Abstract
The severity of allergic diseases may be modified by vitamin D. However, the immune pathways modulated by the active form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], are yet to be fully elucidated. In this study, naturally occurring CD4+ CD25+ cells from the skin-draining lymph nodes (SDLN) of mice treated with topical 1,25(OH)2D3 had an increased ability to suppress T helper type 2 (Th2) -skewed immune responses. CD4+ CD25+ cells transferred from mice treated with topical 1,25(OH)2D3 into ovalbumin (OVA) -sensitized mice challenged intranasally with OVA 18 hr later, significantly suppressed the capacity of airway-draining lymph node (ADLN) cells to proliferate and secrete cytokines in response to further OVA stimulation ex vivo. The CD4+ CD25+ cells from 1,25(OH)2D3-treated mice also reduced airway hyperresponsiveness and the proportions of neutrophils and eosinophils in bronchoalveolar lavage fluid (BALF). To test the effect of 1,25(OH)2D3 on cells able to respond to a specific antigen, CD4+ CD25+ cells were purified from the SDLN of OVA-T-cell receptor (TCR) transgenic mice treated 4 days earlier with topical 1,25(OH)2D3. CD4+ CD25+ cells from OVA-TCR mice treated with 1,25(OH)2D3 were able to alter BALF cell content and suppress ADLN responses to a similar degree to those cells from non-transgenic mice, suggesting that the effect of 1,25(OH)2D3 was not related to TCR signalling. In summary, topical 1,25(OH)2D3 increased the regulatory capacity of CD4+ CD25+ cells from the SDLN to suppress Th2-mediated allergic airway disease. This work highlights how local 1,25(OH)2D3 production by lung epithelial cells may modulate the suppressive activity of local regulatory T cells.
Keywords: asthma, CD4+ CD25+ regulatory T cells, lung, skin, vitamin D
Introduction
Currently, scientists in research laboratories around the world are working to optimize the conditions required to generate regulatory T-cell populations with increased capacity to modulate immune-mediated pathologies (reviewed in refs 1,2), such as asthma. Asthma is a chronic illness of the airways characterized by wheeze, shortness of breath, chest tightness and cough. Dietary and environmental factors can modulate regulatoryT-cell function. Examples of these include vitamin A (reviewed in ref.2), vitamin D3 and ultraviolet B (UVB) irradiation.4,5 One of the immune-driven diseases that can be modulated by environmental factors is asthma. Similarly, chronic treatment of mice with the active form of vitamin D [1,25-dihydroxyvitamin D3; 1,25(OH)2D3)] reduced airway hyperresponsiveness (AHR),6 eosinophilia and interleukin-5 (IL-5) levels in bronchoalveolar lavage fluid (BALF).7 Vitamin D deficiency may contribute towards the asthma epidemic;8 however, the immune pathways modulated by vitamin D are yet to be fully elucidated.
Vitamin D is synthesized from the precursor 7-dehydrocholesterol in the skin following exposure of skin to the UVB irradiation present in sunlight. Further enzymatic conversions in the kidneys and liver and other sites (including the skin) produce the active form of the hormone, 1,25(OH)2D3. Many immune cells express the vitamin D receptor, including T cells, activated B cells and dendritic cells (DC; reviewed in ref.8), and so possess the potential to respond to 1,25(OH)2D3 directly. However, most studies of 1,25(OH)2D3 on immune cells have been performed in vitro, with concentrations of 1,25(OH)2D3 greater than those observed in physiological settings. Vitamin D can regulate the in vitro function of various immune cells, including DC and T cells. Some DC become tolerogenic when cultured with 1,25(OH)2D3. For example, co-stimulatory molecule expression on myeloid DC and Langerhans cells was reduced by 1,25(OH)2D3.9,10 The 1,25(OH)2D3 also has tolerogenic effects on T cells; in particular, IL-10-secreting regulatory CD4+ T cells were induced in vitro by 1,25(OH)2D3 in the presence of dexamethasone.11 In addition, 1,25(OH)2D3 also up-regulates a skin-trophic chemokine receptor (CCR10) on T cells.12 These in vitro observations point to an immunomodulatory role for 1,25(OH)2D3 on DC and T cells.
We have examined the in vivo effects of topically applied 1,25(OH)2D3 on immune cells as local skin concentrations in the order of 2–5 nm 1,25(OH)2D3 may be achieved following UVB irradiation.13,14 We3 and others15 propose that 1,25(OH)2D3 produced in UVB-irradiated skin may initiate some of the immunomodulatory effects of UV irradiation. The 1,25(OH)2D3 was applied to the skin of naive BALB/c mice and the phenotype and function of immune cells were examined in the draining lymph nodes.3 CD4+ T cells from the lymph nodes proliferated less in response to stimulation ex vivo;3 this was the result of an increased suppressive activity of CD4+ CD25+ Foxp3+ cells in the skin-draining lymph nodes (SDLN). Topical 1,25(OH)2D3 augmented the ability of these cells to suppress the proliferation of co-cultured CD4+ CD25− T cells in vitro and also their capacity to suppress T helper type 1 (Th1)/Th17-skewed contact hypersensitivity16 responses upon adoptive transfer into naive recipient mice.3 These results suggested that UVB-induced 1,25(OH)2D3 production in skin affects downstream immune responses by increasing the suppressive activity of CD4+ CD25+ Foxp3+ cells isolated from the SDLN.
As well as being produced by skin epithelial cells,13,14 1,25(OH)2D3 is also synthesized by respiratory epithelial cells.17 Therefore, 1,25(OH)2D3 made in the respiratory environment could modify the activity of local CD4+ CD25+ cells. As a surrogate for 1,25(OH)2D3 produced by airway epithelial cells, we investigated the potential of CD4+ CD25+ cells from the SDLN of 1,25(OH)2D3-treated mice to modulate immune responses in the airways. In addition, we questioned whether the effects of topical 1,25(OH)2D3 on CD4+ CD25+ cells were limited to modulating Th1/Th17-driven immune disease. As in vitro 1,25(OH)2D3 up-regulates skin-homing chemokine receptors (CCR10) on T cells,12 topical 1,25(OH)2D3 treatment may promote the migration of these cells to skin sites and not the respiratory tract. We were uncertain as to whether CD4+ CD25+ cells could regulate Th2 immune responses in the respiratory tract even if transferred immediately before challenge, when signals that promote cell migration into the airways are generated by the lung challenge. Using a classical mouse model of acute asthma induced by the experimental allergen, ovalbumin (OVA), topical 1,25(OH)2D3 augmented the ability of CD4+ CD25+ cells to suppress Th2-driven immune responses when cells were transferred immediately before airway challenge. Hence, topical 1,25(OH)2D3 enhances the ability of CD4+ CD25+ cells to modify Th2-mediated (i.e. OVA-induced airway disease) immune pathologies.
