001 |
| Glucose Metabolism | |||||
| Glucose challenge test (mmol/L) | 8.3 [1.3] | 8.2 [1.4] | 8.2 [1.3] | 8.0 [1.4] | 0.1150 |
| Fasting glucose (mmol/L) | 4.5 [0.5] | 4.6 [0.5] | 4.5 [0.6] | 4.5 [0.6] | 0.1671 |
| AUCgluc on OGTT | 23.0 [3.4] | 23.1 [3.7] | 22.8 [3.9] | 22.3 [3.6] | 0.1960 |
| Glucose tolerance status: | 0.0244 | ||||
| Normal GCT NGT (%) | 10.3 | 14.4 | 11.8 | 20.2 | |
| Abnormal GCT NGT (%) | 48.8 | 47.7 | 48.9 | 47.7 | |
| GIGT (%) | 19.8 | 14.9 | 19.5 | 15.5 | |
| GDM (%) | 21.1 | 23.1 | 19.9 | 16.6 | |
| Insulinogenic Index/HOMA-IR | 10.2 [6.6–5.7] | 9.9 [6.1–14.4] | 10.3 [6.5 –16.9] | 11.1 [6.4–16.7] | 0.5412 |
| ISOGTT | 3.8 [2.7–5.4] | 4.2 [2.8–5.9] | 4.6 [3.0–6.2] | 4.9 [3.1–7.1] | 0.0007 |
Data are presented as mean followed by standard deviation, with the exception of (i) weeks’ gestation, pre-pregnancy BMI, weight gain/week, insulinogenic index/HOMA-IR and ISOGTT (presented as median followed by interquartile range) and (ii) smoking, parity, previous GDM, family history of DM, ethnicity and glucose tolerance status (presented as percentages).
p-values refer to overall differences across groups as derived from ANOVA analysis for continuous variables (parametric test for normally-distributed variables and non-parametric test for skewed variables) or χ2 test for categorical variables
Turning to parameters of glucose metabolism, there were no significant differences between the quartiles with respect to GCT result, fasting glucose and AUCgluc. Nevertheless, glucose tolerance status in pregnancy improved with increasing quartile of pre-gravid total physical activity (p=0.0244). In particular, as total physical activity increased, the prevalence of GDM decreased, while that of normal GCT NGT (ie. completely normal glucose tolerance) rose. Moreover, whereas insulinogenic index/HOMA-IR (beta-cell function) did not differ significantly between the groups, ISOGTT (insulin sensitivity) increased in parallel with quartile of total physical activity (p=0.0007).
Domains of Physical Activity and Glucose Tolerance Status
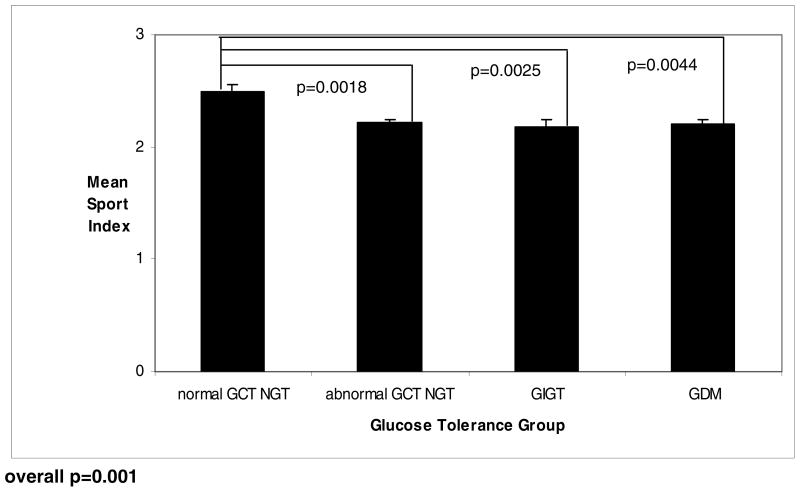
To further elucidate the relationship between pre-gravid physical activity and antepartum glucose homeostasis, mean scores for each domain of physical activity were compared between the glucose tolerance groups. Mean total physical activity score (+/− SD) was highest in women with normal GCT NGT (7.95 +/−0.12), followed in turn by the abnormal GCT NGT group (7.66 +/−0.06), GIGT (7.59 +/−0.1) and GDM (7.57 +/−0.1), respectively (overall p=0.0491) (Figure 1A). Amongst the component domains, mean work score (p=0.9412) and mean non-sport leisure-time score (p=0.5666) both did not differ across the four glucose tolerance groups (data not shown). Importantly, however, mean vigorous/sport activity varied considerably across the groups (overall p=0.001) (Figure 1). Indeed, pre-gravid vigorous/sports activity was significantly higher in women with normal GCT NGT compared to women with (i) abnormal GCT NGT (p=0.0018), (ii) GIGT (p=0.0025), and (iii) GDM (p=0.0044). Furthermore, upon median split of the study participants into 2 groups based on vigorous/sport index score, the odds ratio for abnormal glucose tolerance in pregnancy for women with higher levels of pre-gravid sports activity (ie. >50th percentile) was 0.53 (95% CI: 0.36–0.77), compared to their less sport-oriented peers. With adjustment for age, weeks’ gestation, pre-pregnancy BMI, weight gain in pregnancy, family history of diabetes and ethnicity, this risk reduction remained significant with odds ratio 0.61 (95% CI: 0.39–0.96).
