Abstract
In light of clinical and biological evidence that bile constituents exert preventive effects against colorectal cancer, we evaluated the influence of oral bilirubin and sodium taurocholate (NaTC) on intestinal tumor formation in APCMin/+ mice. Mice received bilirubin and/or bovine serum albumin (BSA) and NaTC in the drinking water for 8 weeks, after which the number, size and location of intestinal adenomas were determined. Tissue specimens were analyzed by light microscopy, TUNEL staining, immunohistochemistry for β-catenin and Ki-67 and quantitative polymerase chain reaction for farnesoid X receptor (FXR)-dependent gene expression. Colon tumor formation also was assessed in azoxymethane (AOM)-treated hyperbilirubinemic Gunn (j/j) and wild-type (+/+) rats. Compared with untreated APCMin/+ mice, the mean number of intestinal adenomas was markedly lower in both bilirubin (10.5 ± 0.9 versus 37.0 ± 5.2; ±SEM; P < 0.001) and NaTC plus BSA (14.3 ± 5.4; P = 0.01)-treated animals. Both treatment groups exhibited reduced levels of cellular proliferation in the ileum (by Ki-67 staining), but no differences in TUNEL staining or the percentage of β-catenin-positive crypts. Bilirubin feeding reduced intestinal inducible nitric oxide synthase expression, but did not alter adenoma multiplicity in APCMin/+ mice or in AOM-treated j/j versus +/+ rats. Mice receiving NaTC manifested increased intestinal expression of the FXR-regulated genes, Shp, FGF15 and IBABP, and a concomitant decrease in cyclin D1 message. Administering NaTC to APCMin/+ mice causes a marked reduction in intestinal adenomas. We postulate that this effect is mediated through activation of FXR, leading to increased Shp expression and consequent downregulation of cyclin D1.
Introduction
Colorectal adenocarcinoma afflicts 6% of the USA population and represents one of the leading causes of cancer-related death (1). Since most colorectal cancers develop within pre-existing adenomatous polyps, efforts at prevention have focused on the detection and removal of these precancerous lesions via colonoscopy (2,3). To supplement and potentially decrease the need for such invasive measures, investigators have sought to identify oral agents capable of suppressing colonic neoplasia in high-risk individuals. While aspirin and non-steroidal anti-inflammatory drugs have shown promising preventive effects (4,5), enthusiasm for their long-term use is tempered by significant gastrointestinal, renal and cardiovascular toxicity (6,7). Hence, the development of an effective and non-toxic chemoprotective agent would constitute an important breakthrough in the management of individuals at increased risk for colorectal cancer.
Although bile salts are generally thought to promote carcinogenesis (8), there is accumulating evidence that certain bile constituents are able to suppress intestinal tumor formation. One example is ursodeoxycholic acid, the oral administration of which has been associated with a reduction in dysplastic intestinal lesions in patients with primary sclerosing cholangitis (9), in carcinogen-treated rats (10) and in mice possessing a nonsense mutation in the adenomatous polyposis coli (Apc) tumor suppressor gene (APCMin/+) (11). Although the mechanism of action remains unclear, investigators have proposed a variety of pathways by which ursodeoxycholate may exert an anti-proliferative effect, including stimulation of histone hypoacetylation (12), downregulation of tumor-associated genes (13,14), induction of adhesion molecules (13) and inhibition of phosphokinases (15,16).
Tumor-suppressive effects also have been ascribed to the bile pigment bilirubin, with large population studies supporting an inverse correlation between serum bilirubin levels and all cancer mortality (17) and, more specifically, the risk of colorectal cancer (18). While bilirubin has been shown to induce apoptosis of colon cancer cells (19,20), the underlying mechanism is ill defined. Given the important role that nitric oxide is believed to play in gastrointestinal carcinogenesis (21) and in light of previous studies demonstrating that bilirubin prevents upregulation of inducible nitric oxide synthase (iNOS) (22), we speculated that bilirubin may exert an anti-proliferative effect by suppressing intestinal nitric oxide production.
In an attempt to further elucidate the modulatory effect of bilirubin on tumorigenesis, we examined the influence of this bile pigment on intestinal tumor formation and iNOS expression in APCMin/+ mice. To promote bilirubin absorption, sodium taurocholate (NaTC) and bovine serum albumin (BSA) were used as constituents of the vehicle (23). Unexpectedly, we found that NaTC causes a marked reduction in intestinal adenomas, an effect that appears to be mediated through activation of the farnesoid X receptor (FXR). Despite its ability to inhibit intestinal iNOS expression, bilirubin did not have a significant impact on adenoma formation.
Materials and methods
Chemicals
Bilirubin (bilirubin IXα) was obtained from Porphyrin Products (Logan, UT). NaTC, BSA, di-n-octylamine and azoxymethane (AOM) were purchased from Sigma Chemical Company (St Louis, MO).
Animal care
APCMin/+ and C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Gender was dictated by supplier availability; however, all mice were of the same sex for each experiment. Wild-type (+/+) and homozygous (j/j) male Gunn rats were from Harlan Sprague Dawley (Indianapolis, IN). Rodents were housed in the Laboratory Animal Medicine Services facility at the University of Cincinnati under controlled conditions (temperature 22 ± 2°C, relative humidity 50 ± 10%, 12 h light–dark cycle). Mice received a high (9%) fat diet (Picolab 5058 Mouse Diet 20; TestDiets Purina Mills, Richmond, IN) to augment adenoma formation (24). Gunn rats were maintained on standard laboratory chow (Harlan Teklad LM-485; Harlan Sprague Dawley). All experiments were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Mouse feeding studies
To assess the effect of bilirubin on intestinal tumor formation in APCMin/+ mice, groups of eight 7-week-old mice were administered no treatment (control), 0.25 mM bilirubin or the bilirubin vehicle (0.5 mM BSA plus 5 mM NaTC) in the drinking water for 9 weeks. This concentration of NaTC has previously been shown to facilitate intestinal absorption of bilirubin in rodents (23), whereas the concentration of BSA was that necessary to effectively solubilize bilirubin, as assessed by absorbance spectrophotometry. To determine the impact of individual vehicle constituents on adenoma formation in APCMin/+ mice, a subsequent experiment included four treatment groups: no treatment (control), 0.5 mM BSA alone, BSA plus bilirubin (0.25 mM) and BSA plus NaTC (5 mM). This latter experiment was terminated at 8 weeks (1 week early) to avoid the premature deaths encountered in the original control group. The effect of the above treatments on intestinal gene expression was determined in C57BL/6J mice (the background strain of APCMin/+). Assessments were made after 2 weeks of feeding, in order to insure that the hepatic bile composition had reached steady state (25). All treatments were protected from light and prepared fresh every 3 days. Animal weight and fluid intake were monitored regularly.