Materials and methods
Mice
Female 8-week-old BALB/c mice were purchased from the Animal Resources Centre (Murdoch, Western Australia). Mice transgenic for the OVA323–339 (ISQAVHAAHAEINEAGR)-specific T-cell receptor-αβ (TCR-αβ; DO11.10) on a BALB/c background were originally purchased from the Jackson Laboratory (Bar Harbor, ME) and bred in-house. Female DO11.10 mice were used between the ages of 8 and 12 weeks. Expression of OVA323–339-specific TCR-αβ on T cells was confirmed by staining lymph node cells with biotinylated anti-DO11.10 TCR monoclonal antibody (KJ1-26; Caltag Laboratories, Burlingame, CA) and then phycoerythrin (PE) -Cy5-conjugated streptavidin (BD Biosciences, Heidelberg, Germany). All experiments were performed according to the ethical guidelines of the National Health and Medical Research Council of Australia with approval from the Telethon Institute for Child Health Research Animal Ethics Committee.
Vitamin D application
A 100-μl aliquot containing 125 ng 1,25-dihydroxyvitamin D3 [1,25(OH)2D3; Sigma Chemical Company, St Louis, MO] diluted in ethanol, propylene glycol and water mixed at a 2 : 1 : 1 ratio was painted onto a clean-shaven 8-cm2 dorsal skin surface of mice. This was equivalent to 37 pmol 1,25(OH)2D3 per cm2 of skin, or 100 μl of 3 μm 1,25(OH)2D3. Alternatively, the vehicle used to dilute 1,25(OH)2D3 was applied in a similar manner. The 1,25(OH)2D3 was stored under argon gas at − 80°; its chemical integrity was routinely verified using a scanning spectrophotometer.
Purification of CD4+ CD25+ cells
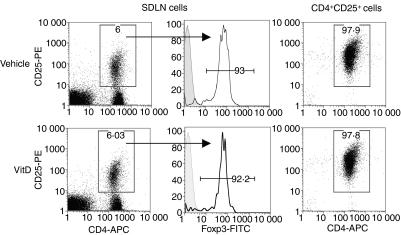
Four days after topical skin treatment, CD4+ CD25+ cells (≥ 95% pure, as determined by flow cytometry) were isolated from the SDLN (inguinal, axillary and brachial lymph nodes) of vehicle-treated or 1,25(OH)2D3-treated mice using the CD4+ CD25+ regulatory T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and the AutoMACS cell separator (Miltenyi Biotec). In one experiment CD4+ CD25+ cells were purified from the SDLN of treated mice by fluorescence-activated cell-sorting (FACS) cells labelled with fluorescein isothiocyanate-conjugated (-FITC) CD3, allophycocyanin-conjugated (-APC) CD4, CD25-PE and propidium iodide (FACS Aria, see below for surface staining). More than 90% of CD4+ CD25+ cells from the lymph nodes of vehicle-treated or 1,25(OH)2D3-treated BALB/c mice express the regulatory cell marker Foxp3 intracellularly (3, Fig. 2).
Figure 2.
CD4+ CD25+ cells from the skin-draining lymph nodes (SDLN) of mice topically treated with vehicle or 1,25(OH)2D3 have similar phenotypes. CD4+ CD25+ cells were purified from the SDLN of BALB/c mice treated topically 96 hr earlier with vehicle or 125 ng 1,25(OH)2D3 (VitD). The proportion of live CD4+ CD25+ cells in the SDLN (left panels) and of isolated CD4+ CD25+ cells (right panels) are shown. The proportions of Foxp3+ cells of the live CD4+ CD25+cells from the SDLN are shown in the middle panel with an isotype control also shown (shaded histogram). Results are representative of cells pooled from the SDLN of BALB/c mice from four individual experiments.
FACS analysis and antibodies
The following anti-mouse monoclonal antibodies were obtained from BD Biosciences: FITC-anti-CD3; PE-anti-CD25; APC-anti-CD25; APC-CD4; and anti-CD16/32. For staining of surface antigens, lymph node cells were incubated with antibodies and staining buffer (phosphate-buffered saline, 0·1% bovine serum albumin, 0·01% sodium azide) for 30 min, washed and immediately acquired on a FACSCalibur flow cytometer (BD Biosciences). For some experiments cells were washed with 1 ml staining buffer with 1 μg/ml propidium iodide (Sigma). A Foxp3 intracellular staining kit (eBiosciences, San Diego, CA) was used to determine intracellular Foxp3 expression. Data were analysed using FlowJo software (v8.3.7) (Treestar Inc., Ashland, OR).
Adoptive transfer of CD4+ CD25+ cells into OVA-sensitized mice
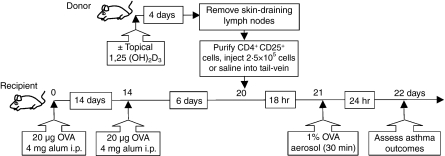
Four days after topical skin treatment, CD4+ CD25+ cells were purified from the SDLN of treated mice and 2·5 × 105 cells in 200 μl 0·9% saline were adoptively transferred through the tail vein of OVA-sensitized and boosted mice 18 hr before OVA aerosol challenge (see below, Fig. 1). In some studies, CD4+ CD25+ cells were labelled with 5 μm carboxyfluorescein succini,idyl ester (CFSE; Molecular Probes, Invitrogen Australia, Mulgrave, Australia) for 10 min at room temperature. After washing three times, cells were resuspended in 0·9% saline and 2·5 × 105 cells were intravenously transferred into naive mice that were sensitized 18 hr later with OVA as described below.
Figure 1.
Experimental timeline for transfer of CD4+ CD25+ cells into ovalbumin (OVA) -sensitized mice. CD4+ CD25+ cells were purified from the skin-draining lymph nodes (SDLN) of ‘donor’ BALB/c or DO11.10 mice treated topically 4 days earlier with vehicle or 125 ng 1,25(OH)2D3. Cells (2·5 × 105) or 0·9% saline (200 μl) were adoptively transferred into ‘recipient’ BALB/c mice intraperitoneally sensitized and boosted earlier with 20 μg OVA and 4 mg Alum adjuvant. These recipient mice were challenged with a 1% OVA in saline aerosol for 30 min, 18 hr after the adoptive transfer. Twenty-four hours later, various asthma outcomes were assessed in the recipient mice.
Sensitization and challenge of mice with OVA
Ovalbumin (Sigma) in an aluminium hydroxide suspension (Alum; Serva, Heidelberg, Germany) was delivered intraperitoneally on day 0 (20 μg OVA in 4 mg aluminium hydroxide per mouse; 200 μl volume) and again on day 14 (Fig. 1). Mice were then challenged on day 21 with a 1% OVA-in-saline aerosol delivered using an ultrasonic nebulizer (UltraNebs; DeVilbiss, Somerset, PA) for 30 min (Fig. 1).
Preparation and culture of single-cell suspensions from airway-draining lymph nodes
Twenty-four hours after OVA challenge, the posterior mediastinal, tracheobrachial and parathymic lymph nodes (airway-draining lymph nodes; ADLN) were removed from mice, pooled within experimental groups, physically disaggregated and cultured at 105 cells/200 μl/well (six replicates per treatment) in RPMI-1640 medium (Gibco, Auckland, New Zealand) with 10% fetal calf serum, 2 mm l-glutamine, 50 mm 2-mercaptoethanol and 5 mg/ml gentamicin. Single-cell suspensions were incubated at 37° with 5% CO2, with OVA (10 μg/ml). Methyl-[3H]thymidine [10 μl (0·25 mCi)/well; Amersham Pharmacia Biotech, Piscataway, NJ] was added at 72 hr and cells were harvested at 96 hr with [3H]thymidine incorporation used as a measure of cellular proliferation. Culture supernatants were also obtained at 96 hr.