Figure 1.
Plot of mean vigorous/sport index, by glucose tolerance group in pregnancy (normal GCT NGT, abnormal GCT NGT, GIGT, GDM)
Domains of Physical Activity and Determinants of Glucose Homeostasis
Having demonstrated an association between pre-gravid physical activity (specifically vigorous/sport index) and glucose tolerance in pregnancy, we next sought to assess the relationships between the domains of physical activity and metabolic factors related to antepartum glucose homeostasis (measures of glycemia, beta-cell function and insulin sensitivity). On Spearman univariate correlation analysis, vigorous/sport index emerged as the domain that best correlated with metabolic parameters (GCT, AUCgluc, ISOGTT) (Table 2). Conversely, ISOGTT was the metabolic parameter that best correlated with the physical activity measures (total physical activity, leisure-time index, vigorous/sport index). Accordingly, the strongest univariate association was between pre-gravid vigorous/sport index and ISOGTT (r=0.21, p<0.0001)
Table 2.
Spearman univariate correlations of glucose metabolism factors with overall physical activity score, and its components: (i) work index, (ii) leisure-time index, and (iii) vigorous/sports index
| Physical Activity Score |
Work Index |
Leisure-time Index |
Sport Index |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Glucose challenge test | −0.09 | 0.0135 | 0 | 0.9035 | −0.05 | 0.1544 | −0.10 | 0.0024 |
| Fasting glucose | −0.06 | 0.1066 | 0.05 | 0.1280 | −0.07 | 0.0455 | −0.08 | 0.0155 |
| AUCgluc on OGTT | −0.08 | 0.0261 | 0.03 | 0.3052 | −0.04 | 0.1907 | −0.11 | 0.0015 |
| Insulinogenic Index/HOMA-IR | 0.01 | 0.7607 | −0.13 | 0.0004 | 0.04 | 0.2050 | 0.09 | 0.0104 |
| ISOGTT | 0.14 | <0.0001 | −0.08 | 0.0218 | 0.13 | 0.0002 | 0.21 | <0.0001 |
Bold indicates p<0.005
As many factors may affect insulin sensitivity, we next sought to determine if pre-gravid sports activity was independently associated with antepartum ISOGTT after adjustment for potential covariates. On multiple linear regression analysis, a model consisting of age, weeks’ gestation, pre-pregnancy BMI, weight gain in pregnancy, family history of T2DM, previous GDM, ethnicity and the 3 domains of physical activity reconciled 30.0% of the variance of dependent variable antepartum ISOGTT (Table 3). Importantly, pre-gravid vigorous/sports activity indeed emerged as a significant independent predictor of ISOGTT (beta=0.60873, t=4.97, p<0.0001). Significant negative determinants were pre-pregnancy BMI (beta=−0.23264, t=−14.62, P<0.0001), weight gain in pregnancy (beta=−0.08406, t=−5.97, p<0.0001), Asian ethnicity (beta=−1.36686, t=−5.10, p<0.0001) and other non-white ethnicity (beta=−1.26223, t=−5.00, p<0.0001), while age was a positive covariate (beta=0.06045, t=3.43, p=0.0006). Neither work index nor non-sport leisure-time activity was significantly related to ISOGTT.
Table 3.
