Abstract
Adjuvants, including antibodies to tumour necrosis factor receptor superfamily members, augment immune responses. One member of this family, glucocorticoid-induced tumour necrosis factor receptor (GITR), is expressed at low levels on naive/resting T cells, B cells and macrophages, but at higher levels on T regulatory cells. The aim of this study was to determine the ability of a rat anti-mouse GITR monoclonal antibody, 2F8, to stimulate murine humoral and cellular immunity in a prime boost model with particular attention to posology and antigen-specific effects. 2F8 enhanced the humoral immune response to ovalbumin and haemagglutinin (HA) compared with controls and this enhancement was equal to or greater than that obtained in mice dosed with standard adjuvants. 2F8 F(ab′)2 fragments were as effective as intact antibody in boosting humoral immunity, indicating that FcR-mediated cross-linking of 2F8 is not required for efficacy. Moreover, the enhanced response was durable and antigen specific. Administration of 2F8 shifted the immune response towards a T helper type 1 response with significant enhancement of immunoglobulin G2a- and G2b-specific anti-HA antibodies, as well as enhanced cellular immunity as measured by ELISPOT. 2F8-treated mice also generated significantly more neutralizing antibodies to HA than control mice. Our findings show that anti-GITR is a robust, versatile adjuvant that, unlike commonly used adjuvants that primarily enhance humoral immunity, enhances both humoral and cellular immunity. These results support the continued development of anti-GITR for such indications as haematological and solid tumours, chronic viral infections, and as a vaccine adjuvant.
Keywords: adjuvant, glucocorticoid-induced tumour necrosis factor receptor, haemagglutinin, T helper type 1 response, tumour necrosis family receptor superfamily
Introduction
Enhancing protective immune responses with minimal adverse side-effects is a major challenge for vaccine development. Attenuated, replication-competent pathogens generally induce a robust protective immunity, but the success of these vaccines is now overshadowed by concern about rare reactogenic adverse side-effects and by the potential to cause disease, particularly in immunocompromised individuals. Inactivated, replication-deficient pathogens and vaccines that are made of well-defined antigens, such as inactivated toxins or recombinant proteins, are less reactogenic and offer important safety advantages. Unfortunately, with few exceptions, these agents are weakly immunogenic and generally ineffective without the co-inoculation of adjuvants that augment immune responses.1–3
Aluminium salts (alum) have been the most widely used vaccine adjuvant in humans since their discovery in the early 20th century.4 More potent adjuvants, such as Freund’s adjuvant and lipopolysaccharide, have since been identified, but local and systemic toxicity have hindered clinical applications. Although the European Medicinal Evaluation Agency has licensed new adjuvants over the last decade,5 alum remains the only adjuvant approved for human use by the US Food and Drug Administration. Alum, which primarily stimulates T helper type 2 (Th2) humoral immunity,6,7 potently induces protective immunity mediated by serum antibodies.8 However, the ability of alum to stimulate Th1 type cell-mediated immunity is weak at best.2,9,10 It has become clear in recent years that cell-mediated immunity is critical for protection against pathogens such as Mycobacterium tuberculosis, human immunodeficiency virus, and hepatitis C virus, as well as for the development of therapeutic vaccines to treat chronically infected patients.11,12 Robust cell-mediated immunity is essential, if not necessarily sufficient, for vaccine induction of acquired immunity against tumour antigens.13,14 For these reasons, a number of strategies have been devised in an effort to enhance cell-mediated responses.15
Co-stimulatory molecules are among the targets being investigated as potential adjuvants to stimulate cell-mediated immunity.16–19 Members of the tumour necrosis family receptor superfamily (TNFRSF) are of particular interest, as costimulation of T cells through several members of this family not only augments effector T-cell function and survival,20–22 but also renders these cells resistant to T regulatory (Treg) cell-mediated immune suppression.23–29 Furthermore, several members of this receptor family augment cell-mediated immunity through their expression on dendritic cells, where they have been shown to regulate both function and survival.30–32
The glucocorticoid-induced tumour necrosis factor receptor (GITR) is a member of the TNFRSF, being member 18 (TNFRSF18). GITR is expressed at low, basal levels in CD4+ CD25− and CD8+ CD25− responder T cells23,24,33 and constitutively at high levels in CD4+ CD25+ Treg cells.24,34 Upon activation, GITR expression is rapidly up-regulated in responder T cells,23,24,33,35–38 and further elevated in Treg cells.23,24,33–38 The cytoplasmic domain of GITR shares significant sequence similarity with OX40, 4-1BB, and CD27, which together form a subgroup within the TNFRSF that regulates several aspects of lymphocyte biology, including activation, differentiation and survival.20,39
The functional consequence of GITR ligation in T cells has been linked to the concurrent level of CD3/T-cell receptor (TCR) stimulation.40 Consistent with the function of costimulatory molecules, in vitro GITR engagement increases CD3/TCR-induced proliferation of T cells and their production of cytokines when CD3/TCR stimulation is suboptimal. GITR also has a complex role in apoptosis, with reports of pro-apoptotic effects28,41,42 in the context of full stimulation, as well as anti-apoptotic effects.38,43–45
Engagement with anti-GITR monoclonal antibody (mAb), soluble GITR ligand (GITRL), or cell-surface-expressed GITRL on transfectants has been shown to augment anti-tumour46–50 and anti-virus immunity51,52 in a number of models. Monoclonal antibodies to other TNFRSF members (4-1BB, CD40, and OX40) also generate strong anti-tumour and anti-virus responses in various experimental models,53–59 which demonstrates an important role for TNFRSF members in the regulation of immune responses.
The timing of anti-GITR exposure relative to antigen presentation appears to be an important variable for capturing its adjuvant effect.46–48 Antibody administration before the second of three vaccinations has been demonstrated to enhance CD8+ T-cell responses against melanoma-specific antigens, whereas administration of antibody with the initial immunization did not.46 Studies to determine which cells are targeted by anti-GITR administration have implicated effector T cells, helper T cells, Treg cells, and natural killer cells.46–50
A recent study with an immunoglobulin M (IgM) anti-GITR mAb demonstrated enhanced costimulation of CD4+ CD25− responder cells and CD4+ CD25+ Treg cells compared with the rat IgG2b anti-GITR mAb, DTA-1. However, the IgM antibody was less efficient at augmenting tumour immunity, possibly because of the enhanced proliferation of CD4+ CD25+ Treg cells.60 These findings serve as reminders that the antibody isotype, in addition to the antigen epitope, will probably be important for clinical development of an anti-GITR mAb.
