Abstract
The synthesis of a functionalized, tetracyclic core of N-methylwelwitindolinone C isothiocyanate is reported. The approach features a convergent coupling between an indole iminium ion and a highly functionalized vinylogous silyl ketene acetal followed by an intramolecular palladium-catalyzed cyclization that proceeds via an enolate arylation.
A series of novel indole alkaloids were isolated in 1994 by Moore and coworkers from the extracts of blue-green cyanobacteria Hapalosiphon wetwitschii and Westiella intracta. 1 These compounds, which were collectively named welwitindolinones, possess a unique skeletal framework and were isolated along with the structurally related fischerindoles and hapalindoles. A putative biogenetic relationship amongst these alkaloids has been proposed.1 These natural products exhibit diverse biological activities, perhaps the most exciting of which is the ability of some to reverse multiple drug resistance (MDR) during chemotherapeutic treatment of cancer.2
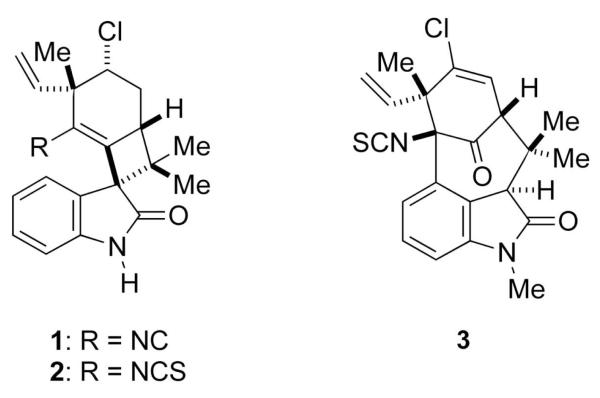
As a result of their novel structures and exciting biological activities, the various welwitindolinones have captured the attention of many groups, whose efforts have been chronicled in a number of accounts.3 Noteworthy are the elegant syntheses of welwitindolinone A isonitrile (1) independently reported by Baran 4 and Wood, 5 and of welwitindolinone A isothiocyanate (2) by Baran.6 Another important member of this family is N-methylwelwitindolinone C isothiocyanate (3)Numerous efforts directed toward the synthesis of this challenging target have not yet reached fruition, 7 but the tetracyclic core has been prepared by several groups.8b,g-k,m-o We now report the details of some of our work in the area.
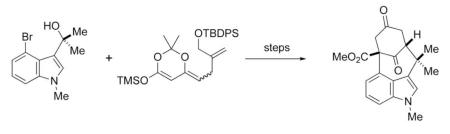
Our initial approach to N-methylwelwitindolinone C isothiocyanate (3)is outlined in retrosynthetic format in Scheme 1. We envisioned that the late stage intermediate 4, which might be elaborated into 3 by a series of refunctionalizations and alkylations, might be accessible via a novel double allylic alkylation of the tricyclic keto ester 5. The synthesis of 5 would then involve the cyclization of 6 via an enolate arylation, and compound 6 might be readily prepared by a Michael addition of N-methyl-4-bromooxindole (7).
Scheme 1.
Initial Retrosynthetic Proposal
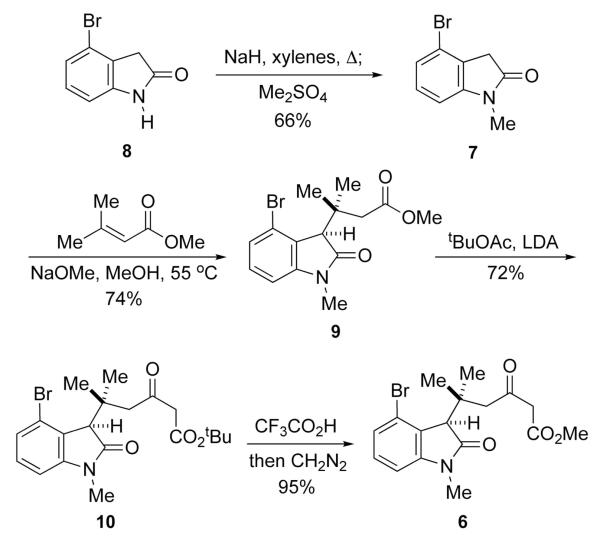
In the event, 4-bromooxindole (8), prepared via a known procedure,8 was selectively N-methylated using a method developed by Bordwell (Scheme 2).9 Warming oxindole 7 in degassed sodium methoxide in methanol with excess methyl 3-methylcrotonate provided the adduct 9 in 74% yield. It was essential to rigorously exclude air in order to avoid extensive formation of the corresponding isatin derivative. The crossed Claisen condensation of 9 with the enolate of tert-butyl acetate afforded ketoester 10. Because the tert-butyl ketoester group was prone to decarboxylation in future reactions, it was converted to the methyl ester 6. The overall yield of 6 from 9 via this two-step procedure was superior to that obtained using methyl acetate in the crossed Claisen condensation with 9.
