Abstract
Purpose: The authors have developed a novel technique using an electronic portal imaging device (EPID) to verify the geometrical accuracy of delivery of dose-rate-regulated tracking (DRRT). This technique, called verification of real-time tracking with EPID (VORTE), can potentially be used for both on-line and off-line quality assurance (QA) of MLC-based dynamic tumor tracking.
Methods: The shape and position of target as a function of time, which is assumed to be known, is projected onto the EPID plane. This projected sequence of apertures as a function of time (target motion) is then used as the reference. The accuracy of dynamic MLC tracking can then be assessed by how well the delivered beam follows this projected target motion without the use of a physical moving phantom. The beam apertures controlled by DRRT (aperture motion) is detected by the EPID as a function of time. The aperture motion is compared to the target motion to evaluate tracking error introduced by DRRT. The accuracy of VORTE was measured using film measurements of ten static fields. The VORTE for dynamic tumor tracking was tested with several target motions, including (1) rigid-body two-dimensional (2-D) cyclic motion in the superior-inferior direction with various period and amplitude; (2) the above 2-D cyclic motion plus cyclic deformation; and (3) 2-D cyclic motion with both deformation and rotation. For each target motion, the controlled aperture motion resulting from DRRT was acquired at ∼8 Hz using EPID in the continuous-acquisition mode. Leaf positions in all captured frames were measured from the EPID and compared to their expected positions. The passing rate of 2 mm criteria for all leaves from all frames was calculated for each of the four patterns of tumor motion. Additionally, the root-mean-square (RMS) deviations of the centroid of the apertures between the designed and delivered beams were calculated for all three cases.
Results: The accuracy of MLC-leaf position determination by VORTE is 0.5 mm (1 standard deviation) by comparison to film measurements. With DRRT, the passing rates using the 2 mm criteria for all acquired frames are 100% for the 2-D displacement, 99% for the 2-D displacement with deformation, and 88% for the 2-D displacement combined with both deformation and rotation. The RMS deviations are 0.6 mm for the 2-D displacement, 1.0 mm for the 2-D displacement with deformation, and 1.1 mm for the 2-D displacement combined with both deformation and rotation.
Conclusions: The VORTE can measure the accuracy of MLC-based tumor tracking without the necessity of employing a moving phantom. Moreover, it can be used for complex target motion (i.e., 2-D displacement combined with deformation and rotation) that is difficult to create with physical moving phantoms. Therefore, the VORTE and the novel QA process illustrated by this study have a great potential for verifying real-time tumor tracking.
Keywords: verification of real-time tumor tracking, multileaf collimator, electronic portal imaging, treatment verification
INTRODUCTION
Real-time tumor tracking is one of the most active research areas under development in recent years. In the near future, it is expected that real-time tumor tracking will be available to treat patients. When it is used in the clinic, patient-specific quality assurance (QA) as well as tracking-system QA are important steps to make sure that treatment delivery using this new technology is safe. However, little work on adequate QA procedures for tumor tracking has been reported.1
Tumor tracking aims at precise targeting of a moving tumor during beam delivery. Since the tumor motion is irregular in terms of period and amplitude, the tracking system should adjust the radiation beam in real time so that the beam maintains synchronization with the tumor motion. The success of tumor tracking depends on how accurately and swiftly the tracking system responds to the changing tumor motion.
To verify the accuracy of tumor tracking, a moving phantom is typically used as a tumor-motion simulator.2, 3, 4, 5 The existing moving phantoms are capable of simulating irregular tumor motion with either rigid-body three-dimensional (3-D) displacement6 or deformable 3-D displacement.7, 8, 9 However, these physical phantoms might not reproduce the expected tumor motion precisely without laborious and time-consuming calibrations. Besides, it is a very challenging task to develop a moving phantom that can mimic complex tumor motion,10 such as 3-D displacement combined with both deformation and rotation.
