Abstract
CD1d-restricted natural killer T (NKT) cells are emerging as critical regulators of the immune response to infectious agents, including Pseudomonas aeruginosa; and therapies to augment NKT-cell activation may represent a novel approach to treat chronic, antibiotic-resistant bacterial infections. We examined the capacity of dendritic cells (DCs) from people with cystic fibrosis (CF) to activate NKT cells. Our study was motivated by three lines of evidence: (i) NKT cells play a critical role in clearing P. aeruginosa infection; (ii) activation of NKT cells requires acidification-dependent processing of glycolipid antigens within the endolysosomal compartment; and (iii) endolysosomal acidification may be reduced in CF. We demonstrated that NKT-cell activation was dependent upon intact organelle acidification as inhibitors of the vacuolar (H+)-ATPases prevented DCs from activating NKT cells with two glycolipid antigens, α-galactosylceramide and galactose-galactosylceramide. In contrast, cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel dysfunction had no significant biological impact on the capacity of DCs to activate NKT cells. Dendritic cells from subjects with CF and DCs treated with the thiazolidinone CFTRinh-172 inhibitor showed no reduction in their ability to activate NKT cells. Based on these data, we find no evidence for an inherent defect in glycolipid antigen presentation to NKT cells in CF subjects.
Keywords: acidification, cystic fibrosis, natural killer T cell, Pseudomonas aeruginosa
Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CF transmembrane conductance regulator (CFTR) gene which encodes a cAMP-regulated chloride channel. There is currently no cure for CF and the median life expectancy for CF patients is 37 years.1–3 Lung disease is the primary cause of morbidity and mortality in CF. Soon after birth, infection with bacterial pathogens begins, triggering an intense neutrophilic response localized to the peribronchial and endobronchial spaces. Intriguingly, only a limited number of bacterial pathogens colonize the airway in CF, with the most clinically relevant being Pseudomonas aeruginosa.4 Although a number of hypotheses have been proposed (reviewed in refs2,4), there is still no clear consensus linking mutations in CFTR to development of chronic infection with P. aeruginosa. In this study we explore the link between CFTR deficiency and lung infection with P. aeruginosa by examining the capacity of CFTR-deficient dendritic cells (DCs) to stimulate natural killer T (NKT) cells.
Natural killer T cells, a specialized type of T cell, are emerging as critical regulators of the immune response to infectious agents.5,6 The NKT cells may be particularly important in CF as evidence suggests that NKT cells play a central role in clearing P. aeruginosa from the lung7 and gastrointestinal tract.8 In contrast to conventional major histocompatibility complex-restricted T cells, NKT cells express a semi-invariant T-cell receptor (iTCR) that recognizes glycolipid antigens presented by CD1d molecules on the surface of antigen-presenting cells, such as DCs and macrophages.5,9 Current knowledge of the glycolipid antigens that activate NKT cells for antimicrobial defence is incomplete, although these glycolipids appear to include both pathogen-derived and host-derived lipids.10–12 For experimental purposes most groups utilize α-galactosylceramide (α-GalCer) and galactose-galactosylceramide (GalGalCer), two well-characterized glycolipids that bind to CD1d and are recognized by the iNKT-cell TCR. Optimal lipid antigen presentation by CD1d, leading to activation of NKT cells, is dependent on endosomal acidification.13–16
Natural killer T-cell biology has been the focus of much recent attention as therapeutic augmentation of NKT-cell activity through the administration of lipid antigens is being trialled as a cancer therapy and may also play a role in managing human infections, such as CF.17,18 Moreover, boosting innate immune responses is particularly attractive in CF as this may allow the eradication of pathogens that are resistant to multiple antibiotics.19
The fact that NKT-cell activation appears to be dependent on endosomal acidification, combined with the observation that NKT cells contribute to clearance of P. aeruginosa from the lung, provides an intriguing (and testable) link between CFTR deficiency and chronic airway infection with P. aeruginosa. For many years it has been suggested that endolysosomal acidification is defective in CF. In what has been coined the ‘defective organelle acidification’ hypothesis, it has been proposed that CFTR provides a conductive pathway for chloride transport across intracellular organelles promoting H+ influx via a counter-ion effect.20–23 Hence in CF, endosomal acidification may be inadequate as H+ pumping is electrically limited by defective CFTR-mediated chloride transport. Although we appreciate that evidence supporting an endosomal acidification defect in CF is far from unanimous,24–29 given that there is potential to develop therapies to boost NKT-cell activity and enhance clearance of infectious agents, in this study we examined endosomal acidification-dependent NKT-cell activation by DCs from patients with CF. Specifically, we hypothesized that DCs with dysfunctional CFTR would be less potent activators of CD1d-restricted NKT cells than DCs with intact CFTR-mediated chloride conductance.
