Abstract
Synapsin III is a neuron-specific phosphoprotein that plays an important role in synaptic transmission and neural development. While synapsin III is abundant in embryonic brain, expression of the protein in adults is reduced and limited primarily to the hippocampus, olfactory bulb, and cerebral cortex. Given the specificity of synapsin III to these brain areas and because it plays a role in neurogenesis in the dentate gyrus, we investigated whether it may affect learning and memory processes in mice. To address this point, synapsin III knockout mice were examined in a general behavioral screen, several tests to assess learning and memory function, and conditioned fear. Mutant animals displayed no anomalies in sensory and motor function or in anxiety- and depressive-like behaviors. Although mutants showed minor alterations in the Morris water maze, they were deficient in object recognition 24 hr and 10 days after training and in social transmission of food preference at 20 min and 24 hr. Additionally, mutants displayed abnormal responses in contextual and cued fear conditioning when tested 1 or 24 hr after conditioning. The synapsin III knockout mice also showed aberrant responses in fear-potentiated startle. Since synapsin III protein is decreased in schizophrenic brain and because the mutant mice do not harbor obvious anatomical deficits or neurological disorders, these mutants may represent a unique neurodevelopmental model for dissecting the molecular pathways that are related to certain aspects of schizophrenia and related disorders.
Keywords: Synapsin III, knockout mice, behavior, explicit memory, conditioned fear
Introduction
The synapsins are a family of three neuron-specific genes, designated synapsin I, II, and III, which encode phosphoproteins that play important roles in the regulation of neurotransmission and neurodevelopment (Greengard et al., 1993; Kao et al., 1998). Synapsin III (Syn3), the most recently identified member of the synapsin family is expressed primarily during early neural development (Ferreira et al., 2000; Pieribone et al., 2002). In hippocampal cultures depleted of Syn3, axon formation is transiently impaired (Ferreira et al., 2000). In adult mice, Syn3 has a widespread distribution throughout the brain, but is particularly abundant in hippocampus (Pieribone et al., 2002). Although Syn3 is a presynaptic protein that is concentrated at nerve terminals, mice lacking this gene display only subtle changes in neurotransmission (Feng et al., 2002). Syn3 is also distributed in the soma of neuronal precursors located in neurogenic regions of the hippocampus and the rostral migratory stream (Pieribone et al., 2000; Kao et al., 2008). Our recent studies have shown that hippocampal neurogenesis was decreased by approximately 30% in the dentate gyrus of adult Syn3 knockout (KO) mice (Kao et al., 2008). These findings point to an early role for Syn3 in neural progenitor cell development of the adult hippocampus.
It is well known that the hippocampus plays an important role in spatial learning and memory, as well as in other cognitive tasks that do not rely upon spatial cues (see Bast, 2007; Eichenbaum & Cohen, 2001). In the present report, we evaluated the status of overall behaviors of Syn3 mice using a neurophysiological screen (Rodriguiz & Wetsel, 2006) and then examined learning and memory in a variety of tests. Spatial learning and memory were investigated using the Morris water maze, while nonspatial learning and explicit memory were studied using the object recognition memory and social transmission of food preference tests. Emotional learning and memory were examined with fear conditioning and fear-potentiated startle. Our findings indicate that Syn3-KO mice have a selective impairment of nonspatial memory whereas spatial memory remains intact. These results suggest that Syn3 is required for specific hippocampal-dependent behaviors and are consistent with prior studies demonstrating a role for Syn3 in this brain region (Kao et al., 2008).
Materials and methods
Animals
The generation of Syn3-KO mice has been described (Feng et al., 2002). The mice were backcrossed to C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) for more than 10 generations to promote greater reliability for behavioral testing (Crawley et al., 1997). C57BL/6J-Syn3 heterozygous animals were intercrossed to generate wild type (WT) and the Syn-3 KO mice. Mice were genotyped by polymerase chain reaction (PCR) using the following procedure. Tail DNAs were submitted to PCR in a buffer containing 20 mM Tris-HCl (pH 8.8), 10 mM (NH4)2SO4, 10 mM KCl, 2 mM MgSO4, 0.1 % Triton X-100, 1 μM reverse primer (5’-CCG CCC TGG ATG TTA AGA TCA GAT-3’), and 0.5 μM each for the WT forward primer (5’-GTC TAG AGC AGA GTT GAA CCT GTG-3’) and the mutant NeoF primer (5’-ACA CTG CTC GAC ATT GGG TGG AAA-3’). The cycling conditions were 95°C for 5 min, 62°C for 2 min, and 72°C for 2 min for the first cycle, followed by 39 cycles of 94°C for 30 sec, 62°C for 1 min, and 72°C 1 min, and a final extension of 72°C for 10 min. Products were resolved on a 1% agarose gel to identify the genotypes (400 bp for WT and 250 bp for Syn-3 KO). Offspring were weaned at 21 days, segregated and housed by genotype and sex, at 3-5 mice per cage. Male and female WT and Syn3-KO littermates were approximately 3-5 months of age when they were behaviorally tested. Animals were maintained in a humidity- and temperature-controlled room under a 14:10 hr light:dark cycle (lights on 0800 hr). All behavioral studies were conducted during the light cycle, between 1100 and 1600 hrs. Animals were naïve for all behavioral tests except in the neurophysiological screen where mice were tested in the following order: zero maze, light-dark emergence test, open field, analyses of orienting and reflexive responses and motor coordination and balance, prepulse inhibition, tail suspension, and sensitivity to foot-shock, where tests were separated from each other by 5-7 days. Except where noted, illumination during testing was indirect and was maintained at ~150-180 lux. Water and laboratory chow were supplied ad libitum. All experiments were conducted with an approved protocol from the Duke University Institutional Animal Care and Use Committee and according to NIH Guide for the Care and Use of Laboratory Animals.
Behavioral studies
Neurophysiological screen
General appearance, orienting and reflexive responses, and motor coordination and balance were evaluated with a neurophysiological screen (Ribar et al., 2000); anxiety-like behaviors were assessed in the elevated zero maze and dark-light emergence tests (Rodriguiz & Wetsel, 2006; Fukui et al., 2007). The zero maze was a 5.5 cm-wide circular platform (34 cm, inner diameter) located 43 cm from the floor with two opposite quadrants (closed areas) enclosed by 11 cm walls on the inner and outer edges of the platform. The closed and open quadrants were equal in area. The maze was illuminated at 40-60 lux; a video camera was suspended 100 cm over the maze. Mice were given free access to the maze for 5 min. The light-dark emergence test was conducted in mouse shuttle-box (MedAssociates, St. Albans, VT) with lighted (~600 lux) and darkened (~5 lux) chambers (20 × 16 × 21 cm/chamber). Mice were placed in the darkened chamber and 5 sec later the door to the adjoining chamber was opened for free access to the apparatus for 5 min. Depressive-like behaviors were examined in a tail suspension test (MedAssociates) over 6 min (Fukui et al., 2007; Rodriguiz et al., 2008; Taylor et al., 2008). Sensitivity to foot-shock was examined as outlined (Grove et al., 2004; Rodriguiz & Wetsel, 2006). Prepulse inhibition (PPI; Ralph et al., 2001), activity in the open field (Pogorelov et al., 2005), and hole-board responses were monitored as noted (Takeda et al., 1998; Kamei et al., 2003; see Supplementary methods).
