Pentagonal molecular architectures possessing five-fold (C5) symmetry exist throughout the chemical world from a wealth of inorganic species with pentagonal, pyramidal, bipyramidal, and prismatic geometries,1 to all-carbon frameworks such as fullerenes and carbon nanotubes bearing curvature-inducing five-membered rings.2 The unique C5 symmetry has also been identified in nanoscale materials3 as well as DNA nanostructures.4 Moreover, a 2D arrangement of pentagonal structures with C5 or quasi-C5 symmetry, distinct from the significantly more common C2, C3, C4 and C6 periodic symmetries, has been a long-term target pursued by crystal engineers that has been meet with limited success.5 Pentagonal architectures are also attractive because of their potential applications in functional materials such as quasicrystals6 and discotic liquid crystals.7
In light of their potentials, the synthesis of discrete pentagonal architectures has remained a formidable challenge. Only a few discrete C5-symmetrical pentagonal organic molecules have been synthesized, generally in low yield and through arduous synthetic work.8 Coordination-driven self-assembly has been extensively explored in the past few decades and shown to be a powerful synthetic strategy for the construction of metallosupramolecular architectures.9 By combining specifically designed organic donor building blocks with directional metal acceptors, a plethora of two-dimensional supramolecular structures, including molecular loops,10 triangles,11 squares,12 and hexagons,13 have been synthesized in high yield. This synthetic methodology provides an efficient and viable means to construct discrete pentagonal structures.
Our group has long endeavored to establish a “molecular library” of metallosupramolecular structures built from the coordination-driven self-assembly of appropriately designed Pt(II) or Pd(II) acceptors and specifically angled donor units in a controllable manner.9a–d According to this design concept, discrete pentagonal entities may be exclusively assembled by the incorporation of five 108° building units with five complementary linear units. However, the scarcity of suitable 108° subunits has complicated the realization of such a design concept. In the few reported examples of supramolecular metal-ligand pentagonal architectures,14 polydentate flexible ligands were used since their coordination to metal centers may lead to a 108° bonding conformation, though an encapsulated anion of specific size must be included to template the assembly process. Furthermore, the difficulty to develop a common methodology to construct a metallosupramolecular pentagon also arises from the internal turning angle of a regular pentagon, 108°, which is close to that of a regular hexagon (120°).15 The small 12° difference between the 120° angle needed for a hexagonal assembly and the 108° angle needed for an analogous pentagon assembly often leads to an equilibrium mixture of pentagon and hexagon suprastructures.
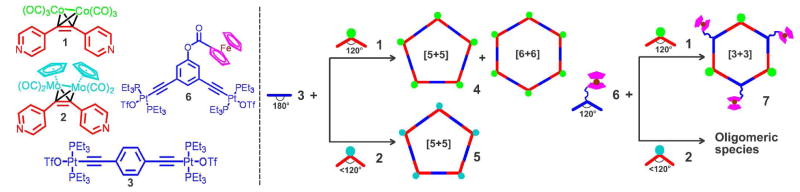
Acetylene units (C≡C) are extensively incorporated into many donor and acceptor building blocks due to their rigid linear conformation. In view of the ready reactivity16 of a wide range of metal carbonyl cluster complexes with acetylene moieties, we envisioned that the steric bulk of a metal carbonyl cluster species adhered to the acetylene moiety may be used as a control factor to adjust the bonding angle of the building block in order to exclusively form a pentagon self-assembly. Two metal carbonyl dipyridine adduct ligands, (4-C5H4N)2C≡CCo2(CO)6 (1) and (4-C5H4N)2C≡CMo2Cp2(CO)4 (2), were synthesized and were combined with a linear acceptor ligand bis[1,4-(trans-Pt(PEt3)2OTf)]ethynylbenzene (3) to investigate the possibility of constructing [5+5] pentagonal metallosupramolecules (Scheme 1).
Scheme 1.
Molecular structures of donor (red) and acceptor (blue) building blocks and their self-assembly into metallacyclic supramolecules.