Materials and methods
Dendritic cell preparation
Blood samples from healthy volunteers and patients diagnosed with CF were obtained at BC Children’s Hospital, Vancouver, Canada. The diagnosis of CF was established by classic clinical features including increased sweat chloride concentrations (> 60 mmol/l). The CF subjects enrolled in this study had the following CFTR mutations: ΔF508/ΔF508 = 4, ΔF508/621 + 1G> T = 1, ΔF508/G85E = 1, ΔF508/unknown = 1, unknown/unknown = 1. Patients receiving systemic immunosuppressive medications such as oral corticosteroids and azithromycin were excluded from the study. All samples were obtained with informed consent with the approval of the University of British Columbia Clinical Research Ethics Board. Peripheral whole blood was collected in sodium heparin Vacutainer® tubes (BD Biosciences, Mississauga, ON, Canada) and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation as previously described.30 Monocytes were purified from PBMCs by magnetic bead separation using a CD14-positive selection kit and an autoMACS™ Pro Separator (Miltenyi Biotec, Auburn, CA) following the manufacturer’s protocols. The CD14-positive cells were resuspended in R10 consisting of RPMI-1640 medium supplemented with 10% (v/v) fetal calf serum (HyClone, Ottawa, ON, Canada), 2 mm l-glutamine, 1 mm sodium pyruvate, 100 U penicillin, 100 μg streptomycin (Invitrogen, Burlington, ON, Canada), 100 ng/ml granulocyte–macrophage colony-stimulating factor and 100 ng/ml interleukin-4 (IL-4; Fitzgerald Industries, Acton, MA). The cells were incubated in six-well plates at 37° and 5% CO2 for 96 hr to allow the cells to differentiate into immature DCs (iDCs).
Quantification of CFTRmessenger RNA expression in human cells
Expression of CFTR messenger RNA (mRNA) in characterized cell populations was quantified by real-time polymerase chain reaction (PCR). The PBMCs from healthy volunteers were prepared as described above, stained with CD14-PECy7 antibody for monocyte isolation and sorted using a FACSAria flow cytometer (BD Biosciences) into CD14-positive populations. Immature DCs were prepared from a fraction of sorted monocytes and differentiated for 96 hr as described above. A bronchial epithelial cell line (NuLi) was used as a positive control for CFTR expression. Messenger RNA was harvested from the cell populations using an RNeasy Plus kit (QIAGEN, Mississauga, ON, Canada) and transcribed to complementary DNA using a SuperScript cDNA synthesis kit (Invitrogen). Expression of CFTR was calculated relative to β-actin and was quantified by SYBR GreenER (Invitrogen) chemistry using a 7300 Real Time PCR System (Applied Biosystems, Foster City, CA). The primers used were, CFTR-forward 5′-ACACGTTGAAAGCAGGTGGGATTC-3′, CFTR-reverse 5′-ACTGCGACAACTGCTATAGCTCCA-3′, ACTB-forward 5′-GTTGCGTTACACCCTTTCTT-3′ and ACTB-reverse 5′-ACCTTCACCGTTCCAGTTT-3′ under standard cycling conditions. The expression of the CFTR gene was calculated relative to iDCs by the 2−ΔΔCT method.
NKT-cell preparation
Human NKT-cell clone BM2a.331 was thawed and mixed in a 1 : 2 ratio with irradiated PBMCs plus 5 μg/ml phytohaemagglutinin (PHA) and allowed to proliferate for a minimum of 14 days in T-cell medium [R10 supplemented with 1% non-essential amino acids (Invitrogen), 2% heat-inactivated human AB serum (Mediatech, Manassas, VA) and 200 U/ml IL-2] at 37° and 5% CO2.