Another issue for verification of tumor tracking is to monitor the response of the tracking system to real-time beam adjustment on a short time scale (faster than a few hundred milliseconds). An electronic portal imaging device (EPID) is capable of obtaining images with typically 8–10 frames per second (FPS), or even up to 50 FPS.11, 12, 13, 14, 15 Thus, the EPID shows great promise as a monitoring tool for real-time tumor-tracking QA. In contrast, film that is typically combined with a moving phantom generates only one integrated image during the tumor tracking. It is not possible to confirm the tracking accuracy on a short timescale using film.
Motivated by these two issues, we have developed a new method, using an EPID to verify the accuracy of MLC-based tumor tracking. This method called the verification of real-time tracking with EPID (VORTE) does not use a moving phantom. The expected shape and position which represent the target are projected onto the EPID. These projected apertures as a function of time play a role of the target motion as a reference. Tumor tracking is simulated to compensate for the target motion, and the delivered radiation beam controlled by the tumor-tracking system is measured by EPID as a function of time. The measured aperture motion is compared to the reference-aperture motion to evaluate the accuracy of tumor tracking. In this study, we aim at proof-of-principle of this VORTE with dose-rate-regulated tracking (DRRT).4, 16 DRRT is a MLC-based tumor-tracking technology with real-time feedback. DRRT adaptively controls MLC motion in real time to track a moving tumor by adjusting the dose rate.
METHODS AND MATERIALS
Principle of the VORTE
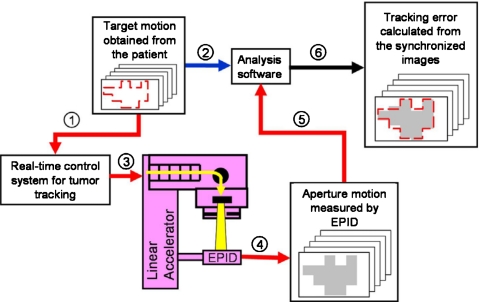
In tumor tracking with MLC, the radiation beam is repositioned by continuously adjusting the beam apertures to follow the irregular tumor motion. The shape and position of the target should be able to be determined in real time to perform tumor tracking. In this study, it is assumed that the target position and shape of the treatment moment are known, and the target is defined as the targeted area to be treated. Fluoroscopic images of EPID are acquired during the treatment with tracking technique. Shapes of apertures acquired from the EPID images are the record of the delivered treatment. Since the motion of the target is known, differences between the target motion and the actual delivery can be compared. It is the principle of the VORTE. Then, treatment is delivered by either the apertures generated on the fly or those designed before treatment for the MLC-based tumor tracking.2, 4, 5 VORTE can be applied for both of the techniques. The DRRT which uses predesigned delivery sequence and adaptively compensate the motion irregularity by changing the dose rate is used for testing the VORTE in this study.4 The VORTE does not use a moving phantom to simulate the target motion; the moving phantom is eliminated. Instead of a moving phantom, the determined target shape or the aperture motion is projected onto the EPID. The projected sequence of apertures as a function of time is then used as the reference (target motion). As shown in Fig. 1, this information on the target motion is an input for the analysis software (step 2). Based on the scenario of the target motion, the real-time control system controls the MLC motion to compensate for the target motion (steps 1 and 3 in Fig. 1). To monitor the motion of the radiation beam resulting from real-time control (aperture motion), the VORTE uses an EPID in continuous-acquisition mode. Depending on the frame rate of EPID, it can generate sequential images of the moving aperture (step 4). For example, a typical clinically available EPID (Portal Vision aS1000, Varian Medical Systems, Palo Alto, CA) can operate at 8–10 FPS (one EPID image every 100–125 ms). As shown in Fig. 1, steps 5 and 6, the analysis software compares the target and measured MLC aperture motions in order to compute the accuracy of the real-time tumor-tracking technique.
Figure 1.