Crystallographic studies have shown that the acetylene moiety adducted by Co2(CO)6 can form a tetrahedral Co2C2 core,16b thus making an angle of 120° between the two pyridine rings in 1. Self-assembly between this 120° donor with the complementary linear acceptor 3 is assumed to construct a [6+6] hexagon. The reaction of 1 with 3 in a 1:1 ratio in CD2Cl2 gave a wine-colored homogeneous solution of 4, whose 31P{1H} NMR spectrum showed a single peak at 16.5 ppm with concomitant 195Pt satellites, upfield shifted by roughly 6.4 ppm compared with 3 (δ = 23.0 ppm) as a result of the coordination of the pyridine rings (Figure S2 in Supporting Information). However, the proton NMR of 4 displayed broad signals in contrast to the sharp peaks of previously reported for discrete hexagon structures,17 implying the possible existence of several species in the mixture (Figure S2). The ESI-TOF mass spectrum indicated that two self-assembled polygons – [5+5] pentagon and [6+6] hexagon – do indeed co-exist in self-assembly 4. Two charge states at m/z = 2040.0 and 1310.3 corresponding to [pentagon – 4CF3SO3]4+ and [pentagon – 6CF3SO3]6+, respectively, were observed and were in good agreement with their theoretical isotopic distributions. The isotropically well-resolved mass peak at m/z = 1952.8, resulting from [hexagon – 5CF3SO3]5+, was found in the mass spectrum as well (Figure 1a).
Figure 1.
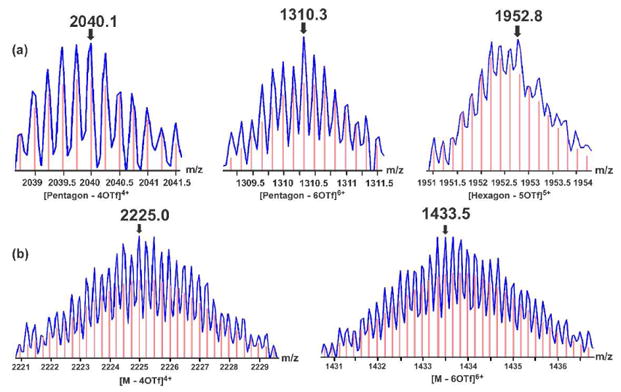
ESI-TOF-MS spectra of (a) self assembly 4 containing both pentagon and hexagon, and (b) two charge states of [5+5] pentagon 5. Red vertical lines are the theoretical abundances.
Mixing molybdenum cluster donor ligand 2 in a 1:1 stoichiometric ratio with 3 generated a homogeneous dark-red solution of 5. A single sharp peak at 16.7 ppm with two 195Pt flanking satellites were observed in the 31P{1H} NMR spectrum of 5 (Figure S3). The proton NMR spectrum of 5 displayed sharp signals with differentiable coupling constants (Figure S3). The signals of the pyridine ring α-protons experienced a small upfield shift of 0.03 ppm, but the β-protons and the hydrogen nuclei on the Cp ring undergo approximately 0.2–0.3 ppm downfield shifts, suggestive of the strong back-donation effect of the molybdenum carbonyl cluster to the pyridine ring.
The ESI-TOF mass spectrum of 5 displayed four peaks corresponding to four charge states of the [5+5] pentagon, including [M – 3CF3SO3]3+ (m/z 3016.6), [M – 4CF3SO3]4+ (m/z 2225.0), [M – 5CF3SO3]5+ (m/z 1750.2, overlapping with the 1+ fragment) and [M – 6CF3SO3]6+ (m/z 1433.5), which were all isotopically well-resolved and agree very well with their respective theoretical distributions (the 4+ and 6+ charge state are illustrated in Figure 1b and the full spectrum is shown in Figure S5). No evidence for any other species such as a [4+4] square, [6+6] hexagonal, or [7+7] heptagonal assembly was found. The exclusive formation of a [5+5] pentagon is also supported by comparing the proton NMR spectrum of pentagon-hexagon mixture 4 and that of 5, wherein the peaks of the former are much broader than the latter (Figure S6).