NKT-cell activation assay
The iDCs and NKT cells were harvested by gentle pipetting, then washed and resuspended in R10. Both iDCs and NKT cells were mixed in a 1 : 1 ratio and incubated in a 96-well plate (5 × 105 each cell type/well). In select experiments the iDCs were pre-treated with the vacuolar-type H+-adenosine triphosphatase (ATPase) inhibitors concanamycin A (2·5 or 12·5 nm) or bafilomycin (100 mm) or the thiazolidinone CFTR inhibitor CFTRinh-172 (10 μm) (Sigma, Oakville, ON, Canada) diluted in dimethylsulphoxide (DMSO). The cells were pre-treated for 30 min at room temperature before addition of NKT cells and glycolipid antigens. CD1d glycolipid antigens α-GalCer or GalGalCer were prepared as described previously.32 Controls consisted of untreated NKT cells alone, NKT cells incubated with 5 μg/ml PHA and NKT cells treated with PHA plus inhibitor or vehicle (DMSO). The cells were incubated at 37° and 5% CO2 and the culture supernatants were harvested after 24 hr. Concentrations of IL-4 and interferon-γ (IFN-γ) were measured by enzyme-linked immunosorbent assay (eBioscience, San Diego, CA).
Statistical analysis
Cytokine production between groups was compared by analysis of variance with Bonferroni post-test for multiple comparisons. P-values < 0·05 were considered significant.
Results
CFTR mRNA is expressed by DCs and other peripheral blood cells
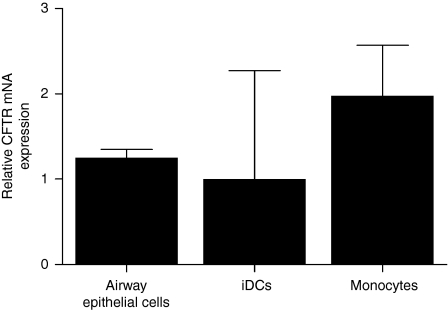
As a first step we examined CFTR mRNA expression in DCs, monocytes and airway epithelial cells. Using quantitative reverse transcription-PCR we demonstrated that immature DCs, monocytes and airway epithelial cells all expressed similar detectable levels of CFTR mRNA (Fig. 1).
Figure 1.
Quantification of CFTR messenger RNA (mRNA) expression by human dendritic cells (iDCs), monocytes and airway epithelial cells. Expression of CFTR mRNA was quantified by real-time polymerase chain reaction and the data were expressed relative to iDCs using the 2−ΔΔCT method. Values represent mean ± SEM of iDCs and monocytes isolated from three healthy donors. The bronchial epithelial cell line (NuLi) was used as a positive control for CFTR expression (n = 3 separate experiments).
Inhibiting DC organelle acidification prevents NKT-cell activation by glycolipid antigens
To validate the critical role that organelle acidification plays in the activation of NKT cells by DCs, we treated the DCs with inhibitors of the vacuolar (H+)-ATPases (V-ATPases). The pH within many intracellular compartments is regulated by V-ATPases and inhibition of V-ATPases prevents endosomal acification.33 For these studies, V-ATPases were blocked with the well-characterized and potent inhibitors concanamycin A and bafilomycin.34
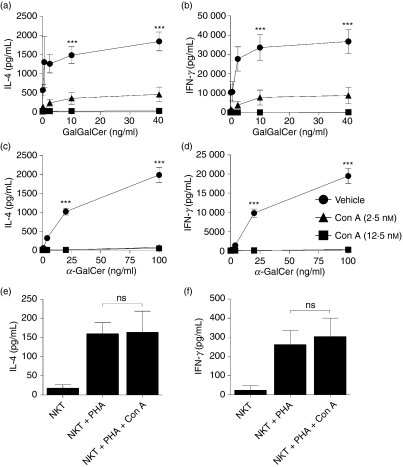
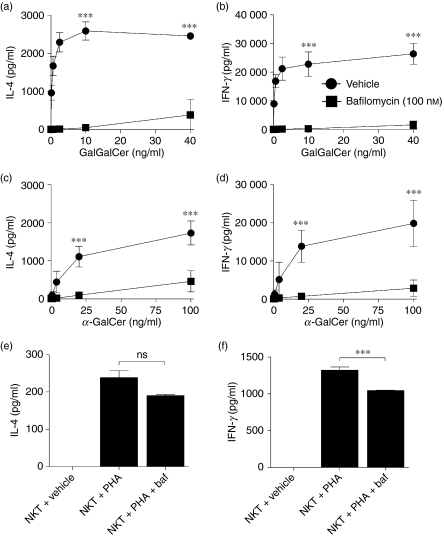
Blockade of V-ATPases with either concanamycin-A or bafilomycin abrogated the ability of the DCs to activate NKT cells with two prototypical glycolipid antigens, α-GalCer and GalGalCer (Figs 2 and 3). Specifically, pre-treatment of DCs with concanamycin A inhibited α-GalCer- and GalGalCer-triggered NKT-cell secretion of IL-4 and IFN-γ by > 97% (P < 0·001) (Fig. 2a–d). Similarly, bafilomycin pre-treatment of DCs inhibited glycolipid-dependent NKT-cell activation by > 73% (P < 0·001) (Fig. 3a–d). Importantly, control experiments demonstrated that V-ATPase inhibition did not directly influence the capacity of NKT cells to secrete IL-4 and IFN-γ. The NKT-cell responses to PHA were not altered significantly by the addition of concanamycin A whereas the addition of bafilomycin only decreased the cytokine output by approximately 20% (Figs 2e,f and 3e,f).