Many potentially confounding results may be understood by considering the model and the antigen that were studied. Successful posology of an anti-GITR mAb requires an understanding of this complex ligand–receptor interaction. To better understand the potential of anti-GITR as an immune adjuvant, we used a simple prime boost protocol to address the effects of the dose and timing of antibody administration, the importance of Fc–FcR interactions and, finally, the effect of the antibody on humoral and cellular immune responses to model antigens. Incomplete Freund’s adjuvant (IFA) and alum were also used as controls in the studies to allow us to gauge the relative potency of any effect attributed to the anti-GITR antibody. We report that anti-GITR antibodies can act as robust adjuvants that augment both Th1 and Th2 responses. Fc–FcR interactions were not required for the observed effects, and the dose of 2F8 required for maximal efficacy varied with the immunogenicity of the antigen.
Materials and methods
Monoclonal antibody preparation
RNA was isolated from acutely rejecting heterotopic heart transplants and mouse GITR (mGITR) was amplified by reverse transcription–polymerase chain reaction (RT-PCR) using standard molecular biology techniques. An mGITR–immunoglobulin (mGITR-Ig) fusion protein was constructed by subcloning the extracellular domains of mGITR together with the human Igγ1 constant region into an expression plasmid. The mGITR-Ig fusion protein was purified from the supernatant of stable Chinese hamster ovary cell transfectants grown in α-minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% Ultralow IgG fetal bovine serum (Invitrogen) and G418 (Invitrogen). The mGITR-Ig fusion protein was purified by Protein A (GE Healthcare, Pittsburgh, PA) chromatography and dialysed into phosphate-buffered saline (PBS; Invitrogen).
The rat anti-mGITR mAb, 2F8 (IgG2a,κ), was produced by Helio® Gene Gun (Bio-Rad Laboratories, Hercules, CA) immunization of rats with mGITR-Ig expression plasmid-coated gold beads every other day for 10 days. Sera from immunized rats were tested for reactivity against purified mGITR-Ig protein by enzyme-linked immunosorbent assay (ELISA). Rats with demonstrated serum immunoreactivity were boosted with recombinant fusion protein 3 days before fusion. Hybridomas were screened by ELISA for immunoreactivity against purified mGITR-Ig, cloned by limiting dilution, and further characterized by flow cytometry.
YAML (555.6), an IgG2a isotype control, was a gift from Professor Herman Waldmann (Sir William Dunn School of Pathology, University of Oxford, Oxford, UK).
The YGITR765 antibody was generated in the laboratory of Professor Herman Waldmann (Sir William Dunn School of Pathology). The rat IgG2b hybridoma was selected for specificity using GITR-transfected cells. The heavy chain was chimerized by grafting the variable region of YGITR765 to the constant regions of a human IgG1 that was modified to remove the N-linked glycosylation site at amino acid 297. The original rat κ light chain was retained and antibodies were expressed in Chinese hamster ovary cells.
Generation of 2F8 F(ab′)2 fragments
The F(ab′)2 fragments were prepared by proteolytic digestion of 2F8 with pepsin (Sigma, St Louis, MO). 2F8 was prepared for digestion by adjusting the concentration to 3·0 mg/ml performing dialysis against 200 mm sodium acetate, pH 4·0 for 4 hr at 2–8°. After dialysis, the 2F8 concentration was adjusted to 2·0 mg/ml with 200 mm sodium acetate, pH 4·0 and mixed at an enzyme to antibody ratio of 1 : 20 with freshly prepared pepsin in 200 mm sodium acetate, pH 4·0. The mixture was then incubated at 37° for 17 ± 1 hr, quenched by the addition of 2·0 m Tris–HCl (20% vol. : vol.), and dialysed against PBS overnight at 2–8°. Purification of the F(ab′)2 fragments from intact antibody or antibody fragments was accomplished by passing the digest over a Protein A affinity column and then loading the flow-through fractions onto a Superdex 200 size exclusion chromatography (SEC) column (GE Healthcare). Fractions from the SEC purification were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and those containing 2F8 F(ab′)2 fragments were pooled, aliquoted and stored frozen at − 20°.
Antibody and antigen administration
BALB/c mice were immunized on day 0 by subcutaneous (s.c.) administration of 100 μg ovalbumin (OVA; Sigma) in saline followed by a boost with an equivalent s.c. dose on day 14. To assess the effect of anti-GITR on the humoral response to OVA, 2F8, 2F8 F(ab′)2, or YAML was administered intraperitoneally on days − 1, 0 and 1 at the doses indicated in the Results section. Anti-OVA serum titres were determined by ELISA on days 21 and 28.
In studies with influenza haemagglutinin (HA) as the primary antigen, mice were immunized s.c. on day 0 with 10 μg HA (A/H3N2/Wyoming/3/2003 or A/H5N1/Vietnam/1203/2004; Protein Sciences, Meriden, CT) in saline or adjuvant followed by an s.c. boost with 5 μg HA in PBS on day 14. Mice that received HA in adjuvant were administered antigen in a 1 : 1 mixture with alum or IFA. 2F8 or YAML was administered intraperitoneally at the primary immunization (days − 1, 0 and 1) or at antigen challenge (days 13, 14 and 15). Anti-HA serum titres were determined on days 21 and 28. For specificity studies, mice previously immunized with HA were subsequently immunized on day 35 with a neoantigen (100 μg OVA, s.c.) and challenged 2 weeks later on day 49. Titres to the neoantigen were assessed on days 56 and 63 by ELISA.
Detection of antigen-specific IgG in serum
Ovalbumin-specific or HA-specific serum antibody titres were determined by ELISA. Nunc-Immuno™ MaxiSorp™ (Thermo Fisher Scientific, Rochester, NY) 96-well plates were coated with OVA (10 μg/ml) or HA (1 μg/ml) in 0·05 m carbonate buffer (pH 9·5) overnight at 4°, followed by blocking with 1% bovine serum albumin in PBS (BSA-PBS) for 2 hr at 37°. Serial 10-fold dilutions of mouse sera prepared in 1% BSA-PBS were added (50 μl/well) to the wells, and plates were incubated for 1 hr at 37°. After three washes with PBS–0·05% Tween 20, goat anti-mouse IgG–horseradish peroxidase (HRP) (Jackson ImmunoResearch, West Grove, PA), rabbit anti-mouse IgG1-HRP, rabbit anti-mouse IgG2b-HRP (Invitrogen), or goat anti-mouse IgG2a-HRP (Southern Biotech, Birmingham, AL) secondary antibodies were added to the wells and plates were incubated for a further 1 hr at 37°. Plates were again washed three times followed by the addition of 50 μl o-phenylenediamine (Sigma) in 0·1 m citrate–phosphate buffer (pH 4·35) plus 0·01% H2O2 to develop the reactions. Reactions were terminated with the addition of 25 μl of 12·5% H2SO4. Titres are expressed as the reciprocal of the serum dilution resulting in an optical density value of 1·5 in the particular ELISA assay. This value was selected as an arbitrary value because it consistently fell in the linear part of the slope of the plotted dilutions when the ELISA was performed.