Our attempts to crystallize the polygonal structures 4 (pentagon and hexagon mixture) and 5 (exclusively pentagon) have so far been unsuccessful. We have therefore used molecular force field simulations to investigate the structural details of the supramolecular pentagon and hexagon composed of cobalt donor 1 and linear di-Pt(II) acceptor 3, as well as the [5+5] pentagon formed by the self-assembly of molybdenum donor 2 with 3. In the case of the pentagonal and hexagonal supramolecules that incorporate 1 with 3, the energies of the two different polygon structures are nearly identical with the [6+6] hexagon being slightly more stable. In the self assembly between 2 and 3, modeling suggests the pentagonal structure is more stable than the hexagon. The modeled suprastructures show that the linear acceptor units in the hexagonal structure must distort away from a 180° orientation in order to fit the complementarity requirement of a [6+6] hexagon whereas the acceptors retain their 180° geometry in the modeled [5+5] pentagonal structure (Figure 2). The formation of a discrete [5+5] pentagon in 5, derived from 2, can also be rationalized by the fact that the primary steric effect of the Cp rings in building block 2 forces the two pyridine rings closer to each other, thus forming a smaller bonding angle than in the related donor 1.
Figure 2.
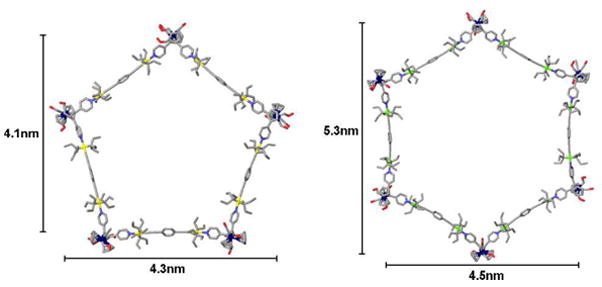
Pentagonal and hexagonal structures composed of molybdenum donor ligand 2 and linear acceptor 3 as obtained from molecular force field modeling.
We also investigated the self-assembly of 1 and 2 with the 120° ferrocenyl acceptor 6 in order to construct a Fe3-Co6-Pt6 and a Fe3-Mo6-Pt6 trimetal [3+3] hexagons, respectively, and substantiate the effects of the bonding angle difference between 1 and 2 in self-assembly(Scheme 1). Preparation of the novel trimetal Fe3-Co6-Pt6 [3+3] hexagon 7 was successfully achieved by mixing 1 with ferrocenyl acceptor 6 in a 1:1 ratio in dichloromethane. The resulting red solution was characterized by 1H and 31P{1H} NMR spectroscopy, with the latter displaying a single sharp peak (Figure S4). The ESI mass spectrum exhibited two charge states of the Fe3-Co6-Pt6 hexagon 7 (m/z = 1335.1 and 1038.3 for +4 and +5, respectively), which were isotropically resolved and were in good agreement with theoretical isotopic distributions (Figure S7). However, our attempt to produce a Fe3-Mo6-Pt6 trimetal [3+3] hexagon using a similar synthetic protocol employing 2 instead of 1 was unsuccessful. This differing reactivity of related donor ligands 1 and 2 further confirms that the bonding angles of the two pyridine rings of each metal carbonyl donor ligand are measurably dissimilar in coordination-driven self assembly because of the difference in the steric bulk of Co2(CO)6 and Mo2Cp2(CO)4.
In conclusion, we have successfully prepared a [5+5] supramolecular pentagon by the self-assembly of a molybdenum carbonyl cluster dipyridyl donor ligand (2) with a linear di-Pt(II) acceptor (3). The roughly 108° bonding angle encoded within 2 directs this coordination-driven self-assembly process to form a single pentagonal metallosupramolecule rather than a pentagon-hexagon mixture, implying that the generality of this method can be extend to construct other pentagonal structures with a variety of functional groups for even more advanced multifunctional materials.
Supplementary Material
Acknowledgments
P.J.S. thanks the NIH (Grant GM-057052) for financial support.