Morris water maze
Spatial learning was examined using the Morris water maze (Lipp & Wolfer, 1998; Rodriguiz & Wetsel, 2006; Rodriguiz et al., 2008; Taylor et al., 2008). Performance was assessed by visual analyses of the tracking profiles for each animal and the swim distance, swim time to find the hidden or visible platform, and swim velocity were determined by Noldus Ethovision (Noldus Information Technology Inc., Leesburg, VA). Testing was conducted in a 120 cm diameter stainless-steel tub filled with opaque water maintained at 25°C. The pool was divided into quadrants labeled northeast (NE), northwest (NW), southeast (SE), and southwest (SW) relative to a camera positioned 140 cm above the center of the pool. A white metal platform (12 cm diameter) was located approximately 1 cm below the surface of the water and 20 cm from the edge of the pool in the NE quadrant. Testing was conducted under four types of conditions: acquisition in which the platform was hidden below the surface of the water in the NE quadrant, reversal where the platform was moved to the SW quadrant, probe trials where the platform was removed from the maze, and testing with the visible platform where it was marked with a 5 × 5 cm flag 24 cm above the surface of the water. All test trials were 1 min in duration. Naïve WT and Syn3-KO mice were used for acquisition and probe testing and additional naïve mice from each genotype were used for visible platform testing. One week prior to testing, all mice were handled daily for 5 min and then were placed in a pan of shallow water (1 cm) for 30 sec to acclimate them to standing in water. On the seventh day after handling, each mouse was placed onto the hidden platform in the NE quadrant for 20 sec and then allowed to swim freely for 60 sec before being returned to the platform for 15 sec. Acquisition testing consisted of 32 trials given across 8 days with 4 trials administered per day. Trials were run in pairs, with each pair separated by 60 min. On days 7 and 8, the hidden platform was moved to the SW quadrant for reversal testing. Probe tests were given at the end of days 3, 6, and 8. For acquisition, reversal, and probe testing, the release point for the animals was randomized across 7 equally spaced points along the perimeter of the maze. For visible-platform testing, naïve mice were trained to swim to a visible platform for 4 trials/day over 2 days. For flag trials, animals were released from the northern-most point on the maze with the location of the platform randomized across quadrants.
Object recognition test
This test was conducted over 10 days as described (Rodriguiz & Wetsel, 2006; Dere et al., 2007) and it consisted of training and a short-term memory (STM) test on day 1, a long-term memory (LTM) test on day 2, and a remote memory (RM) test on day 10. Parenthetically, olfactory cues were removed from the objects by cleaning them and the test environment between mice. Training and test sessions were each 5 min in duration and involved the presentation of two objects made from plastic (4 × 3 × 2 cm) placed into opposite corners of a solid-walled acrylic arena (20 × 20 × 30 cm) and affixed to the floor with double-sided tape. On the first day, mice were exposed to a pair of identical objects and these objects constituted the “familiar” objects for the test. In the STM (20 min), LTM (24 hr), and RM (10 days) tests a single familiar object was compared next to a novel object of the same dimensions. All tests were videotaped and the total time spent with each object was scored by trained observers with Noldus Observer (Noldus Information Technologies) who were blind to the genotypes of the animal. Time spent with an object included direct visual orientation towards an object while being within one-body length of that object, and sniffing, touching, or climbing on the object. Recognition scores were calculated by subtracting the total time with the familiar from time spent with the novel object, and dividing this difference by the total amount of time spent with both objects. Positive scores signified recognition of the novel object, negative scores indicated preferences for the familiar object, and scores approaching “zero” denoted preference for neither object.
Social transmission of food preference
Group-housed mice (n = 4-5/cage) were placed on food restriction and examined in the social transmission of food preference test (Kogan et al., 2000; Rodriguiz & Wetsel, 2006; Rodriguiz et al., 2008; Taylor et al., 2008). Flavored diets (vanilla or chocolate) were prepared by mixing 50 g of ground rodent diet (5001 Lab Diet Formula, Purina Mills Inc., Richmond, IN) with 50 ml of water and 10 ml of either a vanilla- or chocolate-flavored soy-based drink (Ensure, Abbott Laboratories, Abbott Park, IL). Testing was conducted over two days. On the first day, one mouse designated as the demonstrator was placed into a clean 20 × 20 × 30 cm acrylic chamber with a single flat-bottomed 4 cm bowl containing 10 g of a flavored diet. The choice of chocolate or vanilla flavor as the designator diet was randomized across cages and genotypes. After 30 min, the demonstrator was returned to the home-cage where interactions between the demonstrator and testers (i.e., cage-mates) were observed for 20 min. Subsequently, each tester mouse was placed into a clean arena and presented with a simultaneous choice between the familiar demonstrator diet and the alternate novel diet for 30 min. Tester mice were re-examined 24 hr later for retention using the same familiar-novel diet comparison. All test bowls were weighed before and after each test, and any spillage was recovered and noted. Preference for a diet was determined by calculating the difference between the two flavored diets consumed relative to the total amount consumed. Positive preference scores indicated preference for the familiar demonstrator diet, whereas negative scores denoted preferences for the novel or unfamiliar diet. Scores approaching zero indicated no preference for either the demonstrator or novel diet.
Fear conditioning
Animals were examined in context and cued fear conditioning as described (Pillai-Nair et al., 2005; Rodriguiz & Wetsel, 2006; Taylor et al., 2008). In a first set of mice, animals were subjected to a 3-day paradigm consisting of conditioning on day 1, context testing 24 hrs later on day 2, and cued testing 24 hrs later on day 3. All conditioning and testing were conducted in Med-Associates mouse fear conditioning chambers (St. Albans, VT) under ~100 lux illumination. Conditioning consisted of placing the mouse in the test apparatus for 2 min, after which a 30 sec 72-dB 12-kHz tone (CS) was presented for 30 sec, which terminated simultaneously with a 2 sec 0.4 mA scrambled foot-shock (UCS); mice were removed from the conditioning chamber to the home cage 30 sec later. For context testing the next day, animals were returned to the chamber in which they were conditioned for 5 min in the absence of the CS and UCS. For cued testing on the following day, the dimensions, texture, and shape of the conditioning chamber were modified. Mice were introduced into the chamber for 2 min, after which the CS was presented for 3 min. To examine short-term memory processes, a second group of mice was conditioned as described above and assessed in context or cued testing 1 hr later. For all tests, behavior was videotaped and scored with the Noldus Observer (Noldus Information Technology) for freezing behaviors by trained observers who were blind to the genotypes of the mice. Freezing was defined by criteria previously described for mice as the absence of all visible movement except that required for respiration (Anagnostaras et al., 2000).
Fear-potentiated startle
Mice were examined in fear-potentiated startle in a Med-Associates startle apparatus as previously described (Falls et al., 1997; Taylor et al., 2008). Testing was conducted over 5 days. The illumination level in the chambers was <5 lux. On day 1, mice were assessed for acoustic startle responses to 100, 105 and 110 dB startle stimuli. Each stimulus was presented 6 times, in a pseudo-randomized order, for a total of 18 trials with an average intertrial interval (ITI) of 30 sec (range 15-60 sec). On the second day, mice were exposed to each startle stimulus (100, 105 and 110dB) 3 times, followed by 9 trials where the startle stimulus was preceded with a 30 sec 70dB 12kHz tone (CS) and by 9 additional trials where the CS was presented alone. On the third day, the mice were given 10 trials where the CS was followed immediately with a 0.25 sec 0.4mA scrambled foot-shock (UCS). The CS-UCS pairings were separated by 90-180 sec. Forty-eight hrs later, the mice were examined for potentiation of the startle response by the CS using the same procedure described for day 2. Baseline startle responses to the 100, 105, and 100dB startle stimuli on test day 1 were presented in arbitrary startle units (AU). Potentiation of the startle response to the CS before and following conditioning with the UCS were calculated as a ratio of the response to the CS + startle stimuli relative to startle-only responses and were expressed as a percentage.