Multiple linear regression analysis of dependent variable IS OGTT
| Variable |
Parameter Estimate |
Standard Error |
t value |
p |
|---|---|---|---|---|
| Intercept | 9.12806 | 1.30852 | 6.98 | <0.0001 |
| Age | 0.06045 | 0.01865 | 3.43 | 0.0006 |
| Weeks’ gestation | −0.01933 | 0.03321 | −0.58 | 0.5607 |
| Pre-pregnancy BMI | −0.23264 | 0.01616 | −14.62 | <0.0001 |
| Weight gain in pregnancy | −0.08406 | 0.01409 | −5.97 | <0.0001 |
| Family history of diabetes | −0.27675 | 0.16884 | −1.64 | 0.1016 |
| Previous GDM | −0.52425 | 0.40064 | −1.31 | 0.1911 |
| Ethnicity: | ||||
| Asian ethnicity | −1.36686 | 0.26824 | −5.10 | <0.0001 |
| Other non-white ethnicity | −1.26223 | 0.25236 | −5.00 | <0.0001 |
| Work index | 0.08081 | 0.13683 | 0.59 | 0.5550 |
| Leisure-time index | −0.01266 | 0.15438 | −0.08 | 0.9347 |
| Vigorous/Sport index | 0.60873 | 0.12247 | 4.97 | <0.0001 |
model r2 = 30.0%
Next, we tested for interaction amongst the model covariates. There were no significant interactions between vigorous/sport index and either age or weight gain in pregnancy (data not shown). A significant interaction, however, was detected between vigorous/sport index and pre-pregnancy BMI (interaction term: t=−4.25, p<0.0001). Indeed, the univariate association between pre-gravid vigorous/sport activity and ISOGTT in pregnancy was stronger in lean women (pre-pregnancy BMI <25 kg/m2) (r=0.22, p<0.0001) than in those participants with pre-gravid BMI >/= 25 kg/m2 (r=0.12, p=0.0364).
Finally, to elucidate the role of intensity of sport activities, we calculated a mean intensity score for each participant, defined as the mean of the following construct calculated for each sport activity reported by the individual. This construct was the product of (i) the intensity code for the sport activity reported (as determined using the updated Compendium of Physical Activities (22)), (ii) the amount of time per week engaged in the activity, and (iii) the proportion of the year during which this activity was performed. Like the vigorous/sport index, on Spearman univariate analyis, this intensity score was significantly associated with ISOGTT (r=0.17, p<0.0001). Furthermore, on multiple linear regression analysis, this intensity score also emerged as a significant independent predictor of ISOGTT (beta=0.21899, t=3.97, p<0.0001), after adjustment for age, weeks’ gestation, pre-pregnancy BMI, weight gain in pregnancy, family history of diabetes, previous GDM, and ethnicity. These data thus suggest that the intensity of pre-gravid vigorous/sport activity is positively associated with antepartum insulin sensitivity.
DISCUSSION
In this report, we demonstrate that physical activity in the year prior to pregnancy is associated with a reduced risk of GDM and that this relationship is specifically driven by vigorous/sports activity. Indeed, increased pre-gravid vigorous/sports activity was associated with a significantly reduced risk of any degree of glucose intolerance in pregnancy. Furthermore, increased pre-gravid vigorous/sports activity was independently associated with enhanced insulin sensitivity in late 2nd/early 3rd trimester. Taken together, these data support the value of improving insulin sensitivity in women at risk for GDM and suggest that pre-pregnancy vigorous physical activity within the year prior can provide a means of achieving this goal.
A series of recent reports have linked pre-gravid physical activity with risk reduction for GDM (6,13–17). In the Nurses Health Study II, women in the highest quintile of physical activity (specifically vigorous activity) had a ~20% risk reduction for the development of GDM (14). Similarly, in the Project Viva observational cohort, physical activity before pregnancy (again, particularly vigorous activity) was associated with a reduced risk of either GDM or any antepartum glucose intolerance (risk reductions of 44% and 24%, respectively, for vigorous activity) (15). Furthermore, in a cohort of 909 women, Dempsey and colleagues also found that leisure-time physical activity (ie. non-occupational) in the year prior to pregnancy correlated with a lower risk of GDM (13). Our findings of a protective effect of pre-gravid physical activity are thus consistent with these earlier reports, while extending this literature by identifying the specific contribution of vigorous/sports activity in this context. Moreover, as neither the work nor non-sport leisure-time activities of the study subjects are as likely to represent vigorous exertion as their sport activities (eg. as evidenced by the nature of the subjects’ occupations), our findings regarding the specific role of sports exertion are likely consistent with the data from earlier studies describing vigorous activity. Accordingly, intensity of the exertion is likely the mechanism that links pre-pregnant sports activity with a reduced risk of antepartum glucose intolerance.