Cytokine detection in tissue culture supernatants and BALF
Interleukin-2, IL-4, IL-5, IL-10 and interferon-γ (IFN-γ) were detected using rat anti-mouse IL-2, IL-4, IL-5, IL-10 or IFN-γ enzyme-linked immunosorbent assay capture and detection of monoclonal antibodies (BD Biosciences) in a dissociation-enhanced time-resolved fluorescence immunoassay with Europium as the label (sensitivity 25 pg/ml). Recombinant mouse IL-2, IL-4, IL-5, IL-10 or IFN-γ (BD Biosciences) were used as the standards.
Measurement of airway hyperresponsiveness
Twenty-four hours after the aerosol challenge, a modified low-frequency forced oscillation technique was used to measure change in respiratory input impedance (Zrs) in AHR by using increasing doses of methacholine (0·1–30 mg/ml) as described previously.18 Briefly, mice were anaesthetized with xylazine (2 mg/ml; Troy Laboratories, Wetherill Park, NSW, Australia) and ketamine (40 mg/ml; Troy Laboratories) delivered intraperitoneally at a dose of 0·01 ml/g. Mice were tracheostomized and ventilated (flexivent; Scireq, Montreal, Canada) at 450 breaths/min with a tidal volume of 8 ml/kg and a positive end expiratory pressure of 2 cmH2O. Baseline values were obtained by measurement of Zrs five times at 1-min intervals. A 90-second aerosol was delivered with an ultrasonic nebulizer (Devilbiss UltraNeb® Large volume ultrasonic nebulizer, Sunrise Medical, Somerset, PA) and Zrs was measured five times at 1-min intervals. This was repeated with ½ log10 incremental doses of methacholine (0·1–30 mg/ml) and the peak response for each parameter was recorded for analysis. The constant phase model19 was used to partition Zrs into components representing the conducting airway (airway resistance) and the lung parenchyma (tissue damping and tissue elastance).
Broncheoalveolar lung lavage
Twenty-four hours after the aerosol challenge with OVA, BALF was collected by flushing 1·2 ml 0·2% bovine serum albumin in phosphate-buffered saline into the lung. Lavage samples were centrifuged (300 g, 4°, 10 min) and the supernatant was stored at − 20° until cytokine analysis. Cells were then centrifuged onto slides and stained using the DIFF-Quik Stain Set 64851 (Lab Aids, Narrabeen, NSW, Australia) as per the manufacturer’s instructions.
Measuring messenger RNA in CD4+ CD25+ cells
The CD4+ CD25+ cells (≥ 2·5 × 106) were snap-frozen in 1 ml Trizol Reagent (Invitrogen Australia). RNA was extracted according to the manufacturer’s instructions and DNAse-treated using the Turbo DNA-free kit (Ambion, Austin, TX). RNA was further purified using the RNA RT2 qPCR-Grade RNA Isolation kit (SABiosciences, Frederick, MD) and then 100 ng was reversed transcribed into complementary DNA using the RT2 First-Strand kit (SABiosciences). To examine CCR10 messenger RNA (mRNA) expression, a real-time polymerase chain reaction (PCR) was then performed according to the manufacturer’s instructions, using 2 × SuperArray RT2 qPCR Master Mix (SABiosciences) and the Th1-Th2-Th3 RT2 profiler PCR array (PAM034A, 96-wells; SABiosciences). The PCR was performed using the standard two-step cycling conditions on the ABI 7000 SDS (Applied Biosystems, Foster City, CA). Alternatively, using primers designed in-house, the expression of IL-10 and transofrming growth factor-β (TGF-β) were determined in cDNA samples, quantified by real-time PCR using QuantiTect SYBR Green Master Mix (Qiagen, Doncaster, Vic, Australia) on the ABI PRISM 7900HT (Applied Biosystems). Melting-curve analysis was used to assess the specificity of the assay. Expression levels were determined by a standard curve created from serial dilutions of the PCR product and normalized to the reference genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH; as detected on the Th1-Th2-Th3 RT2 profiler PCR array for CCR10) or eukaryotic translation elongation factor 1α.20 In-house-designed primer pairs were: eukaryotic translation elongation factor 1α, 5′-CTGGAGCCAAGTGCTAATATGCC-3′ and 5′-GCCAGGCTTGAGAACACCAGTC-3′; IL-10, 5′-GGTTGCCAAGCCTTATCGGA-3′ and 5′-ACCTGCTCCACTGCCTTGCT-3′; TGF-β 5′-CACTGATACGCCTGAGTG-3′ and 5′-GTGAGCGCTGAATCGAAA-3′.
Statistical analyses
Data were compared using Student’s t-test with the prism statistical analysis program for Macintosh (v4.0b) (GraphPad Prism, GraphPad Software Inc., La Jolla, CA).
Results
Do CD4+ CD25+ cells from 1,25(OH)2D3-treated mice suppress Th2-mediated immune pathologies?
Both naturally occurring21–22 and antigen-induced23 regulatory T cells have been implicated in controlling asthma-associated immune reactions in the airway mucosa, draining lymph nodes and lung parenchyma when transferred before the allergen challenge of pre-sensitized experimental animals. In the current study, CD4+ CD25+ cells were purified from the SDLN of mice topically treated 4 days earlier with 125 ng 1,25(OH)2D3 or vehicle (Fig. 1, donor mice). As shown previously,3 there was no difference in the proportion of CD4+ CD25+ cells in the SDLN of vehicle or 1,25(OH)2D3-treated mice (Fig. 2), such that 2·4 ± 0·5 × 105 or 2·1 ± 0·1 × 105 CD4+ CD25+ cells/mouse (mean ± SEM of n= 4 experiments, Fig. 2) were purified from the SDLN of vehicle-treated or 1,25(OH)2D3-treated BALB/c mice, respectively. More than 90% of the CD4+ CD25+ cells expressed intracellular Foxp3 (Fig. 2) with no difference in the expression of CD25 (Fig. 2) or other T-cell activation/memory/mucosal cell markers as previously reported.3 CD4+ CD25+ cells (2·5 × 105) were adoptively transferred into BALB/c mice, which had been sensitized and boosted earlier with 20 μg OVA and 4 mg Alum (Fig. 1, recipient mice). To control for the effects of cell transfer, a group of mice received 200 μl of 0·9% saline. Eighteen hours after cells were transferred, recipient mice were challenged through the airways with a 1% OVA in saline solution as an aerosol. After a further 24 hr, asthma-related parameters were examined including ADLN cell responses to OVA ex vivo, AHR and the effector cells and cytokines present in the BALF. For all of the tested parameters, there was no difference between the responses observed in the recipients of CD4+ CD25+ cells from vehicle-treated mice and those mice that received saline only (Figs. 3–5).