Statistics
Behavioral analyses were performed with the SPSS-11 statistical programs (SPSS Inc., Chicago, IL) with all results presented as means and standard errors of the mean. Approximately equal numbers of male and female mice were evaluated in each behavioral test. Initially, all statistical analyses were conducted using sex as a variable. Since in all cases no main effects of sex or interactions with sex as a variable were observed, the data were collapsed across sex and analyzed as a single group. Independent measures t-tests were used for analyses of the neurophysiological screen, elevated zero maze, light-dark emergence test, tail suspension, activity in the open field, no-stimulus and pulse-only trials in PPI, and hole-board data. ANOVA was used to analyze contextual fear conditioning data. Repeated-measures ANOVA (RMANOVA) were used to evaluate inhibition of startle responses in PPI for the 4, 8, and 12dB prepulse stimuli, preference scores for STM, LTM, and RM in the object recognition test, responses at 20 min and 24 hr in the social transmission of food preference test, acquisition/reversal curves and probe trial responses in the Morris water maze, freezing in contextual and cued fear conditioning, and fear-potentiated startle. For the social transmission of food preference and the object recognition test, the “within subject effect” was the test interval between training and recall. For cued fear conditioning, the “within subject effects” were the pre-CS freezing compared to freezing during the CS. In fear-potentiated startle, the “within subject effects” were pre- and post-conditioning responses and the three different startle intensities. In all cases, Bonferroni corrected pair-wise comparisons were used as the post hoc tests, and P<0.05 was considered significant.
Results
Syn3-KO mice have normal reflexes and motor responses, but show increased exploration
Syn3-KO mice did not differ from WT controls in regards to general appearance and gross responses (Supplementary Table S1). Motor coordination and balance were also similar between genotypes. Although the latencies to climb up or down a vertical pole, or walk across a horizontal pole were comparable between WT and Syn3-KO animals, mutants covered these distances more rapidly than WT controls [t22=2.234-2.913, Ps<0.036]. In the open field, locomotor activity of Syn3-KO mice was higher than that for WT controls [t18=3.673, P<0.001]; the genotypes were not distinguished by rearing or stereotypical activities (Supplementary Figure S1a-c). While activity in the center was similar between genotypes (Supplementary Figure S1f), mutants had higher levels of activity in the perimeter [t18=2.668-2.843, Ps<0.015] and engaged in more rearing in the corners of the open field [t18=3.601, P<0.002] than WT animals (Supplementary Figure S1d-e, g). When locomotion was examined as distance traveled in the center and peripheral zones, Syn3-KO mice were observed to travel more in the perimeter (WT: 52.3 ±4.60, KO: 69.4 ±5.46 cm; t18=2.270, P<0.036) and less in the center (WT: 47.6 ±4.40, KO: 30.6 ±5.30 cm; t18=2.459, P<0.024) than WT controls. The activity maps supported these findings (Supplementary Figure S1h-i). Similarly in the hole-board test, Syn3-KO animals were more active than WT controls [t17=2.124-4.166, Ps<0.049] (Supplementary Figure S2c-f); however, the total numbers of head-pokes and the latency to first head-poke did not differ between genotypes (Supplementary Figure S2a-b). Of the 16 holes available for exploration, Syn3-KO mice sampled approximately 20% more of these holes than WT mice (Supplementary Figure S2c). Of those holes explored, the mutants were more likely to investigate those located in the center than periphery of the hole-board (Supplementary Figure S2d). Consequently, locomotion was increased in the Syn3-KO animals compared to WT controls and time spent in the center zone was greater (Supplementary Figure S2e-f). Together these findings from the open field and hole-board indicate that the Syn3-KO mice explore their novel environment more than WT animals.
Syn3-KO mice do not display abnormal anxiety- or depressive-like behaviors
In the zero maze, percent time in the open areas, numbers of closed-to-open-area transitions, stretch-attend postures, head-dips, episodes of rearing and freezing, and grooming time were not distinguished by genotype (Supplementary Table S2). Similarly, performance in the dark-light emergence and tail suspension tests was similar between genotypes. These data suggest that the Syn3-KO mice do not display alterations in anxiety- or depressive-like behaviors.
Syn3-KO mice show deficits in PPI
Sensorimotor gating was examined by PPI. Independent measures t-tests failed to discern genotype differences in activity during no-stimulus trials or for the magnitude of startle responses on pulse-only trials (Supplementary Table S3). On prepulse-pulse trials, a RMANOVA revealed a main effect of prepulse intensity [F2,32=64.433, P<0.001] and a significant prepulse intensity by genotype interaction [F2,32=3.756, P<0.034]. Bonferroni corrected pair-wise tests found that compared to the 4db prepulse, WT mice had increased PPI at 8 and 12dB (Ps<0.001) which were different from each other (P<0.008). Syn3-KO mice had increased PPI at 8dB and 12dB (Ps<0.001) compared to the 4 dB prepulse; however, responses between the 8 and 12 dB responses were not significant. Additionally, PPI to the 12dB prepulse was decreased in mutant compared to WT animals (P<0.036). These data show that PPI is abnormal in the Syn3-KO mice.
Spatial learning and memory appear relatively normal in Syn3-KO mice
Spatial learning and memory processes and plasticity of these responses were examined in the Morris water maze. In the visible platform test, no differences in swim distance (Fig. 1a) or swim velocity (Supplementary Table S4) were observed between genotypes. For swim distance a RMANOVA found significant main effects for test trial [F7,147=9.605, P<0.001]. Bonferroni comparisons showed swim distance for all mice was significantly reduced on trials 5, 6, 7 and 8 relative to trial 1 (Ps<0.041); swim velocities did not vary across trials. These findings indicate that sensory and motor functions and motivation are similar between WT and mutant animals.
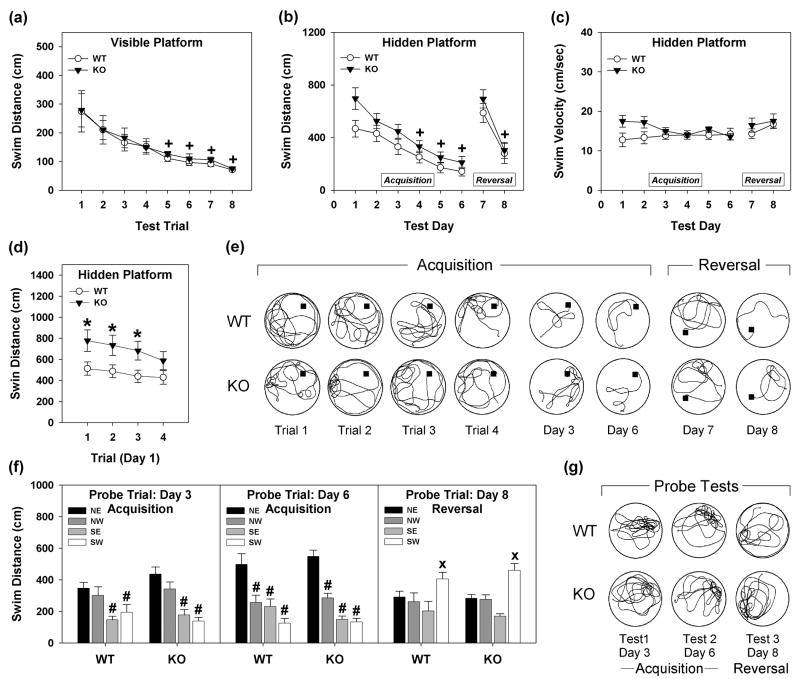
Figure 1. Responses in the Morris water maze by WT and Syn3-KO mice.