An important novel dimension of the current study is the evaluation of the relationships between pre-gravid physical activity and metabolic factors that determine glucose tolerance in pregnancy. Specifically, while not related to beta-cell function, pre-gravid vigorous/sport activity is shown to be associated with improved antepartum insulin sensitivity, thereby suggesting a possible mechanism underlying the GDM risk reduction. Interestingly, we demonstrate a differential effect of pre-pregnancy vigorous/sports activity on insulin sensitivity depending on body habitus, with lean women (BMI < 25 kg/m2) deriving much greater benefit (re insulin-sensitization) than women with BMI >/= 25 kg/m2 (although it is recognized that definitive commentary in this regard is precluded by the absence of data on exercise in pregnancy). This finding may provide an explanation for an earlier observation in Project Viva (15), where vigorous pre-pregnancy exercise significantly reduced the risk of abnormal glucose tolerance in pregnancy in lean women (adjusted odds ratio 0.56, 95% CI 0.36–0.88), but not in women with BMI >/= 25 kg/m2 (adjusted odds ratio 1.03, 95% CI 0.59–1.79). Taken together, these data support the idea that an improvement in insulin sensitivity with pre-gravid vigorous/sports activity may be contributing to the reduced risk of GDM.
It is well recognized that exercise training can improve insulin sensitivity by a variety of mechanisms, such as; (i) enhancing glucose uptake into skeletal muscle (ie. by increasing GLUT4 translocation), (ii) affecting proteins involved in insulin signal transduction (eg. AMP-activated protein kinase (AMPK) and the protein kinase B (Akt) substrate AS160), and (iii) increasing the oxidative capacity of skeletal muscle (by upregulating lipid oxidation and mitochondrial biogenesis) (30). In the current study, it is important to recognize that pre-pregnancy vigorous/sports activity correlated with insulin sensitivity in late 2nd/early 3rd trimester (ie. ~7 months later). This relationship is intriguing, when one considers that there are many factors that are known to affect insulin sensitivity, including genetic determinants, body habitus, and hormonal factors.5,26 Indeed, the effect of multiple factors is consistent with both (i) the relatively modest unadjusted Spearman univariate correlations observed between physical activity domains and ISOGTT in Table 2 and (ii) the identification of several significant correlates of ISOGTT on the multiple linear regression analysis (Table 3). Nevertheless, despite the effects of multiple factors, it is noteworthy that pre-gravid vigorous/sports activity still emerged as an independent determinant of antepartum insulin sensitivity, after adjustment for covariates (Table 3).
The mechanisms underlying this apparent later benefit of pre-gravid exercise are unclear. A likely possibility is that pre-pregnancy exercise patterns may carry on into pregnancy and thereby affect antepartum insulin sensitivity (a possibility that we cannot address in this study, as exercise patterns during pregnancy were not evaluated). Alternatively, the physiologic benefits of pre-gravid exercise may persist into pregnancy. In this context, it is possible that increased insulin sensitivity prior to pregnancy may enable better maintenance of insulin sensitivity in late pregnancy, thereby allowing for appropriate beta-cell compensation and the maintenance of normoglycemia. Such an effect may be particularly important in women at risk of GDM, since this patient population exhibits chronic insulin resistance that is believed to pre-date pregnancy (31,32). Indeed, as increased insulin resistance in 1st trimester (which largely reflects the pre-gravid state) predicts a higher risk of developing GDM later in pregnancy (33,34), it is plausible that improving insulin sensitivity prior to pregnancy would reduce GDM risk.
The insulin-sensitizing adipokine adiponectin is a potential candidate mediator in the relationship between pre-gravid physical activity, insulin sensitivity and risk of GDM. Specifically, low circulating levels of total adiponectin and its high-molecular-weight (HMW) form are related to both GDM and decreased insulin sensitivity (35–37). Exercise can increase both total and HMW adiponectin (38). It is therefore possible that pre-gravid physical activity raises total and HMW adiponectin levels and thereby improves insulin sensitivity and lowers risk of GDM. Consistent with this idea, it should be noted that low total adiponectin in 1st trimester (like insulin resistance) predicts subsequent GDM later in pregnancy (39). Similarly, the current data supports the value of improving insulin sensitivity in women at risk of GDM. Furthermore, following a pregnancy complicated by GDM, insulin-sensitization by either lifestyle modification or thiazolidinedione treatment has been shown to significantly reduce the risk of developing T2DM(32,40). The current study extends this paradigm to the pre-gravid state, at which time it now emerges that insulin-sensitization via vigorous/sports activity may reduce the subsequent risk of GDM.
