Abstract
CD4 T helper cells (Th) are critical in combating pathogens and maintaining immune homeostasis. Since the establishment of the Th1–Th2 paradigm in the 1980s, many types of specialized Th cells, including Th1, Th2, Th17, Th9, follicular helper T and regulatory T, have been identified. We have become accustomed to the idea that different Th cells are ‘committed’ to their paths but recent emerging evidence suggests that under certain conditions, seemingly committed Th cells possess plasticity and may convert into other types of effector cells. In this review, we will first introduce the major sub-types of Th cells that are involved in immune regulation. Then, we will describe in detail the inter-convertibility of Th cells among different sub-types under in vitro and in vivo conditions. Finally, we will discuss our current understanding of the underlying mechanisms on how a particular type of Th cells may convert into other types of Th cells.
Keywords: autoimmunity, CD4/helper T cells, inflammation, regulatory T cells, T cells
Effector T-cell subsets – their characteristics, function and differentiation
Our immune system comprises innate and adaptive immunity. Adaptive immunity is mediated by T and B cells to impose positive as well as negative regulation of immune responses. Effector T cells, also known as helper T (Th) cells, are the key players in steering the immune responses. Th cell differentiation is characterized by the acquisition of cytokine production. Since the establishment of Th1–Th2 paradigm, the function and regulation of effector T cells has been a subject of intense investigation. Owing to years of collective efforts, vast knowledge has been gained in identifying new classes of effector T cells and in understanding their function and regulation.
The Th1 cells, one of the first described Th cells, produce signature cytokine interferon-γ (IFN-γ) along with pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) and TNF-β, to stimulate innate and T-cell immune responses. The most important function of Th1 cells is to promote cell-mediated immunity characterized by cellular cytolytic activity. The Th1 cells are important in protection of the host from the obligate intracellular pathogens. Over-exuberant pro-inflammatory activities of Th1 cells cause tissue damage and elicit unwanted inflammatory disease and self-reactivity including inflammatory bowel disease1 and graft-versus-host disease,2 as well as autoimmune disorders such as insulin-dependent diabetes mellitus3 and rheumatoid arthritis.4 Besides being a Th1 signature cytokine, IFN-γ has been suggested, although debatably, to be important for the differentiation of Th1 cells.5–7 Interleukin-12 (IL-12), however, has been accepted as a potent inducer of Th1 differentiation.8,9 The ultimate outcomes of IFN-γ and IL-12 signalling are to solidify their Th1 function through promoting the expression of Th1-specific transcription factors. T-bet, also known as Tbx21,10 belongs to the T-box family of transcription factors and is the only known T-box gene specifically expressed in the lymphoid system. T-bet is rapidly and specifically induced in developing Th1 cells and is critical for initiating Th1 development. Hence, T-bet is recognized as a master regulator of Th1 differentiation.10
T helper type 2 cells were identified at the same time as Th1 cells in the early 1980s. They are defined as producers of IL-4, IL-5, IL-9, IL-10 and IL-13. The Th2 response is often associated with the humoral response and is important in resistance against extracellular forms of pathogens. The Th2 cells are also important for mucosal immunity in the lung. Aberrant elevation of the Th2 response often leads to chronic inflammatory airway diseases, such as atopic asthma and allergy.11–13 Interleukin-4 is the most potent, if not the only, cytokine known to have the greatest influence in directing Th2 differentiation.14,15 The presence of IL-4, even if endogenously derived, is essential for Th2 differentiation.16 GATA binding protein 3 (GATA-3) is a member of the GATA family of transcription factors. Expression of GATA-3 is sufficient and required for Th2 differentiation.17–19 Therefore, GATA-3 is regarded as the master regulator for Th2 differentiation. Signal transducer and activator of transcription 6 (STAT-6) activated by IL-4 stimulation is the major signal transducer in IL-4-mediated Th2 differentiation in vitro.20–22 One of the mechanisms for STAT-6 to promote Th2 differentiation is through inducing high levels of the transcription factor GATA-3.23