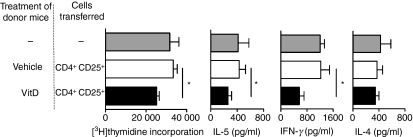
Figure 3.
CD4+ CD25+ cells from the skin-draining lymph nodes (SDLN) of mice topically treated with 1,25(OH)2D3 regulate ex vivo proliferation and cytokine production by airway-draining lymph nodes (ADLN) cells. CD4+ CD25+ cells were purified from the SDLN of BALB/c mice treated topically 4 days earlier with vehicle or 125 ng 1,25(OH)2D3. Cells (2·5 × 105) or 0·9% saline (200 μl) were adoptively transferred into BALB/c mice sensitized and boosted earlier with ovalbumin (OVA). These recipient mice were challenged with an OVA aerosol 18 hr after the adoptive transfer. After a further 24 hr, ADLN cells were pooled from mice within treatment groups and cultured with 10 μg/ml OVA for 96 hr. [3H]thymidine incorporation by ADLN cells is shown for the last 24 hr of a 96-hr culture with interleukin-5 (IL-5), interferon-γ (IFN-γ) or IL-4 levels in supernatants depicted, which were collected after a 96-hr culture. Results are pooled from three independent experiments (n=4 mice/treatment/experiment) and are shown as mean + SEM (*P < 0·05).
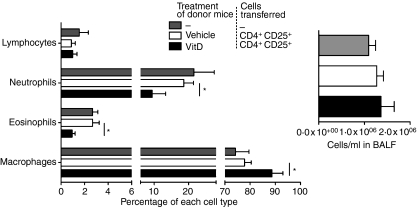
Figure 5.
CD4+ CD25+ cells from the skin-draining lymph nodes (SDLN) of mice topically treated with 1,25(OH)2D3 regulate cells in the bronchoalveolar lavage fluid (BALF). CD4+ CD25+ cells were purified from the SDLN of BALB/c mice treated topically 4 days earlier with vehicle or 125 ng 1,25(OH)2D3. Cells (2·5 × 105) were adoptively transferred into BALB/c mice sensitized and boosted earlier with OVA. These recipient mice were challenged with an OVA aerosol 18 hr after the adoptive transfer. After a further 24 hr, the proportions of lymphocytes, neutrophils, eosinophils and macrophages and the total number of cells were determined in BALF. Results are shown as mean + SEM (n=8 mice/treatment, *P < 0·05).
CD4+ CD25+ cells from 1,25(OH)2D3-treated BALB/c mice reduce cytokine production by, and proliferation of, ADLN cells
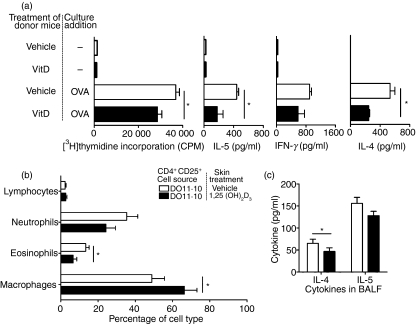
The ADLN cells were removed 24 hr after OVA challenge and cultured for 96 hr with OVA. Results were pooled from three independent experiments (n=4 mice/treatment/experiment). For mice receiving CD4+ CD25+ cells from 1,25(OH)2D3-treated donor mice, the capacity of ADLN cells to proliferate in response to OVA stimulation ex vivo was significantly reduced (Fig. 3). In the supernatants of these proliferating ADLN cells there were significant reductions in both Th1 (IFN-γ) and Th2 (IL-5) cytokine concentrations (Fig. 3). However, levels of IL-2 (data not shown) and IL-4 (Fig. 3) were not modified.
CD4+ CD25+ cells from 1,25(OH)2D3-treated BALB/c mice suppress airway hyperresponsiveness
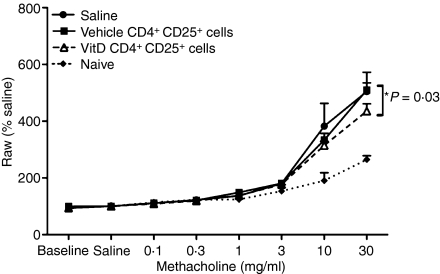
Twenty-four hours after the OVA-aerosol challenge, mice were tested for AHR by a forced oscillation method, using increasing concentrations of the bronchoconstrictor, methacholine.18 Parameters of airway resistance, tissue damping and tissue elastance were measured using a constant-phase model.19 CD4+ CD25+ cells from 1,25(OH)2D3-treated mice suppressed airway resistance by 30% (P = 0·03, one-way t-test, n= 8 mice per treatment, 30 mg/ml methacholine) in comparison to responses observed in recipients of CD4+ CD25+ cells from vehicle-treated mice (Fig. 4). However, airway resistance was greater in recipients of CD4+ CD25+ cells from 1,25(OH)2D3-treated mice than in naive mice, suggesting that other factors control airway resistance. There was no difference in tissue damping or elastance in recipients of CD4+ CD25+ cells from either vehicle-treated or 1,25(OH)2D3-treated mice (data not shown).
Figure 4.
CD4+ CD25+ cells from the skin-draining lymph nodes (SDLN) of mice topically treated with 1,25(OH)2D3 regulate airway hyperresponsiveness. CD4+ CD25+ cells were purified from the SDLN of BALB/c mice treated topically 4 days earlier with vehicle or 125 ng 1,25(OH)2D3. Cells (2·5 × 105) or 0·9% saline (200 μl) were adoptively transferred into BALB/c mice sensitized and boosted earlier with ovalbumin (OVA). These recipient mice were challenged with an OVA aerosol 18 hr after the adoptive transfer. After a further 24 hr, airway resistance to increasing concentrations of the bronchoconstrictor methacholine was determined. Responses were also measured in age-matched naive BALB/c mice. Baseline = values obtained by measurement of Zrs five times at 1-min intervals after stabilization of the mouse on the ventilator; saline = values obtained by measurement of Zrs five times at 1-min intervals after a 90-second saline aerosol; % saline = % increase of saline values for increasing doses of methacholine. Results are shown as mean + SEM (n=8 mice/treatment, *P = 0·03) relative to baseline levels (100%).
CD4+ CD25+ cells from 1,25(OH)2D3-treated BALB/c mice reduce neutrophil and eosinophil numbers in the lungs
The BALF was collected immediately following measurement of AHR. Transfer of CD4+ CD25+ cells did not modify the total number of immune cells in the BALF (Fig. 5) with the proportions of neutrophils and eosinophils in the BALF being similar to those observed in our previous investigations where mice were challenged with a single OVA aerosol.24 However, there were significant reductions in the proportions of neutrophils and eosinophils (Fig. 5) in the BALF of mice that received CD4+ CD25+ cells from 1,25(OH)2D3-treated mice 24 hr before OVA challenge. Similar results were observed in three further experiments (n=4 mice/treatment/experiment) where the average (± SEM) reductions in the proportion of neutrophils and eosinophils were 37% (± 14%) and 45% (± 16%), respectively, in mice that received CD4+ CD25+ cells from 1,25(OH)2D3-treated mice. Even though total BALF cell numbers did not change in recipients of CD4+ CD25+ cells from 1,25(OH)2D3-treated mice, the reduction of granulocyte populations indicates that inflammation was probably reduced in the lungs. The reduction in granulocyte numbers resulted in an increased proportion of macrophages in the BALF of recipients of CD4+ CD25+ cells from 1,25(OH)2D3-treated mice (Fig. 5). Cytokine levels in BALF, including IL-2, IL-4, IL-5 and IL-10, were not significantly altered by any treatment (data not shown). Together, these results indicate that topical 1,25(OH)2D3 increased the ability of CD4+ CD25+ cells isolated from the SDLN to modulate Th2-driven allergic airway disease.