A limitation of this analysis is that the observational nature of the study precludes the exclusion of possible residual confounding by unmeasured factors, despite our having adjusted for several known factors associated with GDM and insulin sensitivity (including age, ethnicity, BMI, weight gain in pregnancy, family history of diabetes and previous GDM). The unmeasured variables include diet, socio-economic status and physical activity during the pregnancy, any of which may be acting upon the relationships reported herein. As such, definitive inference on causality cannot be made with this study. Nevertheless, this analysis is the first report to specifically link pre-gravid vigorous/sports activity with insulin sensitivity in pregnancy, a biologically very plausible association that reconciles several earlier observations, as noted above. Further study is warranted to address the specific biochemical mediators that may be relevant to this relationship.
In summary, physical activity in the year prior to pregnancy is associated with a reduced risk of GDM. This relationship is specifically driven by vigorous/sports activity, which is independently associated with enhanced insulin sensitivity in late pregnancy. Thus, taken together, these data suggest a model in which physical activity prior to pregnancy improves insulin sensitivity and reduces the risk of GDM. As such, these data support the value of improving insulin sensitivity in women at risk for GDM and suggest that vigorous physical activity can provide a means of achieving this goal.
Acknowledgments
We wish to thank the Mount Sinai Hospital Department of Pathology and Laboratory Medicine and Patient Care Services. The study was supported by operating grants (MOP 67063 and 84206) from the Canadian Institutes of Health Research (CIHR). R Retnakaran is supported by a CIHR Clinical Research Initiative New Investigator Award, Canadian Diabetes Association (CDA) Clinician-Scientist incentive funding and a University of Toronto Banting and Best Diabetes Centre New Investigator Award. AJ Hanley holds a Tier II Canada Research Chair in Diabetes Epidemiology and is supported through a CDA Scholarship. B Zinman holds the Sam and Judy Pencer Family Chair in Diabetes Research at Mount Sinai Hospital and University of Toronto.
Footnotes
Conflicts of Interest: None to declare
References
- 1.Kjos SL, Buchanan TA. Gestational diabetes mellitus. New England Journal of Medicine. 1999;341:1749–1756. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan TA, Xiang AH. Gestational diabetes mellitus. Journal of Clinical Investigation. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 4.Retnakaran R, Connelly PW, Sermer M, Zinman B, Hanley AJ. The impact of family history of diabetes on risk factors for gestational diabetes. Clinical Endocrinology. 2007;67:754–760. doi: 10.1111/j.1365-2265.2007.02958.x. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe RM, Black MH, Xiang AH, Allayee H, Lawrence JM, Buchanan TA. Genetics of gestational diabetes mellitus and type 2 diabetes. Diabetes Care. 2007;30 (Suppl 2):S134–140. doi: 10.2337/dc07-s205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegaard HK, Pedersen BK, Nielsen BB, Damm P. Leisure time physical activity during pregnancy and impact on gestational diabetes mellitus, pre-eclampsia, preterm delivery and birth weight: a review. Acta Obstetricia et Gynecologica Scandinavica. 2007;86:290–296. doi: 10.1080/00016340701647341. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29:2223–2230. doi: 10.2337/dc06-0266. [DOI] [PubMed] [Google Scholar]
- 8.Cheung NW, Byth K. Population health significance of gestational diabetes. Diabetes Care. 2003;26:2005–2009. doi: 10.2337/diacare.26.7.2005. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Schulze MB, Solomon CG, Hu FB. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia. 2006;49:2604–2613. doi: 10.1007/s00125-006-0422-1. [DOI] [PubMed] [Google Scholar]
- 10.Bo S, Menato G, Lezo A, Signorile A, Bardelli C, De Michieli F, Massobrio M, Pagano G. Dietary fat and gestational hyperglycaemia. Diabetologia. 2001;44:972–978. doi: 10.1007/s001250100590. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Storlien LH, Jenkins AB, Tapsell LC, Jin Y, Pan JF, Shao YF, Calvert GD, Moses RG, Shi HL, Zhu XX. Dietary variables and glucose tolerance in pregnancy. Diabetes Care. 2000;23:460–464. doi: 10.2337/diacare.23.4.460. [DOI] [PubMed] [Google Scholar]
