Abstract
Rationale
Cocaine use during pregnancy is associated with alterations in the dopamine (DA) system in the fetal brain. However, little is known about the effects of prenatal cocaine exposure on the postnatal dopaminergic system.
Objectives
The objective of the study was to examine DA receptor function in adult monkeys that were prenatally exposed to cocaine.
Materials and methods
Male and female rhesus monkeys (~13 years old) that had been prenatally exposed to cocaine (n=10) and controls (n=10) were used in all studies. First, DA D2-like receptor availability was assessed using positron emission tomography and the D2-like receptor radiotracer [18F]fluoroclebopride (FCP). Next, D3 receptor function was assessed by measuring quinpirole-induced yawning (0.03–0.3 mg/kg). Finally, D1-like receptor function was examined by measuring eye blinking elicited by the high-efficacy D1-like receptor agonist SKF81297 (0.3–3.0 mg/kg).
Results
There were no differences between groups or sexes in D2-like receptor availability in the caudate nucleus, putamen or amygdala. However, quinpirole elicited significantly more yawns in prenatally cocaine-exposed monkeys compared with control monkeys. A significant correlation between gestational dose of cocaine and peak effects of quinpirole was observed. In all monkeys, administration of SKF81297 elicited dose-dependent increases in eye blinks that did not differ between groups.
Conclusions
These findings suggest that prenatal cocaine exposure can have long-term effects on DA D3 receptor function in adults.
Keywords: Prenatal cocaine, D2 receptors, PET imaging, Quinpirole, SKF81297, Rhesus monkey
It has been estimated that over 45,000 infants born each year have been prenatally exposed to cocaine (National Pregnancy and Health Survey 1996). Cocaine use during pregnancy is associated with several physical deficits including reduced body weight, body length, and head circumference at birth (Nair and Watson 1991). However, the effects of cocaine use during pregnancy on postnatal development and long-term neurobiological and behavioral outcomes have been less thoroughly investigated. The present study compared a population of rhesus monkeys that were prenatally exposed to cocaine throughout gestation to control monkeys with nearly identical pharmacological and experimental histories (Morris et al. 1996, 1997). At the start of the present study, these monkeys were adults (13 years old), with minimal drug exposure since birth (see Paule et al. 1996, 2000; Morris et al. 1996). Despite the escalating cocaine intakes of the mothers and the lower infant weights at birth (Morris et al. 1997), over the first 18 months, no differences were observed between cocaine and control groups with respect to postnatal growth (Morris et al. 1996). A particular advantage of using nonhuman primates in prenatal cocaine exposure studies is the relatively long gestational period. In rhesus macaques, the average gestational period is approximately 24 weeks (Silk et al. 1993). Despite this advantage, there are no studies involving prenatal cocaine exposure in rhesus monkeys that have examined the consequences of gestational drug exposure in adults.
For the present studies, the dopamine (DA) neurotransmitter system was examined using several in vivo measures. Within the DA system, there are two superfamilies of DA receptors, the D1-like receptors with two receptor subtypes D1 and D5 and D2-like receptors with D2, D3, and D4 receptor subtypes. Both D1- and D2-like receptors have been shown to be affected by chronic cocaine exposure in adult humans and nonhuman primates (e.g., Moore et al. 1998a, b; Martinez et al. 2004; Nader et al. 2002; Volkow et al. 1999). As it relates to effects on the fetus, elevation of extracellular monoamine concentrations during development may lead to alterations in receptor signaling mechanisms at birth and perhaps throughout life. Since DA is among the first neurochemical pathways to develop in the fetal brain (reviewed in Bhide 2009), the long-lasting effects of cocaine exposure on the dopaminergic system during this crucial development stage are of particular interest.
In the present study, DA D2-like receptor availability was assessed using positron emission tomography (PET) and the tracer [18F]fluoroclebopride (FCP), which does not differentiate between D2-like receptor subtypes (Mach et al. 1996). In adult rhesus monkeys, D2-like receptor availability has been shown to decrease as a consequence of chronic cocaine exposure (Nader et al. 2006). We hypothesized that D2-like receptor availability would be lower in adult monkeys who had been exposed to cocaine throughout gestation. While data suggest that D2-like receptors are reduced due to cocaine exposure, post-mortem studies found D3 receptors to be higher in cocaine overdose victims compared with age-matched controls (Staley and Mash 1996). Thus, we used the D3/D2 agonist quinpirole and the unconditioned behavior yawning to assess D3 receptor function in vivo. Earlier work in rodents has shown that the ascending limb of the quinpirole-elicited yawning dose–response curve, including the peak of thecurve, is mediated by D3 receptors (Collins et al. 2005).