ELISPOT assay
As an assessment of the cellular immune response to HA, the number of HA-specific interferon-γ (IFN-γ) -secreting splenocytes was determined on day 21 using a murine IFN-γ ELISPOT kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Cells (2·5 × 105 or 5 × 105 cells/well) were incubated for 24 hr at 37° in the absence or presence of 5 μg/ml HA. Cells were removed, and the processed plates were analysed by an outside vendor (Zellnet Consulting, Fort Lee, NJ).
Haemagglutination inhibition assay
Serum samples were sent to the laboratory of Stacey Schultz-Cherry (University of Wisconsin–Madison School of Medicine and Public Health, Madison, WI) for haemagglutination analyses. Haemagglutination activity titrations and haemagglutination inhibition (HI) assays were performed as described elsewhere.61 Briefly, serum samples were treated with receptor-destroying enzyme (Denka Seiken Co. Ltd., Tokyo, Japan) at 37° for 18 hr to eliminate non-specific inhibitors of haemagglutination. The receptor-destroying enzyme was heat-inactivated and samples were tested for HA-specific antibodies against the A/H3N2/Wyoming/3/2003 influenza virus. All HI assays were run simultaneously. The HI titres were defined as the reciprocal of the highest dilution of serum that completely inhibited haemagglutination of four HA units of the virus with a 0·5% solution of chicken red blood cells.
Statistical analysis
Treatment groups were compared using one-way analysis of variance (anova) to determine whether the means of the groups differed. Statistically significant anova results were followed by pairwise comparisons using Tukey’s multiple comparison tests to determine which means were different. GraphPad Prism® (GraphPad Software Inc., La Jolla, CA) software was used for all analyses and differences with P < 0·05 were deemed significant.
Results
Anti-mGITR enhances the humoral immune response to OVA
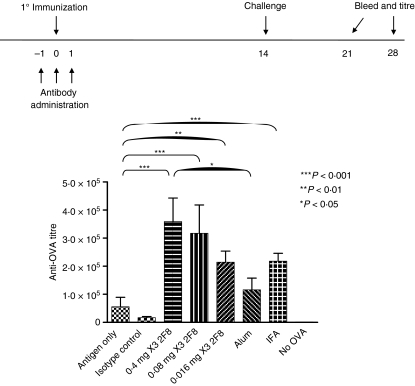
We generated 2F8, an IgG2a rat anti-mGITR mAb that demonstrated agonistic activity on T cells when used in in vitro assays (data not shown). To elucidate the in vivo effect of 2F8 on humoral immunity, mice were administered 2F8 (0·016–0·4 mg) on days − 1, 0 and 1, surrounding the primary immunization with OVA on day 0. Mice were later challenged with OVA on day 14 and anti-OVA titres were analysed on days 21 and 28. To determine background anti-OVA titres, we assessed anti-OVA titres in mice that had not been exposed to OVA. Background levels of anti-OVA were negligible (Fig. 1). Mice treated with 0·4 mg 2F8 generated anti-OVA titres at day 21 that were 7·7-fold and 21-fold greater than mice administered antigen only or an equivalent dose of isotype control mAb (YAML), respectively (Fig. 1). Isotype control rat IgG2a itself had a modest immunosuppressive effect compared with antigen-only-treated mice. Similar results were observed on day 28 (data not shown). However, mice in groups that received 0·08 mg or 0·016 mg 2F8 responded with anti-OVA titres at day 21 that were 5·7-fold and 3·9-fold greater, respectively, than mice that received antigen only (Fig. 1). Again, equivalent results were obtained on day 28. We also compared the adjuvant effect of 2F8 to effects of alum and IFA. Day 21 titres of mice treated with 0·4 or 0·08 mg 2F8 were 1·5-fold greater than mice immunized with OVA in IFA (not significant; n.s.) and 2·5-fold greater than OVA in alum although only the 0·4 mg dose proved to be statistically significantly different (Fig. 1). Mice treated with 0·016 mg 2F8 had anti-OVA titres similar to those of mice immunized with OVA in IFA, but higher than mice that received OVA in alum (n.s.) (Fig. 1).
Figure 1.
2F8 enhances humoral immunity to ovalbumin (OVA). Antibody dosing and immunization schedule are indicated above the graph. OVA mixed with alum or incomplete Freund’s adjuvant (IFA) is included for comparison. Levels of anti-OVA antibodies were determined by enzyme-linked immunosorbent assay as described in the Materials and methods section. Titre is defined in the Materials and methods section. Results represent at least two independent experiments with three to five mice per group. All treated groups were significantly higher than antibody isotype control-treated mice. Significant comparisons are shown, all others were not significant. ***P < 0·001, **P < 0·01, *P < 0·05.
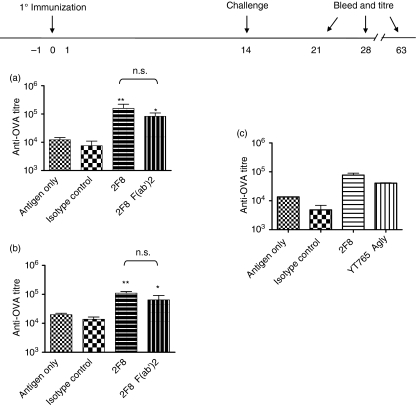
Previous studies found that the rat anti-mGITR, DTA-1 (but not its Fab fragments), abrogated CD4+ CD25+-mediated suppression of T-cell proliferation in vitro.24 To determine whether Fc–FcR interactions are required to enhance humoral immunity, we generated F(ab′)2 fragments of 2F8 and treated mice with 8 mg of 2F8 F(ab′)2 fragments on days − 1, 0 and 1. This dose was selected based on serum antibody level studies that demonstrated comparable exposure (∼ 4 days in serum) of 8 mg 2F8 F(ab′)2 and 0·016 mg intact 2F8 mAb (data not shown), which was the lowest efficacious dose observed in Fig. 1. Mice treated with 2F8 F(ab′)2 generated anti-OVA titres that were 4·8-fold greater than the titres of animals that received antigen only (Fig. 2a), showing the effectiveness of 2F8 F(ab′)2 fragments in augmenting the humoral immune response to OVA. The 2F8-augmented humoral response was durable, as anti-OVA titres on day 63 remained elevated compared with those in the antigen-only group (Fig. 2b). To confirm the results observed with 2F8 F(ab′)2, we used a genetically modified anti-mGITR mAb (YT765) engineered with a human aglycosyl Fc (which does not bind to mouse FcRγI, -II, or -III; H. Waldmann and M. Tone, unpublished observations) in our prime boost model and observed equivalent results (Fig. 2c). These results demonstrate that anti-GITR antibodies augment humoral immunity as well as IFA and can be more effective than alum by means that do not require Fc–FcR interactions.