Footnotes
Supporting Information Available: Synthetic procedures and spectroscopic characterization of compound 2 and assemblies 4, 5 and 7. ESI-TOF-MS of [5+5] pentagon 5. ESI-MS of [3+3] hexagon 7. Molecular modeling results of pentagonal and hexagonal structures composed of 1 and 3. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For some selected samples: Davidovich RL, Stavila Vi, Marinin DV, Voit EI, Whitmire KH. Coord Chem Rev. 2009;253:1316.Zhou B, Denning MS, Kays DL, Goicoechea JM. J Am Chem Soc. 2009;131:2802. doi: 10.1021/ja900055j.
- 2.Fowler P, Manolopoulos D. An Atlas of Fullerenes. 2. Dover Publications; Mineola, NY: 2006. [Google Scholar]
- 3.(a) Eskandari S, Kreman M, Kavanaugh MP, Wright EM, Zampighi GA. Proc Natl Acad Sci USA. 2000;97:8641. doi: 10.1073/pnas.97.15.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pivetta M, Blüm MC, Patthey F, Schneider WD. Angew Chem Int Ed. 2008;47:1076. doi: 10.1002/anie.200704479. [DOI] [PubMed] [Google Scholar]
- 4.Chaput JC, Switzer C. Proc Natl Acad Sci USA. 1999;96:10614. doi: 10.1073/pnas.96.19.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Keller SW, Lopez S. J Am Chem Soc. 1999;121:6306. [Google Scholar]; (b) Moulton B, Lu J, Zaworotko MJ. J Am Chem Soc. 2001;123:9224. doi: 10.1021/ja016637f. [DOI] [PubMed] [Google Scholar]; (c) Thakuria R, Sarma B, Nangia A. Cryst Growth Des. 2008;8:1471. [Google Scholar]
- 6.Man WN, Megens M, Steinhardt PJ, Chaikin PM. Nature. 2005436:993. doi: 10.1038/nature03977. [DOI] [PubMed] [Google Scholar]
- 7.(a) Block MAB, Kaiser C, Khan A, Hecht S. Top Curr Chem. 2005;245:89. [Google Scholar]; (b) Sergeyev S, Pisula W, Geerts YH. Chem Soc Rev. 2007;36:1902. doi: 10.1039/b417320c. [DOI] [PubMed] [Google Scholar]
- 8.(a) Wu YT, Siegel JS. Chem Rev. 2006;106:4843. doi: 10.1021/cr050554q. [DOI] [PubMed] [Google Scholar]; (b) Sessler JL, Anzenbacher P, Jr, Shriver JA, Jursikova K, Lynch V, Marquez M. J Am Chem Soc. 2000;122:12061. doi: 10.1021/jo005610w. [DOI] [PubMed] [Google Scholar]; (c) Zhang W, Moore JS. J Am Chem Soc. 2004;126:12796. doi: 10.1021/ja046531v. [DOI] [PubMed] [Google Scholar]; (d) Qin B, Chen X, Fang X, Shu Y, Yip YK, Yan Y, Pan S, Ong WQ, Ren C, Su H, Zeng H. Org Lett. 2008;10:5127. doi: 10.1021/ol801980h. and references therein. [DOI] [PubMed] [Google Scholar]
- 9.(a) Stang PJ, Olenyuk B. Acc Chem Res. 1997;30:502. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Seidel SR, Stang PJ. Acc Chem Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (d) Northrop BH, Yang HB, Stang PJ. Chem Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Holliday BJ, Mirkin CA. Angew Chem, Int Ed. 2001;40:2022. [PubMed] [Google Scholar]; (f) Cotton FA, Lin C, Murillo CA. Acc Chem Res. 2001;34:759. doi: 10.1021/ar010062+. [DOI] [PubMed] [Google Scholar]; (g) Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:371. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (h) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc Chem Res. 2005;38:351. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (i) Severin K. Chem Commun. 2006:3859. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; (j) Pitt MA, Johnson DW. Chem Soc Rev. 2007;36:1441. doi: 10.1039/b610405n. [DOI] [PubMed] [Google Scholar]