(a) Swim distance in the visible platform test. (b) Swim distances over the 6 days of acquisition and 2 days of reversal testing. (c) Swim velocities during acquisition and reversal testing. (d) Swim distances during the first day of acquisition testing over the 4 test trials. (e) Tracings of swim patterns for representative WT and Syn3-KO mice during acquisition and reversal trials. The apex of the water maze in each tracing is north and the platform location is designated by a filled square. (f) Swim distances during probe trials during acquisition testing on days 3 and 6, and following reversal on day 8. (g) Tracings of swim patterns for representative WT and Syn3-KO mice during probe trials on days 3 and 6 during acquisition and on day 8 for reversal. n = 11-12 mice/genotype; *P<0.05 from the WT controls; +P<0.05 compared to test day 1 for all mice; #P<0.05 compared to NE quadrant; xP<0.05 compared to SW quadrant.
In the hidden platform test during the 6 days of acquisition testing, no statistically significant genotype differences were discerned for swim distance (Fig. 1b) or swim velocity (Fig. 1c). Nevertheless, reductions in swim distances [RMANOVA test day: F5,105=36.553, P<0.001] were observed across test days 4, 5 and 6 compared to day 1 for both genotypes (Ps<0.048) (Fig. 1b). Additionally, there was a tendency for swim velocities of Syn3-KO mice to be faster than those of WT controls on days 1 and 2 of testing (Fig. 1c); however, this difference was not significant. RMANOVA of swim distances on each of the 4 trials on day 1 of acquisition testing (Fig. 1d) found a main effect of test trial [F3,63=4.076, P<0.046] and a significant test trial by genotype interaction [F3,63=5.228, P<0.033]. Bonferroni corrected pair-wise comparisons observed that Syn3-KO mice swam further on day 1 on trials 1-3 than WT animals (Ps<0.052). A RMANOVA for swim velocity supported the genotype effect on this first day [F1,21=4.127, P<0.053] (Supplementary Table S5). To examine responses on day 1 in more detail, the swim distance results were examined in terms of the percent locations (i.e., center or peripheral zones) where the mice were swimming (Supplementary Fig. S3). Although the Syn3-KO animals spent a greater percent time swimming in the periphery than WT mice, the between subjects effect for the RMANOVA only approached significance [F1,121=3.565, P<0.073]. The RMANOVA for within subjects effects found a significant main effect for test trial [F3,63=9.553, P<0.001]; the test trial by genotype interaction was not significant [F3,63=1.678, P<0.181]. Bonferroni tests noted that on trial 4 the percent time swimming in the periphery was significantly less than on trial 1 (P<0.001). Representative tracings for each of these four trials on day 1 (Fig. 1e) also suggested that mutants had a propensity to swim in areas adjacent to and along the perimeter of the pool during initial exploration of the maze.
Plasticity of spatial learning and memory was analyzed by reversal. When the location of the hidden platform was changed from the NE to SW quadrant on days 7 and 8, again no genotype differences were observed for swim distance (Fig. 1b) or swim velocity (Fig. 1c). A RMANOVA for acquisition and reversal testing yielded a significant test day effect [F7,147=13.178, P<0.002]. All mice swam longer distances on day 7 -- the first day of reversal -- compared to the last day of acquisition testing (day 6) and the second day (day 8) of reversal testing (Ps<0.004) (Fig. 1b). Subsequently, no genotype differences were evident by day 8 where swim distances (Fig. 1b) and swim velocities (Fig. 1c) were similar to responses on day 6 of acquisition testing. Additionally, no group distinctions were evident across trials on any day of reversal testing.
Responses on probe trials conducted at the end of days 3 and 6 for acquisition testing and on day 8 for reversal testing demonstrated that all animals developed a clear preference for the NE quadrant and readily switched this preference to the SW quadrant during the reversal test (Fig. 1f). A RMANOVA for swim distance revealed significant within subject effects for quadrant [F3,63=31.125, P<0.001] and a significant quadrant by test day interaction [F6,126=14.113, P<0.001]. Bonferroni comparisons noted that for all mice, swim distances in the SE and SW quadrants were significantly less than that in the NE quadrant on day 3 (Ps<0.031) and by day 6 swim distances in the NE quadrant were markedly greater relative to the SE, SW, and NW quadrants (Ps<0.003). On test day 8, during the reversal testing, swim distance in the SW quadrant was greater than that in all other quadrants for all mice (Ps<0.041). Although Syn3-KO mice swam faster during all probe tests, no significant genotype differences were found as a function of genotype or maze quadrant (Supplementary Table S6). An examination of tracings for probe trials supported this point (Fig. 1g). Together these data demonstrate that while overall performance of WT and Syn3-KO mice are similar, mutants tend to have a slight lag in acquiring the task relative to their WT controls.
Syn3-KO mice are deficient in explicit learning and memory
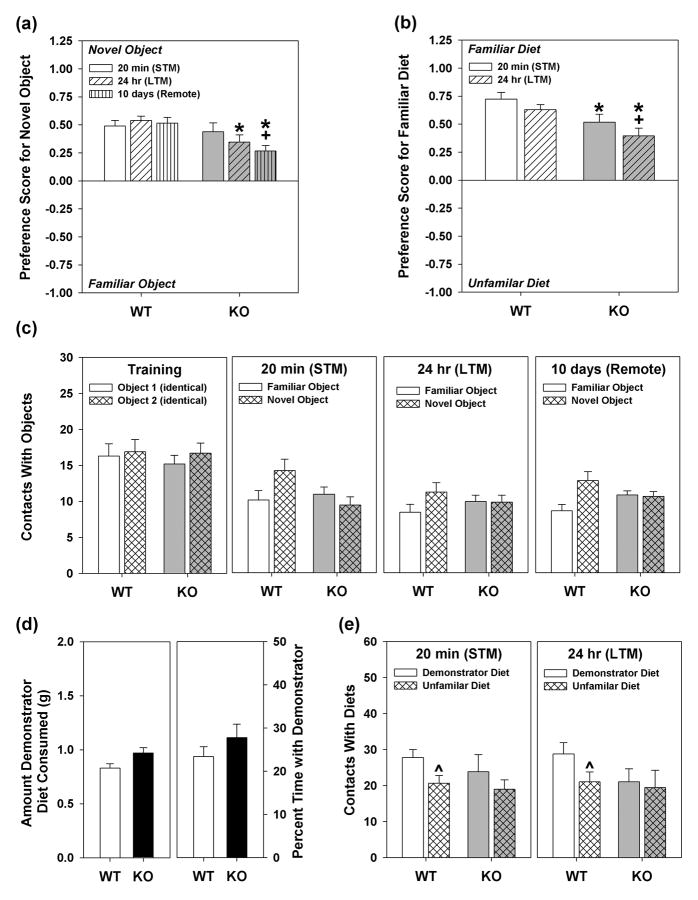
In addition to spatial memory, WT and Syn3-KO animals were examined for deficits in explicit memory using the object recognition and social transmission of food preference tests. In object recognition (Fig. 2a), a RMANOVA revealed a significant test-time by genotype interaction [F2,36=5.235, P<0.034]. Bonferroni tests found no differences between WT and Syn3-KO animals at the 20 min test, but showed WT mice to have higher preferences for the novel object at 24 hr (P<0.049) and at 10 days than mutant animals (P<0.002). Interestingly, Syn3-KO mice had notably lower preference scores at 10 days compared to those at the 20 min test (P<0.047). WT mice demonstrated intact memory processes at all test intervals: 20 min, 24 hrs, and 10 days. Additionally, WT and Syn3-KO mice made similar numbers of contacts with the objects during training and testing at the three time-points (Fig. 2c). Moreover, no differences in the time spent exploring objects were found between genotypes during any of the test times (Supplementary Table S7). Thus, mutant mice show object preferences at 20 min, but these are decreased at the 24 hr and 10 day test times.