CCR10 mRNA levels in CD4+ CD25+ cells are not modified by topical 1,25(OH)2D3
CCR10, a skin-homing chemokine receptor, is up-regulated on T cells after in vitro treatment with 1,25(OH)2D3.12 The expression of CCR10 mRNA was examined for CD4+ CD25+ cells isolated 4 days after topical 1,25(OH)2D3 treatment in three independent experiments. There was no difference in the expression of CCR10 mRNA with 1,25(OH)2D3 treatment of skin (vehicle = 0·007 ± 0·002, 1,25(OH)2D3 = 0·008 ± 0·002 mean ± SEM relative to the housekeeping gene GAPDH). This observation, along with the increased ability of CD4+ CD25+ cells to suppress respiratory immune responses, indicates that topical 1,25(OH)2D3 did not modify or prevent their ability to migrate into the respiratory tract. Levels of mRNA for other immunoregulatory molecules including IL-10, TGF-β and Foxp3 were not modified in CD4+ CD25+ cells isolated from the SDLN 4 days after topical 1,25(OH)2D3 (data not shown). The levels of CCR10 mRNA detected in CD4+ CD25+ cells were comparable with TGF-β and Foxp3 mRNA levels (Ct scores ∼ 30).
CD4+ CD25+ cell migration in vivo is not modified by topical 1,25(OH)2D3
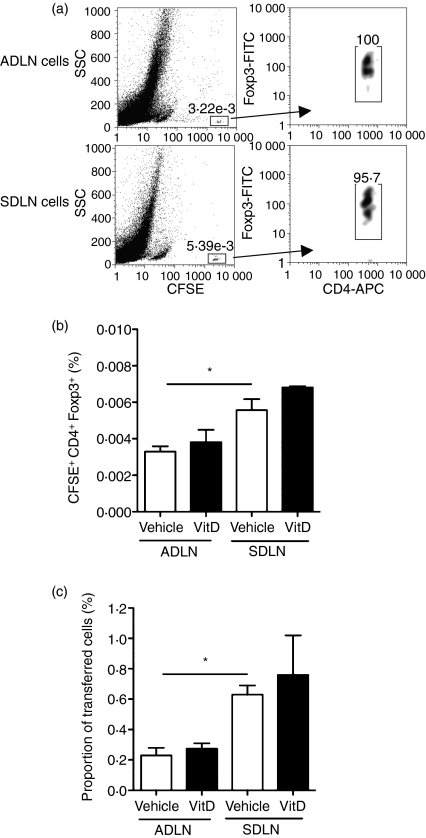
To determine if topical 1,25(OH)2D3 altered the ability of CD4+ CD25+ cells to migrate to airway immune sites, the cells were purified from the SDLN of BALB/c mice 4 days after skin treatment with 1,25(OH)2D3 or vehicle. Cells were labelled with CFSE, and then 105 cells were transferred intravenously into naive mice. Mice were sensitized 18 hr later with OVA and alum with the proportion of transferred cells (CFSE+ Foxp3+ CD4+) in the ADLN and SDLN assessed 3 days later (Fig. 6a). All of the transferred cells expressed high levels of CFSE, indicating that none of them had begun to proliferate 3 days after OVA sensitization. The was no difference in the ability of cells from vehicle or 1,25(OH)2D3-treated mice to migrate into and accumulate in the ADLN or SDLN (Fig. 6b). However, more of the transferred cells were identified in the SDLN than in the ADLN of recipient mice (Fig. 6b) such that about three times the number of transferred CD4+ CD25+ cells were detected in the SDLN than the ADLN of recipient mice (Fig. 6c).
Figure 6.
Topical 1,25(OH)2D3 does not alter migration of CD4+ CD25+ cells to the airway-draining lymph nodes (ADLN). CD4+ CD25+ cells were purified from the skin-draining lymph nodes (SDLN) of BALB/c mice treated topically 4 days earlier with vehicle or 125 ng 1,25(OH)2D3. Cells were labelled with carboxyfluorescein succinimidyl ester (CFSE) and then 1 × 105 intravenously transferred into naive BALB/c mice, which were sensitized 18 hr later with ovalbumin (OVA). Three days after sensitization, (a) the proportions of transferred (CFSE+ Foxp3+ CD4+) cells in the ADLN and SDLN were determined by first gating CFSE+ SSClo cells and then cells that expressed both Foxp3 and CD4. (b) The percentage of CFSE+ Foxp3+ CD4+ cells detected in the ADLN and SDLN and, (c) the proportion of total cells transferred cells. Results for (b) and (c) are depicted as mean + SEM (n=3 mice/treatment, *P < 0·05).
CD4+ CD25+ cells from 1,25(OH)2D3-treated OVA-TCR transgenic mice reduce cytokine production by, and proliferation of, ADLN cells as well as neutrophil and eosinophil numbers in the lungs
For the data described in Figs. 3–5, CD4+ CD25+ cells were derived from 1,25(OH)2D3-treated but otherwise naive BALB/c mice. The effect on CD4+ CD25+ cells expressing the OVA-TCR was examined, where ‘OVA-specific’ CD4+ CD25+ cells were obtained from the SDLN of vehicle or 1,25(OH)2D3-treated OVA-TCR transgenic DO11.10 mice. Approximately 60% of CD4+ CD25+ cells from the SDLN of DO11.10 mice express the OVA-TCR, and this is not modified by topical 1,25(OH)2D3.3 Cells (2·5 × 105) were transferred 18 hr before OVA challenge (Fig. 1). Results were pooled from two independent experiments (n=5 mice/treatment/experiment) and are shown as mean + SEM. As observed following topical treatment of BALB/c mice, there was no difference in the proportion of CD4+ CD25+ cells in the SDLN of vehicle or 1,25(OH)2D3-treated DO11.10 mice, such that 1·1 × 105 or 1·3 × 105 CD4+ CD25+ cells/mouse (mean of n= 2 experiments) were purified from the SDLN of vehicle or 1,25(OH)2D3-treated mice, respectively. Similarly, the expression of CD25 and Foxp3 was not different for CD4+ CD25+ cells from the SDLN of vehicle or 1,25(OH)2D3-treated DO11.10 mice (data not shown).
