How did the study come about?
The metabolic syndrome includes presence of several metabolic risk factors, such as obesity, hypertension, insulin resistance/hyperglycaemia and dyslipidaemia.1 These factors have, separately and jointly, been associated with an increased risk of cardiovascular diseases.2–4 To date, little is known about the metabolic syndrome and cancer risk.5 Recent prospective studies have either focused on the association between the metabolic syndrome and risk of specific cancers, mainly colorectal and prostate cancer, or between a single factor in the metabolic syndrome and cancer risk.5 It remains to be elucidated if factors within the metabolic syndrome act in synergy on the risk of cancer.
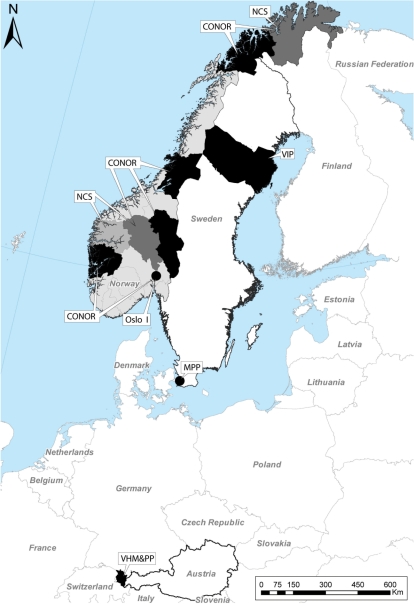
In 2006, we initiated the Metabolic syndrome and Cancer project (Me-Can) in order to create a large pooled cohort to investigate factors of the metabolic syndrome on the association with cancer risk. Existing cohorts in Norway, Austria and Sweden were included in the project. The cohorts were: in Norway, the Oslo study I (Oslo), the Norwegian Counties Study (NCS), the Cohort of Norway (CONOR) and the Age 40 programme (40-years); in Austria, the Vorarlberg Health Monitoring and Prevention Programme (VHM&PP); and in Sweden, the Västerbotten Intervention Project (VIP) and the Malmö Preventive Project (MPP) (Figure 1). The co-ordinating centre is at the Department of Surgical and Perioperative sciences, Umeå University, Sweden, and the Me-Can database is stored at the Oncological Centre, Umeå University, Sweden. The Me-Can project was approved by ethical committees in the respective countries.
Figure 1.
Map with location of subcohorts included in Me-Can. The 40-year cohort includes all counties in Norway (all grey- and black-marked areas in Norway), NCS includes areas  and
and  , the CONOR includes areas
, the CONOR includes areas  and
and  , and further cohorts (marked in black) are; Oslo I, VHM&PP, VIP and the MPP
, and further cohorts (marked in black) are; Oslo I, VHM&PP, VIP and the MPP
The purpose of Me-Can
The aim of Me-Can studies is to investigate the relation between factors in the metabolic syndrome and cancer risk. Key exposure factors are body mass index (BMI), blood pressure, blood glucose and serum levels of total cholesterol and triglycerides. The specific purpose of the Me-Can project is to study the effect of these factors, separately and combined, on the association with risk of cancer overall and of specific cancer sites among men and women, and to use both incident and fatal cancer as endpoints.
Who is in the sample?
All subjects in the Me-Can cohorts underwent one or more health examination(s) and, except for in the VHM&PP, participants were asked to fill in a questionnaire in connection with the examination. The questionnaires covered lifestyle factors and various topics of specific interest for each programme. In the VHM&PP, the examining physician asked about lifestyle issues and answers were recorded; however, tobacco use is the only lifestyle factor with sufficient validity and coverage among participants for use in studies.
Norwegian cohorts
The Norwegian cohorts were all initiated by the Norwegian Institute of Public Health, or the former National Health Screening Service in Norway (now part of the Norwegian Institute of Public Health), and thus, the design of these surveys were similar.
The Oslo study I (Oslo), 1972–73
The Oslo study I is the first of two rounds of surveys conducted among men in Oslo, the capital city of Norway.6,7 All men aged 40–49 years and a random selection (7%) of men aged 20–39 years were invited to attend a health survey programme during the period 1972–73. The purpose was to study the prevention and epidemiology of cardiovascular diseases. The attendance rate in the Oslo study I was 60%; nearly 18 000 individuals participated in the study. Data from the second round of the Oslo study, conducted in 2000, are part of the CONOR study.