Figure 2.
Endosomal acidification blockade using V-type H+ adenosine triphosphatase (ATPase) inhibitor concanamycin A prevents natural killer T (NKT) -cell activation. The human NKT-cell BM2a.3 clone was stimulated with GalGalCer (a, b) or α-GalCer (c, d) at varying concentrations presented by either untreated healthy adult control dendritic cells (DCs) or healthy adult control DCs treated with concanamycin A (2·5 nm and 12·5 nm). Enzyme-linked immunosorbent assays were used to quantify NKT-cell secretion of the cytokines interleukin-4 (IL-4; a, c) or interferon-γ (IFN-γ; b, d). In control experiments, concanamycin A (12·5 nm) did not prevent the activation of NKT cells when stimulated with phytohaemagglutinin (PHA; e, f). Values represent mean ± SEM of 3–11 samples per group. *** signifies P < 0·001 and ns signifies no significant difference.
Figure 3.
Endosomal acidification blockade using V-type H+ adenosine triphosphatase (ATPase) inhibitor bafilomycin prevents natural killer T (NKT) -cell activation. The human NKT-cell BM2a.3 clone was stimulated with GalGalCer (a, b) or α-GalCer (c, d) at varying concentrations presented by either untreated healthy adult control dendritic cells (DCs) or healthy adult control DCs treated with bafilomycin (100 nm). Enzyme-linked immunosorbent assays were used to quantify NKT-cell secretion of the cytokines interleukin-4 (IL-4; a, c) or interferon-γ (IFN-γ; b, d). In control experiments, bafilomycin (100 nm) had a minimal biological effect on the activation of NKT cells when stimulated with phytohaemagglutinin (PHA; e, f). Values represent mean ± SEM of three samples per group. *** signifies P < 0·001 and ns signifies no significant difference.
CFTR inhibition does not alter glycolipid-dependent activation of NKT cells by DCs
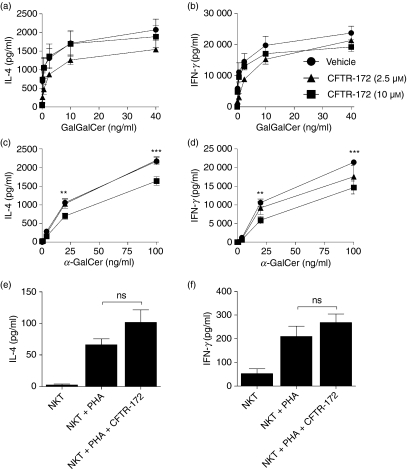
Having established that activation of NKT cells by DCs in our experimental system was sensitive to blockade of organelle acidification, we next examined if NKT-cell activation was influenced by inhibition of CFTR function. The well-characterized thiazolidinone CFTRinh-172 inhibitor was used to block CFTR chloride conductance with high affinity and selectivity.35 Importantly, two independent groups have demonstrated that 10 μm CFTRinh-172 prevented complete endosomal acidification21,22, although a third group failed to reproduce these data.25 Therefore, we hypothesized that CFTR blockade by CFTRinh-172 would decrease the ability of DCs to activate NKT cells following stimulation with glycolipid antigens. However, pre-treatment of DCs from healthy donors with CFTRinh-172 did not alter the NKT-cell responses triggered by GalGalCer (Fig. 4a,b) whereas α-GalCer stimulated cytokine output was statistically significantly reduced, although this biological effect was small compared with the V-ATPase inhibitors (Fig. 4c,d). Importantly, the responsiveness of NKT cells alone to PHA stimulation was unaltered by CFTRinh-172 (Fig. 4e,f).
Figure 4.