Schematic flow diagram for the VORTE. Step 1: The designed-target (target in this study is defined as the targeted area to be treated) motion is used as an input to the real-time control system. Step 2: The same target motion is used as an input to the analysis software. Step 3: Real-time tracking is simulated by positioning the MLC leaves to compensate for the target motion. Step 4: The EPID in the continuous-acquisition mode takes sequential images of the moving apertures controlled during the real-time tracking. Step 5: These sequential images of the aperture motion are used as inputs to the analysis software. Step 6: The analysis software compares the target and aperture motions to evaluate the accuracy of the real-time tracking.
Imager and accelerator
In this study, a Trilogy linear accelerator (Varian Medical Systems, Palo Alto, CA) and aS1000 Portal Vision were used for image acquisition. The aS1000 portal imager has a sensitive area of 30×40 cm2 and a physical pixel pitch of 0.392 mm at the imager plane. A resolution of 0.784 mm at the imager plane was used in our measurements to reduce noise. Images of the aperture motion were acquired at ∼8 Hz in the continuous-acquisition mode (IAS Monitor 7.3.15, Varian Medical Systems, Palo Alto, CA). Images of an 18×18 cm2 square field at collimator angles of 0° and 180°, which was formed only by the jaws, were obtained in the continuous-acquisition mode to find the beam center, using the method published in Ref. 17: The geometrical center of each field was calculated with field edges determined at the 50% of the intensity profile. The beam center was determined to be the average of the two centers. Using the images of the square field, the collimator angle and the tilt of the EPID detector were corrected.18 Gantry angle was 0° for all measurements.
Measurement of the accuracy of the VORTE
Using film as the gold standard for calibration of leaf positions, the accuracy of the VORTE was determined. We designed ten static MLC fields formed by 30 MLC leaves (leaf numbers 16–45). In each MLC field, the positions of each leaf pair were assigned randomly to form a strip field with a size chosen randomly as well within the range of 0.5–15 cm.
The Gafchromic EBT film (International Specialty Products, Wayne, NJ) was located at the isocenter and sandwiched between two solid-water phantoms having thicknesses of 1.5 and 5 cm for the top and bottom, respectively. When each field was set up, 400 MU of the radiation beam was delivered with the nominal dose rate of 400 MU∕min. Keeping the same field setup, the film and phantom were removed for an in-air measurement of the field using EPID at 8 Hz in the continuous-acquisition mode.
Film analysis was performed with an EPSON Expression 10000X1 film scanner (EPSON, Long Beach, CA) and FILMQA™ (ISP, Wayne, NJ) analysis software. The leaf positions for each pair were determined at the 50% of the intensity profile formed by the pair.18 The leaf positions on the EPID images were determined using the same method as the one used for film. The leaf positions measured with the film and EPID were compared to determine the accuracy of the VORTE.
Measurement of the tracking accuracy of DRRT using the VORTE
The VORTE was tested with a real-time tumor-tracking technique, DRRT. The procedure of DRRT is first to generate a preprogrammed MLC sequence in the dose dynamic mode, based on the tumor motion measured prior to treatment. To compensate for irregular tumor motion during the treatment, DRRT controls the delivery speed of the preprogrammed sequence in real time by adjusting the dose rate. The target shape used in this measurement was ellipsoidal in the beam’s eye view at the isocenter plane with diameters of 20 mm in the direction parallel to the leaf motion and 30 mm in the direction perpendicular to the leaf motion. The three preprogrammed MLC sequences were designed with a dose rate of 400 MU∕min: (1) Rigid-body two-dimensional (2-D) displacement with a period of 5 s and amplitudes that are 10 mm peak-to-peak in the direction parallel to the leaf motion and 5 mm in the direction perpendicular to the leaf motion. (2) 2-D displacement with deformation. The same 2-D displacement as for case (1) was combined with deformation so that the target area of the end-of-inhale increases 1.3 times larger than that of the end-of-exhale at the isocenter. (3) 2-D displacement with both deformation and rotation. A 360° rotation for each breathing period was added to case (2). The speed of rotation was uniform with time for each breathing period; for example, if the breathing period is 4 s, then, the speed of rotation is 90° per second.
The live target motion for each preprogrammed sequence described in the previous paragraph was designed to have four different periods as shown in Table 1. DRRT changed the dose rate to compensate for change in period in each of the three cases.