Quantification of macroscopic and microscopic intestinal adenomas in APCMin/+ mice
Following sacrifice, the stomach and intestines were resected en bloc, fixed in 4% paraformaldehyde and sliced longitudinally. Using a dissecting microscope, three blinded observers independently examined the entire intestinal tract (duodenum to colon), recording the number, largest diameter and location of all macroscopic (≥1 mm) adenomas. Sections of the distal ileum (4 cm in length) were subsequently embedded in paraffin and stained with hematoxylin and eosin. The number and size of all microadenomas (≤0.1 mm diameter) in the entire specimen was assessed by light microscopy.
Induction of colon tumors in rats
Wild-type (+/+) and homozygous (j/j) Gunn rats received 10 weekly intraperitoneal injections of AOM (7.5 mg/kg) beginning at 6 weeks of age. Animals were then monitored for an additional 30 weeks, following which the number, diameter and location of colon tumors were independently recorded by two blinded observers.
Serum bilirubin determination
Serum bilirubin levels were determined on a Spectra Max 340 pc ELISA plate reader (Molecular Devices, Sunnyvale, CA) using the Sigma Diagnostics Total Bilirubin Assay, which was adapted to be performed on 20 μl of serum (26). Where indicated, serum bilirubin also was measured by the more sensitive high-performance liquid chromatography method of McDonagh et al. (27) using a Beckman Coulter Gold 126 Solvent Module HPLC system (Fullerton, CA) and a C-18 Ion Pair Analytical Ultrasphere® IP Column (5 μ, 4.6 mm ID and 250 mm length) fitted with a 4.5 cm pre-column (Beckman Coulter). Aliquots of serum (20 μl) were added to 100 μl ice-cold 0.1 M methanolic di-n-octylamine acetate, vortexed and microfuged for 30 s. The supernatant was eluted at room temperature with 5% water/methanolic di-n-octylamine acetate at a flow rate of 1 ml/min. Bilirubin was quantified by absorbance at 450 nm using standard curves generated with bilirubin purified according to the method of McDonagh et al. (28).
TUNEL and immunohistochemical staining of intestinal tissue
Fluorescence microscopy (ex: 350 nm) was employed to visualize apoptotic cells utilizing the TACS TdT In Situ Apoptosis Detection System (R&D Systems, Minneapolis, MN). Immunohistochemical staining for β-catenin was performed with IVIEW DAB (Ventana Medical Systems, Tucson, AZ) using monoclonal mouse anti-β-catenin (BD Transduction Laboratories, San Diego, CA) and a biotinylated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Counterstaining was performed with hematoxylin bluing reagent (Ventana Medical Systems), with isotype IgG (Santa Cruz Biotechnology) serving as control. Staining for Ki-67 was performed with a Discovery XT automated staining module (Ventana Medical Systems) utilizing rabbit anti-mouse Ki67 antibody (diluted 1:1) and biotinylated anti-rabbit secondary antibody, followed by counterstaining with nuclear fast red (Poly Scientific, Bay Shore, NY). Quantification of tissue staining was performed in a blinded manner.
Quantitative polymerase chain reaction analysis of intestinal gene expression
Total RNA was extracted from snap frozen tissue using the RNeasy Mini Kit (QIAGEN, Valencia, CA). Quantitative polymerase chain reaction was performed with an Mx3000P system (Stratagene, Cedar Creek, TX) using the following algorithm: activation at 95°C for 10 min, denaturation for 40 cycles at 95°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 30 s. Primers for murine Shp (sense: 5′-atcctcttcaacccagatgtgcca-3′ and antisense: 5′-taccagaagggtgcctggaatgtt-3′), cyclin D1 (sense: 5′-gcgtaccctgacaccaatct-3′ and antisense: 5′-tggaaagaaagtgcgttgtg-3′), FGF15 (sense: 5′-gattgccatcaaggacgtcag-3′ and antisense: 5′-tcagcccgtatatcttgccg-3′), IBABP (sense: 5′-acttcacctggtcccagtcttact-3′ and antisense: 5′-agatctccaccaacttgtcaccca-3′), iNOS (sense: 5′-ctgctggtggtgacaagcacattt-3′ and antisense: 5′-atgtcatgagcaaaggcgcagaac-3′) and glyceraldehyde-3-phosphate dehydrogenase (sense: 5′-tcaacagcaactcccactcttcca-3′ and antisense: 5′-accctgttgctgtagccgtattca-3′) were utilized.
Statistical analysis
Data were analyzed using a computer-based statistical program (SSI SigmaStat, San Jose, CA). Differences between mean values were evaluated by analysis of variance with Scheffe comparison, and correlations were assessed by Pearson's product-moment and Spearman's rank correlation coefficient.