CD4+ CD25+ cells from 1,25(OH)2D3-treated mice significantly reduced the proliferation and cytokine production (IL-4 and IL-5) by ADLN cells in response to OVA stimulation ex vivo (Fig. 7a) and also diminished the proportions of eosinophils in the BALF of OVA-challenged mice (Fig. 7b). In all treatments there was no change in total BALF cell numbers (data not shown). CD4+ CD25+ cells from 1,25(OH)2D3-treated DO11.10 mice suppressed responses by ADLN cells and in the BALF to a similar degree as those cells from BALB/c mice (Figs 3, 5 and 7). However, the CD4+ CD25+ cells from 1,25(OH)2D3-treated DO11.10 mice were also able to reduce levels of IL-4 but not of IL-5 (Fig. 7c), IL-2 or IL-10 in the BALF (data not shown). These reductions in IL-4 production by ADLN cells and IL-4 accumulation in the BALF of recipients of CD4+ CD25+ cells from 1,25(OH)2D3-treated DO11.10 mice were not observed for recipients of cells from 1,25(OH)2D3-treated BALB/c mice. Hence, 1,25(OH)2D3 has similar but not identical immunoregulatory enhancing effects on CD4+ CD25+ cells from BALB/c and OVA-TCR transgenic mice to increase their ability to suppress Th2-driven immune responses in mouse models of allergic airway disease.
Figure 7.
CD4+ CD25+ cells from the skin-draining lymph nodes (SDLN) of ovalbumin–T-cell receptor (OVA-TCR) transgenic mice topically treated with 1,25(OH)2D3 regulate various asthma parameters. CD4+ CD25+ cells were purified from the SDLN of OVA-TCR transgenic (DO11.10) mice treated topically 4 days earlier with vehicle or 125 ng 1,25(OH)2D3. Cells (2·5 × 105) or 0·9% saline (200 μl) were adoptively transferred into BALB/c mice sensitized and boosted earlier with OVA. These recipient mice were challenged with an OVA aerosol 18 hr after the adoptive transfer. After a further 24 hr, (a) airway-draining lymph node (ADLN) cells were pooled within treatments and cultured with and without (−) 10 μg/ml OVA for 96 hr. [3H]thymidine was added to cultures for the last 24 hr of a 96 hr culture. Interleukin-5 (IL-5), interferon-γ (IFN-γ) or IL-4 concentrations in supernatants were determined after 96 hr of culture. At the same time, (b) the proportions of lymphocytes, neutrophils, eosinophils and macrophages and (c) concentrations of IL-4 and IL-5 were measured in bronchoalveolar lavage fluid (BALF). Results are pooled from two independent experiments (n=5 mice/treatment/experiment) and are shown as mean + SEM (*P < 0·05).
Discussion
In this study CD4+ CD25+ cells from 1,25(OH)2D3-treated mice had an increased capacity to suppress Th2-driven immune responses in a murine model of asthma. CD4+ CD25+ cells from 1,25(OH)2D3-treated mice reduced proliferation and cytokine production by ADLN cells, AHR and the numbers of granulocytes in the BALF of mice. Results were also comparable when CD4+ CD25+ cells were purified using magnetic bead separation or by cell-sorting techniques. It is of note that while the suppressive activity of these cells increased, their numbers did not change in the SDLN. CD4+ CD25+ cells from both BALB/c and OVA-TCR transgenic (DO11.10) mice had an increased ability to suppress aspects of Th2 immunity, indicating that the effects of topical 1,25(OH)2D3 are largely non-antigen-specific and independent of OVA-TCR expression on the CD4+ CD25+ cells. CD4+ CD25+ cells from 1,25(OH)2D3-treated BALB/c or DO11.10 mice were able to suppress Th2 responses to a similar degree. However, 1,25(OH)2D3 did not have identical effects as following topical 1,25(OH)2D3, CD4+ CD25+ cells from OVA-TCR transgenic mice had an additional capacity to suppress IL-4 levels in the BALF, and IL-4 production by ADLN cells ex vivo, perhaps indicating a further effect of 1,25(OH)2D3 on the Th2-regulatory capacity of CD4+ CD25+ regulatory T cells activated through the OVA-TCR.
‘Naturally-occurring’ CD4+ CD25+ regulatory T cells isolated from the lungs and spleens of naive mice can suppress airway inflammation, Th2 cytokine production, and AHR through IL-10 and TGF-β-dependent mechanisms.21,22,25 However, CD4+ CD25+ cells from mice treated topically with 1,25(OH)2D3 did not express increased levels of IL-10 or TGF-β mRNA. In addition, there was no change in the expression of Foxp3 mRNA nor in the intracellular levels of Foxp3 protein in SDLN CD4+ CD25+ cells with topical 1,25(OH)2D3 treatment (Fig. 2 and data not shown). Furthermore, we considered it unlikely that the increased regulatory activity resulted from an increased ability of CD4+ CD25+ cells to proliferate following topical 1,25(OH)2D3. In our previous studies, topical 1,25(OH)2D3 increased the ability of CD4+ CD25+ cells to proliferate when subsequently exposed to OVA.3 The OVA-specific CD4+ CD25+ cells transferred from mice topically treated with 1,25(OH)2D3 proliferated after 3 days to a greater extent in recipient mice primed with OVA.3 However, in the current study using T cells from BALB/c or OVA-TCR transgenic mice, the CD4+ CD25+ cells in the recipient mice were exposed to OVA (administered as an aerosol) for only 24 hr. Furthermore, OVA-TCR+ T cells begin to proliferate 48 hr after OVA priming (S. Gorman, P.H. Hart unpublished data). Using CFSE to label cells, similar numbers of transferred CD4+ CD25+ cells from mice topically treated with vehicle or 1,25(OH)2D3 were identified in the ADLN of recipient mice 72 hr after transfer. These data suggest that topical 1,25(OH)2D3 does not modify the capacity of CD4+ CD25+ cells to migrate to the ADLN. Studies are continuing to investigate the mechanisms by which 1,25(OH)2D3 regulates CD4+ CD25+ cells.