Table 1.
The live target motion with period variations.
| Time (s) | Period (s) |
|---|---|
| 0–15 | 5.0 |
| 15–45 | 10.0 |
| 45–60 | 5.0 |
| 60–70 | 3.3 |
All leaf positions were obtained from the EPID measurements. The leaf positions for each pair were measured at the 50% of the intensity profile formed by the pair. Based on the measured leaf positions using EPID, leaf-by-leaf analysis was performed. Comparing the leaf positions obtained from the designed-target motion (live target motion) and EPID measurement, a positional deviation for each leaf was calculated as a function of time. Combining deviations of all leaves from all image frames, the passing rates were calculated for a positional deviation of less than 1 mm and less than 2 mm.
In addition, the aperture centroid in each image frame was calculated and compared with the one from the designed-target motion. The root-mean-square (RMS) deviation between centroids of the delivered and designed-target apertures over time was calculated.
RESULTS
Accuracy of the VORTE
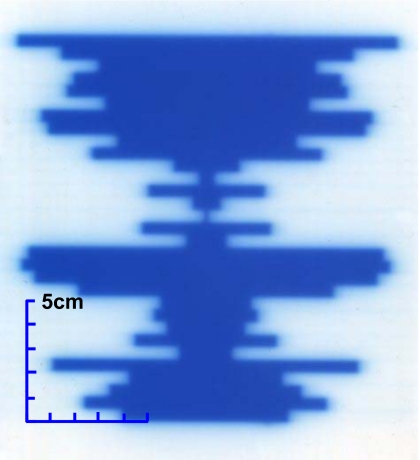
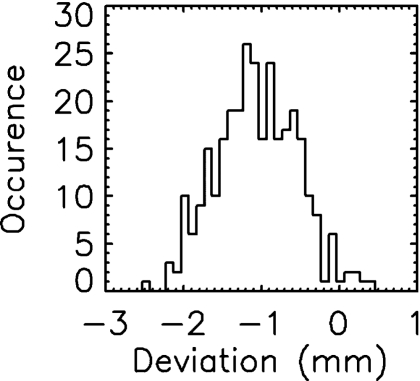
Figure 2 shows the film image of one field designed to measure the accuracy of the VORTE. Each leaf position measured with film was subtracted from the corresponding one measured with EPID. Figure 3 shows leaf positional deviations combined from the measurements of all ten fields. This distribution demonstrates that the VORTE has a spatial accuracy of 0.5 mm (1σ) for measuring each leaf position.
Figure 2.
Film image of a static field used to measure the accuracy of the VORTE.
Figure 3.
Frequency distribution of the deviations in the leaf positions measured with film and the EPID system used for the VORTE. The spatial accuracy of the VORTE, which is defined as a 1σ of the distribution, is 0.5 mm.
Tracking accuracy of DRRT
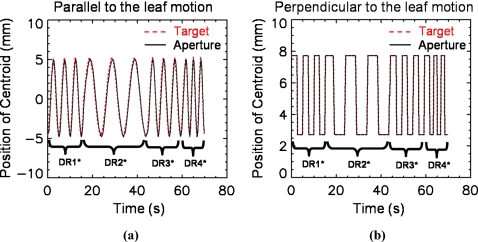
The VORTE was tested with a real-time tumor-tracking technique, DRRT. For a simple experiment, the dose rate was varied four times to adapt the change in the breathing pattern in the target motion, as shown in Fig. 4. The initial dose rate was set to 400 MU∕min when the breathing period was 5 s. When the breathing period was changed to 10 s, the dose rate was decreased to 200 MU∕min. When the period became faster to 3.3 s, the dose rate was increased to 600 MU∕min. Figure 4 shows that the aperture motion follows the target motion well in terms of the centroid for both directions parallel and perpendicular to the leaf motion. The RMS deviations of the centroid are 0.6 mm for the 2-D displacement, 1.0 mm for the 2-D displacement with deformation, and 1.1 mm for the 2-D displacement combined with both deformation and rotation. As the motion becomes more complex, the deviation of the centroid gets larger. DRRT can track the target motion with an accuracy of less than 1.1 mm in the centroid position.