Results
Influence of oral bilirubin on adenoma formation in Min mice
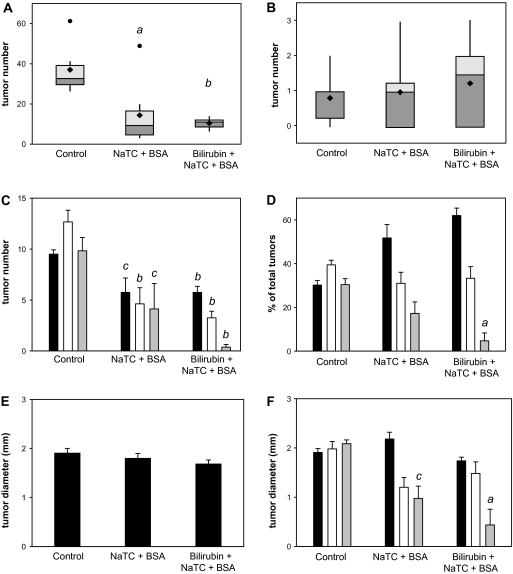
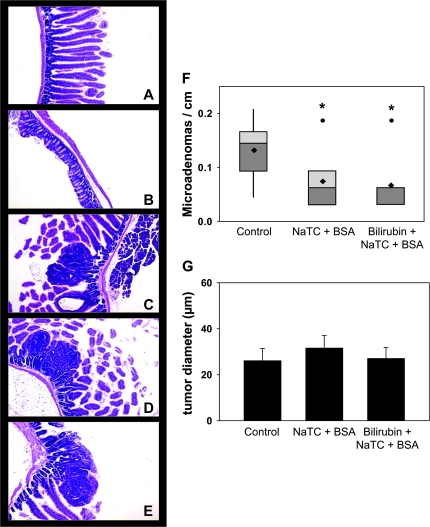
To investigate the effect of bilirubin on intestinal tumor formation, groups of eight male APCMin/+ mice were administered no treatment (control), 0.25 mM bilirubin or the bilirubin vehicle (0.5 mM BSA plus 5 mM NaTC) in the drinking water for 9 weeks. Mean weight and fluid consumption did not differ between the three groups over the course of the experiment. Two mice in the control group died during the final week of treatment and could not be analyzed. The mean number of macroscopic small intestinal adenomas per mouse was markedly lower in bilirubin-treated animals (10.5 ± 0.9; ±SEM; P < 0.001) and, unexpectedly, in vehicle-treated (14.3 ± 5.4; P = 0.01) animals, as compared with the untreated controls (37.0 ± 5.2) (Figure 1A). There were no significant differences in colon adenoma multiplicity between the groups (Figure 1B). While an inhibitory effect of bilirubin and vehicle on adenoma number was observed in all segments of the small intestine (Figure 1C–D), it was most pronounced in the distal third (ileum). Overall, tumor diameter did not differ between groups (Figure 1E); although, ileal tumors were significantly smaller in treated animals (Figure 1F). Microscopic analysis of ileal specimens revealed no inflammation and no gross intergroup differences in the histological appearance of non-dysplastic (Figure 2A and B) or dysplastic (Figure 2C–E) tissue. Consistent with the macroscopic findings, there were significantly fewer microadenomas (≤0.1 mm diameter) per centimeter in APCMin/+ mice fed bilirubin (0.066 ± 0.018) or vehicle (0.074 ± 0.019) versus controls (0.132 ± 0.025) (Figure 2F), although mean tumor diameter was similar (Figure 2G). These data demonstrate that oral bilirubin and vehicle are potent inhibitors of intestinal tumorigenesis in APCMin/+ mice.
Fig. 1.
Effect of oral bilirubin on adenoma formation in APCMin/+ mice. Male APCMin/+ mice received no treatment (control), vehicle (NaTC + BSA) or bilirubin plus vehicle for 9 weeks. Panel A depicts a box (upper and lower quartiles) and whisker (furthest point within 1.5 interquartile ranges from the median) plot of the number of macroscopic adenomas in the small intestine by treatment group. The horizontal line reflects the median, the diamond the mean and the dots data points that lie outside the 10th and 90th percentiles. Panel B displays a similar plot of macroscopic colon adenomas. Panel C shows the number (±SEM) and panel D shows the percent of total adenomas in the upper (black bars), middle (white bars) and lower (gray bars) third of the small intestine. The mean diameter of small bowel adenomas is shown in panel E, whereas panel F displays tumor diameter stratified by tertile of small intestine; aP < 0.01 versus control, bP < 0.001 versus control and cP < 0.05 versus control.
Fig. 2.
Impact of bilirubin on small intestine histology and microadenoma formation. Sections of small (panel A; ×80) and large (panel B; ×80) intestine from bilirubin-treated mice demonstrate normal histological architecture on hematoxylin and eosin staining. Panels C–E display microadenomas in the small intestine of control, vehicle-treated (NaTC + BSA) and bilirubin-treated APCMin/+ mice, respectively (×100). Panel F shows a box and whisker plot of the number of microadenomas per cm of distal small intestine, whereas panel G graphs the average diameter (±SEM) of microadenoma; *P < 0.05 versus control.
Effect of bilirubin on intestinal β-catenin activity, cell proliferation and apoptosis
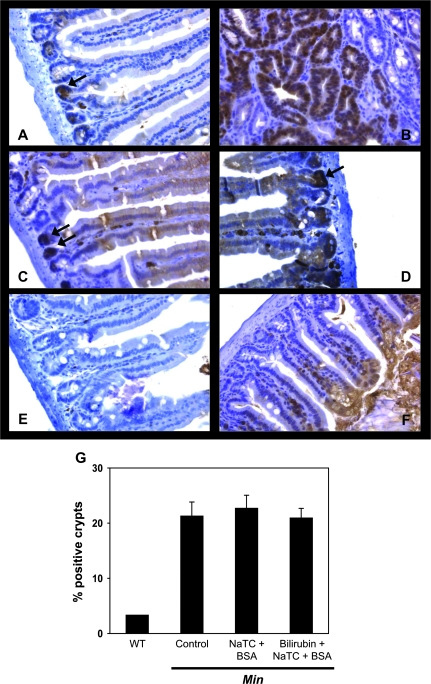
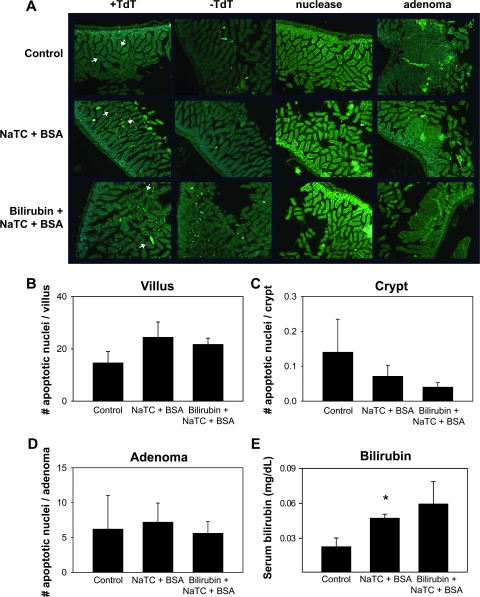
Since loss of Apc heterozygosity, leading to enhanced nuclear translocation of β-catenin, is a seminal event in intestinal adenoma formation in APCMin/+ mice (29), sections of small intestine were analyzed for nuclear accumulation of β-catenin by immunohistochemistry (Figure 3A–F). Consistent with previous reports (30,31), the number of β-catenin-associated crypts was 10-fold higher in APCMin/+ versus C57BL/6J (wild-type) mice (Figure 3G); however, staining was similar in untreated, vehicle-treated and bilirubin-treated animals, indicating that tumor inhibition is effected at a step that is subsequent to Apc loss. There also was no difference in the number of TUNEL-positive cells in the intestinal villi, crypts, or within areas of dysplasia (Figure 4), suggesting that reduced adenoma formation in treated mice was not the result of increased apoptosis.