Figure 2. Object recognition and social transmission of food preference responses of WT and Syn3-KO mice.
(a) Object recognition performance at 20 min, 24 hr, and at 10 days for WT and Syn3-KO animals. (b) Responses by WT and Syn3-KO mice in the social transmission of food preference test. (c) Contacts with objects during training, and for the 20 min, 24 hrs, and 10 day tests. (d) Total diet consumed by the demonstrator and the percent time tester mice spent with the demonstrator after the latter was returned to home cage after diet consumption. (e) Total bowl contacts made by WT and Syn3-KO mice at the 20 min and 24 hr test. n = 10 mice/genotype; *P<0.05 from the WT controls; +P<0.05 compared to the preference at 20 min; ˆP<0.05 compared to the demonstrator diet.
In the social transmission of food preference test, WT and Syn3-KO tester mice were examined for preferences between novel- and familiar-favored diets 20 min following interaction with a demonstrator animal and at 24 hr (Fig. 2b). A RMANOVA revealed significant main effects of test [F1,28=22.114, P<0.001] and a significant test-time by genotype interaction [F1,28=7.874, P<0.009]. Bonferroni corrected pair-wise comparisons showed that Syn3-KO animals had reduced preferences for the familiar diet at 20 min and at 24 hrs compared to WT controls (Ps<0.012). Interestingly, mutants experienced a further decline in memory for the demonstrator diet at 24 hr compared to their performance at the 20 min test (P<0.020). Despite these effects, demonstrator WT and Syn3-KO animals consumed similar amounts of the flavored diet (Fig. 2d, left) and the times spent interacting with the tester animals following food consumption were also similar between genotypes (Fig. 2d, right). Additionally, WT and Syn3-KO mice consumed similar amounts of diets at 20 min (WT = 1.32 ±0.05g; Syn3-KO = 1.26 ±0.06g) and at 24 hr (WT = 1.43 ±0.07g; Syn3-KO = 1.34 ±0.06g). With respect to bowl contacts (Fig. 2e), a RMANOVA found significant main effects for test diet [F1,18=20.178, P<0.001]; the test-diet by test-time by genotype interaction was marginal [F1,28=4.059, P=0.059]. Bonferroni comparisons showed that while no significant differences were discerned between contacts made to the demonstrator and novel diets during the 20 min and 24 hr tests by Syn3-KO animals, WT mice were less likely to contact the novel relative to the demonstrator diet during these test-times (Ps<0.052). Thus, both genotypes of mice demonstrate social transmission of food preference; however, preferences of the familiar diet by the mutant mice are less than those of the WT controls at both test intervals.
Syn3-KO mice display perturbations in conditioned fear
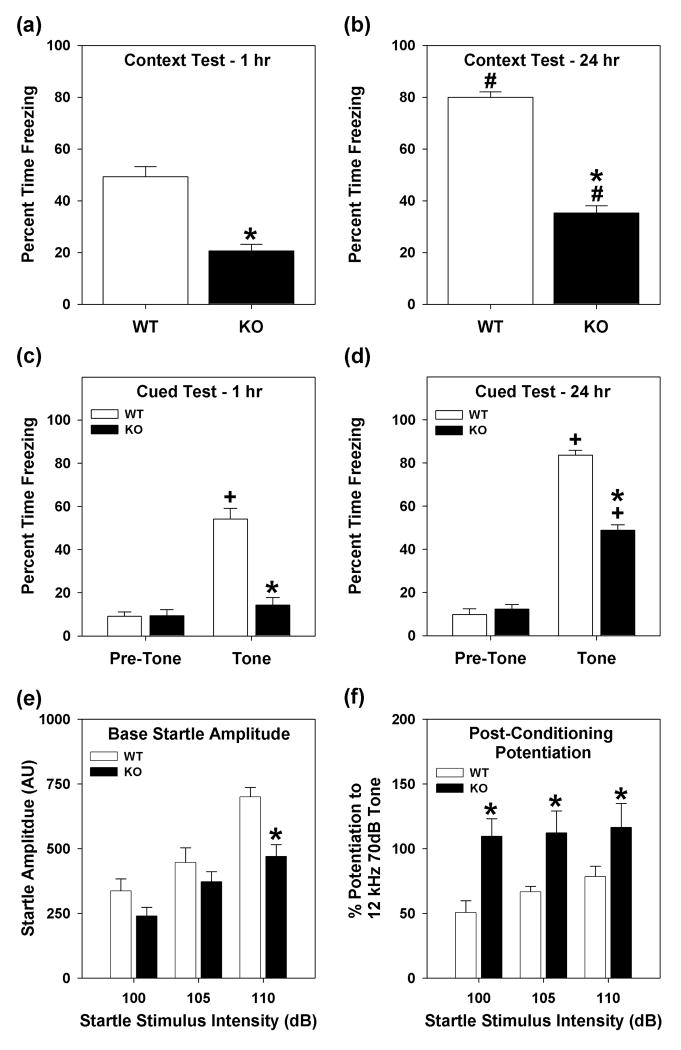
Conditioned fear responses were examined in WT and Syn3-KO mice using fear conditioning and fear-potentiated startle. Prior to testing, groups of naïve WT and Syn3-KO mice were examined for their sensitivities to scrambled foot-shock; no genotype differences were observed (Supplementary Table S8). For fear conditioning, naïve WT and mutant mice showed minimal freezing prior to and during presentation of the CS at conditioning (Supplementary Table S9). Following the CS-UCS pairing, freezing behavior in all mice increased on the conditioning day [RMANOVA: time F2,100=38.311, P<0.001; Bonferroni test: after the CS-UCS presentation compared to before or during the pairing Ps<0.048] but no genotype differences were detected. For contextual fear conditioning (Fig. 3a,b), ANOVA found significant effects for genotype [F1,34=163.691, P<0.001] and test interval [F1,34=62.642, P<0.001]; the genotype by test interval interaction was significant [F1,34=7.700, P<0.009]. Bonferroni comparisons observed that Syn3-KO animals froze considerably less than WT controls at the 1 and 24 hr tests (Ps<0.001). Interestingly, freezing at 24 hr for both WT and mutant animals was increased relative to the 1 hr test (Ps<0.010). In cued fear conditioning (Fig. 3c,d), RMANOVA found significant within subject effects before and during the CS intervals [F1,34=124.159, P<0.001]; the CS-interval by genotype [F1,34=80.532, P<0.001], CS-interval by test-time [F1,34=46,053, P<0.001], and CS-interval by test-time by genotype [F1,34=3.384, P<0.045] interactions were all significant. Bonferroni comparisons demonstrated that prior to CS presentation for the 1 hr and 24 hr tests, WT and Syn3-KO animals did not differ in freezing behaviors. Subsequently, WT mice increased freezing during the CS compared to the pre-CS interval at the 1 and 24 hr tests (Ps<0.001). By comparison, freezing did not change for Syn3-KO animals during CS presentation at 1 hr. Both WT and mutant mice froze more during the 24 hr than at the 1 hr test (Ps<0.003); however, freezing was higher in WT than the Syn3-KO mice at the 1 and 24 hr time-points (Ps<0.001).
