Abstract
Over 1 billion pounds of organophosphorus (OP) chemicals are manufactured worldwide each year, including 70 million pounds of pesticides sprayed in the US. Current methods to monitor environmental and occupational exposures to OPs such as chlorpyrifos (CPS) have limitations, including low specificity and sensitivity, and short time windows for detection. Biomarkers for the OP tricresyl phosphate (TCP), which can contaminate bleed air from jet engines and cause an occupational exposure of commercial airline pilots, crewmembers and passengers, have not been identified.
The aim of our work has been to identify, purify, and characterize new biomarkers of OP exposure. Butyrylcholinesterase (BChE) inhibition has been a standard for monitoring OP exposure. By identifying and characterizing molecular biomarkers with longer half-lives, we should be able to clinically detect TCP and OP insecticide exposure after longer durations of time than are currently possible.
Acylpeptide hydrolase (APH) is a red blood cell (RBC) cytosolic serine proteinase that removes N-acetylated amino acids from peptides and cleaves oxidized proteins. Due to its properties, it is an excellent candidate for a biomarker of exposure. We have been able to purify APH and detect inhibition by both CPS and metabolites of TCP. The 120-day lifetime of the RBC offers a much longer window for detecting exposure. The OP-modified serine conjugate in the active site tryptic peptide has been characterized by mass spectrometry.
This research uses functional proteomics and enzyme activities to identify and characterize useful biomarkers of neurotoxic environmental and occupational OP exposures.
Keywords: Butyryl cholinesterase, Acylpeptide hydrolase, Biomarkers of OP exposure, Mass spectrometry, Affinity purification, Immunomagnetic beads
1 Introduction
Biomarkers are used in many aspects of health surveillance to identify disease presence, track disease progression, monitor drug delivery or metabolism, or monitor chemical exposure. There is a recognized need to expand and improve biomarker identification and quantification. Exposures to the xenobiotic organophosphates (OPs) range from low-level, chronic exposure during pesticide application (e.g., on farms, in residences, or in the workplace) to high-dose, acute exposures including release of nerve agents or toxic industrial OPs. OPs can have both rapid and chronic toxicity, due to their action on specific esterases and lipases, most notably acetylcholinesterase (AChE) and neuropathic target esterase (NTE). In each of the above examples, a rapid and accurate assessment of the OP to which the person was exposed, degree of exposure, and time period of exposure will help direct therapy to the victim and assess the level of threat to others. Traditionally, metabolites for specific OPs can be measured in the urine after an exposure, such as 3,5,6-trichloro-2-pyridinol for chlorpyrifos (CPS). However, there are several problems with this method. Usually, the metabolite is excreted for only a short period of several days after a significant exposure. In addition, due to the widespread use of CPS and environmental persistence of its breakdown products, the metabolites appear at a high background level in the general population (Hill, 1995). Their appearance does not indicate whether the individual was exposed to the harmful parent compound, or the harmless breakdown product.
Human plasma can serve as an ideal source for biomarkers of exposure, due to the ease of sample collection and the wide range of proteins held within the plasma compartment (Anderson and Anderson, 1977). In terms of monitoring OP exposure, the esterases present in plasma are ideal targets for assessing their inhibition and modification. Initial attempts at biomarker discovery focused on the major plasma esterases, including AChE, butyrylcholinesterase (BChE), and albumin (Black et al. 1999; Peeples et al. 2005). AChE is present in only trace amounts in plasma (Li et al. 2005), but also exists as membrane-bound protein on red blood cells (RBCs), where its function is unknown. In humans, measurement of RBC AChE inhibition has been one standard method of detecting OP exposure (Holmstedt, 1959), with the caveat that inhibition of AChE on the RBCs usually overestimates inhibition of AChE in the central nervous system (CNS) depending on the pharmacokinetics of the agent and passage of time since exposure. In addition, there are other difficulties with this method. The first difficulty is due to inter-individual variability. While the intraindividual coefficient of variation (CV) is about 10%, inter-individual CV is between 10 and 40% (Lotti, 1995). Measuring the individual’s pre-exposure activity levels would improve precision, but this is only practical in certain situations, such as monitoring agricultural worker exposure during the growing season. Pre-exposure activity levels are rarely available for cases of non-agricultural exposure. Second, measuring AChE activity levels from RBCs will not identify the specific inhibitory OP agent.