Fig. 3.
Immunohistochemical staining of small intestinal tissue for β-catenin. Shown are representative non-dysplastic (panel A) and dysplastic (panel B) sections of small intestine from untreated APCMin/+ mice, and non-dysplastic tissue from vehicle-treated (panel C) or bilirubin (panel D)-treated APCMin/+ mice stained for β-catenin (×320). Positively stained nuclei are indicated by the arrows. Specimens from untreated APCMin/+ mice probed with an isotype antibody (panel E), and from C57BL/6J (wild-type) mice stained for β-catenin (panel F) serve as controls. Panel G plots the percentage of small intestinal crypts (±SEM) staining positive for β-catenin in wild-type (WT), untreated APCMin/+ mice (control) and APCMin/+ mice treated with vehicle (NaTC + BSA) or bilirubin.
Fig. 4.
TUNEL staining for intestinal apoptosis. The first column (+TdT) of panel A displays representative TUNEL-stained sections of small intestinal tissue from untreated (control), vehicle-treated (NaTC + BSA) and bilirubin-treated APCMin/+ mice (×80). The arrows highlight positive nuclei. The second column (−TdT) shows negative controls in which terminal deoxynucleotidyl transferase was omitted, whereas sections treated with nuclease serve as positive controls (third column). The fourth column depicts TUNEL staining within adenomas. Panels B–D display the mean number of apoptotic nuclei (±SEM) per villus, crypt and microadenoma, respectively. Mean serum bilirubin levels (±SEM), measured colorimetrically, are presented in panel E; *P < 0.05 versus control.
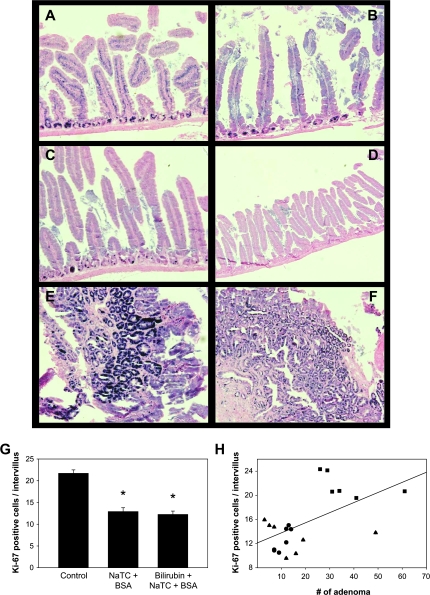
To assess whether the treatments altered cell proliferation, intestinal specimens were stained for the Ki-67 antigen (32), which is abundantly expressed in the intestinal crypts of untreated APCMin/+ mice (Figure 5A). Staining, as quantified by the number of Ki-67-positive nuclei per intervillus unit (33), was significantly less in mice receiving vehicle and/or bilirubin (Figure 5B, C and G) and was highly correlated (P = 0.007) with the number of macroscopic adenomas (Figure 5H). Although not formally measured, treated mice also appeared to exhibit reduced Ki-67 within adenomas (Figure 5E and F). Taken together, these findings support that both bilirubin and vehicle inhibit intestinal tumorigenesis by suppressing epithelial cell proliferation. A potential explanation as to why both treatments are equally effective is derived from the unexpected observation that serum bilirubin concentrations are increased to a similar degree in APCMin/+ mice fed vehicle (47 ± 3 μg/dl; P = 0.04) or bilirubin (59 ± 19 μg/dl; P = 0.06) as compared with untreated animals (23 ± 7 μg/dl) (Figure 4E). We postulate that bilirubin levels are elevated in vehicle-treated mice because NaTC facilitates the absorption of endogenous bilirubin normally present in the intestine (34).
Fig. 5.
Ki-67 staining for cellular proliferation. Ki-67-stained sections of small intestine from untreated (panel A), vehicle-treated (panel B) and bilirubin-treated (panel C) APCMin/+ mice are displayed (×200). Panel D shows tissue from an untreated APCMin/+ mouse probed with an isotype antibody as control (×100). Panels E and F depict staining of adenomas from untreated and bilirubin-treated mice (×200), respectively. The mean number of Ki-67-positive cells per intervillus unit (n = 30 units per animal; ±SEM) is stratified by treatment group in panel G, while panel H plots the number of macroscopic intestinal adenomas versus Ki-67 staining for individual untreated (filled squares), vehicle-treated (filled triangles) and bilirubin-treated (closed circles) APCMin/+ mice. The line reflects a linear fit of the data (correlation coefficient: 0.578; P = 0.007); *P < 0.001 versus control.
Assessment of colon tumor formation in Gunn rats
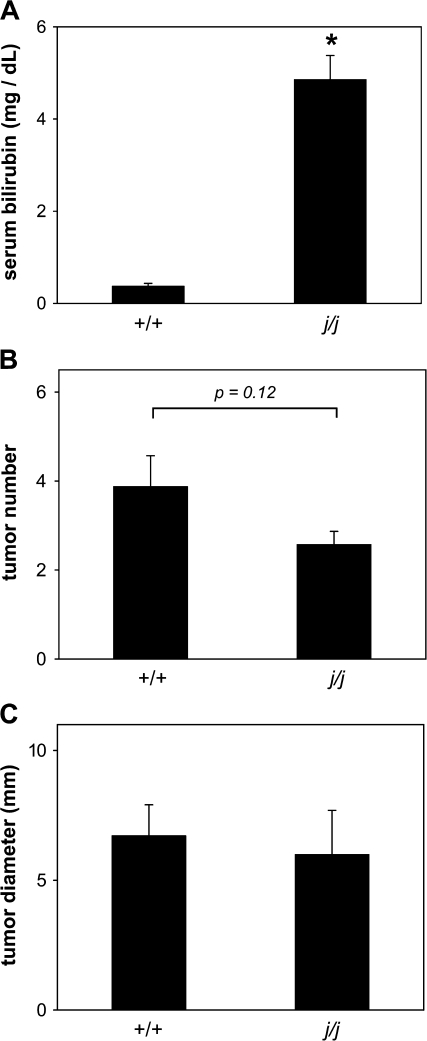
To establish whether bilirubin was the primary effector of the reduced number of intestinal adenomas observed in vehicle-treated APCMin/+ mice, we employed an animal model that obviates the need for oral bilirubin. The homozygous (j/j) Gunn rat possesses a mutation in the uridine diphosphate-glucuronosyltransferase 1A gene locus, which results in persistent hyperbilirubinemia due to impaired bilirubin conjugation (Figure 6A). Dysplasia was induced by administering AOM to groups of eight male wild-type (+/+) and j/j rats. The number (Figure 6B) and diameter (Figure 6C) of colon tumors was assessed at 30 weeks. One j/j animal was excluded from the analysis because a facial lesion necessitated premature sacrifice. Although a trend toward lower numbers of tumors in j/j (2.6 ± 0.3) versus +/+ (3.9 ± 0.7) rats was found, the difference was not statistically significant (P = 0.12), suggesting that bilirubin is not a potent inhibitor of intestinal tumorigenesis.