- 12.Radesky JS, Oken E, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Gillman MW. Diet during early pregnancy and development of gestational diabetes. Paediatric and Perinatal Epidemiology. 2008;22:47–59. doi: 10.1111/j.1365-3016.2007.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempsey JC, Sorensen TK, Williams MA, Lee IM, Miller RS, Dashow EE, Luthy DA. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. American Journal of Epidemiology. 2004;159:663–670. doi: 10.1093/aje/kwh091. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Solomon CG, Manson JE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Archives of Internal Medicine. 2006;166:543–548. doi: 10.1001/archinte.166.5.543. [DOI] [PubMed] [Google Scholar]
- 15.Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstetrics & Gynecology. 2006;108:1200–1207. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudra CB, Williams MA, Lee IM, Miller RS, Sorensen TK. Perceived exertion in physical activity and risk of gestational diabetes mellitus. Epidemiology. 2006;17:31–37. doi: 10.1097/01.ede.0000184474.33629.cd. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal R, Rafique G, Badruddin S, Qureshi R, Cue R, Gray-Donald K. Increased body fat percentage and physical inactivity are independent predictors of gestational diabetes mellitus in South Asian women. European Journal of Clinical Nutrition. 2007;61:736–742. doi: 10.1038/sj.ejcn.1602574. [DOI] [PubMed] [Google Scholar]
- 18.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1-hour on oral glucose tolerance test in pregnancy resembles gestational diabetes in predicting postpartum metabolic dysfunction. Diabetes Care. 2008;31:1275–1281. doi: 10.2337/dc08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008 Jul 15; doi: 10.2337/dc08-0972. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Kriska AM, Casperson CJ. A collection of physical activity questionnaires for health related research. Medicine & Science in Sports & Exercise. 1997;29(Suppl 1):S1–S203. [PubMed] [Google Scholar]
- 22.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Medicine & Science in Sports & Exercise. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 23.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 25.Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24:1602–1607. doi: 10.2337/diacare.24.9.1602. [DOI] [PubMed] [Google Scholar]
- 26.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 27.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R, Murphy MB, Shamoon H The Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabetic Medicine. 1995;12:931. doi: 10.1111/j.1464-5491.1995.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiologica. 2008;192:127–135. doi: 10.1111/j.1748-1716.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 31.Clark CM, Jr, Qiu C, Amerman B, Porter B, Fineberg N, Aldasouqi S, Golichowski A. Gestational diabetes: should it be added to the syndrome of insulin resistance? Diabetes Care. 1997;20:867–871. doi: 10.2337/diacare.20.5.867. [DOI] [PubMed] [Google Scholar]
- 32.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 33.Ozcimen EE, Uckuyu A, Ciftci FC, Yanik FF, Bakar C. Diagnosis of gestational diabetes mellitus by use of the homeostasis model assessment-insulin resistance index in the first trimester. Gynecological Endocrinology. 2008;24:224–9. doi: 10.1080/09513590801948416. [DOI] [PubMed] [Google Scholar]
- 34.Smirnakis KV, Martinez A, Blatman KH, Wolf M, Ecker JL, Thadhani R. Early pregnancy insulin resistance and subsequent gestational diabetes mellitus. Diabetes Care. 2005;28:1207–1208. doi: 10.2337/diacare.28.5.1207. [DOI] [PubMed] [Google Scholar]
- 35.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 36.Retnakaran R, Hanley AJ, Raif N, Hirning CR, Connelly PW, Sermer M, Kahn SE, Zinman B. Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia. 2005;48:993–1001. doi: 10.1007/s00125-005-1710-x. [DOI] [PubMed] [Google Scholar]
- 37.Retnakaran R, Connelly PW, Maguire G, Sermer M, Zinman B, Hanley AJ. Decreased high molecular weight adiponectin in gestational diabetes: implications for the pathophysiology of type 2 diabetes. Diabetic Medicine. 2007;24:245–252. doi: 10.1111/j.1464-5491.2007.02077.x. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary VB, Jorett AE, Marchetti CM, Gonzalez F, Phillips SA, Ciaraldi TP, Kirwan JP. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. American Journal of Physiology Endocrinology and Metabolism. 2007;293:E421–427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 39.Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. Journal of Clinical Endocrinology and Metabolism. 2004;89:2306–11. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]
- 40.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle modification or metformin. New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]