The Norwegian Counties Study, 1974–88
Screening for cardiovascular disease prevention was performed in the Norwegian counties, Finnmark, Sogn og Fjordane and Oppland in three time periods: 1974–78, 1977–83 and 1985–88.8,9 All residents in these counties aged 35–49 years and a random sample of 20–34-year-old subjects were invited to the screening in 1974–78. The sample invited to the second and third screening was a combination of previous participants and new cohorts. The attendance rate in the screening periods and the counties was between 78 and 90% and altogether, approximately 93 000 individuals participated in the NCS.
The Cohort of Norway, 1994–2003
The CONOR was established by the Norwegian Ministry of Health and is a research collaboration between the Norwegian Institute of Public Health and the Universities of Bergen, Oslo, Tromsø and Trondheim.10 The purpose of the establishment of CONOR was to set up a cohort that included core survey data and stored blood samples for future research on aetiological factors for a variety of diseases. A standardized protocol was used for all common measurements in surveys included in CONOR. Several surveys from different regions in Norway, and within different age groups, provide data for the CONOR; the first data are from the year 1994. The attendance rate in CONOR surveys was on average 56% (range: 30–76% between surveys) and altogether, 173 236 individuals participated in the CONOR. A smaller proportion of participants had two or more health examinations.
The 40-year cohort (40-year), 1985–99
The Age 40-programme was ongoing in Norway between 1985 and 1999 and by 1993, the programme covered all counties in Norway.11 Every 3 years, all 40–42-year-old residents of a county were invited to a health examination for monitoring of risk factors of cardiovascular diseases and epidemiological research. In some counties, broader age groups were invited. The attendance rate was on average 69% for the whole period 1985–99, and the number of participants was nearly 420 000. The attendance rate has declined over time; it was 62% in the period 1994–99. During this period, 140 000 persons participated in the Age 40-programme, and observations from these years will be used as baseline in Me-Can studies.
The Austrian cohort
The Vorarlberg Health Monitoring and Prevention Programme, 1985–2005
The VHM&PP is a population-based risk factor surveillance programme in Vorarlberg, the westernmost province in Austria.12 The purpose of the programme was to prevent chronic diseases, foremost cardiovascular diseases and cancer, and it was routinely performed by the Agency for Social and Preventive Medicine. All adult residents in the region were invited by written invitations, television, radio or newspapers, to participate in a health examination up to once a year. Since 1985, more than two-thirds of the population of the province participated in the programme. Data from the years 1985–2003 are included in Me-Can, and during these years the attendance rate in the VHM&PP was 66% and roughly 176 000 persons participated in the programme.
Swedish cohorts
The Västerbotten Intervention Project, 1985 and ongoing
The VIP is an ongoing project aiming for prevention of diabetes and cardiovascular disease in residents of Västerbotten county in the north of Sweden.13,14 Since 1985, all residents have been invited for a health check-up at 40, 50 and 60 years of age, and during the first 10 years of the project, residents were also invited at the age of 30. The attendance rate has been 60% on average over the years. By the end of 2006, approximately 86 000 men and women had participated in the VIP.
The Malmö Preventive Project, 1974–92
Middle-aged men and women in the city of Malmö in southern Sweden, were invited to a screening programme for prevention of cardiovascular disease and alcohol abuse.15,16 Screenings were carried out between 1974 and 1992, to which all residents within predefined birth cohorts, born between 1921 and 1949, were invited. The attendance rate was on average 71% over the years. A total of 33 346 men and women participated in the baseline screening, and 5722 of these men (born 1926–38) and 387 women (born in 1931) participated in a second screening in 1981–89. The examination at the second screening was similar to that of the first screening.
What has been measured?
In all Me-Can cohorts, measurements of height, weight and systolic and diastolic blood pressure have been performed, and blood/plasma/serum levels of glucose, total cholesterol and triglycerides have been analysed. However, glucose levels were not measured throughout all years and in all surveys in Norway, and before the year 2001, triglycerides were not measured consequently in all participants in the VIP. There are no other data available on metabolic factors or factors related to the MetS with sufficient coverage in all Me-Can cohorts.