Figure 3. Conditioned fear responses of WT and Syn3-KO mice.
(a-b) Percent freezing responses in contextual fear conditioning at 1 (a) and 24 hr (b). (c-d) Percent freezing responses prior to and during presentation of the CS in cued fear conditioning at 1 (c) and 24 hr (d). (e) Baseline startle amplitudes on day 1 of fear-potentiated startle. (f) Fear-potentiated startle following CS-UCS conditioning. n = 10 mice/genotype; *P<0.05 from the WT controls; #P<0.05 compared to 1 hr context test; +P<0.05 compared to pre-CS freezing.
During scoring of videotapes for fear conditioning it was observed that the mutants appeared to freeze for shorter durations than the WT controls. Examination of the frequencies and durations of freezing bouts during contextual and cued fear conditioning revealed significant genotype effects (Table 1). For the context test, bouts of freezing were increased for Syn3-KO animals [ANOVA: genotype F1,37=40.605, P<0.001; test-interval [F1,37=32.459, P<0.001] and the durations of these bouts were decreased in the 1 and 24 hr tests compared to those in the WT mice [ANOVA: genotype F1,37=104.252, P<0.001]. For the frequencies of freezing bouts in cued fear conditioning, RMANOVA found significant main effects of the pre-CS/CS interval [F1,34=39.116, P<0.001], and the pre-CS/CS interval by 1-hr/24-hr interval [F1,34=37.525, P<0.001] and the pre-CS/CS interval by 1-hr/24-hr test interval by genotype [F1,34=16.847, P<0.001] interactions were significant. Bonferroni comparisons observed that Syn3-KO mice had fewer freezing bouts during the pre-CS (P<0.008) and CS (P<0.002) intervals at 1 hr tests but they were increased at 24 hr (Ps<0.001) relative to WT controls. In all cases, the frequencies of freezing bouts increased during the CS compared to the pre-CS intervals during both the 1 and 24 hr tests (Ps<0.001). Although prior to CS presentation the durations of freezing were similar for the two genotypes, only WT controls showed any changes in their durations of freezing upon CS exposure. For the durations of freezing bouts in cued conditioning, RMANOVA noted significant main effects of pre-CS/CS [F1,34=22.009, P<0.001] and a significant pre-CS/CS interval by genotype interaction [F1,34=24.414, P<0.001]. Bonferroni comparisons reported that the duration of freezing per bout was similar for the genotypes for the pre-CS at the 1 and 24 hr tests. However, WT controls always showed increases in the duration of freezing per bout when the CS was presented compared to the pre-CS interval (Ps<0.001), whereas Syn3-KO mice did not show any appreciable changes. It should be noted that none of the mice engaged in escape-like behaviors, such as darting or running during testing. Together, these findings suggest that contextual and cued fear conditioning are aberrant in the Syn3-KO animals.
Table 1.
Bouts and durations of freezing in contextual and cued fear conditioning for WT and Syn3-KO mice
| Bouts of freezing1 | WT2 | KO2 |
|---|---|---|
| Context test (1 hr) | 15.6 ±1.3 | 27.1 ±2.33 |
| Context test (24 hr) | 27.1 ±2.3 | 40.9 ±2.23 |
| Cued test, pre-CS (1 hr) | 4.1 ± 0.6 | 2.0 ± 0.53 |
| Cued test, CS (1 hr) | 20.0 ±1.5 | 11.7 ±1.93 |
| Cued test, pre-CS (24 hr) | 4.3 ±1.1 | 7.5 ±1.13 |
| Cued test, CS (24 hr) | 24.1 ±1.6 | 35.3 ±1.83 |
| Duration of immobility bout4 | ||
| Context test (1 hr) | 9.7 ± 0.9 | 2.2 ± 0.943 |
| Context test (24 hr) | 10.1 ± 1.1 | 2.6 ± 0.23 |
| Cued test, pre-CS (1 hr) | 2.8 ± 0.8 | 2.4 ± 0.63 |
| Cued test, CS (1 hr) | 5.3 ± 0.8 | 2.2 ± 1.43 |
| Cued test, pre-CS (24 hr) | 3.4 ± 1.3 | 2.5 ± 0.23 |
| Cued test, CS (24 hr) | 6.4 ± 1.4 | 2.5 ± 0.83 |
Number of freezing bouts.
n=9-10 mice/genotype/test.
Ps<0.05, WT versus KO mice.
Duration of immobility bout is presented as sec/bout.
Since the percent time spent freezing was reduced in contextual and cued fear conditioning in Syn3-KO mice (Fig. 3a-d), amygdala functioning was evaluated in fear-potentiated startle. For day 1 of baseline responses (Fig, 3e), RMANOVA revealed significant main effects of stimulus intensity [F2,36=16.134, P<0.001] and a significant stimulus intensity by genotype interaction [F2,36=5.170, P<0.011]. Syn3-KO mice had significantly overall lower responses to the startle stimuli on day 1 than WT mice and Bonferroni comparisons showed that startle responses of WT and mutant animals did not differ at 100 and 105dB, but responses at 110dB were decreased for the Syn3-KO animals (P<0.001). Examination of pre-conditioning potentiation (Supplementary Table S10) revealed no significant genotype differences when the CS was presented before the startle stimuli. When post-conditioning potentiation was examined 24 hrs after CS-UCS pairing (Fig. 3f), RMANOVA revealed significant effects of stimulus intensity [F2,36=6.345, P<0.001]; the stimulus intensity by genotype interaction was also significant [F2,36=3.308, P<0.050]. Bonferroni comparisons revealed that mutants had higher startle potentiation at each stimulus intensity relative to the WT controls (Ps<0.043). Collectively, these data confirm that Syn3-KO mice are perturbed in conditioned fear.
Discussion
Syn3 is widely distributed throughout the developing brain and its concentrations decline after the first week of life (Ferreira et al., 2000). In adult brain, levels of Syn3 are lower than those for synapsin I and II (Kao et al., 1998). Nonetheless, in adults the highest concentrations are found in hippocampus with lower levels in the olfactory bulb and cerebral cortex (Pieribone et al., 2002; www.brainatlas.com). Like other synapsins, Syn3 is localized to presynaptic nerve terminals; however, Syn3 is also found at other presynaptic sites as well as in cell soma and growth cones (Ferreira et al., 2000). As may be anticipated, Syn3 is involved in early axonal outgrowth (Feng et al., 2002). It can also control the size of the recycling pool of transmitter and the strength of synaptic depression. Intriguingly, adult Syn3-KO mice have marked reductions in neurogenesis in the dentate gyrus (Kao et al., 2008). These results suggest that Syn3 may play an important, yet unknown role in hippocampally-based behaviors. To demonstrate that the mutants did not harbor gross deficits in behavior, animals were first evaluated in a neurophysiological screen. No genotype differences in anxiety- or depressive-like responses were identified. Similarly, no anomalies were detected for gross sensory or motor function. However, locomotion in the open field was increased in Syn3-KO animals and this was particularly evident by their increased thigmotactic activity. Mutant also reared in the corners more than WT controls. In the hole-board test, locomotion in Syn3-KO mice was increased and they explored more holes than WT animals. Nevertheless, the latency to head-poke and the total number of head-pokes in hole-board did not differ between the two genotypes, suggesting that the increased activity of mutants does not reflect impulsivity or unregulated motor responses. These data suggest that the increased activity in the open field and hole-board tests reflects an increase in exploration by the Syn3-KO mice when they are placed into a novel environment.