T helper type 17 is a newly identified class of effector T cells that produce IL-7A and F. Th17 cells play critical role in the induction and propagation of autoimmunity. Interleukin-17 expression has been associated with autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, psoriasis and inflammatory bowel disease as well as allergic responses.24,25 Transforming growth factor-β (TGF-β) and IL-6 act co-operatively and non-redundantly to promote Th17 commitment.26–28 Interleukin-21 is an IL-2 family member that has been recently found to be highly produced by Th17 cells. It can substitute for IL-6 to induce Th17 cells along with TGF-β.29–31 Retinoic acid-related orphan receptors (ROR) are the key transcription factors in Th17 differentiation. Both ROR-α and ROR-γt are critical and somewhat redundant in promoting Th17 differentiation.32,33
Follicular helper T (Tfh) cells express high levels of CXCR5 and cytokine IL-10 and IL-21, which are not typically associated with other types of Th cells.34–37 Interleukin-21 has been found to be critical for Tfh differentiation.35,37 Bcl-6 was recently reported to be a transcription factor that can be up-regulated by IL-21 signalling and is critical for Tfh generation.38
Regulatory T (Treg) cells form a subset of CD4 T cells that either develop in the thymus (naturally occurring Treg; nTreg) or are differentiated from naive T cells in the presence of TGF-β following T-cell receptor stimulation (induced Treg; iTreg). Unlike other Th cells, which promote an immune response, Treg cells are immune-suppressive. The most prominent function of Treg cells is maintaining self-tolerance and immune homeostasis.39,40 Disruption of Treg cell function contributes to a plethora of autoimmune and inflammatory pathologies. It is also suggested that Treg cells are important for tempering immune responses against infectious agents and in re-establishing immune homeostasis following pathogen clearance.41 A recent study suggests that Treg cells can also facilitate immune response against herpes simplex virus infection by recruiting natural killer, dendritic and T cells to the site of infection.42 Foxp3 is a transcription factor highly and specifically expressed in Treg cells. Foxp3 is recognized as the master regulator for Treg function controlling the expression of a wide array of genes including cytokines and surface molecules.43
Malleability of Th cells is dictated by the cytokine milieu and beyond…
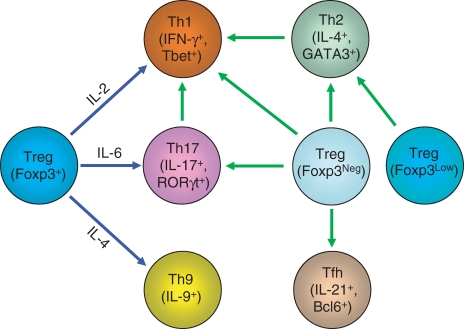
Historically, Th cells are deemed terminally differentiated cell lineages ‘committed’ to their path. Certainly, most in vitro differentiation models suggest that this is the case and such ‘commitments’ provide simplified experimental models that allow us to understand how Th cells function and are regulated. T cells in our body often have to mount different responses towards different insults at short notice. It is certainly advantageous for Th cells to be able to convert from one type of Th cells to another type to efficiently fight against ongoing immunological insults. Recently, accumulating evidence suggests that Th cells are functionally ‘plastic’ and can be converted into other types of Th cells under in vitro as well as in vivo conditions (Fig. 1).
Figure 1.
Inter-conversion among T helper (Th) cell subsets. Under the influence of cytokines, regulatory T (Treg) cells convert into other types of Th cells in vitro (connected by blue lines). In addition, through yet-to-be-defined mechanisms, Th1, Th2, Th17, follicular helper T (Tfh) and Treg cells with no (Foxp3Neg) or reduced (Foxp3Low) Foxp3 expression can convert into other Th cells in vivo (connected by green lines). IFN-γ, interferon-γ; IL, interleukin; ROR, retinoic acid-related orphan receptors.