As it relates to D1 receptors, Jones et al. (2000) demonstrated that prenatal cocaine exposure induced early desensitization of DA D1-like receptors in fetal rabbit anterior cingulate cortex and caudate nucleus that occurred without alterations of the receptor protein itself, suggesting that the D1-like receptors become uncoupled from their G-protein (Lidow 1998; Jones et al. 2000). Importantly, D1-like receptor alterations in rabbits and rodents prenatally exposed to cocaine have been shown to persist into adolescence and adulthood (Bayer et al. 2000; Stanwood and Levitt 2007). Therefore, in the present study, D1-like receptor function was investigated by assessing the ability of the high-efficacy agonist SKF 81297 to elicit eye blinking (Jutkiewicz and Bergman 2004). For these studies, there was a near-equal distribution of male and female monkeys, so the effects of prenatal cocaine exposure and sex were factors in all analyses.
Materials and methods
Subjects
Twenty adult rhesus monkeys (Macaca mulatta), born between 1993 and 1995 and raised at the FDA facility in Little Rock, AR, until their arrival at Wake Forest University in 2007, served as subjects. Ten monkeys (six male and four female) were prenatally exposed to cocaine, and ten monkeys (five male and five female) were controls, as described previously (Morris et al. 1996, 1997). Briefly, mothers of cocaine-exposed monkeys received intramuscular injections of escalating doses of cocaine three times per day for the entire course of gestation; the mean cumulative cocaine intake was 1131.5 (±56.1 mg/kg SEM; Morris et al. 1996). At 6 months of age, all monkeys were housed individually in the same colony room and began behavioral training involving an operant test battery (Morris et al. 1997). Other than their prenatal drug histories, all monkeys had identical experimental histories, including acute exposure to cocaine, amphetamine, haloperidol, quinpirole, SCH-23390, spiperone, and MK-801 (see Paule et al. 1996; Morris et al. 1997; personal communication from M. Paule). At the time of the present studies, there were no significant differences between the prenatally cocaine-exposed and control monkeys in age (12.4±0.3 vs. 12.9± 0.3 years, respectively) or weight (7.8±0.7 vs. 6.4±0.4 kg, respectively). Monkeys were individually housed in stainless-steel cages with water available ad libitum and had visual and auditory contact with each other. During a 2-month quarantine, a free-feeding weight was determined and monkeys’ body weights were maintained at approximately 95% of that value throughout these studies (LabDiet Monkey Chow and fresh fruit). Each monkey was fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate Products) using a specially designed stainless-steel pole that attached to the collar. All experimental and environmental enrichment protocols were approved by the Wake Forest University Institutional Animal Care and Use Committee. The experiments are listed in the order that each animal was tested.
Menstrual phase determination
Since we have previously shown that D2 receptor availability differs across the menstrual cycle (Czoty et al. 2009), all experiments were conducted in the follicular phase. Menstrual cycle was assessed by daily vaginal swabs. Days of bleeding were recorded as indicative of menses. PET scans were scheduled to occur during the follicular phase (days 2–12). To confirm cycle phase, on the day of a PET study, 3 ml of blood was drawn from the femoral vein and analyzed for progesterone at the Biomarkers Core Laboratory of the Yerkes National Primate Research Center of Emory University in Atlanta, GA (see Czoty et al. 2009 for details).
Experiment 1: effects of prenatal cocaine exposure on D2 receptor availability
Magnetic resonance imaging (MRI) scans were acquired for each monkey. Twenty minutes before the MRI, subjects were anesthetized with ketamine [15–20 mg/kg, intramuscular (i.m.)] and transported to the MRI facility. Anesthesia was maintained during the scanning procedure with ketamine supplements when necessary. T1-weighted images of the entire brain were acquired with a 1.5-Tesla GE Signa NR scanner (GE Medical Systems). Images were used to anatomically define regions of interest (ROIs), including the caudate nucleus, putamen, amygdala, and cerebellum, for later co-registration with PET images.