Hippocampal function was examined in the Morris water maze, object recognition, and social transmission of food preference tests (Morris et al., 1986; Crawley & Paylor, 1997; Logue et al., 1997; Lipp & Wolfer, 1998; Wolfer et al., 1998; Holmes et al., 2002; Ross & Eichenbaum, 2006; Dere et al., 2007). The Syn3-KO mice swam further and faster on the first day of acquisition testing than WT controls. During this first day, mutants swam further on each of the first 3 trials. This propensity may be attributed to their search strategy and not to sensory, motor, or motivational factors since no genotype differences were observed with the visible platform. An examination of the swim profiles on this first day and visual inspection indicates that Syn3-KO mice seem to be orienting themselves relative to the perimeter of the maze, rather than using a more generalized search strategy as the WT controls. This response may reflect the proclivity of the Syn3-KO animals to explore areas more intensely where novel or appealing stimuli are located. Alternatively, the mutants may be using a different strategy to learn the task across trials on day 1. Regardless, the Syn3-KO mice learned the location of the hidden platform and despite differences in responses during early acquisition testing, no genotype differences emerged.
When the location of the hidden platform was moved from the NE to SW quadrant, no genotype differences were observed. This finding is interesting because Syn3-KO mice show a 30% reduction in the proliferation of neural progenitor cells in the dentate gyrus (Kao et al., 2008), a brain area known to play an important role in navigation and learning in the water maze (Morris et al., 1990; Logue et al., 1997; Lipp & Wolfer, 1998; Eichenbaum & Cohen, 2001; Holmes et al., 2002). One reason why decreased neurogenesis may not exert a more discernable effect is that the reduction was modest and that there was an increase in survival of progenitor cells and neuronal differentiation was enhanced (Kao et al., 2008). These changes probably served compensatory roles and ameliorated any potential deficiencies on this test.
In the object recognition task, explicit STM seemed to be intact in the mutants. However, by 24 hrs and at 10 days performance declined and they appeared to be deficient relative to WT controls. Given that the overall numbers of contacts with the novel and familiar objects were similar between genotypes at these latter test times, the reduced preference scores for the mutants cannot be attributed to motivational differences in object exploration or neophobia. This contention is further supported by the fact that both genotypes spent similar amounts of time exploring the objects during each test interval. Together, these results indicate that the Syn3-KO mice are deficient in LTM and RM and at least hippocampal-cortical interactions may be perturbed.
In the social transmission of food preference test, Syn3-KO mice showed reduced preferences for the familiar demonstrator diet at 20 min and 24 hr compared to those of WT controls. These responses cannot be due to motivational distinctions since both genotypes consumed similar amounts of food at both test intervals. It is unlikely also that the deficits in social transmission are due to deficiencies in gross olfaction since the mutants can still discriminate odors in this test. Nevertheless, STM is selectively impaired in the social transmission but not in the object test. These differences may be due to several factors. First, the object recognition test relies upon a propensity to explore novelty (Eichenbaum & Cohen, 2001; Holmes et al., 2002; Dere et al., 2007). Results from the open field and hole-board tests indicate the mutants are attracted to novelty. Since the object test is biased towards novelty (i.e., preference for the novel object), it is not surprising that the mutants perform better on this than the social transmission task -- especially at the 20 min interval when they first encounter the novel object. Second, social transmission of food preference is highly dependent upon learning from a social partner (Holmes et al., 2002; Vale-Martinez et al., 2002). Although WT and Syn3-KO mice spent similar amounts of time investigating and interacting with the demonstrator animal, the tester must be exposed to the demonstrator in the familiar home cage and then to recall information in a novel environment. Hence, the context of testing may affect responses of the mutant animals. Finally, while both the object recognition and social transmission tasks require an intact hippocampus, successful performance on the tests relies upon somewhat different brain circuits and areas (Bunsey & Eichenbaum, 1995; Winocur et al., 2001; Eichenbaum & Cohen, 2001; Holmes et al., 2002;). Hence, Syn3 seems to play a selective role in learning and memory functions where spatial learning and memory are largely intact, but processes underlying explicit memory are affected.
Animals were examined also in tests of conditioned fear. In contextual and cued fear conditioning, WT and Syn3-KO mice showed increases in freezing behavior in the 1 and 24 hr tests. This enhancement suggests that both genotypes develop conditioned fear and that consolidation of emotional memory occurs over time. However, the mutants spent less time freezing on both tests than WT controls. These genotype distinctions in freezing cannot be ascribed to differences in sensitivity to noxious stimuli because both genotypes responded similarly to varying intensities of foot-shock. Instead, the patterns of responding appear to be different. For instance, Syn3-KO mice displayed more bouts of freezing than WT controls except for the 1 hr cued fear conditioning test when it was decreased. By comparison, for WT mice the duration of freezing during each bout was enhanced at the 1 and 24 hr tests for both contextual and cued fear conditioning. As a result, only WT controls showed changes in their durations of freezing during CS presentation. When other activities such as rearing, rapid walking, jumping, or running were examined, it was found that these incidences were rare and occurred at the same frequencies in both genotypes. However, results from the neurophysiological screen, open field, hole-board, and water maze indicate that the Syn3-KO mice have enhanced motor activity and this propensity may compete or interfere with their ability to engage in protracted freezing behavior. Nevertheless, responses in the hole-board test gave no evidence of impulsivity. In another test of conditioned fear, fear-potentiated startle, Syn3-KO mice showed increased potentiation to each of the startle intensities. These findings suggest that conditioned fear may be enhanced in Syn3-KO mice and that their reductions in freezing behavior in fear conditioning may be due to their inability to express higher levels of freezing than the WT controls.
Although the Syn3-KO mice show deficiencies in explicit memory and abnormalities in conditioned fear, the mechanisms underlying the behavioral changes are unclear. For example, the phenotype may be due to neurodevelopmental deficits induced by the lack of Syn3. In this regard Syn3 is known to be involved in neural progenitor cell survival, neuronal differentiation, and early axonal outgrowth (Feng et al., 2002). Alternatively or in addition, the abnormal behaviors may be attributed to the direct absence of Syn3 in mature neurons. In this case Syn3 is known to participate in dentate neurogenesis and neurotransmitter secretion (Feng et al., 2002; Kao et al., 2008). Hence, the behavioral deficits in Syn3-KO mice may be ascribed to a number of different mechanisms that will be the subject of future investigations.
Our findings with Syn3 mice may have relevance for schizophrenia. SYN3 polymorphisms have been identified with increased frequency in schizophrenia (Porton et al., 2004; Lachman et al., 2005) and the levels of protein are reduced in the hippocampus (Vawter et al., 2002) and dorsolateral prefrontal cortex of individuals with schizophrenia (Porton and Wetsel, 2007). Moreover, consistent with our findings in Syn3-KO mice (Kao et al., 2008), adult hippocampal neurogenesis is decreased in postmortem brains of individuals with schizophrenia (Reif et al., 2006). Although the disorder now includes cognitive dysfunction (see Gold & Weinberger, 1995), it should be emphasized that these symptoms are quite variable (Mohamed et al., 1999; Brewer et al., 2006; Keefe et al., 2006). Hence, it is possible that some of the abnormalities in activity, PPI, and learning and memory in Syn3-KO mice may share some similarities to those in patients with schizophrenia or schizotypal personality disorder. Since schizophrenia is recognized as a developmental disorder (Yung and McGorrey, 1996) and because Syn3 expression is most pervasive in developing brain (Ferreira et al., 2000; Pieribone et al., 2002), investigations of the Syn3 mice may provide unique insights into basic mechanisms that subserve cognition in schizophrenia and related psychiatric disorders.