Figure 2.
2F8-enhanced humoral immunity to ovalbumin (OVA) is durable and does not require Fc–FcR interactions. Antibody dosing and immunization schedules are indicated above the graph; 8 mg of 2F8 F(ab′)2 fragments (a and b), 0·4 mg 2F8 (a, b, and c), or 0·4 mg YT765 (c) were used in the studies. Anti-OVA titres determined by enzyme-linked immunosorbent assay were analysed on day 21 (a and c) and day 63 (b). Results represent at least two independent experiments with three to five mice per group. Titres are compared against both the antigen-only and isotype control groups. **P < 0·001, *P < 0·01.
Anti-mGITR enhances the humoral immune response to influenza HA
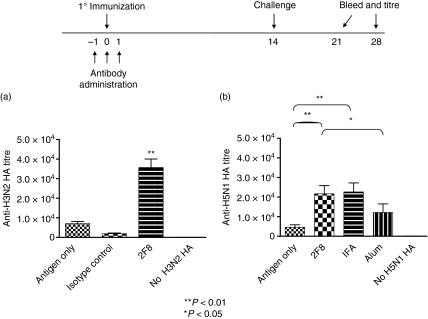
To assess the ability of 2F8 to augment the humoral immune response to a clinically relevant antigen, we further examined its effect on anti-HA titres using the influenza virus A/H5N1/Vietnam and A/H3N2/Wyoming subtypes. Mice were immunized with HA (day 0) during a 3-day course of 2F8 administration (days – 1, 0 and 1) and boosted with HA on day 14. As observed with the OVA antigen, mice that had not been exposed to HA had negligible anti-HA titres (Fig. 3). Doses of 0·4 mg 2F8 augmented the humoral response to both H3N2 HA (Fig. 3a) and H5N1 HA (Fig. 3b), with day 21 anti-HA titres 5-fold and 6·3-fold greater than antigen-only or isotype control mAb groups, respectively. Furthermore, when H5N1 HA was used, these doses of 2F8 were as effective as IFA in augmenting the humoral immune response to HA and yielded titres that were significantly greater than in HA-alum-treated mice (Fig. 3b). Unlike the results seen with OVA, however, there was a pronounced therapeutic-antibody dose-dependent effect on anti-HA titres. Mice treated with 0·08 mg 2F8 had 2·8-fold higher anti-HA titres than antigen-only mice, whereas mice treated with 0·016 mg and 0·0032 mg 2F8 experienced minimal and no increases, respectively, over titres generated by antigen-only mice (data not shown).
Figure 3.
2F8 enhances humoral immunity to (a) H3N2 haemagglutinin (HA) and (b) H5N1 HA. Antibody dosing and immunization schedules are indicated above the graph; 0·4 mg 2F8 was used in these studies. HA mixed with alum or incomplete Freund’s adjuvant (IFA) is included for comparison. Levels of anti-HA antibodies were determined by enzyme-linked immunosorbent assay, as described in the Materials and methods section. Results represent at least two independent experiments with three to five mice per group. In (a), 2F8 is compared against both antigen-only and isotype control groups. **P < 0·01, *P < 0·05.
The augmented humoral response retains specificity for the antigen administered with 2F8 antibody
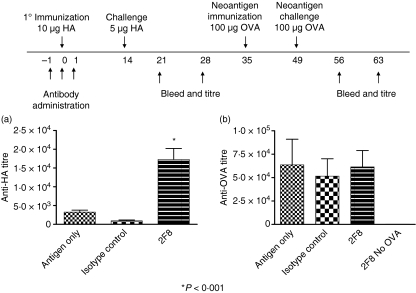
We next assessed whether 2F8 augmented humoral responses in an antigen-specific manner and whether the timing of antibody exposure relative to antigen was important. To this end, mice were immunized with HA on day 0 of a 3-day course of 2F8 (0·4 mg on days – 1, 0 and 1) and boosted with HA on day 14. Anti-HA titres were determined on days 21 and 28. Mice were allowed to recover for an additional week before immunization with OVA as a neoantigen on day 35. Mice were challenged 2 weeks later with OVA and their serum was analysed for anti-OVA titres on day 63. Anti-HA titres were also determined on days 56 and 63 to assess the longevity of the augmented immune response. As previously observed with OVA, anti-HA titres were still elevated on day 63 compared with those in mice administered either an isotype control antibody or antigen only. Hence, 2F8 induced a long-lived humoral response (Fig. 4a). Administration of a neoantigen (OVA) induced an immune response, as 2F8-treated mice that had not been immunized with OVA had negligible anti-OVA titres. Anti-OVA titres on day 63 were similar in the three groups that received OVA (Fig. 4b) and were equivalent to anti-OVA titres observed in mice that received antigen only in the studies depicted in Fig. 1. Consequently, the effect of 2F8 on the humoral immune response appears to be specific for the antigen delivered during the administration of the 2F8 mAb. Prior administration of anti-GITR did not affect subsequent responses to neoantigens, as the response to a neoantigen after the anti-GITR antibody had been cleared was comparable to the response generated in the absence of antibody.
Figure 4.
2F8-enhanced humoral immunity is specific for the antigen administered during antibody dosing. Antibody dosing and immunization schedules are indicated above the graph; 0·4 mg 2F8 was used in these studies. Mice were allowed to rest for 2 weeks before immunization with the neo-antigen, ovalbumin (OVA). No additional antibody was administered to any of the mice. Anti-haemagglutinin (HA) (a) and anti-OVA (b) titres were determined by enzyme-linked immunosorbent assay on day 56. Results represent at least two independent experiments with three to five mice per group. *P < 0·001.