Cystic fibrosis transmembrane conductance regulator (CFTR) inhibition does not alter natural killer T (NKT) -cell activation. The human NKT cell BM2a.3 clone was stimulated with GalGalCer (a, b) or α-GalCer (c, d) at varying concentrations presented by either untreated healthy adult control DCs or healthy adult control DCs treated with CFTR-172inh inhibitor (2·5 μm and 10 μm). Enzyme-linked immunosorbent assays were used to quantify NKT-cell secretion of the cytokines interleukin-4 (IL-4; a, c) or interferon-γ (IFN-γ; b, d). In control experiments, CFTR-172inh (10 μm) had no impact on the activation of NKT cells when stimulated with phytohaemagglutinin (PHA; e, f). Values represent mean ± SEM of three to five samples per group. **P < 0·01, ***P < 0·001 and ns signifies no significant difference.
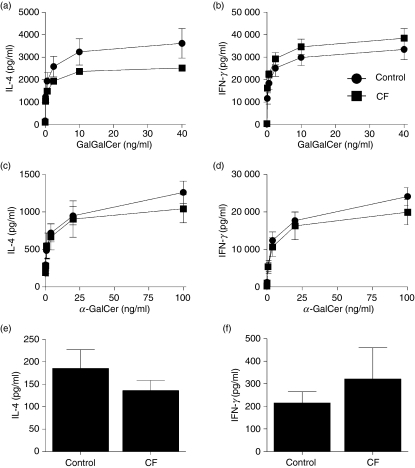
DCs from CF patients are able to activate NKT cells
To model the human situation, we isolated DCs from patients with CF and healthy age-matched controls and assessed the capacity of these DCs to activate NKT cells following exposure to glycolipid antigens. Consistent with the lack of impact of CFTRinh-172 on NKT-cell activation (Fig. 4), DCs from CF patients and controls were indistinguishable in their ability to activate NKT cells following stimulation with GalGalCer and α-GalCer (Fig. 5a–d). We also examined the capacity of CF and control DCs to present self-antigens to NKT cells. It has been demonstrated previously that NKT cells will generate weak responses to CD1d-presented endogenous self-antigens in the absence of added antigen or microbial products.31 However, the baseline cytokine secretion of NKT cells co-cultured with DCs without glycolipid was not significantly altered if DCs were from CF patients or healthy controls (IL-4: control = 185 ± 41 pg/ml versus CF = 135 ± 22 pg/ml; IFN-γ: control = 215 ± 49 pg/ml versus CF = 321 ± 138 pg/ml).
Figure 5.
Cystic fibrosis dendritic cells (CF DCs) are able to activate natural killer T (NKT) cells. The human NKT cell BM2a.3 clone was stimulated with GalGalCer (a, b) or α-GalCer (c, d) at varying concentrations presented by either DCs derived from CF patients (n = 5) or healthy child controls (n = 4–5). Enzyme-linked immunosorbent assays were used to quantify NKT-cell secretion of the cytokines interleukin-4 (IL-4; a, c) or interferon-γ (IFN-γ; b, d). Values represent mean ± SEM of four or five samples per group.
Discussion
Pseudomonas aeruginosa is the predominant and most clinically relevant respiratory pathogen infecting patients with CF.36 The unique tropism of P. aeruginosa for the CF respiratory tract has not been adequately explained and although a variety of hypotheses have been proposed, there is still no widely accepted unifying explanation for the peculiar propensity of P. aeruginosa to infect the CF airway (reviewed in refs2,4). Understanding the immune mechanisms that facilitate chronic P. aeruginosa infection of the CF lung may lead to the development of novel therapies. The ability to boost dysfunctional immune responses may be particularly effective in combating P. aeruginosa as this organism frequently develops resistance to multiple antimicrobial agents.