In vitro, a different cytokine milieu directs CD4 T cells to differentiate into different types of Th cells. Cytokine-driven T-cell differentiation in vitro has been a fruitful approach to understanding the regulation and the function of various Th lineages. Evidence is available to suggest that Treg cells are able to convert into other types of Th cells under culturing conditions. The Treg-to-Th17 conversion has been studied extensively. In an earlier study, IL-6 was shown to abrogate nTreg cell suppressive activity.44 In the ensuing years, IL-6 was found to be important in suppression of Treg generation and to promote Th17 differentiation. The TGF-β promotes the generation of Foxp3-expressing Treg cells from naive T cells. However, IL-6 antagonizes TGF-β-induced Treg generation and promotes Th17 differentiation of naive T cells.27,28 These findings raise the possibility that Treg cells may be able to convert into Th17 cells upon IL-6 stimulation because TGF-β is highly expressed by Treg cells. Indeed, freshly isolated nTreg cells produce large amounts of TGF-β upon stimulation in vitro. In the presence of IL-6, Foxp3-expressing cells were found to express IL-17, suggesting that nTreg can be converted into Th17-like cells.45,46 Not only nTreg cells are able to be converted into Th17 cells, The TGF-β-induced iTreg cells were also shown to turn into IL-17-producing cells upon IL-6 stimulation.46 Conversion of Treg to Th17 cells was concomitant with down-regulation of Foxp3, whose expression has been shown to suppress RORγt expression and to shut down the Th17 programme.45,47 Therefore the down-regulation of Foxp3 seems to be important in licensing Treg cells to Th17 differentiation. Interleukin-6-dependent signalling is sufficient to both suppress Foxp3 expression and promote IL-17 expression. It is specifically required for the down-regulation of Foxp3, as STAT-3-deficient mice failed to repress Foxp3 following IL-6 stimulation. Hence, IL-6 signalling appears to be the molecular switch between Treg and Th17.45 Although IL-17 expression in Treg cells coincided with Foxp3 down-regulation, the total abolishment of Foxp3 was not required for Th17 conversion of Treg cells, because cells expressing both Foxp3 and IL-17 were found.45,46 Hence, Foxp3 expression and IL-17 expression are not mutually exclusive.
Some studies have addressed the potential of Foxp3-expressing cells to produce IFN-γ and to become Th1 cells in vitro with controversial findings. In one study, Treg cells were found not to be able to produce IFN-γ even under Th1 polarizing conditions.46 However, in other studies, Foxp3-expressing cells were found to produce IFN-γ under Th1 conditions in Foxp3-expressing and Foxp3-non-expressing populations (references 48,49 and our unpublished observations). In the absence of large amounts of exogenous IL-2, Foxp3-expressing cells display an anergic phenotype and could not be activated. This may contribute to the absence of cytokine production recorded in the first study. However, when a high dose of exogenous IL-2 was provided in the culture to force Treg cells to exit the anergic state, Treg cells generated substantial amounts of IFN-γ to become Th1 cells. Therefore, the experimental conditions used in these studies might account for the different results obtained. Aforementioned findings suggest that Treg cells are able to convert into Th1 cells in vitro under certain conditions. While Treg cells possess great plasticity to convert into Th1 and Th17 cells, no evidence has been presented to show that Treg cells can be converted into Th2 cells under culture conditions. When CD4 T cells were stimulated in the presence of IL-4 and TGF-β, the TGF-β-induced Foxp3 expression was blunted.50 However, activated T cells did not become IL-4-producing T cells. Instead, they produced IL-9.26,51 Therefore, it remains unknown whether Treg are able to become IL-4-producing cells in vitro.
Although in vitro studies are informative, whether Th cells possess plasticity and how it is regulated in vivo are of ultimate importance for our understanding of Th cell function and regulation. Recently, emerging evidence suggests that under certain conditions, Th cells indeed possess plasticity and may convert into other types of effector cells. Long-lived Th1 effector/memory cells are able to turn off IFN-γ expression in vivo, appearing to be ready to ‘re-differentiate’.52 It is an interesting finding in that most previous studies suggested that Th1 and Th2 differentiation is counter-regulatory and self-reinforcing.53 Hence, IFN-γ and IL-12 act on activated naive CD4 T cells to direct Th1 differentiation, whereas IL-4 directs Th2 differentiation through the induction of transcriptional programmes dominated by central ‘master regulators’. Activation of STAT-1 by IFN-γ and of STAT-4 by IL-12 drive optimal expression of T-bet, a central transcription factor for Th1 programming, whereas activation of STAT-6 by IL-4 up-regulates GATA-3, which is central to Th2 programming. These developmental programmes are enforced through the production of autocrine cytokines by differentiated effector cells via feed-forward loops. Early skewing towards one pathway therefore becomes self-reinforcing to mature Th1 or Th2 cells with functional programmes that are largely ‘fixed.’ Although no evidence has been presented to show the inter-conversion between Th1 and Th2 cells in vitro, it would be interesting to know whether such a conversion could occur in vivo in light of the fact that IL-4 and IFN-γ expression is not absolutely mutually exclusive as cells that simultaneously produce IL-4 and IFN-γ can be readily detected.