Figure 4.
Verification of DRRT tracking with the VORTE. The track of the aperture centroid (solid line) was controlled using DRRT to synchronize with the target centroid (dotted line). (a) The target and aperture motions over time in the direction parallel to the leaf motion. (b) The target and aperture motions over time in the direction perpendicular to the leaf motion. (DR=dose rate. DR1 is 400 MU/min, DR2 200 MU/min, DR3 400 MU/min, and DR4 600 MU/min.)
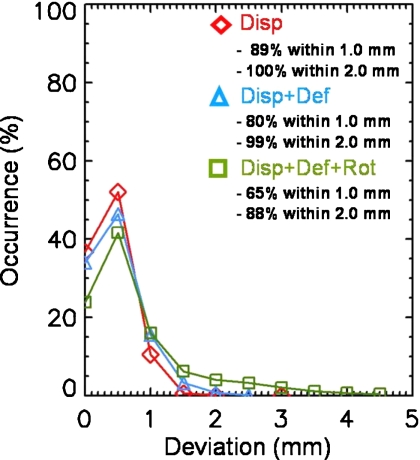
Figure 5 shows the leaf-by-leaf analysis for DRRT. The overall tendency of the distribution of the positional deviations and passing rates is very similar to the case without DRRT shown in Fig. 3. As can be seen in Fig. 5, the passing rate for a deviation of less than 2 mm is 100% for the 2-D displacement, 99% for the 2-D displacement with deformation, and 88% for the 2-D displacement combined with both deformation and rotation.
Figure 5.
Distribution of deviations in the leaf positions for all leaves from all frames using DRRT, our real-time tumor-tracking technique.
One of the great advantages of the VORTE is that the leaves that show a large deviation and the cause of the deviation can be identified. Table 2 shows a sample of the leaves that have a deviation of >2 mm for the 2-D displacement case. For example, leaf number 28 on the A side of the MLC shows a positional deviation of 2.6 mm for a designed-leaf speed of 21.0 mm∕s at the time of 1.1 s. The deviation for this leaf was resulted from its high MLC speed in the design.
Table 2.
Identification of the leaves that show a positional deviation of >2 mm for the 2-D displacement case.
| Deviation >2 mm for 2-D displacement | |||
|---|---|---|---|
| Leaf ID | Time (s) | Deviation (mm) | Designed leaf speed (mm∕s) |
| 28 B | 1.1 | 2.6 | 21.0 |
| 28 A | 5.7 | −2.6 | 21.1 |
| 35 A | 8.7 | −2.1 | 24.6 |
| 35 A | 12.1 | −3.1 | 22.9 |
| 28 B | 48.3 | −2.6 | 25.0 |
DISCUSSION
We propose here a novel method to evaluate the accuracy of tumor tracking in the absence of a moving phantom. This method that we call VORTE uses target motions and the fluoroscopic mode of an EPID for monitoring the motion of the tracking aperture. We have demonstrated the feasibility of the VORTE as a verification tool for tumor tracking for several tumor-motion patterns: Rigid 2-D displacement, deformable 2-D displacement, and deformable 2-D displacement combined with rotation. Our measurements show that the VORTE has an accuracy of 0.5 mm (1σ) for measuring each leaf position.
The advantages of the VORTE are (1) it does not require a moving phantom to simulate the tumor motion and (2) it can monitor the aperture motion with a high frame rate (i.e., from ∼8 to 50 Hz). It is difficult, at best, to simulate a complex tumor motion such as 2-D displacement combined with deformation and rotation using any existing moving phantom. The VORTE is a good way to overcome this obstacle.