It may be expected that CD4+ CD25+ cells from DO11.10 (that express the OVA-TCR) would inhibit respiratory responses to OVA challenge more effectively than those from BALB/c mice; however, this does not appear to be the case. In a recent study, OVA-TCR transgenic CD4+ CD25+ cells transferred intratracheally into recipient mice suppressed aspects of ragweed-induced allergic airway disease including airway hyperreponsiveness to a similar extent as that observed by these cells when transferred into OVA-sensitized and challenged mice.26 These results suggest that antigen-specific TCR is not required for the capacity of CD4+ CD25+ regulatory T cells to suppress allergic airway disease and that non-antigen-specific cells may suppress as well as those that are antigen specific. The OVA-TCR+ cells were closely associated with CD8+ T cells and major histocompatibility complex class II+ cells in the lungs of ragweed-sensitized mice, indicating that the transferred cells could indeed migrate to the lungs despite their antigen specificity.26
CD4+ CD25+ cells from both BALB/c and DO11.10 mice treated with 1,25(OH)2D3 had an increased capacity to suppress the influx of both eosinophils and neutrophils into the lungs of the recipient mice. However, the mechanism by which this occurs is not known. In other studies depletion or transfer of CD4+ CD25+ cells affected eosinophil numbers in the BALF and lungs of mice sensitized and challenged with OVA.27,28 CD4+ CD25+ cells also affected the recruitment of neutrophils into the kidneys and airways of mice with injury29 or OVA-induced inflammation,30 respectively. The effects on neutrophils may be dependent on IL-10 secreted by CD4+ CD25+ cells29 but in vitro studies examining the effects of lipopolysaccharide-activated or TCR-activated CD4+ CD25+ cells on neutrophils have shown that TGF-β and contact-dependent mechanisms may also be important.31
CD4+ CD25+ (Foxp3+) cells are generally considered regulatory cells; however, in this study 2·5 × 105 CD4+ CD25+ cells from untreated mice were not sufficient to modify asthma responses in recipient mice after intravenous transfer. Topical treatment with 1,25(OH)2D3 significantly enhanced the regulatory ability of CD4+ CD25+ cells such that the same number of CD4+ CD25+ cells could suppress the experimental asthma responses. Similarly, others have adoptively transferred (intravenously) 5 × 105 CD4+ CD25+ cells from non-transgenic mice (e.g. BALB/c) and found no effects on OVA-induced asthma responses.21 These cells suppressed only when transferred through the trachea.21 In contrast, in models of asthma using house dust mite (Der p I;22) or cockroach25 antigens, intravenous transfer of 1 × 105 to 5 × 105 CD4+ CD25+ cells from naive mice modified lung and airway inflammation, AHR and Th2 cytokine production. It is possible that the inability of ≤ 5 × 105 CD4+ CD25+ cells from naive mice to suppress allergic airway disease following intravenous transfer may be specific to when OVA is used as an allergen.
In other studies,32 CD4+ CD25+ cells from OVA-TCR transgenic mice modulated allergic airway disease following the intravenous transfer of 5 × 105 cells. Some of these cells were derived from the spleens of mice, as also performed for other studies using non-antigen-specific CD4+ CD25+ cells.22,25 It is unlikely that splenic CD4+ CD25+ cells have an increased regulatory capacity in respiratory models. We did not isolate CD4+ CD25+ cells from the spleens of mice topically treated with 1,25(OH)2D3 because results from our previous studies indicated that the suppressive capacity of CD4+ CD25+ cells from the spleen was not modified by topical 1,25(OH)2D3.3
The aim of this study was to determine whether 1,25(OH)2D3 applied in vivo could stimulate CD4+ CD25+ cells in lymph nodes to regulate allergic airway disease. To detect a difference in responses, low numbers (2·5 × 105) of CD4+ CD25+ cells were adoptively transferred. We were actually aiming for none or a limited amount of suppression by CD4+ CD25+ cells from control mice, so increasing the window for the detection of enhanced regulatory ability by CD4+ CD25+ cells from 1,25(OH)2D3-treated mice. Low numbers of cells (2·5 × 105) were transferred because we had previously found that this number of cells from 1,25(OH)2D3-treated mice was able to suppress contact hypersensitivity responses, even though CD4+ CD25+ cells from non-treated (vehicle) mice did not suppress.3 In our experiments, in consideration of cell numbers and their potency, we have effectively ‘concentrated’ the effects of topical 1,25(OH)2D3 by transferring CD4+ CD25+ cells from approximately two donor mice into one recipient mouse.
Cells were transferred 18 hr before OVA challenge (1% OVA aerosol, Fig. 1) because previous studies have indicated that CD4+ CD25+ cells from naive mice can suppress elements of allergic airway disease if transferred the day before the first respiratory challenge with allergen.21,22 The timing of cell transfer is an important factor to consider as the transfer of 2·5 × 105 CD4+ CD25+ cells from donor mice topically treated with 1,25(OH)2D3 before the initial OVA sensitization of the recipient mice (18 hr before the first OVA/Alum injection, Fig. 1) had no effect on the asthma outcomes tested (data not shown). Transferring the cells immediately before the respiratory challenge with OVA may enable the migration of the cells to the challenge site, and their subsequent interactions with cells such as DC and Th2 effector cells, which mediate allergic airway disease and AHR in the trachea and respiratory tract.23
In other studies, a single topical application of 1,25(OH)2D3 did not regulate OVA-induced allergic airway disease, such that it was necessary to transfer (intravenously) CD4+ CD25+ cells disaggregated from the SDLN. From this report, and highlighting the importance of this study, the ability of respiratory epithelial cells to synthesize 1,25(OH)2D317 has implications for the ability of ‘local’ CD4+ CD25+ cells to modulate DC and Th2 cell interactions which mediate allergic airway disease in the respiratory tract. The production of 1,25(OH)2D3 by respiratory epithelial cells highlights the dual role that 1,25(OH)2D3 has to (i) modulate immune cells that regulate adaptive immune responses (such as regulatory T cells), and to (ii) promote antimicrobial activity through production of cathelicidin during infection.17
In vivo treatment of OVA-primed mice with 1,25(OH)2D3 (chronic intraperitoneal injection) down-regulates the in vivo homing ability of CD4+ T cells to ADLN.33 In these studies, purified CD4+ T cells were labelled with CFSE, and cells were injected intravenously into 1,25(OH)2D3-treated or untreated control mice. After 3·5 hr, the accumulation of labelled cells in the spleen was unaffected by the various pre-treatments. In contrast, migration of cells to the lymph nodes of 1,25(OH)2D3-treated mice was significantly decreased.33 In our studies, topical 1,25(OH)2D3 did not imprint the cells with chemokine receptor expression that prevented their migration to sites such as the airways. In contrast to findings with unfractionated T cells,12 expression of mRNA for the skin-trophic chemokine receptor CCR10 was not modified in CD4+ CD25+ cells isolated from the SDLN of mice 4 days after topical 1,25(OH)2D3. However, following intravenous transfer and subsequent OVA sensitization, more CD4+ CD25+ cells from the SDLN migrated to the SDLN than the ADLN, but importantly 1,25(OH)2D3 did not alter the migratory patterns of these cells. Upon OVA aerosol challenge, sufficient CD4+ CD25+ cells migrated to the airway lymphatic tissue and presumably other respiratory sites where those from 1,25(OH)2D3-treated mice significantly regulated allergic airway disease.
The potential of topical 1,25(OH)2D3 to augment the ability of CD4+ CD25+ regulatory T cells to suppress Th1/Th17 and Th2-driven immune responses may provide additional treatment options for the control of autoimmune and allergic diseases. Calcipotriene, a non-calcaemic analogue of 1,25(OH)2D3, is already used topically to treat psoriasis.34 In addition, with dexamethasone, vitamin D3 induces IL-10-secreting regulatory T cells,11,35 which potentially augment therapeutic responses in steroid-resistant asthma patients.35 The apparent prevalence of vitamin D insufficiency around the world makes it important for us to better understand the immunomodulatory effects of vitamin D. In this study, in which 1,25(OH)2D3 was applied topically to the experimental animal and not to cells in culture, 1,25(OH)2D3 increased the immunoregulatory capacity of CD4+ CD25+ regulatory T cells, which in turn could modulate Th2-driven immune responses in a mouse model of asthma. It is proposed that sufficient levels of vitamin D are required for the optimal suppressive activity of CD4+ CD25+ regulatory T cells.