2F8 administration during antigen challenge augments humoral immune responses
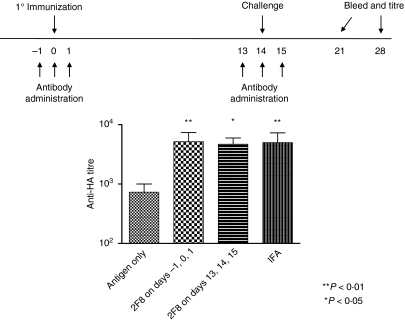
The timing of antibody administration was critical for effectiveness when anti-mGITR antibodies were investigated as adjuvants in mouse tumour models.46–49 To determine if the observed augmentation of humoral immunity by 2F8 depended on the timing of antibody administration, we compared the effect of 0·4 mg 2F8 administered at the time of HA immunization with that of 0·4 mg 2F8 administered at the time of the HA boost. Mice were immunized with HA (day 0) and boosted (day 14) during a 3-day course of 2F8 administration (days 13, 14 and 15). Anti-HA titres were measured on days 21 and 28 (Fig. 5). No statistical difference in day 21 anti-HA titres was observed between groups receiving 2F8 only at the time of immunization and those receiving 2F8 only at the time of boost (7·2-fold and 5·6-fold increases, respectively). Anti-HA titres were equivalent to those of IFA-treated mice. These results demonstrate that 2F8 is effective at augmenting the humoral immune response when delivered at the time of antigen boost or during the primary immunization.
Figure 5.
The effect of the timing of antibody administration on humoral immunity. Antibody dosing and immunization schedule are indicated above the graph. Mice received 0·4 mg 2F8 either during the primary immunization or during the antigen challenge. Anti-haemagglutinin (HA) titres were determined by enzyme-linked immunosorbent assay on day 21. Titres of mice treated with 2F8 on days − 1, 0 and 1 versus mice treated on days 13, 14 and 15 are shown. Mice treated with a mixture of incomplete Freund’s adjuvant (IFA) and HA are included for comparison. Results represent at least two independent experiments with three to five mice per group. **P < 0·01, *P < 0·05.
2F8 shifts the immune response to a Th1-type response
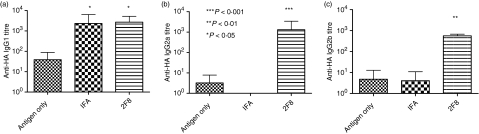
The immune response to HA in BALB/c mice has been characterized as a predominantly Th2 response.62,63 Alum and IFA have been shown to preferentially augment the Th2 response while having little effect on the Th1 response.6,7,64,65Figures 1 and 3 demonstrate that 0·4 mg of 2F8 enhanced total IgG anti-OVA and anti-HA titres when compared with alum but responses were similar after treatment with IFA. We decided to compare the immune responses induced by 2F8 with those induced by IFA in more detail. The IgG1-, IgG2a- and IgG2b-specific anti-HA titres were analysed in mice immunized with HA alone (antigen only), HA mixed with IFA, and HA plus 2F8 dosed on days − 1, 0 and 1. All mice were administered HA on day 0. Consistent with the literature, HA induced a predominant IgG1 response (antigen only; Fig. 6a–c). The IgG1-specific titres were augmented by IFA compared with HA alone (Fig. 6a). 2F8-treated mice induced comparable IgG1-specific titres to IFA-treated mice; both 2F8-treated and IFA-treated mice produced titres that were larger than those produced by mice treated with HA alone (P < 0·05). Haemagglutinin alone or IFA mixed with HA generated poor IgG2a-specific and IgG2b-specific anti-HA antibodies. However, 2F8-treated mice generated robust IgG2a-specific and IgG2b-specific anti-HA titres, which indicates a major contribution from a Th1-type response (Fig. 6b,c). These findings suggest that, although humoral immune responses to HA seemed comparable in 2F8 and IFA, the type of response is quite different, as IFA induces a predominately Th2-type response. In contrast, 2F8 shifts the immune response toward a Th1-type response.
Figure 6.
2F8 enhances T helper type 1 humoral immunity; 0·4 mg 2F8 was used in these studies. Haemagglutinin (HA) mixed with incomplete Freund’s adjuvant (IFA) is included for comparison. Levels of immunoglobulin G1 (IgG1) -specific (a), IgG2a-specific (b) and IgG2b-specific (c) anti-HA antibodies were determined by enzyme-linked immunosorbent assay, as described in the Materials and methods section. Results represent at least two independent experiments with three to five mice per group. In (a), comparison is to the antigen-only group. In (b) and (c), 2F8 is compared with the antigen-only and IFA groups. ***P < 0·001, **P < 0·01, *P < 0·05.
Anti-mGITR enhances the cellular immune response against influenza HA
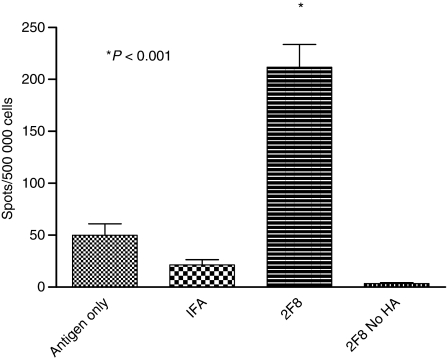
The anti-HA-specific antibody isotype data suggest that 2F8 induced a predominately Th1-type humoral response. To explore the role of 2F8 in cellular immunity, we analysed the number of IFN-γ-producing cells by ELISPOT assays using day 21 splenocytes from mice in each group. 2F8-treated mice had on average 4·3 times as many IFN-γ-producing cells as antigen-only mice and 9·9 times more than IFA-treated mice (Fig. 7). The ranges for 2F8-treated mice were 132–276 spots versus 5–97 and 3–40 spots for antigen-only-treated and IFA-treated mice, respectively. The effect was specific for HA, because neither naive mice administered 2F8 without antigen (data not shown) nor 2F8-plus-antigen-treated mice splenocytes stimulated with medium alone (2F8 No HA) (Fig. 7) produced HA-specific IFN-γ-producing cells. Hence, in contrast to IFA, which augments only humoral IgG1 responses to HA, these data demonstrated that 2F8 enhances both humoral (IgG1, IgG2a, IgG2b) and cellular immune responses.
Figure 7.