Many animals, including pig and rodent species, have abundant carboxylesterase (CE) in plasma. In humans, CE is not free in plasma, but can be purified from a membrane-bound form on monocytes (Saboori and Newcombe, 1990). While perhaps not an ideal biomarker, CE can be inhibited by an array of OPs, including the active metabolites of CPS and tricresyl phosphate (TCP), two OPs we have been interested in.
2 Modification of Blood and Plasma Biomarkers
Owing to its freely soluble presence in plasma, BChE has been a popular target for biomarker research. Newer methods have been developed that can identify and quantify OP exposure based on BChE modification. Polhuijs and colleagues developed a novel method involving fluoride ions. When incubated with 2 M potassium fluoride at pH 4, inhibited BChE released the inhibiting OP and yielded free enzyme plus the phosphofluoridate form of the OP (Polhuijs et al. 1997). In this case, sarin was regenerated and was analyzed using gas chromatography/mass spectrometry (GC/MS). A further advance was made analyzing peptic digests of uninhibited and inhibited BChE with electrospray liquid chromatography/tandem mass spectrometry (LC/MS/MS) (Fidder et al. 2002). Using this method, they could detect methylphosphonic acid residues adducted to the active site serine, at position 192. They repeated the experiments with other OPs, including more commonly-used pesticides. In addition to this work on BChE, highly sensitive and specific MS peptide capture methods have been developed using magnetic beads (Whiteaker et al. 2007). Their protocol involved digesting the biomarker of interest with trypsin, incubating the target peptides with beads coupled to anti-peptide antibody, then eluting and analyzing the captured peptides with LC/MS/MS. The sample could be spiked with a known amount of a stable isotope of biomarker peptide or protein (utilizing 13C and 15N), which provided an extra peak of known magnitude next to the desired sample and appeared as a doublet. This rapid and precise enrichment can enable detection down to ng/mL concentrations.
OP modification of BChE coupled with MS detection was a major advance in diagnosing OP exposure, especially in nerve agent attacks. However, BChE has somewhat limited utility because of its 11-day half-life in plasma. Another serine esterase, acylpeptide hydrolase (APH), was proposed in 2000 as both a diagnostic and therapeutic target for OPs (Richards et al. 2000). APH was first isolated in the liver (Tsunasawa et al. 1975; Gade and Brown, 1978), then in the circulating RBC cytosol and the brain (Yamin et al. 2007). In the RBC cytosol, APH removes N-acetyl amino acids from the ends of peptides (Fujino et al. 2000). APH serves a critical function in protecting the cytosol from denatured and oxidized proteins (Shimizu et al. 2004), and loss of this function by inhibition is lethal (Yamaguchi et al. 1999). Due to its presence in the RBC, which has a lifespan of 120 days and no protein synthetic capability, OP-modified APH should be measurable for several weeks, depending on the level of exposure. Casida and colleagues characterized the inhibition profile of APH using a large array of OPs (Quistad et al. 2005). They administered sufficient di-isopropyl fluorophosphate intraperitoneally into mice to inhibit RBC APH by 100% and plasma BChE by 80% at 4 hours post-injection, and brain AChE by 40% at 8 hours post-injection. While the BChE activity returned to baseline by day 4 post-injection, only 20% of the APH activity returned at this time, with no further follow-up past 4 days. These data suggest that APH inhibition and modification should be detectable significantly longer post-exposure than OP-modified plasma BChE.
Many other secondary targets of OPs have been identified and could also serve as biomarker candidates (Casida and Quistad, 2004). One biomarker property that we have sought is detectable inhibition by TCP metabolites. TCP is of important historical interest, since it caused paralysis afflicting tens of thousands of Americans during Prohibition (Parascandola, 1995). Many over-the-counter medicinals were tinctures, or alcoholic extracts of certain plant parts, such as leaves and roots. Unfortunately, many adulterants were added to either dilute the preparation or improve the taste. One popularly abused tincture was Jamaica ginger, or jake, which had a 70% alcohol content. TCP was used as an adulterant in the manufacture of a popular brand, since it was cheap, soluble in alcohol, and improved the strong taste of ginger.