Scheme 2.
Preparation of Ketoester 6
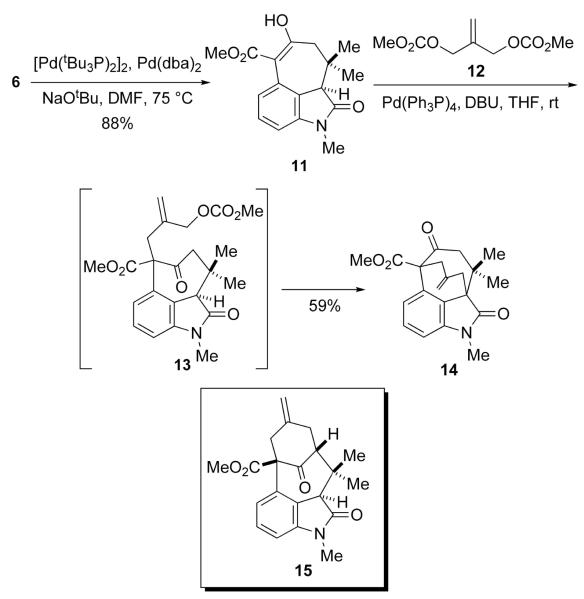
The next stage of the synthesis required the palladium catalyzed cyclization of the enolate of the ketoester 6 (Scheme 3). Although use of bis-tert-butyl-2-biphenyl phosphine as a ligand 10 provided the cyclized β-ketoester, which existed in its enol form 11, in 58% yield, significant amounts of unreacted 6 were invariably recovered; the structure of 11 was established by X-ray crystallography. On the other hand, use of a combination of commercially available tri-tert-butylphosphine palladium dimer 11 and either palladium bisdibenzylideneacetone or chlorobisallylpalladium dimer a ratio of 2:1 as suggested by Fu12> gave the enol 11 88% yield. These reactions are sensitive to the presence of oxygen, and the best results were obtained with a freezepump-thaw protocol.
Scheme 3.
Attempted Preparation of Tetracycle 15
The synthetic plan then anticipated the conversion of 11 into the tetracyclic intermediate 15 via a palladium catalyzed double allylic alkylation using the known bisallylic carbonate 12. However, when 11 was allowed to react with 12 in the presence of base and a number of Pd(0) catalysts, none of the desired 15 was obtained. Somewhat surprisingly 14 was the only product isolated.13 This alternate mode of cyclization is presumably the consequence of the more acidic proton on the oxindole ring. A change of strategy that would obviate this deleterious cyclization was thus warranted.
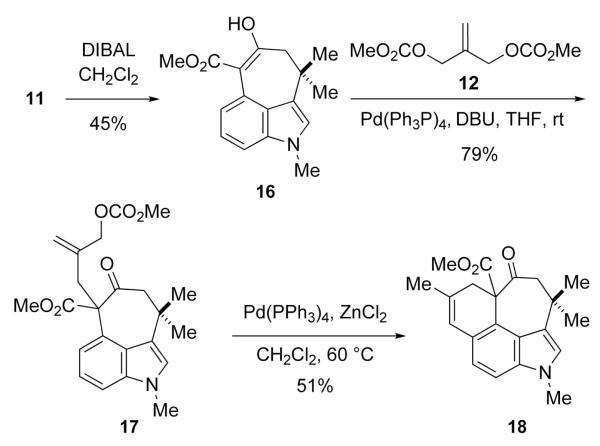
Reasoning that the undesired cyclization mode would not be accessible to the indole analog of 13, 11 was tranformed into 16, which like 11 existed in its enolic form as confirmed via X-ray crystallography, in modest yield by reduction and dehydration (Scheme 4). Palladium-catalyzed alkylation of 16 with 12 afforded allylic carbonate 17; however, all attempts using various bases to enolize the ketone function in 17 under the reaction conditions were unsuccessful. Interestingly, when 17 was treated with ZnCl2 in the presence of a Pd(0) catalyst, 18 was formed in 51% yield. 14 When this reaction was performed in the absence of the palladium catalyst, 18 was again isolated, albeit in only 28% yield.