Fig. 6.
Serum bilirubin levels and colon tumor formation in AOM-treated Gunn rats. Panel A displays mean serum bilirubin levels (±SEM) in AOM-treated wild-type (+/+) and Gunn (j/j) rats as determined by colorimetric assay. The average number and diameter of colon tumors are shown in panels B and C, respectively; *P = 0.001.
Effect of vehicle constituents on adenoma formation in APCMin/+ mice
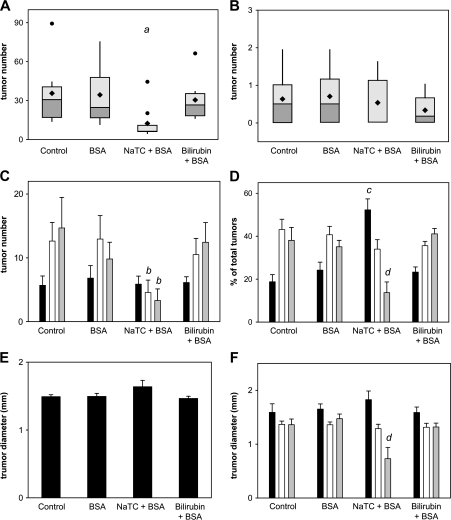
In order to validate our findings and ascertain which constituent of the vehicle is the primary effector of tumor suppression, groups of eight female APCMin/+ mice were administered no treatment (control), BSA alone (0.5 mM), BSA plus NaTC (5 mM) or BSA plus bilirubin (0.25 mM) as described above, except that treatment was shortened to 8 weeks to avoid the premature fatalities encountered in the control group of the prior experiment. It was again observed that oral NaTC plus BSA is associated with a substantial reduction in the number of small intestinal adenomas per mouse (13.3 ± 4.8; P = 0.02) as compared with untreated (35.6 ± 8.6), BSA-treated (34.6 ± 8.1) or bilirubin plus BSA-treated (30.8 ± 5.8) animals (Figure 7A). Also consistent with our previous findings, the impact of NaTC plus BSA on adenoma multiplicity (Figure 7C and D) and diameter (Figure 7E and F) was most pronounced in the ileum, whereas colon tumor burden was unaffected (Figure 7B). These data implicate NaTC as the constituent responsible for tumor suppression and further demonstrate that the observed effects are independent of mouse gender.
Fig. 7.
Influence of vehicle constituents on macroscopic adenoma formation. The number of macroscopic adenomas in the small intestine (panel A) and colon (panel B) of female APCMin/+ mice administered no treatment (control), BSA, NaTC plus BSA or bilirubin plus BSA are displayed as box and whisker plots (as described in Figure 1). Panels C and D stratify the number and percent of total adenomas (±SEM), respectively, by localization to the upper (black bars), middle (white bars) or lower (gray bars) third of the small intestine. The mean diameter of small intestinal adenomas is shown in panel E, with data grouped by tertile of small intestine in panel F; aP = 0.02 versus all other groups, bP < 0.05 versus middle and lower tertile for all other groups, cP = 0.001 versus upper tertile for all other groups and dP < 0.02 versus lower tertile for all other groups.
Influence of vehicle constituents on intestinal gene expression
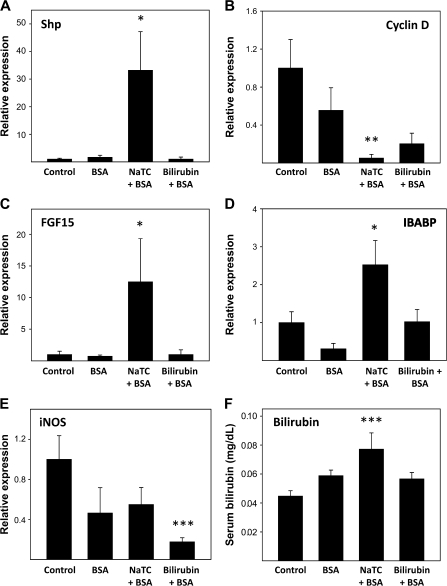
It is thought that adenoma formation in APCMin/+ mice results from unrestrained cellular proliferation caused by excessive β-catenin activity, which leads to overexpression of cell cycle promoters such as cyclin D1 and c-myc (35). It has been previously demonstrated that NaTC feeding enriches murine bile in deoxycholic acid (36), a potent activator ligand for FXR (37). As FXR activation has been shown to inhibit tumorigenesis (38–41), potentially by inducing short heterodimer partner (Shp), a negative regulator of cyclin D1 (42), we postulated that NaTC-induced suppression of adenoma formation in APCMin/+ mice is mediated by FXR. To test this hypothesis, we employed quantitative polymerase chain reaction to assess for expression of FXR-regulated genes in the ileum of groups of 10 female C57BL/6J mice (the background strain of APCMin/+) that were administered no treatment (control), BSA (0.5 mM) alone, BSA plus NaTC (5 mM) or BSA plus bilirubin (0.25 mM) in the drinking water for 2 weeks. Only those animals receiving NaTC plus BSA exhibited significantly higher levels of message for Shp (Figure 8A) and lower levels of cyclin D1 (Figure 8B). Notably, expression of Shp-independent targets of FXR (43), FGF15 (Figure 8C) and IBABP (Figure 8D), also was increased by this treatment. These data are consistent with the hypothesis that the tumor-suppressive effects of NaTC occur through FXR-mediated downregulation of cyclin D1.