In the early 1930s, the National Institutes of Health, then a division of the National Health Service, identified the ortho isomer of TCP, tri-orthocresyl phosphate (TOCP), as the cause of the neural toxicity, including wrist drop and foot drop (Parascandola, 1995). The latter inspired many songs and stories of the era involving jake leg or jake walk blues. In 1954, Aldridge recognized that TOCP itself was a poor inhibitor of cholinesterases, but became a much more potent inhibitor after incubation with rat liver (Aldridge, 1954). Later, Casida and colleagues determined that the active metabolite responsible for the toxicity of TOCP was saligenin cyclic-o-tolyl phosphate (Casida et al. 1961). Phenyl saligenin phosphate (PSP) is an analogue of this metabolite, which we use in its place since it is easier to obtain. TCP is currently of interest because of its continued use as a plasticizer and an additive in jet-engine lubricants and hydraulic fluids. In the US, over 20 million pounds of TCP is used annually, and it is a concern due to continued occupational exposure during its manufacture and use, despite its known toxicity (Winder and Balouet, 2002). Hundreds of crewmembers have reported exposure to cabin fumes, resulting in memory loss and cognitive dysfunction of sufficient severity to result in lost time at work and even permanent removal from the workforce. To date, there have been no published biomarkers that enable detection beyond several days after exposure to TCP or TOCP. One focus of our research is to identify biomarkers that can identify common pesticide exposure as well as TCP exposure.
3 Identification and Characterization of Biomarkers of OP Exposure from Human Blood
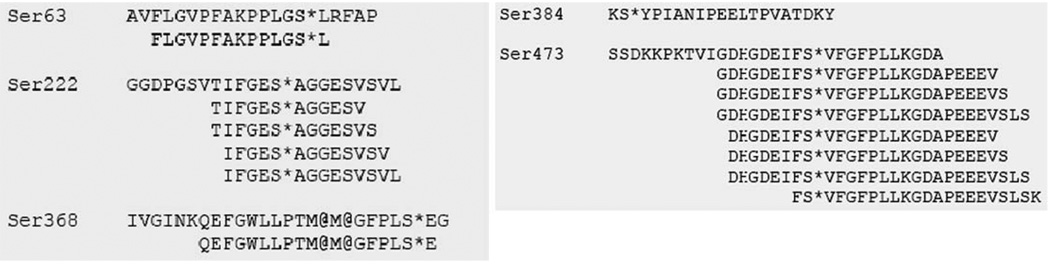
To test the feasibility of identifying peptides modified by triaryl phosphates, we inhibited pig carboxylesterase (PCE) using a mixture of TCP isomers that inhibit PCE without bioactivation. After incubating with TCP, modified (inhibited) PCE was digested with trypsin and the peptides were analyzed by tandem mass spectrometry on a linear ion trap, with multidimensional protein identification technology (MudPIT). The tryptic digests were loaded onto a nanoflow chromatography column and then eluted using a reverse-phase gradient. The effluent was electrosprayed into the mass spectrometer and the eluting peptides were detected and fragmented automatically by data-dependent acquisition. The resulting MS/MS spectra provided evidence that identified modified aged and un-aged peptide residues of PCE fragments. The aged peptide modifications resulted from the loss of one cresyl group thus generating a negative charge, a necessity for the neurotoxicity that is associated with TCP. This was identified as a 170-Da shift of the peptide containing the active site serine residue, 222 (Fig. 1) (Furlong et al. 2005).
Fig. 1.