PET data were acquired using a GE Advance NXi PET scanner (~4.8 mm3 resolution) and the radiotracer [18F] FCP, which binds with high affinity to D2-like receptors (Mach et al. 1993) with a test/retest variability of ~2% (Nader et al. 1999). Methodological details regarding the data acquisition protocol, blood sampling, and metabolite analysis for FCP have been described previously (Mach et al. 1996; Nader et al. 1999). Approximately 30 min before the scan, monkeys were anesthetized with ketamine (10 mg/kg, i.m.), intubated, and maintained throughout the scan by inhaled isoflurane (1.5%). This induction protocol does not alter D2 receptor availability as measured with FCP (Nader et al. 1999). Catheters were placed in an external artery and vein by percutaneous sticks, and saline was delivered to the monkey throughout the scan. Body temperature was maintained at 38°C, and vital signs (heart rate, blood pressure, respiration rate, and temperature) were monitored throughout the scanning procedure. A paralytic (0.07 mg/kg vecuronium bromide) was administered intravenously (i.v.) and respiration was maintained by a ventilator. Supplemental doses of vecuronium bromide (0.1 mg/h) were administered throughout the study.
A 5-min transmission scan was acquired in 2D mode. Next, the monkey received a bolus dose of [18F]FCP (2–5 mCi) followed by a 3-ml flush with heparinized saline, and a 180-min dynamic acquisition scan was acquired. Twenty-six frames were acquired over 3 h (5×1 min, 5× 2 min, 5×5 min, 8×10 min, and 3×20 min) in 3D mode (i.e., septa retracted). Image reconstruction of 3D data was done using the 3D-reprojection method (Rogers et al. 1987) with full quantitative corrections. Once the scanning was compete, the transmission scan data were smoothed transaxially using a 4-mm Gaussian filter and segmented (Bettinardi et al. 1999). Emission data were corrected for attenuation and reconstructed into 129×128 matrices using a Hanning filter with a 4-mm cutoff transaxially and a ramp filter with an 8.5-mm cutoff axially.
The first five frames of each study’s PET image data were then added together. This summed image represents tracer uptake in the early part of the study and approximates a blood flow image. The image was then registered to the animal’s MRI using the AIR algorithm (Woods et al. 1993) after extracting the brain image from the MRI, using the method of Smith (2002). Time–activity curves were generated for radiotracer concentrations in ROIs defined on each subject’s co-registered MRI. Distribution volume ratios (DVR) were calculated for each ROI using the cerebellum as the reference region and the graphical method of Logan et al. (1996). The DVR thus served as an index of specific FCP binding in each ROI. For all regions, the right and left sides’ DVRs did not differ and were averaged.
Experiment 2: effects of prenatal cocaine exposure on quinpirole-induced yawning
A quinpirole dose–response curve was determined for each monkey. Before each experimental session, the monkey was placed in a primate chair and given an injection of saline (1.0 ml) or quinpirole (0.03, 0.1, or 0.3 mg/kg, i.m.); doses were tested in random order with at least 2 days between testing. These doses of quinpirole do not induce hypothermia in our monkeys (unpublished observations), an effect described as D2 receptor-mediated in rodents (Boulay et al. 1999a, b; Chaperon et al. 2003; Collins et al. 2007). Immediately after the injection, occurrences of yawning were counted for 30 min. Full extension of the jaws, withdrawal of the lips, and exposure of the teeth characterized yawning (Code and Tang 1991). Sessions were videotaped, and two people who were blind to the monkeys’ prenatal history scored these sessions with an inter-observer variability of <5%.
Experiment 3: effects of prenatal cocaine exposure on SKF 81297-induced eye blinking
Monkeys were seated in a primate chair in a testing room. Following a 15-min acclimation period, saline (1.0 ml) was administered into the saphenous vein and blinking was counted during the last 2.5 min of the following 15-min period. Subsequently, cumulative doses of SKF 81297 (0.3, 1.0, and 3.0 mg/kg, i.v.) were administered, and blinking was counted in the last 2.5 min of the 15-min period following each dose. Total session length was 75 min. Sessions were videotaped, and two people, one of whom was blind to the monkeys’ prenatal drug history, scored these sessions with an inter-observer variability of <8%.
Drugs
Quinpirole (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline to a concentration of 1.0 mg/ml. SKF 81297 (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline to a concentration of 5.0 mg/ml. All doses are expressed as the salt.
Statistical analysis
In experiment 1, for each ROI, data were analyzed using a two-way repeated measures analysis of variance (ANOVA) with group (prenatal cocaine and control) and sex as factors. In experiments 2 and 3, three-way repeated measures ANOVAs with group, sex, and dose (quinpirole or SKF 81297) as factors were conducted. In all cases, significance was accepted at the 95% level of confidence (p<0.05).