Fig. 8.
Effect of vehicle constituents on intestinal gene expression. Female C57BL/6J mice received no treatment (control), BSA, NaTC plus BSA or bilirubin plus BSA for 2 weeks, after which message levels for Shp (panel A), cyclin D1 (panel B), FGF15 (panel C), IBABP (panel D) and iNOS (panel E) in the ileum were determined by quantitative polymerase chain reaction. Expression is normalized to glyceraldehyde-3-phosphate dehydrogenase and plotted relative to control (±SEM). Panel F displays mean serum bilirubin levels as measured by high-performance liquid chromatography; *P < 0.05 versus all other groups, **P < 0.05 versus control and BSA and ***P < 0.05 versus control.
Since iNOS inhibitors attenuate tumor formation in APCMin/+ mice (44,45) and since bilirubin blocks iNOS upregulation (22), we also quantified ileal expression of iNOS in treated by quantitative polymerase chain reaction. Despite lower levels of iNOS message in C57BL/6J mice receiving bilirubin plus BSA (Figure 8E), similarly treated APCMin/+ mice did not exhibit a reduced tumor burden (Figure 7A). Counterintuitively, only mice fed NaTC plus BSA (but ‘not’ bilirubin plus BSA) had increased levels of serum bilirubin (Figure 8F), suggesting that oral NaTC promotes enterohepatic cycling of endogenous bilirubin, whereas BSA-bound bilirubin is poorly absorbed, presumably because efficient digestion of albumin in the proximal gastrointestinal tract (46) leads to precipitation of bilirubin within the lumen (47). Thus, the fact that iNOS levels are reduced in animals receiving oral bilirubin, but not NaTC, implies that the lumenal (rather than serum) concentration of bilirubin modulates intestinal iNOS expression.
Discussion
The impetus for investigating the influence of bilirubin on intestinal tumorigenesis was derived from epidemiological (17,18) and in vitro (19,20) data suggesting that this bile pigment exerts a chemopreventive effect. Unexpectedly, we found that oral administration of the bilirubin vehicle (comprised BSA and NaTC) to APCMin/+ mice caused a marked reduction in tumor multiplicity and further showed that NaTC is the principal effector of tumor suppression. These results are surprising in light of fact that taurocholate has previously been shown to ‘promote’ tumor formation in rodent models (8). Specifically, Ho et al. (48) reported that feeding 0.2% taurocholic acid to C57BL/6J mice injected with the carcinogen N-methyl-N′-nitro-N-nitrosoguanidine increased the number and histological stage of intestinal adenocarcinoma. Taurocholate also was found to augment the formation of N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric tumors in Wistar rats (49).
The incongruity between our results and those of these other investigators could potentially reflect differences in the animal models employed. While the merits of available models of colorectal cancer have been debated (50), the APCMin/+ mouse is considered relevant to human disease because it possesses a mutation similar to that commonly found in individuals with sporadic and inherited colorectal cancer syndromes (51). There have been two prior studies of the effect of bile acids on intestinal adenoma formation in APCMin/+ mice. Mahmoud et al. (30) reported an increased number of duodenal tumors in animals fed a diet containing 0.5% chenodeoxycholate; however, overall intestinal tumor burden was actually reduced by 33% (P = 0.10). Jacoby et al. (11) found a similar 31% decrease in the number of intestinal adenomas in APCMin/+ mice receiving ursodeoxycholic acid. We observed a substantially larger (∼65%) inhibitory effect of taurocholate on tumor multiplicity, which may reflect our use of higher doses of bile salt or, potentially, a unique mechanism of action.
In this regard, feeding taurocholate to mice previously has been shown to enrich hepatic bile in deoxycholic acid (36), a potent activator ligand for FXR (37). Evidence that FXR acts as a tumor suppressor is derived from studies demonstrating that loss of FXR is associated with increased intestinal dysplasia and tumor formation in both APCMin/+ and in AOM-treated C57BL/6 mice (40,41). Conversely, gain of FXR function was found to inhibit colon cancer cell growth in a xenograft mouse model (41). There are data to support that FXR impedes tumorigenesis by inducing apoptosis (41), suppressing inflammation (38–41) and/or inhibiting cell proliferation (38–40), the latter potentially through the induction of Shp, a negative regulator of cyclin D1 (42) and inhibitor of carcinoma cell growth (52). We found that mice fed NaTC exhibit increased ileal expression of Shp, as well as other FXR-dependent genes, FGF15 and IBABP, confirming that oral taurocholate activates FXR in the murine small intestine. We further show that NaTC-fed mice exhibit reduced intestinal cyclin D1 expression and cellular proliferation (by Ki-67 staining), in the absence of alterations in cellular apoptosis or β-catenin activity. These findings are consistent with the hypothesis that taurocholate inhibits cell cycling through Shp-dependent downregulation of cyclin D1. Notably, NaTC decreases intestinal tumor formation to an extent that is similar to what has been reported in APCMin/+ mice possessing a simultaneous deletion of the cyclin D1 gene (53); however, our data in no way exclude a contribution of other mechanisms, such as augmented innate immunity (54), to NaTC-mediated tumor suppression.
Inhibitors of iNOS have been shown to reduce tumor formation in APCMin/+ mice (44,45). Since bilirubin prevents iNOS upregulation (22), we postulated that it would suppress tumorigenesis by decreasing iNOS levels in the intestine. Our finding that mice fed bilirubin plus BSA exhibit reduced intestinal iNOS expression, yet tumor multiplicity remains unaltered, argues against this hypothesis. While these data suggest that bilirubin is not a potent anti-proliferative agent, interpretation is confounded by the inability to establish an appropriate control group. Serum bilirubin levels in vehicle-treated mice are equivalent to those in animals receiving vehicle ‘plus’ bilirubin (Figure 4H), most probably because NaTC facilitates uptake of the endogenous bilirubin that is normally present in the gut (55). In the absence of a solubilizing agent such as NaTC (23,47), intestinal absorption of bilirubin is negligible because it rapidly precipitates within the lumen (56), as occurred when BSA alone was used as the vehicle. In an attempt to circumvent these problems, we assessed tumor formation in hyperbilirubinemic (Gunn) rats. Although the 33% reduction in colon tumors in j/j versus +/+ animals was not significant, the limitations of the model, including low sensitivity (due to the small number of tumors per animal), the possibility that the Gunn mutation alters AOM metabolism and potential compensatory responses to the genetic defect, make firm conclusions difficult.