Modified porcine carboxylesterase (PCE) peptides. Aged peptides identified with a mass shift of 170 Da on serine are indicated by S*. Ser222 is the catalytic serine, with Ser368, Ser384, and Ser473 located nearby. (Adapted with permission from Fig. 2 in Furlong et al. 2005)
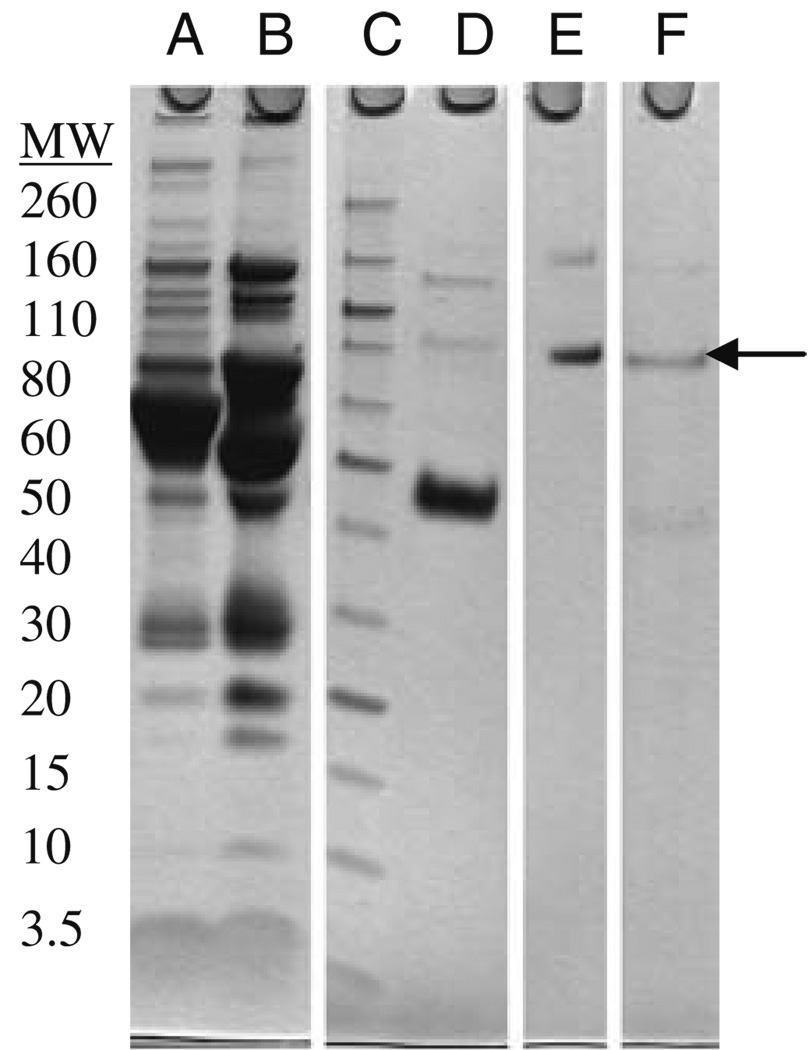
We next sought to apply this approach to human plasma BChE. Our first aim was to generate sufficient human plasma BChE for the required experiments. BChE has been isolated from human plasma by a variety of methods (Lockridge and La Du, 1978; Ralston et al. 1983; Grunwald et al. 1997; Mehrani, 2004; Lockridge et al. 2005). Most recent methods have used the procainamide affinity column chromatographic technique first described by Lockridge and La Du in 1978. We attempted to simplify the methods using more available anion exchange and hydrophobic interaction chromatography (HIC). In addition, since our laboratory routinely processes quantities of plasma for purification of paraoxonase 1 (PON1), we wanted to design a protocol for purifying both proteins from the same starting material. De-identified plasma was treated with Cibacron Blue Agarose to separate the HDL-associated proteins from BChE. The eluted BChE fractions were pooled and further resolved with ion exchange chromatography. Consecutive octyl-HIC columns were used for further purification before a final purification step with Superdex gel filtration column. Figure 2 shows an SDS-PAGE analysis of the purification steps.
Fig. 2.