When a moving phantom is used for verification of tumor tracking, film is the typical monitoring tool used. Film, however, produces only one integral image of the tracking aperture that is not able to show the accuracy of the tracking as a function of time. The VORTE, on the other hand, can monitor the accuracy of the tracking system for every real-time adjustment because it uses an EPID, which can monitor the aperture motion with a high frame rate. Furthermore, the VORTE can monitor the accuracy of the motion of each leaf as a function of time, which is impossible with film. For example, the VORTE can identify the leaves that show a large deviation during the real-time tracking.
The VORTE employs the same method to measure MLC-leaf position using EPID that has been previously reported for IMRT verification.20 However, the purpose of the VORTE is different from that of IMRT verification—The VORTE aims at verification of tumor tracking in which MLC motion is frequently controlled in real time. Thus, the target motion that real-time tumor tracking tries to adapt for must be designed to serve as the reference to evaluate the accuracy of real-time tracking system. The VORTE system can be used for testing the tracking method itself, the pretreatment QA, and the post-treatment QA, depending on when we acquire the target motion information. If a motion pattern of the patient is acquired during simulation phase, we can test the tracking accuracy before the treatment by acquiring the fluoroscopic EPID images while a dry run of tracking the target motion which has been acquired already. Then, we can determine the discrepancy between the delivered and the target position. The expected tracking error of the treatment of the patient can be calculated. Fluoroscopic EPID images also can be acquired during patient treatment, and then the VORTE can be used for post-treatment verification. In addition, since the VORTE does not require a moving phantom, it is possible to use the VORTE during treatment for in vivo verification, if the calculation process is fast enough.
Since the VORTE employs an EPID as a detector system, this new method can measure leaf trajectories only when the treatment beam is on. Another limitation in using an EPID is that a dead time in frame acquisition can result in missing image frames or reducing an EPID signal. This effect can be worse when leaf speed is high. This adverse effect will hinder to the accurate determination of leaf positions.
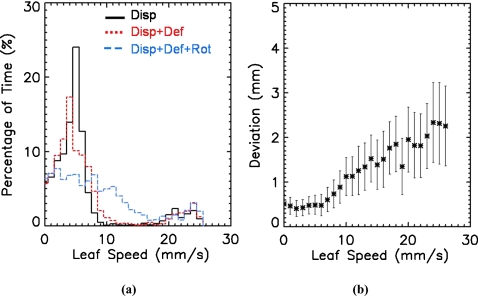
The tracking accuracy of the most complex tumor motion, such as deformable 2-D displacement with rotation, was observed to be lower in our measurements. This is caused mainly by two factors: One is complex tumor motion that requires a higher leaf speed than simple motion, such as 2-D displacement, as shown in Fig. 6a. The average leaf speed is 6.5±5.9 mm∕s for the 2-D displacement, 6.9±6.3 mm∕s for the deformable 2-D displacement, and 8.9±6.7 mm∕s for the deformable 2-D displacement combined with rotation. Figure 6b shows that the positional deviation of a MLC leaf becomes larger as the leaf speed gets higher. Sonke et al.18 and Ploeger et al.19 made the same observation that the faster the leaf speed, the larger is the leaf positional uncertainty. Thus, the leaf positional uncertainty resulting from a high leaf speed degrades the tracking accuracy for complex tumor motion in our measurements. Since MLC is part of the MLC-based tumor tracking system, this uncertainty is attributed to the tracking system. The other factor contributing to lower tracking accuracy is measurement uncertainty of the VORTE system for the high leaf speed that could affect the measured leaf position, since a leaf speed of 20 mm∕s is ∼2.5 mm per image if the image acquisition is 8 frames per second. In this study, uncertainty of the MLC positions and the VORTE system for leaves with high speeds was not discriminated.
Figure 6.
MLC-leaf speed analysis. (a) Distribution of the MLC-leaf speed for the 2-D displacement (solid line), the deformable 2-D displacement (dotted line), and the deformable 2-D displacement combined with rotation (dashed line). (b) MLC-leaf speed versus positional deviation of the MLC leaves. The higher the MLC-leaf speed, the larger is the positional deviation of the leaves.