Results
Experiment 1: effects of prenatal cocaine exposure on D2 receptor availability
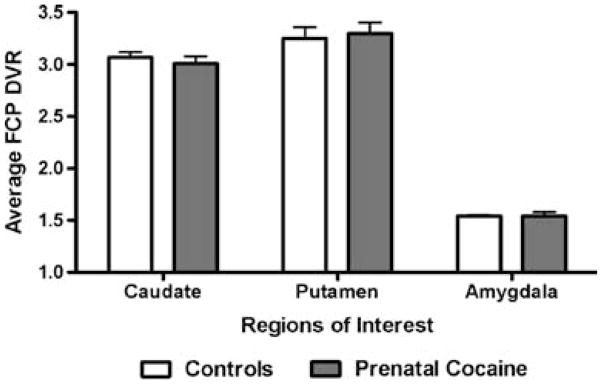
For all monkeys, there was a high level of uptake of [18F]FCP in all three regions of interest and a linear rate of washout, as shown previously (e.g., Morgan et al. 2002). In the cerebellum, [18F]FCP uptake was low with a rapid rate of washout (not shown). For both groups of monkeys, [18F]FCP DVRs in the caudate nucleus and putamen were higher than DVRs observed in the amygdala (Fig. 1). There were no significant main effects of prenatal cocaine exposure or sex and no group × sex interactions for any ROI.
Fig. 1.
Distribution volume ratios (DVRs) of [18F]FCP in control (open bars) and prenatally cocaine-exposed (shaded bars) monkeys in the caudate nucleus, putamen, and amygdala. Each bar represents mean±SEM values from ten monkeys
Experiment 2: effects of prenatal cocaine exposure on quinpirole-induced yawning
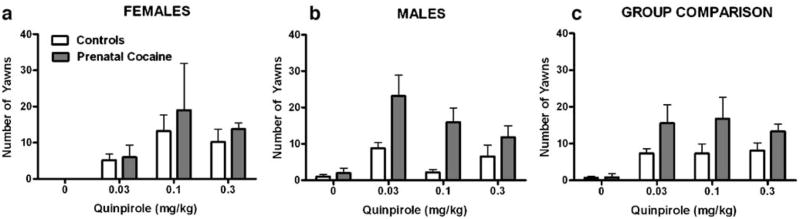
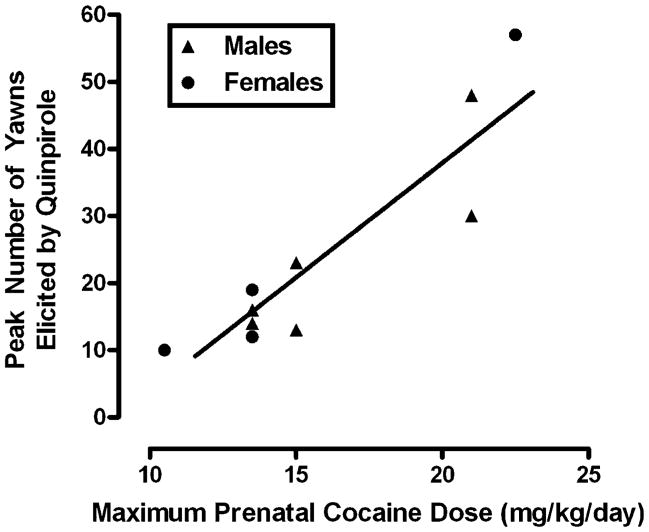
Using a 3-way repeated measures ANOVA, there was a significant effect of quinpirole dose [F(3, 48)=7.13, p<0.001], and group [F(1, 16)=8.51, p<0.01] and a significant sex x quinpirole dose interaction [F(3, 48)=3.42, p<0.05] (Fig. 2). All quinpirole doses elicited more yawns in the prenatal cocaine-exposed monkeys compared with controls (Fig. 2, right panel). Male monkeys, irrespective of their prenatal condition, yawned more following 0.03 mg/kg quinpirole compared with female monkeys whose quinpirole curve peaked at 0.1 mg/kg (Fig. 2, left and middle panels). For control and prenatally cocaine-exposed monkeys (male and female), all quinpirole doses elicited more yawns than saline, but the dose–response curves were relatively flat (Fig. 2, right panel). Finally, combining the data for all prenatally cocaine-exposed monkeys (Fig. 3) revealed a significant positive correlation between the maximal effect of quinpirole and maximal daily in utero cocaine exposure (r2=0.84, p<0.0005). However, when cumulative gestational cocaine dose was used in the analysis, the correlation was not significant.