SDS-PAGE analysis of the purification steps of butyrylcholinesterase (BChE). (a) Original whole plasma, (b) Flow-through after Cibacron Blue, (c) Molecular weight markers (listed in kDa), (d) Active BChE fractions after two octyl-HIC columns, (e) Active BChE fractions after Superdex 200, (f) Our internal BChE standard. Arrow indicates BChE mobility
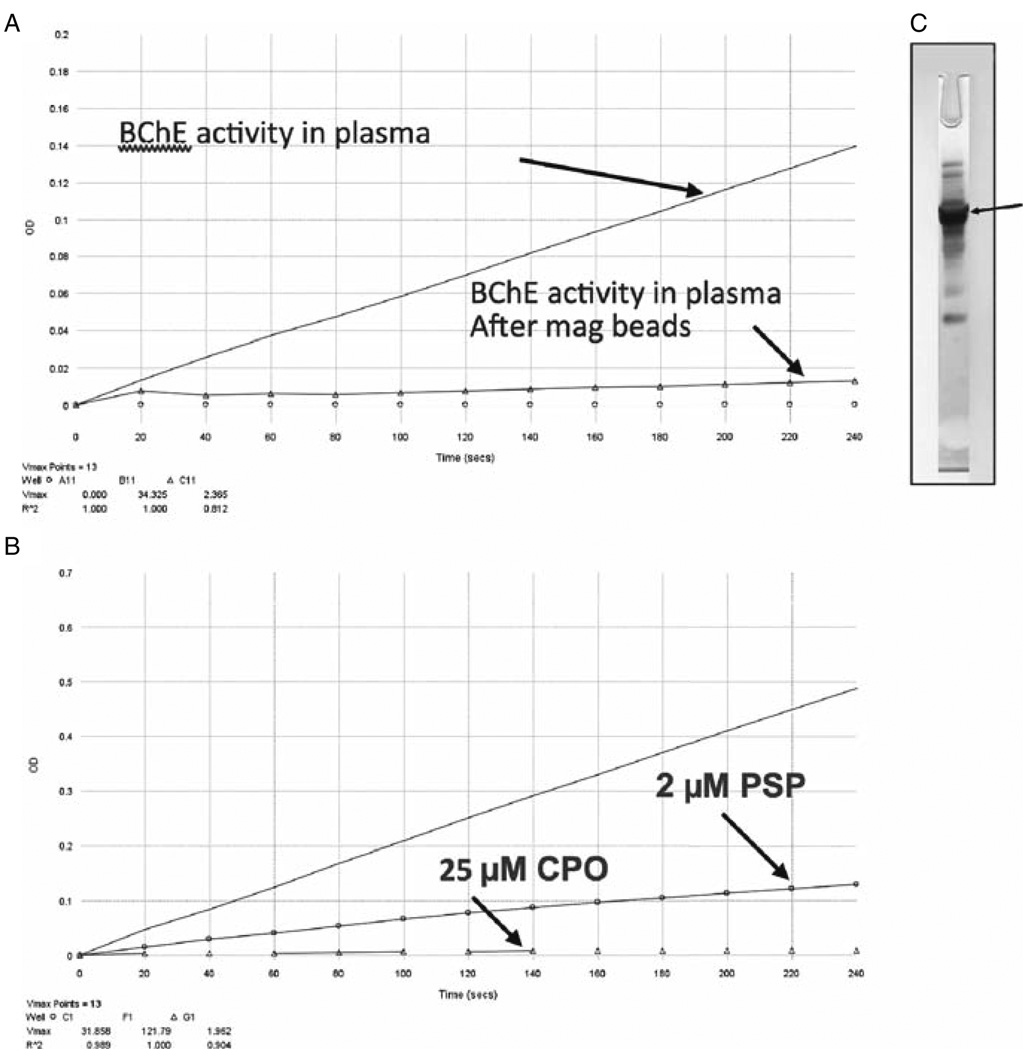
The purified BChE was used to raise antibodies for rapid processing of subject samples. These rapid protocols will utilize immunomagnetic bead (IMB) separation procedures. Further rapid purification protocols will make use of IMB procedures. In preliminary studies, we linked tosyl-activated magnetic beads (Dynal Dynabeads, Invitrogen) with commercial human anti-BChE antibody. After separating the plasma fraction from a human whole blood sample, we added several OPs to inhibit BChE activity, then incubated both inhibited and uninhibited plasma samples with magnetic beads. Figure 3a shows a 93% depletion of BChE activity following incubation of plasma with IMB. Figure 3b shows BChE inhibition by two different OPs, PSP and chlorpyrifos oxon (CPO). After BChE was bound to the beads, the beads were magnetically separated and eluted in formic acid. The BChE appears as the dark band in the silver-stained gel (Fig. 3c).