We are investigating the dosimetric accuracy of real-time tracking using VORTE. The dose delivery expected from each designed-target aperture can be calculated using a treatment planning system. The use of an EPID in the fluoroscopic mode with a high frame rate can enable one to measure the actual delivered dose.21, 22, 23 Performing a gamma analysis for each frame, it is possible to judge the accuracy of tumor tracking dosimetrically as a function of time. This task will be the next step in our study of the applications of our VORTE.
CONCLUSION
We have developed and tested a new method called VORTE to verify the accuracy of real-time tumor tracking. This novel method can provide measurements having a spatial accuracy of 0.5 mm with a temporal resolution of about 100–120 ms. In this study, we have demonstrated that the VORTE has great potential as a verification tool for real-time tumor tracking with MLC.
ACKNOWLEDGMENTS
This study was supported in part by NIH Grant No. 1R01CA133539-01A2 and Varian Medical Systems.
References
- Dieterich S. and Pawlicki T., “Cyberknife image-guided delivery and quality assurance,” Int. J. Radiat. Oncol., Biol., Phys. 71, S126–S130 (2008). 10.1016/j.ijrobp.2007.08.081 [DOI] [PubMed] [Google Scholar]
- Keall P. J. et al. , “Geometric accuracy of a real-time target tracking system with dynamic multileaf collimator tracking system,” Int. J. Radiat. Oncol., Biol., Phys. 65, 1579–1584 (2006). 10.1016/j.ijrobp.2006.04.038 [DOI] [PubMed] [Google Scholar]
- D’Souza W., Shahid N., and Yu C., “Real-time intra-fraction-motion tracking using the treatment couch: A feasibility study,” Phys. Med. Biol. 50, 4021–4033 (2005). 10.1088/0031-9155/50/17/007 [DOI] [PubMed] [Google Scholar]
- Yi B., Han-Oh S., Berman B. L., Lerma F., and Yu C., “Real-time tumor tracking with preprogrammed dynamic multileaf collimator motion and adaptive dose-rate regulation,” Med. Phys. 35(9), 3955–3962 (2008). 10.1118/1.2965261 [DOI] [PubMed] [Google Scholar]
- Sawant A., Venkat R., Srivastava V., Carlson D., Povzner S., Cattell H., and Keall P., “Management of three-dimensional intrafraction motion through real-time DMLC tracking,” Med. Phys. 35(5), 2050–2061 (2008). 10.1118/1.2905355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick M., Starkschall G., Balter P., Antolak J., Guerrero T., Nelson C., Keall P., and Mohan R., “A novel platform simulating irregular motion to enhance assessment of respiration-correlated radiation therapy procedures,” J. Appl. Clin. Med. Phys. 6(1), 13–21 (2005). 10.1120/jacmp.2023.25319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani R., Hub M., Kessler M., and Balter J., “Technical note: A physical phantom for assessment of accuracy of deformable alignment algorithm,” Med. Phys. 34, 2785–2788 (2007). 10.1118/1.2739812 [DOI] [PubMed] [Google Scholar]
- Kashani R., Lam K., Litzenberg D., and Balter J., “Technical note: A deformable phantom for dynamic modeling in radiation therapy,” Med. Phys. 34, 199–201 (2007). 10.1118/1.2400612 [DOI] [PubMed] [Google Scholar]
- Serban M., Heath E., Stroian G., Collins D., and Seuntjens J., “A deformable phantom for 4D radiotherapy verification: Design and image registration evaluation,” Med. Phys. 35, 1094–1102 (2008). 10.1118/1.2836417 [DOI] [PubMed] [Google Scholar]
- Dieterich S., Cleary K., D’Souza W., Murphy M., Wong K., and Keall P., “Locating and targeting moving tumors with radiation beams,” Med. Phys. 35(12), 5684–5694 (2008). 10.1118/1.3020593 [DOI] [PubMed] [Google Scholar]