Fig. 2.
Yawning induced by quinpirole (0.03–0.3 mg/kg) in female (a) and male (b) control (open bars) and prenatally cocaine-exposed (shaded bars) adult rhesus monkeys. c Represents mean data (male and female) for each group. Data are represented as the mean±SEM number of yawns in a 30-min observation period
Fig. 3.
Relationship between peak number of yawns elicited by quinpirole and maximal cocaine dose received in utero (from Morris et al. 1996). Different symbols represent males (triangles) and females (circles)
Experiment 3: effects of prenatal cocaine exposure on SKF 81297-induced eye blinking
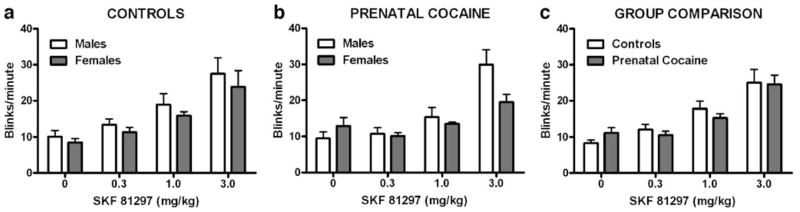
Following saline administration, rates of blinking ranged from 4.8–17.2 blinks per minute but did not differ between prenatally cocaine-exposed and control monkeys (Fig 4). A repeated measures three-way ANOVA revealed a significant main effect of SKF 81297 dose [F(3,39)=45.80, p<0.001] and SKF 81297 dose × sex interaction [F(3,39)=3.11, p<0.05]. For both groups and across all SKF81297 doses, males blinked more than females.
Fig. 4.
Effects of SKF 81297 on rate of eye blinking in control (a) and prenatally cocaine-exposed (b) male (filled bars) and female (open bars) monkeys. c Represents mean data (male and female) for each group. Each point represents mean±SEM values
Discussion
The purpose of the present studies was to determine if there were long-term alterations in dopamine function in adult monkeys that were exposed to cocaine in utero. Ten monkeys (male and female) prenatally exposed to cocaine were compared to ten age-matched control monkeys who had nearly identical postnatal experimental histories. There were no differences between groups in D1-like receptor function, as assessed by SKF 81297-elicited eye blinks, or in D2-like receptor availability as determined with PET imaging. In contrast, the D3/D2 receptor agonist quinpirole elicited significantly more yawns in monkeys prenatally exposed to cocaine compared with control monkeys. Furthermore, a significant correlation was observed between maximal daily gestational dose of cocaine and peak effects of quinpirole. These findings suggest long-lasting effects of prenatal cocaine exposure on DA D3 receptor function.
Accumulating evidence suggests that chronic cocaine exposure can produce significant reductions in DA D2-like receptor availability in adult humans and animals (e.g., Volkow et al. 1999; Martinez et al. 2004; Nader et al. 2002, 2006). However, earlier work suggested that the effects of chronic cocaine on fetal DA receptor densities may be different from those observed in adults. For example, Fang et al. (1997) observed significantly higher levels of D2-like receptor densities in the fetal monkey striatum following gestational cocaine exposure. Data from the present PET imaging study suggest that any changes in D2-like receptor availability that may have occurred in utero or in the developing brain have recovered in adulthood. Compared with the Fang et al. (1997) rhesus monkey study, the present study involved longer in utero treatments (approximately 6 months), full-term pregnancy, and 13 years of abstinence. Future longitudinal PET imaging experiments conducted at multiple points during a monkey’s lifespan following in utero cocaine exposure would directly address the time course of recovery.
No significant sex differences were observed in D2-like receptor availability in any of the regions of interest. This is consistent with the lack of sex differences seen in striatal D2/D3 receptor binding using [18F]-fallypride in adolescent rhesus monkeys (Christian et al. 2009) and with previous reports of women and men showing equivalent D2-like receptor availability (Farde et al. 1995; Pohjalainen et al. 1998; Munro et al. 2006). However, it has been suggested that female sex hormones may enhance presynaptic dopamine turnover (Laakso et al. 2002), and the radiotracer used in this experiment (FCP) is sensitive to fluctuations in menstrual cycle phase (Czoty et al. 2009). In addition, sex differences have been reported in a study using [11C] raclopride and PET in healthy men and women of ages ranging from 19–82 years old (Pohjalainen et al. 1998). Therefore, it remains possible that differences in D2-like receptor availability in males and females may have been observed at earlier time points or may yet be seen as these monkeys age.