Interleukin-17-producing memory T cells isolated from mice retained a Th17 phenotype even when they were cultured with IL-12 or IL-4, indicating that at least a subset of Th17 cells achieve phenotypic stability via mechanisms undefined in vitro. Nevertheless, it is clear that Th17 precursors do transition to IFN-γ-producing cells in vivo. Transferring Th17 precursors isolated from IL-17F reporter mice into immunodeficient hosts induced colitis that was associated with a shift in the cytokine phenotype of transferred T cells – reduction (but not extinction) of IL-17A expression, loss of IL-17F expression, and induction of IFN-γ expression.54 This finding is in agreement with a previous study showing that Th17-polarized cells converted into Th1 cells in an antigen-specific model of ocular inflammation.55 In addition, two independent studies reported that Th17 to Th1 conversion led to the onset of diabetes in transfer models.56,57 Transferring Th17-polarized, islet-reactive T-cell receptor transgenic BDC2.5 T cells induced insulitis and diabetes, associating with the observation that a substantial fraction of transferred T cells become Th1 cells.
Accumulating evidence has been presented to show that Treg cells can convert into effector T cells in vivo. In a mouse model where an enhanced green fluorescent protein (EGFP) expression cassette was knocked into the first exon of the endogenous Foxp3 gene, cells that lack functional Foxp3 protein but with active Foxp3 promoter activities were therefore marked with GFP. Loss of functional Foxp3 resulted in Scurfy-like phenotype in the mice. However, GFP-expressing cells were readily detected with an elevated percentage compared with healthy mice. These GFP-expressing cells expressed Treg signature genes at reduced levels. Although GFP-expressing cells could not suppress the immune response, they are anergic to T-cell receptor stimulation. As a consequence, cells that do not express Foxp3 remain to be Treg lineage but with abrogated immune suppressive function. These Treg cells became effector T cells in vivo, producing IFN-γ, IL-4 and IL-17. Therefore, with the loss of Foxp3 expression, Treg cells could be generated but functionally dysregulated by losing suppressive activity and gaining Th1, Th2 and Th17 effector function.58 The Treg cells were also shown to down-regulate Foxp3 expression and convert into effector T cells under homeostatic conditions in vivo. Zhou et al.59 generated a BAC transgenic mouse model where Cre and EGFP were expressed in Foxp3-expressing Treg cells. By crossing such a mouse with a yellow fluorescent protein (YFP) Cre-reporter mouse strain, they tracked the T cells that are expressing and used to express Foxp3. They found that, in vivo, a small percentage of CD4 T cells expressed YFP but not GFP, indicating that these T cells used to express Foxp3 but shut down the Foxp3 expression at the time of assay. Therefore, down-regulation of Foxp3 expression of Treg cells normally occurs in vivo. They referred to these YFP+ GFP− cells as exFoxp3 Treg cells. In addition, they found that exFoxp3 Treg cells expressed IFN-γ and IL-17, suggesting their conversion into Th1 and Th17 cells. Moreover, exFoxp3 Treg cells were able to promote inflammation and contributed to the onset of type 1 diabetes upon transfer. Therefore, Treg-to-Th conversion could be important for directing inflammatory responses. Evidence for Treg-to-Tfh conversion in vivo is also available. Upon being transferred into CD3−/− mice where T cells were absent, Foxp3-expressing Treg cells down-regulate Foxp3 expression in Peyer’s patch. The transferred Foxp3-non-expressing Treg cells but not Foxp3-expressing Treg cells clustered around germinal centres and expressed CXCR5, IL-21 and Bcl6, a phenotype resembling Tfh.60 In the aforementioned studies, Treg-to-Th conversion corresponded with the loss of Foxp3 expression, suggesting that conversion and Foxp3 expression might be mutually exclusive, and questioning the intrinsic ability of Treg cells to become other types of Th cells in vivo. By knocking a luciferase and EGFP expressing cassette into the 3′-untranslated region of the endogenous Foxp3 gene locus, we serendipitously generated a mouse model whose Treg cells expressed Foxp3 at a reduced level.61 Reduction of Foxp3 expression resulted in a systemic autoimmune syndrome similar to what has been observed in Scurfy mice. Interestingly, reduction of Foxp3 led to a preferential conversion of Treg cells into IL-4-producing Th2 cells.61 Conversion of Treg cells to Th2 might be essential for directing Th2 responses as reduced Foxp3 expression in Treg cells has been associated with Th2-type immune disorders.62,63 Hence, in vivo, Foxp3 expression and Treg-to-Th conversion is not mutually exclusive. Nevertheless, the expression level of Foxp3 limits the potential of Treg cells to become all types of Th cells. It remains to be