A description of measurement methods is shown in Table 1. Anthropometric measurements were conducted in a similar way in all Me-Can cohorts, with participants wearing light indoor clothes and no shoes, whereas different methods were used in the cohorts for measurement of blood pressure. In the Norwegian cohorts, participants were not requested to fast before the health examination, and thus, most blood samples were drawn in a non-fasting state. In the VHM&PP and the MPP, participants were asked to fast overnight before the health examination, which also became standard procedure in 1992 in the VIP. In the VHM&PP, plasma glucose was measured after an oral glucose tolerance test (OGTT) during the first 3 years, and from 1988, fasting glucose was measured. Glucose levels were measured in serum in the Norwegian cohorts, in plasma in the VHM&PP and the VIP, and in whole blood in the MPP. Determination of lipid levels was performed in serum in all cohorts.
Table 1.
Measurement methods in Me-Can cohorts
| Norway |
Austria |
Sweden |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Oslo | NCS | CONOR | 40-year | VHM&PP | VIP | MPP | |
| Height | No shoes | No shoes | No shoes | No shoes | No shoes | No shoes | No shoes | |
| Weight | Light clothes | Light clothes | Light clothes | Light clothes | Light clothes | Light clothes | Light clothes | |
| Blood pressure | ||||||||
| Number of measurements | 2a | 2a,b | 3a | 3a | 1 | 1 | 1–2 (mean value was recorded) | |
| Rest before measurement | 4 min, 1 min between each measurement | 4 min, 1 min between each measurementb | 2 min, 1 min between each measurement | 2 min, 1 min between each measurement | 5 min | 5 min | 10 min | |
| Position | Sitting | Sitting | Sitting | Sitting | Sitting | Supine | Supine | |
| Instrument | Mercury sphygmanometer | Mercury sphygmanometer | Automatic device | Automatic device | Mercury sphygmanometer | Mercury sphygmanometer | Mercury sphygmanometer | |
| Fasting status before measurement | Non-fasting | Non-fasting | Non-fasting | Non-fasting | Fasting from 1988 | Fasting from 1992 | Fasting | |
| Glucose | ||||||||
| Substance | Serum | Serum | Serum | Serum | Plasma | Plasma | Whole blood | |
| Method | Non-enzymaticc | Non-enzymaticc | Enzymaticc | Enzymaticc | Enzymatic | Enzymatic | Enzymatic | |
| Cholesterol and triglycerides | ||||||||
| Substance | Serum | Serum | Serum | Serum | Serum | Serum | Serum | |
| Method | Non-enzymaticd | Non-enzymatic Enzymatic from year 1980d | Enzymaticd | Enzymaticd | Enzymatic | Enzymatic | Enzymatic | |
aIn accordance with previous studies in Norway,10,17 the second blood pressure out of two measurements is used in Me-Can studies, and the mean value of the second and third measurement is used if three measurements were recorded.
bFrom 1985, i.e. the third screening in the NCS, blood pressure was measured as described for the CONOR and 40-year cohort.
cLevels from the non-enzymatic method yielded 0.8–1.1 mmol/l higher levels than the enzymatic method.