The PET radiotracer used in the present study does not differentiate between D2, D3, and D4 subtypes of the D2-like receptor superfamily. Thus, it is conceivable that prenatal cocaine exposure could have long-term effects on subtypes of this superfamily which would be obscured by opposite adaptations in another subtype. For example, in vitro receptor autoradiography studies have shown lower D2-like receptor densities (e.g., Moore et al. 1998b; Nader et al. 2002) and higher D3 receptor densities (e.g., Staley and Mash 1996) in cocaine-exposed individuals compared with age-matched controls. To determine if there were differences in D3 receptor function, the D3/D2 receptor agonist quinpirole was used to examine the sensitivity of behavior related to this subtype in both groups of monkeys and as a function of sex. Collins et al. (2005, 2007) have shown that the ascending limb of the quinpirole dose–response curve is mediated by D3 receptors while the descending limb is mediated by D2 receptors. Based on previous experiments in rhesus monkeys (Martelle et al. 2007), the dose range of quinpirole administered in the present study is situated on the ascending limb of the dose–response curve and therefore is thought to assess primarily D3 receptor function. The greater ability of quinpirole to elicit yawning in the prenatally cocaine-exposed monkeys is similar to results from Moody et al. (1992), who demonstrated that rat pups exposed to cocaine throughout gestation exhibited a supersensitivity to the stimulating effects of quinpirole with respect to behaviors such as forward locomotion, rearing, and directed oral movements compared with control pups. Additionally, when all monkeys prenatally exposed to cocaine were used in the analysis, we found that D3 receptor sensitivity correlated with the maximum daily dose of cocaine each individual monkey received in utero. Taken together, the present results provide evidence for long-term neuropharmacological consequences of prenatal cocaine exposure on D3 receptor function under conditions in which no difference in D2-like receptors was observed using PET imaging. The combination of effects lead to interesting hypotheses regarding differential sensitivity to the reinforcing effects of cocaine. For example, because PET imaging studies in monkeys have shown a relationship between D2-like receptor availability and cocaine reinforcement (see Nader et al. 2008), the PET imaging data would suggest no differences between prenatally cocaine-exposed and control monkeys in vulnerability to cocaine reinforcement. However, D3 receptor sensitivity has been associated with impulsivity (e.g., Dodd et al. 2005; Sokoloff et al. 2006), which would suggest differential sensitivity of cocaine-exposed monkeys compared with controls in acquisition of cocaine self-administration. Additional behavioral studies in these monkeys, including assessing the reinforcing effects of cocaine, will provide important information as to the long-term consequences of prenatal cocaine exposure and the role of D2-like receptor subtypes in these behavioral outcomes.
In an effort to more fully characterize DA receptor activity in vivo, functional studies of the D1 receptor were also undertaken in these same monkeys. D1-like receptor densities have previously been shown to be affected by chronic cocaine exposure in adult monkeys (Moore et al. 1998b) and not necessarily in a manner similar to the effects of cocaine on D2-like receptors (Nader et al. 2002). Fang et al. (1997) reported that cocaine treatment from gestational day 22 to 70 resulted in significant increases in D1-like receptor densities in day 70 fetal monkey striatum. In rodent and rabbit models, several studies suggest that prenatal cocaine exposure uncoupled the D1 receptor from its G-protein resulting in an attenuation of D1 receptor signaling (Friedman et al. 1996; Wang et al. 1995; Lidow 1998; Jones et al. 2000; Unterwald et al. 2003). However, there are no data assessing D1-like receptor function in adults who had been prenatally exposed to cocaine. In the present study, no differences in potency or effects of SKF 81297-elicited eye blinks were observed in adult monkeys prenatally exposed to cocaine versus controls. Because it has been argued that this unconditioned behavior is a sensitive measure of D1 signaling (Jutkiewicz and Bergman 2004), these data suggest that any functional differences in D1-like receptor sensitivity observed in prenatally cocaine-exposed animals shortly after birth are no longer apparent in these animals as adults. It should be noted that under other conditions in socially housed monkeys, SKF 81297-elicited eye blinking did not differentiate monkeys based on social rank (Czoty et al. 2004), even though differences in sensitivity to cocaine reinforcement were observed (Czoty et al. 2005). It remains possible that other functional measures of D1-like receptor activity (e.g., drug discrimination or drug self-administration) may yield differential sensitivity due to prenatal cocaine exposure. The present findings are also the first to note sex differences in sensitivity to the D1-like agonist effects elicited by SKF 81297. It is important to note that D3 receptor function (quinpirole-elicited yawning) was also differentially affected by sex. The present findings add to a growing body of evidence for sex differences in the behavioral effects of drugs. Taken together, these findings indicate that prenatal cocaine exposure can have long-lasting effects on DA receptor function and that males and females are equally sensitive to these perturbations.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants R01 DA25120, R37 DA10584 and K31 DA024485. The authors report no conflict of interest and would like to acknowledge the technical assistance of Tonya Calhoun, Kimberly Black, Holly Smith, and Whitney Wilson. The authors also thank Dr. Merle Paule for providing information related to the histories of these monkeys and Dr. Anthony Liguori for statistical consultation.