2F8 augments cellular immunity. Total splenocytes from mice treated with saline, incomplete Freund’s adjuvant (IFA), or 2F8 were prepared on day 21, cultured with or without haemagglutinin (HA), and assessed for the number of interferon-γ-secreting cells. Results represent at least two independent experiments with three to five mice per group. *P < 0·001.
2F8-treated mice produce more neutralizing antibodies to HA than control mice
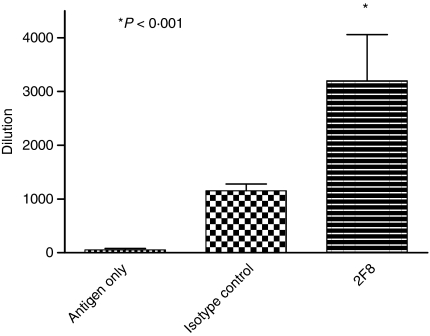
To assess whether anti-HA titres produced neutralizing antibodies to HA, we analysed day 21 sera using both an HI and a microneutralization assay. Serum samples from mice treated with 0·4 mg 2F8 inhibited haemagglutination at dilutions ranging from 1 : 640 to 1 : 5120 (Fig. 8). Serum samples from mice that were administered antigen only were effective at dilutions ranging from 1 : 20 to 1 : 120 (Fig. 8). Interpretation of the microneutralization assay results confirmed conclusions from the HI assay (data not shown). Hence, not only do 2F8-treated mice have augmented anti-HA titres, but the anti-HA immunoglobulin repertoire consists of more neutralizing antibodies than in isotype control antibody and antigen-only mice.
Figure 8.
2F8 administration augments neutralizing antibodies to haemagglutinin (HA). Serum samples from day 21 bleeds were analysed for neutralizing antibodies to HA. Results represent at least two independent experiments with three to five mice per group. 2F8 is compared with antigen-only and isotype control groups. *P < 0·001.
Discussion
The adjuvant potential of co-stimulatory molecules, including TNFRSF members, has received increased attention as a possible route to augmentation of cellular and humoral immune responses. Agonist antibodies and soluble ligands to 4-1BB, OX40 and GITR, among others, have shown efficacy in a number of anti-tumour and anti-virus preclinical models.46–59 However, the same molecular agonists have been reported to induce widely differing, and even opposing, functions in other readout systems.60,66,67
To better understand what adjuvant opportunities exist for anti-GITR antibodies, we generated an agonistic rat anti-mGITR mAb, 2F8, and evaluated its effect on immune responses to a set of model antigens using a simple prime boost protocol. 2F8 induced a robust and durable augmentation of humoral immunity against OVA and HA antigens, with elevated antibody titres for up to 2 months after the primary immunization.
In contrast to the whole antibody, ‘blocking’ (Fab) fragments of the anti-mGITR mAb DTA-1 were unable to attenuate CD4+ CD25+-mediated suppression of responder T-cell proliferation in vitro,24 confirming that GITR signalling, rather than blockade of GITR–GITRL interactions, is essential for abrogation of Treg-cell suppression. However, these experiments did not address the importance of FcR-mediated cross-linking of the antibody. Although we did not test Fab fragments of 2F8, mice treated with 2F8 F(ab′)2 fragments not only showed augmented serum titres to antigen, but their humoral responses were similar to those of mice treated with intact 2F8 antibody. These results were confirmed using a different anti-mGITR mAb, YT765, with an intact but functionally disabled Fc. This finding – that FcR-mediated cross-linking of the anti-mGITR antibody is not required for efficacy – has significant implications for the clinical development of anti-GITR antibodies. Indeed, from a safety perspective, the data indicate that it is possible, and may be preferable, to develop an anti-GITR mAb that is genetically modified to minimize Fc–FcR interactions that have potential for eliciting cytokine release and mediating immune effector functions such as antibody-dependent cellular cytotoxicity and complement-mediated lysis.68 Additionally, from a manufacturing perspective, generation of F(ab′2) fragments for clinical use is more laborious and more costly because of the chemical modifications needed to extend the half-life of antibody fragments. In contrast, large-scale mammalian cell culture fermentation and down-stream processing are well-established, safe methods for the production of therapeutic antibodies. Furthermore, the removal of the N-linked glycosylation site prevents the heterogeneity of carbohydrates often observed with glycosylated antibodies, thereby eliminating the glycoform variability within the Fc region that may be introduced during manufacturing development. We did not address the effect of other amino acid substitutions to glycosylated antibodies that have been demonstrated to reduce Fc–FcR interactions, but we assume that anti-GITR antibodies designed with such mutated Fc regions would also act as adjuvants, although the issue of carbohydrate heterogeneity in manufacturing the antibody would still apply.
The timing of anti-GITR exposure relative to antigen presentation was demonstrated to be an important variable for capturing its adjuvant effect in other studies.46–48 In our studies, 2F8 was found to be equally efficacious when administered at the time of induction or at challenge. These findings suggest that GITR–GITRL interactions can modulate both primary and secondary immune responses and are consistent with reports demonstrating that anti-GITR is able to co-stimulate naive and memory T cells. The results support the potential versatility of anti-GITR as an adjuvant and highlight a difference between targeting GITR versus other TNFSFR family members, such as OX40 and 4-1BB, which have been implicated predominantly in memory responses.