In summary, we show that NaTC causes a pronounced reduction in the number of intestinal adenomas in APCMin/+ mice. We postulate that this effect is mediated through the activation of FXR and downstream suppression of cyclin D1, resulting in reduced epithelial cell proliferation. As FXR is downregulated in human colon cancers (57), therapeutic strategies directed at augmenting FXR expression may prove effective for chemoprevention (41). While oral bilirubin reduces intestinal iNOS expression, its impact on tumorigenesis remains uncertain.
Funding
National Institutes of Health (CA119006) to S.D.Z.; Ohio Division Supported Pilot Research Grant, American Cancer Society to S.D.Z.; Cancer Research and Prevention Foundation to S.D.Z.
Acknowledgments
The authors wish to gratefully acknowledge Dr Anthony McDonagh for advice regarding serum bilirubin measurement, Jay Card and Dr Jiang Wang for assistance with microscopy and image acquisition, Dr Keith Stringer for guidance with immunohistochemical staining and Drs Elizabeth Mann and Mitchell Cohen for technical support with the performance of high-performance liquid chromatography. We also would like to extend a special thanks to Judith Mouch's ninth grade biology class at Indian Hill High School for their creative ideas and insights. A preliminary report of this work was published in abstract form (U.A., P.K., D.L.H.S. and S.D.Z. Effect of bile constituents on intestinal adenoma formation in Min mice. Hepatology 2005; 42: 459A).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AOM
azoxymethane
- Apc
adenomatous polyposis coli
- BSA
bovine serum albumin
- FXR
farnesoid X receptor
- NaTC
sodium taurocholate
- Shp
short heterodimer partner
References
- 1.Greenlee RT, et al. Cancers statistics, 2000. CA Cancer J.Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Burt RW. Colon cancer screening. Gastroenterology. 2000;119:837–853. doi: 10.1053/gast.2000.16508. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N. Engl. J. Med. 1993;329:1977–1983. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Moran EM. Epidemiological and clinical aspects of nonsteroidal anti-inflammatory drugs and cancer risks. J. Environ. Pathol. Toxicol. Oncol. 2002;21:193–201. [PubMed] [Google Scholar]
- 5.Giardiello FM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N. Engl. J. Med. 2002;346:1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks P. Use and benefits of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:9S–13S. doi: 10.1016/s0002-9343(97)00204-0. [DOI] [PubMed] [Google Scholar]
- 7.Kerr DJ, et al. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N. Engl. J. Med. 2007;357:360–369. doi: 10.1056/NEJMoa071841. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein H, et al. Bile acids as carcinogens in human gastrointestinal cancers. Mut. Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Tung BY, et al. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann. Intern. Med. 2001;134:89–95. doi: 10.7326/0003-4819-134-2-200101160-00008. [DOI] [PubMed] [Google Scholar]
- 10.Earnest DL, et al. Chemoprevention of azoxymethane-induced colonic carcinogenesis by supplemental dietary ursodeoxycholic acid. Cancer Res. 1994;54:5071–5074. [PubMed] [Google Scholar]
- 11.Jacoby RF, et al. Ursodeoxycholate/sulindac combination treatment effectively prevents intestinal adenomas in a mouse model of polyposis. Gastroenterology. 2004;127:838–844. doi: 10.1053/j.gastro.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Akare S, et al. Ursodeoxycholic acid modulates histone acetylation and induces differentiation and senescence. Int. J. Cancer. 2006;119:2958–2969. doi: 10.1002/ijc.22231. [DOI] [PubMed] [Google Scholar]
- 13.Wali RK, et al. Ursodeoxycholic acid and F6-D3 inhibit aberrant crypt proliferation in the rat azoxymethane model of colon cancer: roles for cyclin D1 and E-cadherin. Cancer Epidemiol. Biomarkers Prev. 2002;11:1653–1662. [PubMed] [Google Scholar]
- 14.Khare S, et al. Ursodeoxycholic acid inhibits Ras mutations, wild-type ras activation, and cyclooxygenase-2 expression in colon cancer. Cancer Res. 2003;63:3517–3523. [PubMed] [Google Scholar]
- 15.Wali RK, et al. Mechanism of action of chemoprotective ursodeoxycholate in the azoxymethane model of rat colonic carcinogenesis: potential roles of protein kinase C-α, -β II, and -ζ. Cancer Res. 1995;55:5257–5264. [PubMed] [Google Scholar]
- 16.Im E, et al. Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells. J. Nutr. 2004;134:483–486. doi: 10.1093/jn/134.2.483. [DOI] [PubMed] [Google Scholar]
- 17.Temme EHM, et al. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 2001;12:887–894. doi: 10.1023/a:1013794407325. [DOI] [PubMed] [Google Scholar]
- 18.Zucker SD, et al. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827–835. doi: 10.1002/hep.20407. [DOI] [PubMed] [Google Scholar]
- 19.Keshavan P, et al. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int. J. Cancer. 2004;112:433–445. doi: 10.1002/ijc.20418. [DOI] [PubMed] [Google Scholar]
- 20.Rao P, et al. Bilirubin exhibits a novel anti-cancer effect on human adenocarcinoma. Biochem. Biophys. Res. Commun. 2006;342:1279–1283. doi: 10.1016/j.bbrc.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal M, et al. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G626–G634. doi: 10.1152/ajpgi.2001.281.3.G626. [DOI] [PubMed] [Google Scholar]
- 22.Wang WW, et al. Bilirubin inhibits iNOS expression and nitric oxide production in response to endotoxin. Hepatology. 2004;40:424–433. doi: 10.1002/hep.20334. [DOI] [PubMed] [Google Scholar]
- 23.Lester R, et al. Intestinal absorption of bile pigments. I. The enterohepatic circulation of bilirubin in the rat. J. Clin. Invest. 1963;42:736–746. doi: 10.1172/JCI104766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasan HS, et al. Dietary fat influences on polyp phenotype in multiple intestinal neoplasia mice. Proc. Natl Acad. Sci. USA. 1997;94:3308–3313. doi: 10.1073/pnas.94.7.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy JR, et al. Effect of bile acid composition and manipulation of enterohepatic circulation on leptin gene regulation. Metabolism. 1998;47:285–291. doi: 10.1016/s0026-0495(98)90258-x. [DOI] [PubMed] [Google Scholar]