Contributor Information
Lindsey R. Hamilton, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, 546 NRC, Medical Center Blvd., Winston-Salem, NC 27157-1083, USA
Paul W. Czoty, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, 546 NRC, Medical Center Blvd., Winston-Salem, NC 27157-1083, USA
H. Donald Gage, Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Michael A. Nader, Email: mnader@wfubmc.edu, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, 546 NRC, Medical Center Blvd., Winston-Salem, NC 27157-1083, USA. Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, NC, USA
References
- Bayer LE, Brown A, Mactutus CF, Booze RM, Strupp BJ. Prenatal cocaine exposure increases sensitivity to the attentional effects of the dopamine D1 agonist SKF81297. J Neurosci. 2000;20:8902–8908. doi: 10.1523/JNEUROSCI.20-23-08902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinardi V, Pagani E, Gilardi MC, et al. An automatic classification technique for attenuation correction in positron emission tomography. Eur J Nucl Med. 1999;26:447–458. doi: 10.1007/s002590050410. [DOI] [PubMed] [Google Scholar]
- Bhide PG. Dopamine, cocaine, and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin Cell Dev Biol. 2009;20:395–402. doi: 10.1016/j.semcdb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology. 1999a;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ. Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology. 1999b;38:555–565. doi: 10.1016/s0028-3908(98)00213-5. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Tricklebank MD, Unger L, Neijt HC. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology. 2003;44:1047–1053. doi: 10.1016/s0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Christian BT, Vandehey NT, Fox AS, et al. The distribution of D2/D3 receptor binding in the adolescent rhesus monkey using small animal PET imaging. Neuroimage. 2009;44:1334–1344. doi: 10.1016/j.neuroimage.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code RA, Tang AH. Yawning produced by dopamine agonists in rhesus monkeys. Eur J Pharmacol. 1991;201:235–238. doi: 10.1016/0014-2999(91)90351-p. [DOI] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, et al. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, et al. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EA, Gage HD, Nader MA. Characterization of dopamine D1 receptor function in socially housed cynomolgus monkeys. Psychopharmacology. 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34:548–554. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol. 2005;62:1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- Fang Y, Janowsky A, Ronnekleiv OK. Cocaine exposure in fetal rhesus monkey: consequences for dopamine D1- and D2-like receptor binding densities. Brain Res Dev Brain Res. 1997;104:163–174. doi: 10.1016/s0165-3806(97)00151-x. [DOI] [PubMed] [Google Scholar]
- Farde L, Hall H, Pauli S, Halldin C. Variability in D2-dopamine receptor density and affinity: a PET study with [11C]reclopride in man. Synapse. 1995;20:200–208. doi: 10.1002/syn.890200303. [DOI] [PubMed] [Google Scholar]
- Friedman E, Yadin E, Wang HY. Effects of prenatal cocaine on dopamine receptor-G protein coupling in mesocortical regions of the rabbit brain. Neuroscience. 1996;70:739–747. doi: 10.1016/s0306-4522(96)83011-9. [DOI] [PubMed] [Google Scholar]
- Jones LB, Stanwood GD, Reinoso BS, et al. In utero cocaine-induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci. 2000;20:4606–4614. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Bergman J. Effects of dopamine D1 ligands on eye blinking in monkeys: efficacy, antagonism, and D1/D2 interactions. J Pharmacol Exp Ther. 2004;1999:649–661. doi: 10.1124/jpet.104.071092. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Bergman J, et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Soc Biol Psychiatry. 2002;52:759–763. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Nonhuman primate model of the effect of prenatal cocaine exposure on cerebral cortical development. Ann NY Acad Sci. 1998;846:182–193. [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Mach RH, Luedtke RR, Unsworth CD, et al. 18F-labeled benzamides for studying the dopamine D2 receptor with positron emission tomography. J Med Chem. 1993;36:3707–3720. doi: 10.1021/jm00075a028. [DOI] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RL, et al. Comparison of two fluorine-18 labeled benzamide derivatives that bind reversibly to dopamine D2 receptors: in vitro binding studies and positron emission tomography. Synapse. 