- James H. V., Atherton S., Budgell G. J., Kirby M. C., and Williams P. C., “Verification of dynamic multileaf collimation using an electronic portal imaging device,” Phys. Med. Biol. 45, 495–509 (2000). 10.1088/0031-9155/45/2/316 [DOI] [PubMed] [Google Scholar]
- Partridge M., Evans P. M., van Herk M., Ploeger L. S., Budgell G. J., and James H. V., “Leaf position verification during dynamic beam delivery: A comparison of three applications using electronic portal imaging,” Med. Phys. 27, 1601–1609 (2000). 10.1118/1.599026 [DOI] [PubMed] [Google Scholar]
- Pasma K. L., Dirkx M. L. P., Kroonwijk M., Visser A. G., and Heijmen B. J. M., “Dosimetric verification of intensity modulated beams produced with dynamic multileaf collimation using an electronic portal imaging device,” Med. Phys. 26, 2373–2378 (1999). 10.1118/1.598752 [DOI] [PubMed] [Google Scholar]
- Pasma K. L., Kroowijk M., de Boer J. C. J., Visser A. G., and Heijmen B. J. M., “Accurate portal dose measurement with a fluoroscopic electronic portal imaging device (EPID) for open and wedged beams and dynamic multileaf collimation,” Phys. Med. Biol. 43, 2047–2060 (1998). 10.1088/0031-9155/43/8/004 [DOI] [PubMed] [Google Scholar]
- Althof V. G. M., de Boer J. C. J., Huizenga H., Stroom J. C., Visser A. G., and Swanenburg B. N., “Physical characteristics of a commercial electronic portal imaging device,” Med. Phys. 23, 1845–1855 (1996). 10.1118/1.597836 [DOI] [PubMed] [Google Scholar]
- Han-Oh S., Yi B., Berman B., Lerma F., and Yu C., “Usefulness of guided breathing for dose-rate-regulated tracking,” Int. J. Radiat. Oncol., Biol., Phys. 73, 594–600 (2009). 10.1016/j.ijrobp.2008.09.015 [DOI] [PubMed] [Google Scholar]
- Samant S., Zheng W., Parra N., Chandler J., Gopal A., Wu J., Jain J., Zhu Y., and Sontag M., “Verification of multileaf collimator leaf positions using an electronic portal imaging device,” Med. Phys. 29(12), 2900–2912 (2002). 10.1118/1.1515760 [DOI] [PubMed] [Google Scholar]
- Parent L., Seco J., Evans P., Dance D., and Fielding A., “Evaluation of two methods of predicting MLC leaf positions using EPID measurements,” Med. Phys. 33(9), 3174–3182 (2006). 10.1118/1.2335490 [DOI] [PubMed] [Google Scholar]
- Sonke J., Ploeger L. S., Brand B., Smitsmans M., and Herk M. V., “Leaf trajectory verification during dynamic intensity modulated radiotherapy using an amorphous silicon flat panel imager,” Med. Phys. 31(2), 389–395 (2004). 10.1118/1.1639125 [DOI] [PubMed] [Google Scholar]
- Ploeger L. S., Smitsmans M., Gilhuijs K., and Herk M. V., “A method for geometrical verification of dynamic intensity modulated radiotherapy using a scanning electronic portal imaging device,” Med. Phys. 29(6), 1071–1079 (2002). 10.1118/1.1481513 [DOI] [PubMed] [Google Scholar]
- Lee L., Mao W., and Xing L., “The use of EPID-measured leaf sequence files for IMRT dose reconstruction in adaptive radiation therapy,” Med. Phys. 35(11), 5019–5029 (2008). 10.1118/1.2990782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamonti C., Casati M., and Bucciolini M., “Pretreatment verification of IMRT absolute dose distributions using a commercial a-Si EPID,” Med. Phys. 33(11), 4367–4378 (2006). 10.1118/1.2357834 [DOI] [PubMed] [Google Scholar]
- Lee C., Menk F., Cadman P., and Greer P., “A simple approach to using an amorphous silicon EPID to verify IMRT planar dose map,” Med. Phys. 36(3), 984–992 (2009). 10.1118/1.3075817 [DOI] [PubMed] [Google Scholar]