Scheme 4.
Attempted Preparation of an Indolic Tetracycle
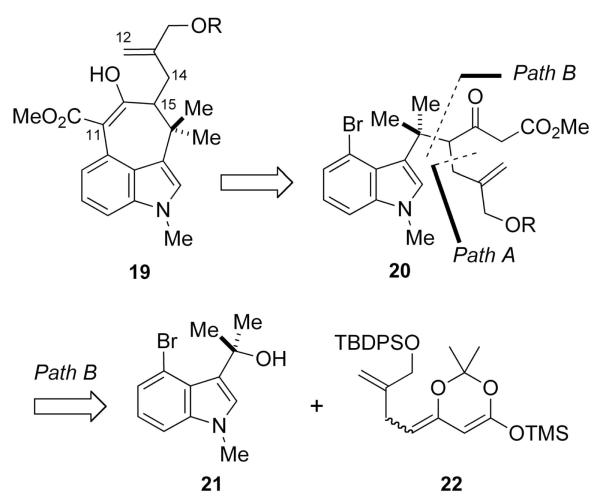
Because the problem we encountered involved forming the C(14)–C(15) bond after forming the C(11)–C(12) bond, it occurred to us that reversing the order of these two bond constructions might be a viable alternative. This revised approach then dictated the intermediacy of a substrate such as 19, which could be formed by cyclization of the β-keto ester 20 (Scheme 5). Our first attempts to prepare 20 involved the alkylation of the dianion of 10 with a suitably substituted allyl halide (Path A), but these efforts were to no avail. We also envisioned that 20 might be accessed by Path B, a process that would involve capture of the stabilized carbocation generated from 21 with a π-nucleophile such as 22. At the time we conceived of this approach there was little precedent for such a construction.15 Shortly after we had conducted this reaction, Rawal reported a similar process using a N-protected indole in his work directed toward the welwitindolinones.7g, m Since our original discovery, we have found this reaction to be more generally useful for preparing heteroaryl propanoic acid derivatives.16
Scheme 5.
A Revised Retrosynthetic Analysis
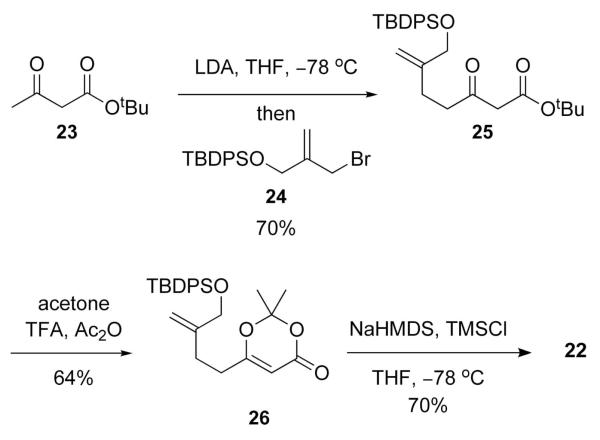
In order to examine the feasibility of forming 20 via Path B, the vinylogous silyl ketene acetal 22 was first prepared (Scheme 6). Accordingly, the bis-anion of tert-butylacetoacetate (23) was alkylated with the allylic bromide 24, which was easily prepared in two steps from the corresponding diol, to furnish 25. Dioxanone formation to give 26 and subsequent silylation of the dienolate derived from 26 gave 22, which because of instability was used without further purification, as inconsequential mixture (1.5:1) of isomeric olefins.
Scheme 6.
Preparation of Vinylogous Silyl Ketene Acetal 22.