Data management and selection of subjects/observations for Me-Can studies
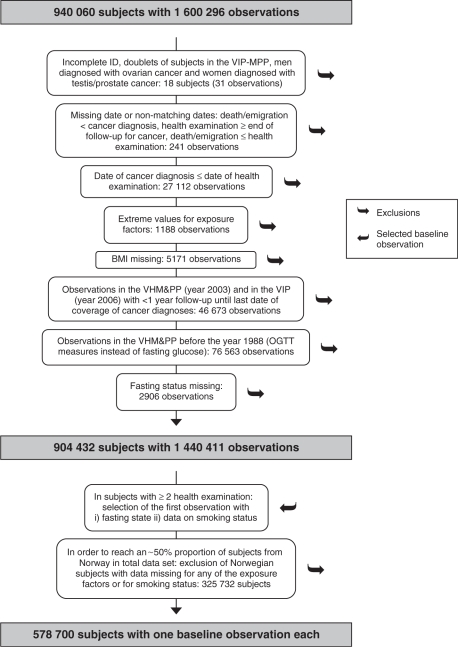
Altogether, the Me-Can study file includes 940 060 subjects with data from 1 600 296 health examinations, and after data cleaning and selection of subjects and observations, data from a total of 578 700 subjects will be used in Me-Can studies on the metabolic syndrome and cancer risk (Figure 2). From the original data files, subjects with an incomplete personal identity number were excluded, and of subjects included in both the VIP and the MPP, the subject in the doublet with the least complete data was excluded. One man with ovarian cancer recorded and seven women with prostate or testicular cancer recorded, were excluded. Missing data for day and month were substituted with Day 15 for missing day and June 30 for missing month and day, and observations with dates that did not match were excluded. Observations with a malignant cancer before the health examination were also excluded at baseline. Observations with extreme values for exposure factors were excluded as follows: height <100 or >250 cm, weight <35 or >250 kg, BMI <15 or >60 kg/m2, systolic blood pressure <75 mmHg, diastolic blood pressure <40 mmHg, systolic blood pressure <diastolic blood pressure, glucose <1 mmol/l, cholesterol <0.5 or >20 mmol/l, triglycerides <0.05 or >35 mmol/l. We also excluded observations with data missing for height and weight. Further, we excluded examinations (observations) that had been performed <1 year before the end of follow-up for incident cancer, and examinations in the VHM&PP that had been performed before 1988, during which post-load glucose had been measured instead of fasting glucose. Observations with data missing for fasting status were also excluded. A total of 904 432 subjects with 1 440 411 observations remained eligible for use in studies of the metabolic syndrome and cancer risk. In subjects with several observations available, we selected the first observation with a fasting blood sample and with smoking status reported as the baseline observation. After this selection, 70% of subjects in the study file were from the Norwegian cohorts. A policy imposed by the Norwegian Institute of Public Health restricts Me-Can studies not to exceed ∼50% of subjects from the Norwegian cohorts in the total study population. Thus, to reduce the proportion, we further excluded subjects from Norway without data on all the exposure factors (BMI, blood pressure, glucose, cholesterol and triglycerides) and those without data on smoking status. Missing data for glucose was the cause for exclusion in 99% of subjects. The final data set to be used in Me-Can studies includes 578 700 subjects, 289 866 (50%) men and 288 834 (50%) women (Table 2). Of these subjects, data from two or more health examinations are available for 207 890 (36%) subjects, which will be used to calculate random error in exposure factors to assess more accurate estimates of associations with cancer risk.20
Figure 2.
Description of data cleaning and selection of subjects/observations for Me-Can studies on the metabolic syndrome and cancer risk
Table 2.
Description of baseline observations in Me-Can studies of the metabolic syndrome and cancer risk
| Norway |
Austria |
Sweden |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Oslo | NCS | CONOR | 40-year | VHM&PP | VIP | MPP | Total |
| Year of health examination (range) | 1972–73 | 1974–83 | 1995–2003 | 1994–99 | 1988–2002 | 1985–2005 | 1974–1992 | 1972–2005 |
| Subjects (n) | ||||||||
| Men | 16 760 | 25 952 | 52 181 | 60 676 | 73 213 | 38 843 | 22 241 | 289 866 |
| Women | 25 072 | 57 687 | 68 211 | 86 671 | 40 669 | 10 524 | 288 834 | |
| All | 16 760 | 51 024 | 109 868 | 128 887 | 159 884 | 79 512 | 32 765 | 578 700 |
| Age, mean (SD) | ||||||||
| Men | 44.1 (5.6) | 40.3 (6.9) | 47.7 (14.7) | 41.5 (2.0) | 42.6 (14.8) | 47.6 (9.6) | 43.7 (6.6) | 43.9 (11.1) |