addressed whether Treg cells with reduced Foxp3 expression are able to convert into Th cells other than Th2 cells. It appears that the expression levels of Foxp3 control Treg function – high level of Foxp3 expression endows T cells with suppressive activity, reduced level of Foxp3 expression leads to Th2 conversion, and loss of Foxp3 expression licenses Treg cells to become Th1, Th2, Th17 as well as Tfh cells. As a consequence, Foxp3 acts as a rheostat controlling the diverse function of Treg cells in vivo. The mechanisms underlying such phenomena are important questions that warrant being addressed in the future. In addition, as Foxp3 down-regulation is a critical trigger for Treg-to-Th conversion in vivo, what triggers Foxp3 down-regulation or alternatively, what maintains high levels of Foxp3 expression, are questions that need to be addressed. Furthermore, the biological function of Treg conversion during normal and aberrant immune responses still waits to be revealed. While Treg cells function like effector T cells, they nevertheless phenotypically resemble naive T cells with certain features of activation. Most Treg-to-Th conversion studies were performed by using freshly isolated nTreg but not from in vitro differentiated iTreg cells. Therefore, it is reasonable to believe that returning to or maintaining a non-activated naive state of Th cells may be a pre-requisite for their conversion into other Th cell types.
While the signal transduction network controlling Th conversion remains to be unveiled, studies on epigenetic control of Th differentiation have provided important mechanistic insights for the plasticity of Th cells. T-cell lineage specification is accompanied by epigenetic modifications of key cytokine and transcription factor gene loci. Such epigenetic change provides a basis for the heritability of gene expression patterns acquired by differentiating T cells.64,65 Histone methylation or acetylation, DNA methylation and higher order chromatin structure each contribute to regulation of the accessibility of cis elements that bind lineage-specifying transcription factors and ultimately the expression of lineage-specific genes. The growing recognition of instability among the functional Th cells, as demonstrated in particular by plasticity of Treg programmes, implies greater malleability in T-cell epigenetic modifications than had previously been thought. It suggests that conversion of Treg cells is probably reflected in the reversal of epigenetic modifications induced during initial differentiation of these cells. A global genome analysis of permissive and repressive histone methylation marks of naive, Th1, Th2, Th17, nTreg and iTreg cells was recently reported, providing a mechanistic basis for aspects of Treg cell conversions.49 Trimethylations of histone H3 were identified at the proximal promoters of key T-cell lineage transcription factors or cytokine genes. H3K4me3 and H3K27me3 modifications were used to mark the genes that are expressed or repressed respectively. The promoters for the genes that are highly expressed in certain Th lineages correlated with permissive H3K4me3 configuration. However, the promoters for the genes that were not expressed were not necessarily associated with high amounts of repressive H3K27me3 configuration. While only a small number of lineage-specific H3-methylation islands were found for Th cells, a large number of the unique H3K4me3 and H3K27me3 islands were found in nTreg cells, underlying its greater potential for diverse functions. Indeed, although the promoters for IFN-γ, IL-4 and IL-17a were not marked with H3K4me3 for active expression, they were barely marked with H3K27me3 for active suppression either. In addition, transcription factors T-bet, GATA-3 and ROR-γt that master Th1, Th2 and Th17 differentiation respectively were not marked with suppressive configuration in Treg cells. Hence, at the epigenetic level, Treg cells did not shut down the genetic programme for other Th cells and remained permissive for their differentiation.49
With many years of collective efforts, we have achieved tremendous progress in understanding the function and regulation of Th cells during normal and pathological immune responses. Recent evidence suggests that one type of Th cell may convert into other types of Th cells to suit various immune responses. Therefore, understanding the biological meaning and the regulation of the plasticity of Th cells should enhance our understanding of the aetiology of autoimmunity and inflammatory diseases. By generating reporter mice for different subtypes of helper T cells, we have started to unravel the diverse function of Treg cells in vitro and in vivo. Future studies are needed to investigate the molecular mechanisms underlying the plasticity of Th cells and to address how Th cell conversion might impact immune responses and the development of various immune disorders.