Fig. 3.
(a) Removal of butyrylcholinesterase (BChE) after incubation of human plasma with magnetic beads bound to anti-BChE antibody. (b) BChE inhibition after incubating human plasma with 2 µM phenyl saligenin phosphate (PSP) and 25 µM chlorpyrifos oxon (CPO). (c) Purified BChE (arrow), after magnetic bead capture, elution, and analysis by silver staining of SDS-PAGE
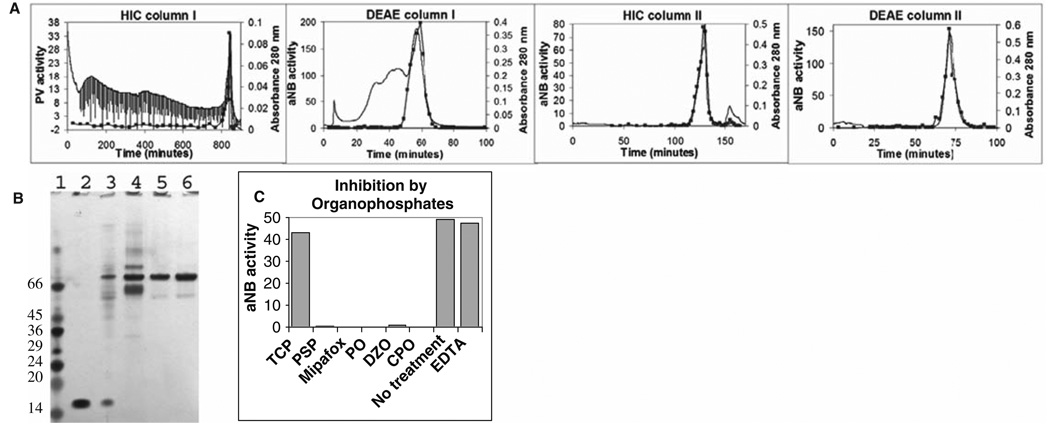
The next biomarker candidate that we purified and characterized was the RBC acylpeptide hydrolase (APH). Vose et al. (2007) characterized an RBC lysophosphatidylcholine hydrolase as a promising candidate for identifying OP-delayed neurotoxicants. We designed a protocol that would purify a soluble lipase from RBCs. Using a combination of hydrophobic interaction chromatography (HIC) and anion exchange chromatography (DEAE), we purified an RBC esterase to near homogeneity (20,000 fold) (Fig. 4a). Figure 4b shows an SDS-PAGE silver-stain analysis of each step of the purification procedure. Purification of this esterase has been carried out with samples as small as one millilitre of RBC lysate. The purified esterase was further gel-purified, excised, and digested with trypsin. Peptides were separated and characterized by reverse phase LC/MS/MS. The esterase was identified as acetyl peptide hydrolase (APH) by mass spectrometry. Quistad et al. (2005) had previously proposed APH as a sensitive biomarker for exposure to specific OP compounds. To determine the inhibitor specificity of the purified APH, we incubated it with a variety of OPs, including TCP and PSP. The purified APH was inhibited by CPO, diazoxon (DZO), paraoxon (PO), PSP, and the classical NTE inhibitor, mipafox, but not TCP (Fig. 4c). However, since APH was inhibited by PSP, an analogue of the active TOCP metabolite generated in vivo by hepatic transformation, we suspect that a TCP exposure containing any of the ortho analogs (mono-, di- or tri-orthocresyl phosphate) will result in APH inhibition in actual cases of exposure. The identity of the aged OP on the active site serine of APH was verified by mass spectrometry (Fig. 5).
Fig. 4.