| Women | 40.3 (7.0) | 47.4 (15.2) | 41.5 (1.9) | 42.8 (16.0) | 47.5 (9.6) | 49.6 (7.5) | 44.1 (12.3) | |
| All | 44.1 (5.6) | 40.3 (7.0) | 47.5 (15.0) | 41.5 (1.9) | 42.7 (15.4) | 47.6 (9.6) | 45.6 (7.4) | 44.0 (11.7) |
| Fasting status (%) | ||||||||
| <4 h | 81 | 78 | 78 | 79 | 0 | 3 | 0 | 42 |
| 4 to <8 h | 10 | 19 | 16 | 17 | 0 | 7 | 0 | 10 |
| ≥8 h | 9 | 3 | 6 | 4 | 100 | 90 | 100 | 48 |
| Data coverage (%) | ||||||||
| BMI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Blood pressure | 100 | 100 | 100 | 100 | 100 | 99 | 100 | 100 |
| Glucose | 100 | 100 | 100 | 100 | 100 | 99 | 100 | 100 |
| Cholesterol | 100 | 100 | 100 | 100 | 100 | 99 | 100 | 100 |
| Triglycerides | 100 | 100 | 100 | 100 | 100 | 85 | 100 | 98 |
Follow-up
Nationwide registers in Norway, Austria and Sweden will be used for follow-up of subjects. Linkages have been performed to the cancer registers, which have an almost complete coverage of cancer cases in Norway21 and Sweden,22 and the cancer register in Austria has also shown a high coverage of diagnosed cancers.23 The cohorts have also been linked to the respective National Cause of Death Register, and in Norway and Sweden, to the Register of the Total Population and Population Changes, for vital status (data not available in Austria). To reduce the possibility of reverse causation, follow-up in Me-Can studies will consequently start 1 year after the baseline examination, which with the current linkage dates resulted in 37 000 incident cancers and 13 000 fatal cancers during follow-up. The Me-Can project has the statistical power of 80%, with an alfa level of 5%, to detect a relative risk for the fifth versus first quintile of 1.05 for all cancer sites combined (37 000 cases), 1.13 for sites with 5000 cases (prostate, breast), 1.33 for sites with 1000 cases (kidney, endometrium) and 1.79 for sites with 250 cases (oesophagus, liver). The power can be increased by using models that utilize all data, i.e. continuous levels, for estimation.
What are the main strengths and weaknesses?
The main strength of the Me-Can project is the large data set from a number of population-based surveys, with almost complete coverage of data for exposure factors in all cohorts, and the access of data on repeated health examinations for a substantial number of the subjects. Another strength is the use of high-quality national registers for follow-up of subjects. Limitations of the project are the lack of detailed data on possible confounders and on tumour characteristics and cancer treatment, and that measurement methods of exposure factors differed between the cohorts.
Where can I find out more?
Further information about cohorts in Me-Can can be found in the reference list, and for the Norwegian cohorts, at the Norwegian Institute of Public Health web-page: http://www.fhi.no/eway/default.aspx?pid=233&trg=MainArea_5661&MainArea_5661=5631:0:15,3570:1:0:0:::0.
Funding
World Cancer Research Fund (Grant 2007/09 to Me-Can); Swedish Cancer Society (Grant 2007/693 to Me-Can; Austrian National Bank (Grant ONEB-12737 to VHM&PP).
Acknowledgements
We thank the study participants and the personnel who collected and/or handled the data in the cohorts. We also thank all other personnel who contributed to the existence of the cohorts: in Norway, the screening team at the former National Health Screening Service of Norway, now the Norwegian Institute of Public Health, the contributing research centres delivering data to CONOR; in the Vorarlberg Health Monitoring and Prevention Program, Elmar Stimpfl, data base manager for the VHM&PP, Karin Parschalk at the cancer registry, and Elmar Bechter and Hans-Peter Bischof, medical doctors at the Health Department of the Vorarlberg State Government; in the VIP; Åsa Ågren, project data base manager at the Medical Biobank, Umeå University, Sweden.
Conflict of interest: None declared.