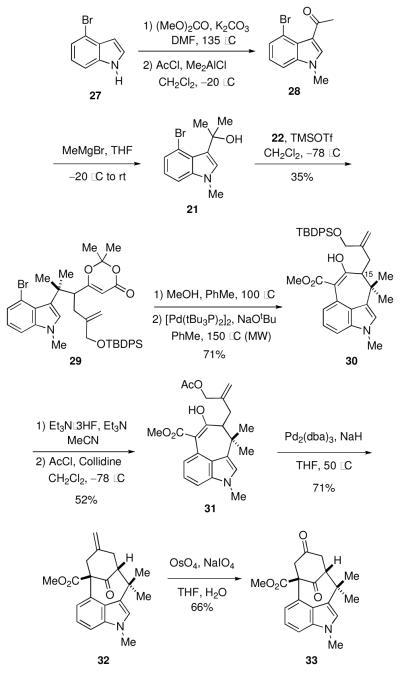
The requisite indole fragment 29 was then prepared from 4-bromoindole (27) by a procedure developed by Rapoport (Scheme 7). 17 N-Methylation of 27 with dimethylcarbonate18 followed by acetylation at C(3) of 27 afforded ketone 28. 19 Reaction of 28 with methylmagnesium bromide provided the unstable tertiary alcohol 21, which was immediately treated with the crude vinylogous silyl ketene acetal 22 in the presence of TMSOTf to form 29 in 35% yield over two steps. Heating 29 with methanol unveiled an intermediate ketoester 20 (R = TBDPS), which underwent palladium catalyzed cyclization to give 30 in 71% yield from 29. The silyl ether was cleaved with triethylamine hydrofluoride, but the subsequent acetylation of the resulting alcohol was complicated by competing reaction with the enolized ketone to give an enol acetate. After some experimentation, we discovered that selective acetylation of the alcohol could be achieved by the action of acetyl chloride in the presence of collidine at –78 °C to furnish 31 in 52% overall yield from 30. Cyclization of the sodium enolate derived from 31 was achieved utilizing Pd2(dba)3 to deliver the desired tetracycle 32 in 71% yield. Oxidative cleavage of the exocyclic olefin under Johnson-Lemieux conditions gave the dione 33.
Scheme 7.
Preparation of the Welwitindolinone C Skeleton
In preparing 33, we have thus developed a facile entry to the tetracyclic scaffold found in N-methylwelwitindolinone C isothiocyanate (3). The synthesis features the coupling of an indole-stabilized carbocation with a vinylogous silyl ketene acetal as a π -nucleophile together with a palladium-catalyzed enolate arylation and a palladium-catalyzed allylic alkylation. Efforts toward the application of this approach and variants thereof to the total synthesis of 3 are in progress and will be reported in due course.
Supplementary Material
Figure 1.
Structures of Welwitindolinone A Isocyanate (1), Welwitindolinone A Isothiocyanate (2) and N-Methylwelwitinolidone C Isothiocyanate (3).
Acknowledgment
We thank the National Institutes of Health (GM 25439), the Robert A. Welch Foundation, Pfizer, Inc., Merck Research Laboratories, and Boehringer Ingelheim Pharmaceuticals for their generous support of this research. We are also greatful to Vince Lynch (The University of Texas) for X-ray crystallography, Steve Sorey (The University of Texas) for NMR spectroscopy, and Bob Fu (The University of Texas) for helpful discussions and technical assistance.
Footnotes
Supporting Information Available Experimental procedures and 1H and 13C NMR spectra for all new compounds, plus CIF files representing X-ray coordinates for compounds 11 and 16 are included. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J. Am. Chem. Soc. 1994;116:9935–9942. [Google Scholar]
- (2).Smith CD, Zilfou JT, Stratmann K, Patterson GML, Moore RE. Mol. Pharmacol. 1995;47:241–247. [PubMed] [Google Scholar]
- (3).Reviews: Avendanó C, Menédez JC. Curr. Org. Synth. 2004;1:65–82. Menendez JC. Top. Heterocycl. Chem. 2007;11:63–101. Brown LE, Konopelski JP. Org. Prep. Proced. Int. 2008;40:411–445.