Purification of acylpeptide hydrolase (APH) from human RBCs. (a) Four-step purification using hydrophobic interaction chromatography (HIC) and anion exchange chromatography (DEAE) resulting in >95% purity. (b) Silverstained SDS-PAGE analysis of purification steps; lane 1, molecular weight markers; lane 2, RBC extract; lane 3, HIC column I; lane 4, DEAE column I; lane 5, HIC column II; and lane 6, DEAE column II. (c) Inhibition by 25–40 µM OP compounds
Fig. 5.
The sequence of the acylpeptide hydrolase (APH) active site peptide with the active site serine (587) after tryptic digestion and modification with phenyl saligenin phosphate (PSP)
4 Conclusions
Expanding the identification and characterization of biomarkers beyond BChE is necessary for detecting and treating poisonous OP exposure. The approaches described here have included the standard biomarker BChE, as well as several others such as APH. The approach involves the development of rapid protocols for extraction of the target biomarker protein from a sample, digesting with the appropriate enzyme and identifying the OP-modified peptide by mass spectrometry. Additional directions that have been underway include expressing recombinant, active biomarker proteins in an E. coli system to provide heavy isotope-labeled standards to use in quantifying the degree of modification. We feel these methods are optimal for filling a void of diagnosing and treating long-term exposures to several ubiquitous OPs.
Acknowledgments
This work was supported by NIH Grants R01ES09883, P42ES04696, and NIEHS P30ES07033 (UW CEEH), as well as funding from Pilot Unions, Flight Attendant Unions, the Royal Australian Air Force, the Norwegian Union of Energy Workers (SAFE) and NYCO S.A. We thank Dr. Marian Ehrich for the kind gift of PSP, and Dr. Oksana Lockridge for BChE and anti-BChE antibodies.
References
- Aldridge WN. Tricresyl phosphates and cholinesterase. Biochem J. 1954;56(2):185–189. doi: 10.1042/bj0560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L, Anderson NG. High resolution two-dimensional electrophoresis of human plasma proteins. Proc Natl Acad Sci USA. 1977;74(12):5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RM, Harrison JM, Read RW. The interaction of sarin and soman with plasma proteins: the identification of a novel phosphonylation site. Arch Toxicol. 1999;73(2):123–126. doi: 10.1007/s002040050596. [DOI] [PubMed] [Google Scholar]
- Casida JE, Eto M, Baron RL. Biological activity of a tri-o-cresyl phosphate metabolite. Nature. 1961;191:1396–1397. doi: 10.1038/1911396a0. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17(8):983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Lanenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol. 2002;15(4):582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- Fujino T, Watanabe K, Beppu M, Kikugawa K, Yasuda H. Identification of oxidized protein hydrolase of human erythrocytes as acylpeptide hydrolase. Biochim Biophys Acta. 2000;1478(1):102–112. doi: 10.1016/s0167-4838(00)00004-2. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Cole TB, MacCoss M, Richter R, Costa LG. Biomarkers for Exposure and of Sensitivity to Organophosphorus [OP] Compounds. Proceedings of the BALPA Air Safety and Cabin Air Quality International Aero Industry Conference; April 20–21; London: Imperial College; 2005. [Google Scholar]
- Gade W, Brown JL. Purification and partial characterization of alpha-N-acylpeptide hydrolase from bovine liver. J Biol Chem. 1978;253(14):5012–5018. [PubMed] [Google Scholar]
- Grunwald J, Marcus D, Papier Y, Raveh L, Pittel Z, Asani Y. Large-scale purification and long-term stability of human butyrylcholinesterase: a potential bioscavenger drug. J Biochem Biophys Methods. 1997;34(2):123–135. doi: 10.1016/s0165-022x(97)01208-6. [DOI] [PubMed] [Google Scholar]
- Holmstedt B. Pharmacology of organophosphorus cholinesterase inhibitors. Pharmacol Rev. 1959;11:567–688. [PubMed] [Google Scholar]