References
- 1.Aguilar-Salinas CA, Rojas R, Gomez-Perez FJ, et al. The metabolic syndrome: a concept hard to define. Arch Med Res. 2005;36:223–31. doi: 10.1016/j.arcmed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–89. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–78. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 4.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program – Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 5.Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169:1505–22. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leren P, Askevold EM, Foss OP, et al. The Oslo study. Cardiovascular disease in middle-aged and young Oslo men. Acta Med Scand Suppl. 1975;588:1–38. [PubMed] [Google Scholar]
- 7.Lund Haheim L, Wisloff TF, Holme I, Nafstad P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am J Epidemiol. 2006;164:769–74. doi: 10.1093/aje/kwj284. [DOI] [PubMed] [Google Scholar]
- 8.Bjartveit K, Foss OP, Gjervig T. The cardiovascular disease study in Norwegian counties. Results from first screening. Acta Med Scand Suppl. 1983;675:1–184. [PubMed] [Google Scholar]
- 9.Tverdal A, Foss OP, Leren P, Holme I, Lund-Larsen PG, Bjartveit K. Serum triglycerides as an independent risk factor for death from coronary heart disease in middle-aged Norwegian men. Am J Epidemiol. 1989;129:458–65. doi: 10.1093/oxfordjournals.aje.a115157. [DOI] [PubMed] [Google Scholar]
- 10.Naess O, Sogaard AJ, Arnesen E, et al. Cohort profile: cohort of Norway (CONOR) Int J Epidemiol. 2008;37:481–85. doi: 10.1093/ije/dym217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aires N, Selmer R, Thelle D. The validity of self-reported leisure time physical activity, and its relationship to serum cholesterol, blood pressure and body mass index. A population based study of 332,182 men and women aged 40-42 years. Eur J Epidemiol. 2003;18:479–85. doi: 10.1023/a:1024682523710. [DOI] [PubMed] [Google Scholar]
- 12.Ulmer H, Kelleher C, Diem G, Concin H. Long-term tracking of cardiovascular risk factors among men and women in a large population-based health system: the Vorarlberg Health Monitoring & Promotion Programme. Eur Heart J. 2003;24:1004–13. doi: 10.1016/s0195-668x(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 13.Weinehall L, Hallgren CG, Westman G, Janlert U, Wall S. Reduction of selection bias in primary prevention of cardiovascular disease through involvement of primary health care. Scand J Prim Health Care. 1998;16:171–76. doi: 10.1080/028134398750003133. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl B, Weinehall L, Asplund K, Hallmans G. Screening for impaired glucose tolerance. Results from a population-based study in 21,057 individuals. Diabetes Care. 1999;22:1988–92. doi: 10.2337/diacare.22.12.1988. [DOI] [PubMed] [Google Scholar]
- 15.Berglund G, Eriksson KF, Israelsson B, et al. Cardiovascular risk groups and mortality in an urban swedish male population: the Malmo Preventive Project. J Intern Med. 1996;239:489–97. doi: 10.1046/j.1365-2796.1996.483819000.x. [DOI] [PubMed] [Google Scholar]
- 16.Berglund G, Nilsson P, Eriksson KF, et al. Long-term outcome of the Malmo preventive project: mortality and cardiovascular morbidity. J Intern Med. 2000;247:19–29. doi: 10.1046/j.1365-2796.2000.00568.x. [DOI] [PubMed] [Google Scholar]
- 17.Tverdal A. Systolic and diastolic blood pressures as predictors of coronary heart disease in middle aged Norwegian men. Br Med J (Clin Res Ed) 1987;294:671–73. doi: 10.1136/bmj.294.6573.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stensvold I, Tverdal A, Urdal P, Graff-Iversen S. Non-fasting serum triglyceride concentration and mortality from coronary heart disease and any cause in middle aged Norwegian women. Br Med J. 1993;307:1318–22. doi: 10.1136/bmj.307.6915.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Cardiovascular Disease Study in Norwegian Counties. Results from Second Screening. Oslo: National Health Screening Service; 1988. National Health Screening Service, Health Services of Finnmark, Sogn og Fjordane, and Oppland counties, Ullevål Hospital, Central Laboratory, Oslo. [Google Scholar]
- 20.Wood AM, White I, Thompson SG, Lewington S, Danesh J. Regression dilution methods for meta-analysis: assessing long-term variability in plasma fibrinogen among 27,247 adults in 15 prospective studies. Int J Epidemiol. 2006;35:1570–78. doi: 10.1093/ije/dyl233. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Registry of Norway. Cancer in Norway 2006. [1 December 2008, date last accessed]. http://www.kreftregisteret.no/no/Generelt/Publikasjoner/Cancer-in-Norway/Cancer-in-Norway-2006/
- 22.Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register - a sample survey for year 1998. Acta Oncol. 2008;3:1–7. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 23.Rapp K, Schroeder J, Klenk J, et al. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49:945–52. doi: 10.1007/s00125-006-0207-6. [DOI] [PubMed] [Google Scholar]