For comparison, we included two widely used commercial adjuvants, IFA and alum, in our studies. The IFA is a potent inducer of TH2 and B-cell responses in mice and alum is a component of several vaccines licensed for human use. Like IFA, alum skews the immune response to a predominantly Th2 response and is a poor inducer of Th1 cell-mediated immunity.5 2F8-treated mice injected with OVA had humoral responses that were two-fold higher than mice treated with a mixture of antigen and IFA and three-fold higher than antigen-treated and alum-treated mice (0·4 mg 2F8 versus IFA or alum, Fig. 1). Our studies using HA also demonstrated a significant increase in humoral immunity in mice treated with 2F8 when compared with alum-treated mice (Fig 3b); however, the response was comparable to that in IFA-treated mice. Analysis of specific antibody isotype responses to HA in mice treated with IFA or 2F8 revealed that the humoral IgG response in the IFA-treated mice was predominantly IgG1. 2F8-treated mice produced statistically significant increases in IgG2a- and IgG2b-specific anti-HA antibodies (Fig. 6), which is indicative of a Th1-type immune response.69 The enhanced Th1 immunity is of particular importance because IgG2a antibodies have high affinities for complement70 and activating Fc receptors when compared with IgG1 antibodies.71 The latter interaction has been demonstrated to activate Fc effector functions such as antibody-dependent cellular cytotoxicity72 and opsonophagocytosis by macrophages73, which contribute to viral clearance of influenza-infected hosts74 and enhance tumour immunity.75,76 Anti-GITR administration not only produced IgG2a- and IgG2b-specific titres that were higher than those observed in IFA-treated mice, but the anti-GITR-treated mice also generated a specific and prolific cellular immune response, whereas the IFA-treated mice did not. The exact mechanism(s) by which anti-GITR enhances both humoral and cellular responses are currently under investigation. However, these findings agree with literature demonstrating that anti-GITR ligation can significantly induce Th1 and Th2 cytokine production by naive CD4+ CD25− T cells and up-regulate T-bet and GATA3, key transcription factors for Th1 and Th2 responses, respectively.77
In addition, serum from 2F8-treated mice had enhanced anti-HA neutralizing antibodies as determined by HI and microneutralization assays. These observations are particularly meaningful given the fact that protection against influenza viruses is enhanced by a robust neutralizing response to HA.16 H5N1 HA is reported to be a poorly immunogenic antigen,78 whereas OVA induces robust humoral immunity. Varying the dose of anti-GITR with a constant dose of antigen demonstrated that the more potent the antigen, the lower the dose of adjuvant antibody needed to augment that immunity. These data reveal the importance of antigen immunogenicity in harnessing the full benefit of the adjuvant antibody and suggest that in oncological indications with weak tumour antigens, higher levels of adjuvant antibody may be required. Alternatively, indications with stronger tumour-specific antigens such as mutated self or viral antigens should be targeted. The data also suggest that combination therapy with vaccines in oncology may be an appropriate path of development.
Some studies using the DTA-1 (anti-GITR) antibody in mice have found that the mice developed colitis, gastritis, vitiligo, and had increased anti-double-stranded DNA autoantibodies, indicative of autoimmunity.24,33,46,47 Although we have not performed full toxicology studies with 2F8, we have administered 2F8 to over 500 mice in numerous studies and did not observe any overt autoimmunity, morbidity, or mortality attributable to the antibody (data not shown). This safety profile, coupled with the ability of anti-GITR to augment both humoral and cellular immunity, supports the development of anti-GITR in indications such as virology and oncology, as well as a vaccine adjuvant. Our findings show that careful consideration must be paid to the amount and timing of mAb administration and that the immunogenicity of the antigen(s) being targeted is important when optimizing mAb dosing. Additional studies to determine the lowest effective dose, optimal dosing schedule, and mechanism(s) of action are ongoing.
Acknowledgments
The authors would like to thank Claire McCall for assistance in preparing this manuscript and Irina Apostolou for critical review of the manuscript.
Disclosures
Jose F Ponte, Paul Ponath, Reema Gulati, Michael Slavonic, Michael Paglia, Adam O'Shea, Louis Vaickus and Michael Rosenzweig are or were employees of Tolerx, Inc. Herman Waldmann is a co-founder of Tolerx, Inc., and is a member of the Board of Directors. Masahide Tone has no conflict of interest.
References
- 1.Fraser CK, Diener KR, Brown MP, Hayball JD. Improving vaccines by incorporating immunological coadjuvants. Expert Rev Vaccines. 2007;6:559–78. doi: 10.1586/14760584.6.4.559. [DOI] [PubMed] [Google Scholar]
- 2.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–96. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 3.Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis. 2000;30(Suppl. 3):S266–70. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 4.Glenny ATPC, Waddington H, Wallace V. The antigenic value of toxoid precipitated by potassium-alum. J Pathol Bacteriol. 1926;29:38–45. [Google Scholar]
- 5.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 7.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134–45. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J. 2001;20:63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RK, Siber GR. Adjuvants for human vaccines – current status, problems and future prospects. Vaccine. 1995;13:1263–76. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 10.Lindblad EB. Aluminium adjuvants – in retrospect and prospect. Vaccine. 2004;22:3658–68. doi: 10.1016/j.vaccine.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Sela M, Hilleman MR. Therapeutic vaccines: realities of today and hopes for tomorrow. Proc Natl Acad Sci U S A. 2004;101(Suppl. 2):14559. doi: 10.1073/pnas.0405924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandepapeliere P. Therapeutic vaccination against chronic viral infections. Lancet Infect Dis. 2002;2:353–67. doi: 10.1016/s1473-3099(02)00289-x. [DOI] [PubMed] [Google Scholar]
- 13.Dredge K, Marriott JB, Todryk SM, Dalgleish AG. Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer Immunol Immunother. 2002;51:521–31. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda H, Chamoto K, Tsuji T, Suzuki Y, Wakita D, Takeshima T, Nishimura T. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004;95:697–703. doi: 10.1111/j.1349-7006.2004.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moingeon P, Haensler J, Lindberg A. Towards the rational design of Th1 adjuvants. Vaccine. 2001;19:4363–72. doi: 10.1016/s0264-410x(01)00193-1. [DOI] [PubMed] [Google Scholar]
- 16.Barr TA, Carlring J, Heath AW. Co-stimulatory agonists as immunological adjuvants. Vaccine. 2006;24:3399–407. doi: 10.1016/j.vaccine.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Hodge JW, Greiner JW, Tsang KY, et al. Costimulatory molecules as adjuvants for immunotherapy. Front Biosci. 2006;11:788–803. doi: 10.2741/1837. [DOI] [PubMed] [Google Scholar]
- 18.Serghides L, Vidric M, Watts TH. Approaches to studying costimulation of human antiviral T cell responses: prospects for immunotherapeutic vaccines. Immunol Res. 2006;35:137–50. doi: 10.1385/IR:35:1:137. [DOI] [PubMed] [Google Scholar]
- 19.Ward RC, Kaufman HL. Targeting costimulatory pathways for tumor immunotherapy. Int Rev Immunol. 2007;26:161–96. doi: 10.1080/08830180701365941. [DOI] [PubMed] [Google Scholar]