Supplementary Material
Acknowledgments
We wish to thank Jiechun Zhou, Liping Du, Santowana Ayral, and Natalia Pogorelov for breeding and maintaining the mice for these studies. We also thank Rosalie Bateson, Michael Cools, Caroline Kim, Yuxin Ma, Lavender Ning, and Nancy Wang for assistance in behavioral testing. Special thanks to Fayin Li of CleverSys Incorporated for developing specialized behavioral recognition configurations for the analyses of the hole-board videos. These studies were supported in part by NIH grant MH 070898 (BP), an APA Diversity Fellowship (RMR), Howard Hughes fellowships for high school (RB, LN, NW) and undergraduate students (JWG), and NIH grant MH082441 (WCW).
References
- Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18:253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, Cornblatt B, McGorry PD. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Paired associate learning in rats: critical involvement of the parahippcampal region. Behav Neurosci. 1995;107:740–747. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, de Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:674–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Reflection: Memory Systems of the Brain. Oxford University Press; New York: 2001. [Google Scholar]
- Falls WA, Carlson S, Turner JG, Willott JF. Fear-potentiated startle in two strains of inbred mice. Behav Neurosci. 1997;111:855–861. [PubMed] [Google Scholar]
- Feng J, Chi P, Blanpied TA, Xu Y, Magarinos AM, Ferreira A, Takahashi RH, Kao H-T, McEwen BS, Ryan TA, Augustine GJ, Greengard P. Regulation of neurotransmitter release by synapsin III. J Neurosci. 2002;22:4372–4380. doi: 10.1523/JNEUROSCI.22-11-04372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Kao H-T, Feng J, Rapoport M, Greengard P. Synapsin III: Developmental expression, subcellular localization, and role in axon formation. J Neurosci. 2000;20:3736–3744. doi: 10.1523/JNEUROSCI.20-10-03736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Weinberger DR. Cognitive deficits and the neurobiology of schizophrenia. Curr Opin Neurobiol. 1995;5:225–230. doi: 10.1016/0959-4388(95)80030-1. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM. Abi2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for relevance memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Kamei J, Matsunawa Y, Miyata S, Tanaka S, Saitoh A. Effects of nociception on the exploratory behavior of mice in the hole-board test. Eur J Neurosci. 2003;489:77–87. doi: 10.1016/j.ejphar.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Kao H-T, Porton B, Czernik AJ, Feng J, Yiu G, Häring M, Benfenati F, Greengard P. A third member of the synapsin gene family. Proc Natl Acad Sci USA. 1998;95:4667–4672. doi: 10.1073/pnas.95.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H-T, Li P, Chao HM, Janoschka S, Pham K, Feng J, McEwen BS, Greengard P, Pieribone VA, Porton B. Early involvement of Synapsin III in neural progenitor cell development in the adult hippocampus. J Comp Neurol. 2008;507:1860–1870. doi: 10.1002/cne.21643. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res. 2006;88:26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Stopkova P, Rafael MA, Saito T. Association of schizophrenia in African Americans to polymorphism in synapsin III gene. Psychiatr Genet. 2005;15:127–132. doi: 10.1097/00041444-200506000-00009. [DOI] [PubMed] [Google Scholar]
- Lipp HP, Wolfer DP. Genetically modified mice and cognition. Curr Opin Neurobiol. 1998;8:272–280. doi: 10.1016/s0959-4388(98)80151-7. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:1191–1197. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum: dissociating components allocentric spatial learning. Eur J Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Porton B, Rendon R, Greengard P, Kao H-T. Expression of synapsin III in nerve terminals and neurogenic regions of the adult brain. J Comp Neurol. 2002;454:105–114. doi: 10.1002/cne.10417. [DOI] [PubMed] [Google Scholar]
- Pillai-Nair N, Panicker AK, Rodriguiz RM, Miller K, Demyanenko GP, Huang J, Wetsel WC, Maness PF. NCAM-Secreting transgenic mice display abnormalities in GABAergic interneurons and alterations in behavior. J Neurosci. 2005;25:4659–4671. doi: 10.1523/JNEUROSCI.0565-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorelov VM, Rodriguiz RM, Insco ML, Caron MG, Wetsel WC. Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology. 2005;30:1818–1831. doi: 10.1038/sj.npp.1300724. [DOI] [PubMed] [Google Scholar]
- Porton B, Ferreira A, DeLisi LE, Kao H-T. A rare polymorphism affects a mitogen-activated protein kinase site in synapsin III: possible relationship to schizophrenia. Biol Psychiat. 2004;55:118–125. doi: 10.1016/j.biopsych.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Porton B, Wetsel WC. Reduction of synapsin III in the prefrontal cortex of individuals with schizophrenia. Schizophr Res. 2007;94:366–370. doi: 10.1016/j.schres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knockout mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Ribar T, Rodriguiz RM, Khiroug L, Wetsel WC, Augustine GJ, Means AR. Cerebellar defects in Ca2/calmodulin kinase IV deficient mice. J Neurosci. 2000;RC107:1–5. doi: 10.1523/JNEUROSCI.20-22-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz RM, Wetsel WC. Assessment of cognitive deficits in mutant mice. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. CRC Press; Boca Raton, FL: 2006. pp. 223–282. [PubMed] [Google Scholar]
- Rodriguiz RM, Gadnidze K, Ragnauth A, Dorr N, Yanagisawa M, Wetsel WC, Devi LA. Animals lacking endothelin converting enzyme-2 are deficient in learning and memory. Genes Brain Behav. 2008;7:418–426. doi: 10.1111/j.1601-183X.2007.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Tsuji M, Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect anxiogenic and/or anxiolytic state in mice. Eur J Pharmacology. 1998;350:21–29. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Rodriguiz RM, Greene RI, Daniell X, Henry SC, Crooks KR, Kotloski R, Tessarollo L, Phillips LE, Wetsel WC. Behavioral characterization of P311 knockout mice. Genes Brain Behav. 2008;7:786–795. doi: 10.1111/j.1601-183X.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale-Martinez A, Baxter MG, Eichenbaum H. Selective lesions of basal forebrain cholinergic neurons produce anterograde and retrograde deficits in social transmission of food preference task in rats. Eur J Neurosci. 2002;16:983–998. doi: 10.1046/j.1460-9568.2002.02153.x. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Thatcher L, Usen N, Hyde TM, Kleinman JE, Freed WJ. Reduction of synapsin in the hippocampus of patients with bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:571–578. doi: 10.1038/sj.mp.4001158. [DOI] [PubMed] [Google Scholar]
- Winocur G, McDonald RM, Moscovitch M. Anterograde and retrograde amnesia in rats with large hippocampal lesions. Hippocampus. 2001;11:18–26. doi: 10.1002/1098-1063(2001)11:1<18::AID-HIPO1016>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp H-P. Spatial memory and learning in transgenic mice: fact or artifact? News Physiol Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Aust N Z J Psychiatry. 1996;30:587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.