- 26.Zucker SD, et al. Mechanism of indinavir-induced hyperbilirubinemia. Proc. Natl Acad. Sci. USA. 2001;98:12671–12676. doi: 10.1073/pnas.231140698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonagh AF, et al. Hepatobiliary excretion of biliverdin isomers and C10-substituted biliverdins in MRP2-deficient (TR)- rats. Biochem. Biophys. Res. Commun. 2002;293:1077–1083. doi: 10.1016/S0006-291X(02)00325-X. [DOI] [PubMed] [Google Scholar]
- 28.McDonagh AF, et al. The ready isomerization of bilirubin IXα in aqueous solution. Biochem. J. 1972;129:797–800. doi: 10.1042/bj1290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luongo C, et al. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54:5947–5952. [PubMed] [Google Scholar]
- 30.Mahmoud NN, et al. Administration of an unconjugated bile acid increases duodenal tumors in a murine model of familial adenomatous polyposis. Carcinogenesis. 1999;20:299–303. doi: 10.1093/carcin/20.2.299. [DOI] [PubMed] [Google Scholar]
- 31.Kongkanuntn R, et al. Dysregulated expression of β-catenin marks early neoplastic change in Apc mutant mice, but not all lesions arising in Msh2 deficient mice. Oncogene. 1999;18:7219–7225. doi: 10.1038/sj.onc.1203181. [DOI] [PubMed] [Google Scholar]
- 32.Hu R, et al. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- 33.Valentin-Vega YA, et al. Mdm4 loss in the intestine epithelium leads to compartmentalized cell death but no tissue abnormalities. Differentiation. 2009;77:442–449. doi: 10.1016/j.diff.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brink MA, et al. Bilirubin cycles enterohepatically after ileal resection in the rat. Gastroenterology. 1996;110:1945–1957. doi: 10.1053/gast.1996.v110.pm8964422. [DOI] [PubMed] [Google Scholar]
- 35.Wood PA, et al. Period 2 mutation accelerates Apc Min/+ tumorigenesis. Mol. Cancer Res. 2008;6:1786–1793. doi: 10.1158/1541-7786.MCR-08-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolters H, et al. Effects of bile salt flux variations on the expression of hepatic bile salt transporters in vivo in mice. J. Hepatol. 2002;37:556–563. doi: 10.1016/s0168-8278(02)00247-7. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 38.Yang F, et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 39.Kim I, et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maran RRM, et al. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J. Pharmacol. Exp. Ther. 2009;328:469–477. doi: 10.1124/jpet.108.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modica S, et al. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology. 2008;48:289–298. doi: 10.1002/hep.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiang JY. Bile acids: regulation of synthesis. J. Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn B, et al. Suppression of intestinal polyposis in ApcMin/+ mice by inhibiting nitric oxide production. Cancer Res. 2001;61:8357–8360. [PubMed] [Google Scholar]
- 45.Kohno H, et al. A specific inducible nitric oxide synthase inhibitor, ONO-1714, attenuates inflammation-related large bowel carcinogenesis in male ApcMin/+ mice. Int. J. Cancer. 2007;121:506–513. doi: 10.1002/ijc.22736. [DOI] [PubMed] [Google Scholar]
- 46.Yamada C, et al. Digestion and gastrointestinal absorption of the 14-16-kDA rice allergens. Biosci. Biotechnol. Biochem. 2006;70:1890–1897. doi: 10.1271/bbb.60054. [DOI] [PubMed] [Google Scholar]
- 47.Vitek L, et al. Enterohepatic cycling of bilirubin as a cause of ‘black’ pigment gallstones in adult life. Eur. J. Clin. Invest. 2003;33:799–810. doi: 10.1046/j.1365-2362.2003.01214.x. [DOI] [PubMed] [Google Scholar]
- 48.Ho SB, et al. Experimental model of upper intestinal adenocarcinoma induced by N-methyl- N′-nitro- N-nitrosoguanidine in C57BL/6 mice. Cancer Lett. 1995;91:177–183. doi: 10.1016/0304-3835(95)03748-l. [DOI] [PubMed] [Google Scholar]
- 49.Salmon RJ, et al. Effect of taurocholic acid feeding on methyl-nitro-N-nitroso-guanidine induced gastric tumors. Cancer Lett. 1984;22:315–320. doi: 10.1016/0304-3835(84)90168-x. [DOI] [PubMed] [Google Scholar]
- 50.Corpet DE, et al. Point: from animal models to prevention of colon cancer: systematic review of chemoprevention in Min mice and choice of the model system. Cancer Epidemiol. Biomarkers Prev. 2004;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 51.Shoemaker AR, et al. Studies of neoplasia in the Min mouse. Biochim. Biophys. Acta. 1997;1332:F25–F48. doi: 10.1016/s0304-419x(96)00041-8. [DOI] [PubMed] [Google Scholar]
- 52.He N, et al. Epigenetic inhibition of nuclear receptor small heterodimer partner is associated with and regulates hepatocellular carcinoma growth. Gastroenterology. 2008;134:793–802. doi: 10.1053/j.gastro.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Wilding J, et al. Cyclin D1 is not an essential target of β-catenin signaling during intestinal tumorigenesis, but it may act as a modifier of disease severity in multiple intestinal neoplasia (Min) mice. Cancer Res. 2002;62:4562–4565. [PubMed] [Google Scholar]
- 54.Vavassori P, et al. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez NM, et al. Ursodeoxycholic acid and cholesterol induce enterohepatic cycling of bilirubin in rodents. Gastroenterology. 1998;115:722–732. doi: 10.1016/s0016-5085(98)70152-0. [DOI] [PubMed] [Google Scholar]
- 56.Van der Veere CN, et al. Rapid association of unconjugated bilirubin with amorphous calcium phosphate. J. Lipid Res. 1995;36:1697–1707. [PubMed] [Google Scholar]
- 57.De Gottardi A, et al. The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differentially expressed in colon cancer. Dig. Dis. Sci. 2004;49:982–989. doi: 10.1023/b:ddas.0000034558.78747.98. [DOI] [PubMed] [Google Scholar]