- 20.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 21.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14:265–73. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 22.Kober J, Leitner J, Klauser C, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38:2678–88. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji HB, Liao G, Faubion WA, Abadia-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172:5823–7. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+ CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 25.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–20. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 26.Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, Kwon BS. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75:785–91. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 27.Robertson SJ, Messer RJ, Carmody AB, Mittler RS, Burlak C, Hasenkrug KJ. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J Immunol. 2008;180:5267–74. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4+CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–51. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 29.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–10. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167:2479–86. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 31.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox RA, Chapoval AI, Gorski KS, et al. Cutting edge: expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262–7. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 33.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–22. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 34.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 35.Gurney AL, Marsters SA, Huang RM, et al. Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr Biol. 1999;9:215–18. doi: 10.1016/s0960-9822(99)80093-1. [DOI] [PubMed] [Google Scholar]
- 36.Kwon B, Yu KY, Ni J, et al. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J Biol Chem. 1999;274:6056–61. doi: 10.1074/jbc.274.10.6056. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Miyagawa J, Moriwaki M, et al. Analysis of expression profiles of islet-associated transcription and growth factors during beta-cell neogenesis from duct cells in partially duct-ligated mice. Pancreas. 2003;27:345–55. doi: 10.1097/00006676-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002;100:350–2. doi: 10.1182/blood-2001-12-0276. [DOI] [PubMed] [Google Scholar]
- 39.So T, Lee SW, Croft M. Tumor necrosis factor/tumor necrosis factor receptor family members that positively regulate immunity. Int J Hematol. 2006;83:1–11. doi: 10.1532/IJH97.05120. [DOI] [PubMed] [Google Scholar]
- 40.Tone MTY, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci U S A. 2003;100:15059–64. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muriglan SJ, Ramirez-Montagut T, Alpdogan O, et al. GITR activation induces an opposite effect on alloreactive CD4+ and CD8+ T cells in graft-versus-host disease. J Exp Med. 2004;200:149–57. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spinicelli S, Nocentini G, Ronchetti S, Krausz LT, Bianchini R, Riccardi C. GITR interacts with the pro-apoptotic protein Siva and induces apoptosis. Cell Death Differ. 2002;9:1382–4. doi: 10.1038/sj.cdd.4401140. [DOI] [PubMed] [Google Scholar]
- 43.Esparza EM, Arch RH. Glucocorticoid-induced TNF receptor functions as a costimulatory receptor that promotes survival in early phases of T cell activation. J Immunol. 2005;174:7869–74. doi: 10.4049/jimmunol.174.12.7869. [DOI] [PubMed] [Google Scholar]
- 44.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:6216–21. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan Y, Brown LE, Deliyannis G, et al. Responses against complex antigens in various models of CD4 T-cell deficiency: surprises from an anti-CD4 antibody transgenic mouse. Immunol Res. 2004;30:1–14. doi: 10.1385/IR:30:1:001. [DOI] [PubMed] [Google Scholar]
- 46.Cohen AD, Diab A, Perales MA, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8+ T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–12. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176:6434–42. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 48.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–82. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+ CD25+ CD4+ regulatory T cells. J Exp Med. 2005;202:885–91. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou P, L’Italien L, Hodges D, Schebye XM. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J Immunol. 2007;179:7365–75. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
- 51.Dittmer U, He H, Messer RJ, et al. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 52.La S, Kim E, Kwon B. In vivo ligation of glucocorticoid-induced TNF receptor enhances the T-cell immunity to herpes simplex virus type 1. Exp Mol Med. 2005;37:193–8. doi: 10.1038/emm.2005.26. [DOI] [PubMed] [Google Scholar]
- 53.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 54.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 55.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–7. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 56.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–9. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 57.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002;168:3777–85. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 58.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112:559–66. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao C, Jin H, Hu Y, et al. Enhanced protective efficacy and reduced viral load of foot-and-mouth disease DNA vaccine with co-stimulatory molecules as the molecular adjuvants. Antiviral Res. 2007;76:11–20. doi: 10.1016/j.antiviral.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Nishioka TNE, Iida R, Morita A, Shimizu J. In vivo expansion of CD4+ Foxp3+ regulatory T cells mediated by GITR molecules. Immunol Lett. 2008;121:97–104. doi: 10.1016/j.imlet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Robert G, Webster SK, World Health Organization Global Influenza Programme . WHO manual on animal influenza diagnosis and surveillance. Geneva: World Health Organization, Department of Communicable Disease Surveillance and Response; 2002. [Google Scholar]
- 62.Benne CA, Harmsen M, van der Graaff W, Verheul AF, Snippe H, Kraaijeveld CA. Influenza virus neutralizing antibodies and IgG isotype profiles after immunization of mice with influenza A subunit vaccine using various adjuvants. Vaccine. 1997;15:1039–44. doi: 10.1016/s0264-410x(96)00287-3. [DOI] [PubMed] [Google Scholar]
- 63.Hocart MJ, Mackenzie JS, Stewart GA. The immunoglobulin G subclass responses of mice to influenza A virus: the effect of mouse strain, and the neutralizing abilities of individual protein A-purified subclass antibodies. J Gen Virol. 1989;9:2439–48. doi: 10.1099/0022-1317-70-9-2439. [DOI] [PubMed] [Google Scholar]
- 64.Shibaki A, Katz SI. Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund’s adjuvant. Exp Dermatol. 2002;11:126–34. doi: 10.1034/j.1600-0625.2002.110204.x. [DOI] [PubMed] [Google Scholar]
- 65.Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–60. [PubMed] [Google Scholar]
- 66.Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu YX. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:1457–65. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 67.Sytwu HK, Lin WD, Roffler SR, et al. Anti-4-1BB-based immunotherapy for autoimmune diabetes: lessons from a transgenic non-obese diabetic (NOD) model. J Autoimmun. 2003;21:247–54. doi: 10.1016/s0896-8411(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 68.Tao MH, Morrison SL. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989;143:2595–601. [PubMed] [Google Scholar]
- 69.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–47. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 70.Neuberger MS, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11:1012–6. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- 71.Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76:231–48. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 72.Kipps TJ, Parham P, Punt J, Herzenberg LA. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med. 1985;161:1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–29. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 74.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–8. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 75.Aurisicchio L, Peruzzi D, Conforti A, et al. Treatment of mammary carcinomas in HER-2 transgenic mice through combination of genetic vaccine and an agonist of Toll-like receptor 9. Clin Cancer Res. 2009;15:1575–84. doi: 10.1158/1078-0432.CCR-08-2628. [DOI] [PubMed] [Google Scholar]
- 76.Durso RJ, Andjelic S, Gardner JP, et al. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin Cancer Res. 2007;13:3999–4008. doi: 10.1158/1078-0432.CCR-06-2202. [DOI] [PubMed] [Google Scholar]
- 77.Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, Liew FY. Glucocorticoid-induced TNFR family-related protein (GITR) activation exacerbates murine asthma and collagen-induced arthritis. Eur J Immunol. 2005;35:3581–90. doi: 10.1002/eji.200535421. [DOI] [PubMed] [Google Scholar]
- 78.Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]