Acknowledgments
Y.Y.W. is supported by a K99/R00 award from National Institutes of Health/National Institute of Allergy and Infectious Disease. We are grateful to R. Zhai for critical reading and helpful comments.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–51. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu HZ, Li GL, Lim YK, Chan SH, Yap EH. Kinetics of interferon-γ secretion and its regulatory factors in the early phase of acute graft-versus-host disease. Immunology. 1999;98:379–85. doi: 10.1046/j.1365-2567.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang B, Andre I, Gonzalez A, Katz JD, Aguet M, Benoist C, Mathis D. Interferon-γ impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:13844–9. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung BP, McInnes IB, Esfandiari E, Wei XQ, Liew FY. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J Immunol. 2000;164:6495–502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon γ-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–71. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swihart K, Fruth U, Messmer N, et al. Mice from a genetically resistant background lacking the interferon γ receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med. 1995;181:961–71. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenner CA, Guler ML, Macatonia SE, O’Garra A, Murphy KM. Roles of IFN-γ and IFN-α in IL-12-induced T helper cell-1 development. J Immunol. 1996;156:1442–7. [PubMed] [Google Scholar]
- 8.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 9.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–92. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 11.Wierenga EA, Snoek M, de Groot C, Chretien I, Bos JD, Jansen HM, Kapsenberg ML. Evidence for compartmentalization of functional subsets of CD2+ T lymphocytes in atopic patients. J Immunol. 1990;144:4651–6. [PubMed] [Google Scholar]
- 12.Parronchi P, Macchia D, Piccinni MP, et al. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci USA. 1991;88:4538–42. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 14.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–806. [PubMed] [Google Scholar]
- 15.Le GrosG, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–9. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–55. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 18.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci USA. 2004;101:1993–8. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Min B, Hu-Li J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–65. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 21.Shimoda K, van Deursen J, Sangster MY, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–3. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, Tanaka T, Shi W, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–30. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 23.Kurata H, Lee HJ, O’Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677–88. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 24.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-β‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 27.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 28.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 29.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 31.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 32.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 35.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–49. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suto A, Kashiwakuma D, Kagami S, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–79. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–37. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–5. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 40.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 41.Belkaid Y. Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol. 2008;38:918–21. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–4. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–62. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 44.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 45.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+ CD25– Foxp3– T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J Immunol. 2007;178:6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Lopes JE, Chong MM, et al. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng WP, Chang C, Lai JJ. Immune suppressive activity and lack of T helper differentiation are differentially regulated in natural regulatory T cells. J Immunol. 2009;183:3583–90. doi: 10.4049/jimmunol.0900146. [DOI] [PubMed] [Google Scholar]
- 49.Wei G, Wei L, Zhu J, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–67. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantel PY, Kuipers H, Boyman O, et al. GATA3-driven Th2 responses inhibit TGF-β1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3– effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–60. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 53.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 54.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–13. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. pii: 37865. doi: 10.1172/JCI37865. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–24. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–5. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–92. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 61.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–70. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 62.Joetham A, Matsubara S, Okamoto M, Takeda K, Miyahara N, Dakhama A, Gelfand EW. Plasticity of regulatory T cells: subversion of suppressive function and conversion to enhancement of lung allergic responses. J Immunol. 2008;180:7117–24. doi: 10.4049/jimmunol.180.11.7117. [DOI] [PubMed] [Google Scholar]
- 63.Provoost S, Maes T, van Durme YM, et al. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009;64:1539–46. doi: 10.1111/j.1398-9995.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 64.Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat Immunol. 2003;4:616–23. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 65.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]