1996;24:322–333. doi: 10.1002/(SICI)1098-2396(199612)24:4<322::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, et al. Effects of two novel D3-selective compounds, NGB 2904 and CJB 090, on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, et al. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuro-psychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Moody CA, Frambes NA, Spear LP. Psychopharmacological responsiveness to the dopamine agonist quinpirole in normal weanlings and in weanling offspring exposed gestationally to cocaine. Psychopharmacology. 1992;108:256–262. doi: 10.1007/BF02245109. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse. 1998a;28:1–9. doi: 10.1002/(SICI)1098-2396(199801)28:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, et al. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998b;30:88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morris P, Binienda Z, Gillam MP, et al. The effect of chronic cocaine exposure during pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol. 1996;18:147–154. doi: 10.1016/0892-0362(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Morris P, Binienda Z, Gillam MP, et al. The effect of chronic cocaine exposure throughout pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol. 1997;19:47–57. doi: 10.1016/s0892-0362(96)00187-0. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Nader MA, Grant KA, Gage HD, et al. PET imaging of dopamine D2 receptors with [18F]fluoroclebopride in monkeys: effects of isoflurane- and ketamine-induced anesthesia. Neuro-psychopharmacology. 1999;21:35–46. doi: 10.1016/S0893-133X(98)00101-8. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;21:589–596. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV. PET imaging studies of dopamine receptors in primate models of addiction. Phil Trans Royal Soc B. 2008;363:3223–3232. doi: 10.1098/rstb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair BS, Watson RR. Cocaine and the pregnant woman. J Reprod Med. 1991;36:862–867. [PubMed] [Google Scholar]
- Paule MG, Gillam MP, Binienda Z, Morris P. Chronic cocaine exposure throughout gestation in the rhesus monkey. Pregnancy outcomes and offspring behavior. Ann NY Acad Sci. 1996;801:301–309. doi: 10.1111/j.1749-6632.1996.tb17450.x. [DOI] [PubMed] [Google Scholar]
- Paule MG, Gillam MP, Allen RR, Chelonis JJ. Effects of chronic in utero exposure to cocaine on behavioral adaptability in rhesus monkey offspring when examined in adulthood. Ann NY Acad Sci. 2000;914:412–417. doi: 10.1111/j.1749-6632.2000.tb05215.x. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, et al. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry. 1998;155:768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- Rogers JG, Harrop R, Kinahan PE. The theory of three-dimensional image reconstruction for PET. IEEE Trans Med Imaging. 1987;6:239–243. doi: 10.1109/TMI.1987.4307832. [DOI] [PubMed] [Google Scholar]
- Silk J, Short J, Roberts J, Kusnitz J. Gestation length in rhesus macaques (Macaca mulatta) Intern J Primatol. 1993;14:95–104. [Google Scholar]
- Smith S. Fast robust automated brain extraction. Hum Brain Map. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Prenatal exposure to cocaine produces unique developmental and long-term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci. 2007;27:152–157. doi: 10.1523/JNEUROSCI.4591-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies. Results from the 1992 National Pregnancy and Health Survey. 1996 A report. [Google Scholar]
- Unterwald EM, Ivkovic CM, et al. Prenatal exposure to cocaine decreases adenylyl cyclase activity in embryonic mouse striatum. Brain Res Dev Brain Res. 2003;147:67–75. doi: 10.1016/s0165-3806(03)00058-0. [DOI] [PubMed] [Google Scholar]
- Volkow D, Wang GJ, Fowler JS, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- Wang HY, Runyan S, Yadin E, Friedman E. Prenatal exposure to cocaine selectively reduces D1 dopamine receptor-mediated activation of striatal Gs proteins. J Pharmacol Exp Ther. 1995;273:492–498. [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]