Acknowledgments
We thank Dr Deborah Strickland from the Telethon Institute for Child Health Research for her critical appraisal of this manuscript. This study was funded by grants from the National Health and Medical Research Council of Australia (No. 458612, P.H.H. and D.J.T.), the Cancer Council of Western Australia (P.H.H. and S.G.) and the University of Western Australia (S.G.).
Glossary
Abbreviations:
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- ADLN
airway-draining lymph nodes
- AHR
airway hyperresponsiveness
- BALF
bronchoalveolar lavage fluid
- DC
dendritic cells
- SDLN
skin-draining lymph nodes
Disclosures
None of the authors have any financial or other conflicts of interest or are associated with a company or institution that might benefit from the publication.
References
- 1.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–5. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyara M, Wing K, Sakaguchi S. Therapeutic approaches to allergy and autoimmunity based on Foxp3+ regulatory T-cell activation. J Allergy Clin Immunol. 2009;123:749–55. doi: 10.1016/j.jaci.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, Hart PH. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+ CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–83. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 4.Gorman S, Tan JW-Y, Yerkovich ST, Finlay-Jones JJ, Hart PH. CD4+ T cells in lymph nodes of UVB-irradiated mice suppress immune responses to new antigens both in vitro and in vivo. J Invest Dermatol. 2007;127:915–24. doi: 10.1038/sj.jid.5700600. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells – from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 6.Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1α,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-β. J Immunol. 2008;180:5211–21. doi: 10.4049/jimmunol.180.8.5211. [DOI] [PubMed] [Google Scholar]
- 7.Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585–92. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 8.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacyoid dendritic cells. J Immunol. 2007;178:145–53. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 10.Fujita H, Asahina A, Komine M, Tamaki K. The direct action of 1α,25(OH)2-vitamin D3 on purified Langerhans cells. Cell Immunol. 2007;245:70–9. doi: 10.1016/j.cellimm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin-10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann B, Sauter W, Knuschke P, Dressler S, Meurer M. Demonstration of UVB-induced synthesis of 1α,25-dihyroxyvitamin D3 (calcitriol) in human skin by microdialysis. Arch Dermatol Res. 2003;295:24–8. doi: 10.1007/s00403-003-0387-6. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, Reeve VE, Mason RS. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707–15. doi: 10.1038/sj.jid.5700597. [DOI] [PubMed] [Google Scholar]
- 15.Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP. Expansion of antigen-specific regulatory T cells with the topical vitamin D analog calcipotriol. J Immunol. 2009;182:6071–8. doi: 10.4049/jimmunol.0804064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. IL-17 and IFN-γ mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J Immunol. 2009;183:1463–70. doi: 10.4049/jimmunol.0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hantos Z, Collins RA, Turner DJ, Janosi TZ, Sly PD. Tracking of airway and tissue mechanics during TLC manoeuvers in mice. J Appl Physiol. 2003;95:1695–705. doi: 10.1152/japplphysiol.00104.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hantos Z, Adamicza A, Govaerts E, Daroczy B. Mechanical impedances of lungs and chest wall in the cat. J Appl Physiol. 1992;73:427–33. doi: 10.1152/jappl.1992.73.2.427. [DOI] [PubMed] [Google Scholar]
- 20.Hamalainen HK, Tubman JC, Vikman S, Kyrola T, Ylikoski E, Warrington JA, Lahesmaa R. Identification and validation of endogenous reference genes for expression profiling of T helper cell differentiation by quantitative real-time RT-PCR. Anal Biochem. 2001;299:63–70. doi: 10.1006/abio.2001.5369. [DOI] [PubMed] [Google Scholar]
- 21.Joetham A, Takada K, Taube C, et al. Naturally occurring lung CD4+CD25+ T cell regulation of airway allergic responses depends on IL-10 induction of TGF-β. J Immunol. 2007;178:1433–42. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 22.Leech MD, Benson RA, de Vries A, Fitch PM, Howie SEM. Resolution of Der pI-induced allergic airway inflammation is dependent on CD4+ CD25+ Foxp3+ regulatory T cells. J Immunol. 2007;179:7050–8. doi: 10.4049/jimmunol.179.10.7050. [DOI] [PubMed] [Google Scholar]
- 23.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+ CD25+ regulatory T cells. J Exp Med. 2006;203:2649–60. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGlade JP, Gorman S, Zosky GR, Larcombe AN, Sly PD, Finlay-Jones JJ, Turner DJ, Hart PH. Suppression of the asthmatic phenotype by ultraviolet B-induced antigen-specific regulatory cells. Clin Exp Allergy. 2007;37:1261–3. doi: 10.1111/j.1365-2222.2007.02750.x. [DOI] [PubMed] [Google Scholar]
- 25.McGee HS, Agrawal DK. Naturally occurring and inducible T-regulatory cells modulating immune response in allergic asthma. Am J Respir Crit Care Med. 2009;180:211–25. doi: 10.1164/rccm.200809-1505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joetham A, Takeda K, Okamoto M, Taube C, Matsuda H, Dakhama A, Gelfand EW. Antigen specificity is not required for modulation of lung allergic responses by naturally occurring regulatory T cells. J Immunol. 2009;183:1821–7. doi: 10.4049/jimmunol.0900303. [DOI] [PubMed] [Google Scholar]
- 27.Kearley J, Robinson DS, Lloyd CM. CD4+ CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodelling. J Allergy Clin Immunol. 2008;122:617–24. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudousquie C, Pellaton C, Barbier N, Spertini F. CD4+ CD25+ T cell depletion impairs tolerance induction in a murine model of asthma. Clin Exp Allergy. 2009;39:1415–26. doi: 10.1111/j.1365-2222.2009.03314.x. [DOI] [PubMed] [Google Scholar]
- 29.Kinsey GR, Sharm R, Huang L, Li L, Vergis AL, Ye H, Ju S-T, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia–reperfusion injury. J Am Soc Nephrol. 2009;20:1744–53. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suto A, Nakajuma H, Kagami S-I, Suzuki K, Saito Y, Iwamoto I. Role of CD4+ CD25+ regulatory T cells in T helper 2 cell-mediated allergic inflammation in the airways. Am J Respir Crit Care Med. 2001;164:680–7. doi: 10.1164/ajrccm.164.4.2010170. [DOI] [PubMed] [Google Scholar]
- 31.Lewkowicz P, Lewkowicz N, Sasiak A, Tchorzewski H. Lipopolysaccharide-activated CD4+ CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J Immunol. 2006;177:7155–63. doi: 10.4049/jimmunol.177.10.7155. [DOI] [PubMed] [Google Scholar]
- 32.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+ CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1538–47. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur J Immunol. 2004;34:1068–76. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 34.Segaert S, Duvold LB. Calcipotriol cream: a review of its use in the management of psoriasis. J Dermatol Treat. 2006;17:327–37. doi: 10.1080/09546630600999219. [DOI] [PubMed] [Google Scholar]
- 35.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]