- (4).Baran PS, Richter JM. J. Am. Chem. Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r. [DOI] [PubMed] [Google Scholar]
- (5)(a).Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J. Am. Chem. Soc. 2006;128:1448–1449. doi: 10.1021/ja057640s. [DOI] [PubMed] [Google Scholar]; (b) Reisman SE, Ready JM, Weiss MM, Hasuoka A, Hirata M, Tamaki K, Ovaska TV, Smith CJ, Wood JL. J. Am. Chem. Soc. 2008;130:2087–2100. doi: 10.1021/ja076663z. [DOI] [PubMed] [Google Scholar]
- (6).Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. J. Am. Chem. Soc. 2008;130:17938–17954. doi: 10.1021/ja806981k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7)(a).Konopelski JP, Deng H, Schiemann K, Keane JM, Olmstead MM. Synlett. 1998:1105–1107. [Google Scholar]; (b) Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J. Am. Chem. Soc. 1999;121:6326–6327. [Google Scholar]; (c) Deng H, Konopelski JP. Org. Lett. 2001;3:3001–3004. doi: 10.1021/ol016379r. [DOI] [PubMed] [Google Scholar]; (d) Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835–6838. [Google Scholar]; (e) López-Alvarado P, García-Granda S, Àlvarez-Rúa C, Avendaño C. Eur. J. Org. Che. 2002:1702–1707. [Google Scholar]; (f) Ready JM, Reisman SE, Hirata M, Weiss MM, Tamaki K, Ovaska TO, Wood JL. Ang. Chem. Int. Ed. 2004;43:1270–1272. doi: 10.1002/anie.200353282. [DOI] [PubMed] [Google Scholar]; (g) MacKay JA, Bishop RL, Rawal VH. Org. Lett. 2005;7:3421–3424. doi: 10.1021/ol051043t. [DOI] [PubMed] [Google Scholar]; (h) Baudoux J, Blake AJ, Simpkins NS. Org. Lett. 2005;7:4087–4089. doi: 10.1021/ol051239t. [DOI] [PubMed] [Google Scholar]; (i) Greshock TJ, Funk RL. Org. Lett. 2006;8:2643–2645. doi: 10.1021/ol0608799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Lauchli R, Shea KJ. Org. Lett. 2006;8:5287–5289. doi: 10.1021/ol0620747. [DOI] [PubMed] [Google Scholar]; (k) Boissel V, Simpkins NS, Bhalay G. Tetrahedron Lett. 2009;50:3283–3286. [Google Scholar]; (l) Boissel V, Simpkins NS. Chem. Commun. 2009:1398–1400. doi: 10.1039/b820674k. [DOI] [PubMed] [Google Scholar]; (m) Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351. doi: 10.1021/ol9007684. [DOI] [PubMed] [Google Scholar]; (n) Trost BM, McDougall PJ. Org. Lett. 2009;11:3782–3785. doi: 10.1021/ol901499b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Brailsford JA, Lauchli R, Shea KJ. Org. Lett. 2009;11:5330–5333. doi: 10.1021/ol902173g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kosuge T, Ishida H, Inaba A, Nukaya H. Chem. Pharm. Bull. 1985;33:1414–1418. doi: 10.1248/cpb.33.2890. [DOI] [PubMed] [Google Scholar]
- (9).Bordwell FG, Fried HE. J. Org. Chem. 1991;56:4218–4223. [Google Scholar]
- (10).Fox JM, Huang S, Chieffi A, Buchwald SL. J. Am. Chem. Soc. 2000;122:1360–1370. [Google Scholar]
- (11).Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 1999;121:1473–1478. [Google Scholar]
- (12).Littke AF, Schwarz L, Fu GC. J. Am. Chem. Soc. 2002;124:6343–6348. doi: 10.1021/ja020012f. [DOI] [PubMed] [Google Scholar]
- (13).The conversion of enol 11 into 14 under similar conditions has been previously reported. Holubec AA. PhD Dissertation. Yale University; 2000. We thank a referee for calling this reference to our attention.
- (14).For related cyclizations of 4-substituted indoles, see: Fillion E, Dumas AM. J. Org. Chem. 2008;73:2920–2923. doi: 10.1021/jo702591p. and references therein.
- (15).For examples, see: Comins DL, Stroud ED. Tetrahedron Lett. 1986;27:1869–1872. Muratake H, Natsume M. Tetrahedron. 1990;46:6331–6342. Grieco PA, Handy ST. Tetrahedron Lett. 1997;38:2645–2648.
- (16).Fu T-H, Bonaparte A, Martin SF. Tetrahedron Lett. 2009;50:3253–3256. doi: 10.1016/j.tetlet.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Moyer MP, Shiurba JF, Rapoport H. J. Org. Chem. 1986;51:5106–5110. This material is also commercially available.
- (18).Jiang X, Tiwari A, Thompson M, Chen Chem. Z., Cleary TP, Lee TBK. Org. Process Res. Dev. 2001;5:604–608. [Google Scholar]
- (19).Okauchi T, Itonaga M, Minami T, Owa T, Kitoh K, Yoshino H. Org. Lett. 2000;2:1485–1487. doi: 10.1021/ol005841p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.