- 10.(a) Sun SS, Stern CL, Nguyen ST, Hupp JT. J Am Chem Soc. 2004;126:6314. doi: 10.1021/ja037378s. [DOI] [PubMed] [Google Scholar]; (b) Ghosh S, Mukherjee PS. Organometallics. 2008;27:316. [Google Scholar]
- 11.(a) Qin Z, Jennings MC, Puddephatt RJ. Inorg Chem. 2002;41:3967. doi: 10.1021/ic020227q. [DOI] [PubMed] [Google Scholar]; (b) Cotton FA, Lin C, Murillo CA. Proc Natl Acad Sci USA. 2002;99:4810. doi: 10.1073/pnas.012567599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J Am Chem Soc. 2003;125:5193. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]; (d) Martin-Redondo MP, Scoles L, Sterenberg BT, Udachin KA, Carty AJ. J Am Chem Soc. 2005;127:5038. doi: 10.1021/ja050155c. [DOI] [PubMed] [Google Scholar]; (e) Weilandt T, Troff RW, Saxell H, Rissanen K, Schalley CA. Inorg Chem. 2008;47:7588. doi: 10.1021/ic800334k. [DOI] [PubMed] [Google Scholar]; (f) Ghosh S, Mukherjee PS. Inorg Chem. 2009;48:2605. doi: 10.1021/ic802254f. [DOI] [PubMed] [Google Scholar]
- 12.(a) Angaridis P, Berry JF, Cotton FA, Murillo CA, Wang X. J Am Chem Soc. 2003;125:10327. doi: 10.1021/ja036095x. [DOI] [PubMed] [Google Scholar]; (b) Caskey DC, Shoemaker RK, Michl J. Org Lett. 2004;6:2093. doi: 10.1021/ol049539i. [DOI] [PubMed] [Google Scholar]; (c) Ni ZH, Tao J, Wernsdorfer W, Cui AL, Kou HZ. Dalton Trans. 2009:2788. doi: 10.1039/b814860k. [DOI] [PubMed] [Google Scholar]; (d) Theilmann O, Saak W, Haase D, Beckhaus R. Organometallics. 2009;28:2799. [Google Scholar]
- 13.(a) Coronado E, Galan-Mascaros JR, Gavina P, Marti-Gastaldo C, Romero FM, Tatay S. Inorg Chem. 2008;47:5197. doi: 10.1021/ic8000569. [DOI] [PubMed] [Google Scholar]; (b) Ghosh K, Zhao Y, Yang HB, Northrop BH, White HS, Stang PJ. J Org Chem. 2008;73:8553. doi: 10.1021/jo801692v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Hasenknopf B, Lehn JM, Kneisel B, Baum G, Fenske D. Angew Chem Int Ed Engl. 1996;35:1838. [Google Scholar]; (b) Hasenknopf B, Lehn JM, Boumediene N, Dupontgervais A, Vandorsselaer A, Kneisel B, Fenske D. J Am Chem Soc. 1997;119:10956. [Google Scholar]; (c) Campos-Fernandez CS, Clerac R, Koomen JM, Russell DH, Dunbar KR. J Am Chem Soc. 2001;123:773. doi: 10.1021/ja002960r. [DOI] [PubMed] [Google Scholar]; (d) Campos-Fernández CS, Schottel BL, Chifotides HT, Bera JK, Bacsa J, Koomen JM, Russell DH, Dunbar KR. J Am Chem Soc. 2005;127:12909. doi: 10.1021/ja052108q. [DOI] [PubMed] [Google Scholar]; (e) Satake A, Tanak H, Hajjaj F, Kawai T, Kobuke Y. Chem Commun. 2006:2542. doi: 10.1039/b603360a. [DOI] [PubMed] [Google Scholar]
- 15.Caulder DL, Raymond KN. Acc Chem Res. 1999;32:975. [Google Scholar]
- 16.(a) Abel EW, Stone FGA, Wilkinson G. Comprehensive Organometallic Chemistry II. Chapter 1 Vol. 8. Pergamon; Oxford: 1995. [Google Scholar]; (b) Song LC, Jin GX, Wang HT, Zhang WX, Hu QM. Organometallics. 2005;24:6464. [Google Scholar]
- 17.Yang HB, Das N, Huang F, Hawkridge AM, Díaz DD, Arif AM, Finn MG, Muddiman DC, Stang PJ. J Org Chem. 2006;71:6644. doi: 10.1021/jo0608117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.