- Li B, Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70(11):1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Lockridge O, La Du B. Comparison of atypical and usual human serum cholinesterase. Purification, number of active sites, substrate affinity, and turnover number. J Biol Chem. 1978;253(2):361–366. [PubMed] [Google Scholar]
- Lockridge O, Schopfer LM, Winger G, Woods JH. Large scale purification of butyrylcholinesterase from human plasma suitable for injection into monkeys; a potential new therapeutic for protection against cocaine and nerve agent toxicity. J Med Chem Biol Radiol Def. 2005;3:5095. doi: 10.1901/jaba.2005.3-nihms5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti M. Cholinesterase inhibition: complexities in interpretation. Clin Chem. 1995;41:1814–1818. [PubMed] [Google Scholar]
- Mehrani H. Simplified procedures for purification and stabilization of human plasma butyrylcholinesterase. Process Biochem. 2004;39(7):877–882. [Google Scholar]
- Parascandola J. The Public Health Service and Jamaica ginger paralysis in the 1930s. Public Health Reports. 1995;110(3):361–363. [PMC free article] [PubMed] [Google Scholar]
- Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci. 2005;83(2):303–312. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- Polhuijs M, Langenberg JP, Benschop HP. New method for retrospective detection of exposure to organophosphorus anticholinesterases: application to alleged sarin victims of Japanese terrorists. Toxicol Appl Pharmacol. 1997;146(1):156–161. doi: 10.1006/taap.1997.8243. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Casida JE. Blood acylpeptide hydrolase activity is a sensitive marker for exposure to some organophosphate toxicants. Toxicol Sci. 2005;86(2):291–299. doi: 10.1093/toxsci/kfi195. [DOI] [PubMed] [Google Scholar]
- Ralston JS, Main AR, Kilpathrick BF, Chasson AL. Use of procainamide gels in the purification of human and horse serum cholinesterases. Biochem J. 1983;211(1):243–250. doi: 10.1042/bj2110243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards PG, Johnson MK, Ray DE. Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol Pharmacol. 2000;58(3):577–583. doi: 10.1124/mol.58.3.577. [DOI] [PubMed] [Google Scholar]
- Saboori AM, Newcombe DS. Human monocyte carboxylesterase. Purification and kinetics. J Biol Chem. 1990;265(32):19792–19799. [PubMed] [Google Scholar]
- Shimizu K, Kiuchi Y, Ando K, Hayakawa M, Kikugawa K. Coordination of oxidized protein hydrolase and the proteasome in the clearance of cytotoxic denatured proteins. Biochem Biophys Res Commun. 2004;324(1):140–146. doi: 10.1016/j.bbrc.2004.08.231. [DOI] [PubMed] [Google Scholar]
- Tsunasawa S, Narita K, Ogata K. Purification and properties of acylamino acid-releasing enzyme from rat liver. J Biochem. 1975;77(1):89–102. [PubMed] [Google Scholar]
- Vose SC, Holland NT, Eskenazi B, Casida JE. Lysophosphatidylcholine hydrolases of human erythrocytes, lymphocytes, and brain: sensitive targets of conserved specificity for organophosphorus delayed neurotoxicants. Toxicol Appl Pharmacol. 2007;224(1):98–104. doi: 10.1016/j.taap.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker JR, Zhao L, Zhang HY, Feng LC, Piening BD, Anderson L, Paulovich AG. Antibody-based enrichment of peptides on magnetic beads for mass-spectrometry-based quantification of serum biomarkers. Anal Biochem. 2007;362(1):44–54. doi: 10.1016/j.ab.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder C, Balouet JC. The toxicity of commercial jet oils. Environ Res. 2002;89(2):146–164. doi: 10.1006/enrs.2002.4346. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Kambayashi D, Toda J, Sano T, Toyoshima S, Hojo H. Acetylleucine chloromethyl ketone, an inhibitor of acylpeptide hydrolase, induces apoptosis of U937 cells. Biochem Biophys Res Commun. 1999;263(1):139–142. doi: 10.1006/bbrc.1999.1289. [DOI] [PubMed] [Google Scholar]
- Yamin R, Bagchi S, Hildebrant R, Scaloni A, Widom RL, Abraham CR. Acyl peptide hydrolase, a serine proteinase isolated from conditioned medium of neuroblastoma cells, degrades the amyloid-beta peptide. J Neurochem. 2007;100(2):458–467. doi: 10.1111/j.1471-4159.2006.04251.x. [DOI] [PubMed] [Google Scholar]