The aim of this study was to assess a possible link between CFTR dysfunction and P. aeruginosa infection through an examination of acidification-dependent NKT-cell immune function in patients with CF. Our study was motivated by three lines of evidence: (i) NKT cells play a critical role in clearing P. aeruginosa infection;7,8 (ii) activation of NKT cells by antigen-presenting cells requires acidification-dependent processing of glycolipid antigens within the endolysosomal compartment;13–16 and (iii) endolysosomal acidification may be reduced in CF.20–23 Combined, these previous studies raise the testable possibility that acidification-dependent NKT-cell activation might be deficient in patients with CF, helping to explain the tropism of P. aeruginosa for the CF airway. Testing this hypothesis was particularly timely beause therapeutic activation of NKT cells with the exogenous glycolipids is currently being tested as a cancer treatment; and if NKT-cell activation were found to be deficient in patients with CF, a similar immunotherapy approach might be useful to help clear P. aeruginosa from the CF airway.17
We have specifically documented CFTR expression in human monocytes and DCs (Fig. 1), consistent with previous reports from studies in humans and mice that some monocytes/macrophages express CFTR at the mRNA and protein level.21,37 In addition, recent work by Xu and colleagues confirmed CFTR mRNA and protein expression in mouse DCs.38
In our model system we confirmed that glycolipid-mediated activation of NKT cells by DCs was dependent on organelle acidification. Pre-treatment of DCs with the well-characterized V-ATPase inhibitors, concanamycin A and bafilomycin, almost completely abolished NKT-cell responses to glycolipid antigens (Figs 2 and 3), as reported by other groups.13–16 In contrast, CFTR chloride channel dysfunction had no significant biological effect on the capacity of DCs to activate NKT cells following exposure to the classic glycolipid antigens, α-GalCer and GalGalCer (Figs 4 and 5). Although we found that the highest concentrations of CFTRinh-172 decreased NKT-cell activation by 10–30% (Fig. 4), these small changes are unlikely to be immunologically significant in vivo. Most importantly, we validated these findings in the human system and showed that DCs from patients with CF had no defects in their ability to activate NKT cells.
Why did we fail to support our original hypothesis? Why did functional CFTR deficiency fail to reduce the capacity of DCs to activate NKT cells following exposure to glycolipid antigens? The most likely answer is that CF is not associated with an immunologically relevant defect in organelle acidification. There is long-standing interest concerning the possibility of defective organellar acidification in CF cells (as recently reviewed in ref.39). Defective acidification of the trans-Golgi, endosomes and pre-lysosomes in CF was initially reported in 1991 by al-Awqati’s group.20 Since this initial provocative observation, multiple groups using an array of experimental approaches have both supported20–23 and refuted24–29 the observation that organelle acidification is defective in CF, and the debate continues. Rather than join in this divided biochemical debate, we investigated a clinically relevant immunological function that is dependent on endolysosomal acidification – namely, glycolipid-dependent activation of NKT cells by DCs. Our data demonstrate that the ability of DCs to activate NKT cells with glycolipid antigens was not dependent on CFTR function. Importantly, the results were similar regardless of whether CFTR dysfunction was achieved pharmacologically, using CFTRinh-172, or naturally, using DCs from CF patients with pathological CFTR mutations.
In conclusion, we have shown that DCs from patients with CF have no defect in their ability to activate NKT cells in response to classic glycolipid antigens. Therefore, it is unlikely that a failure to fully activate NKT cells contributes to the susceptibility of CF patients to develop chronic pulmonary infection with P. aeruginosa, and people living with CF are unlikely to benefit from therapies designed to augment NKT-cell activation.
Acknowledgments
We wish to acknowledge the contribution of all volunteer subjects and their families in providing blood samples. We thank the patients with CF and the CF Clinic Staff at BC Children’s Hospital and St Paul’s Hospital who generously facilitated sample acquisition. This work was funded by operating grants from the Canadian Cystic Fibrosis Foundation and BC Lung Association. S.E.T. was supported by a Career Development Award from the Canadian Child Health Clinician Scientist Program (CCHCSP) – a CIHR Strategic Training Program.
Disclosure
The authors declare no competing interests related to this manuscript.
References
- 1.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–82. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 2.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–9. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 3.Davies JC, Alton EW, Bush A. Cystic fibrosis. BMJ. 2007;335:1255–9. doi: 10.1136/bmj.39391.713229.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 5.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–17. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieuwenhuis EE, Matsumoto T, Exley M, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–93. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwenhuis EE, Matsumoto T, Lindenbergh D, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241–50. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spada FM, Borriello F, Sugita M, Watts GF, Koezuka Y, Porcelli SA. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur J Immunol. 2000;30:3468–77. doi: 10.1002/1521-4141(2000012)30:12<3468::AID-IMMU3468>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 10.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 11.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen D, Milland J, Mouhtouris E, Vaughan H, Pellicci DG, McConville MJ, Godfrey DI, Sandrin MS. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 2008;6:e172. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briken V, Moody DB, Porcelli SA. Diversification of CD1 proteins: sampling the lipid content of different cellular compartments. Semin Immunol. 2000;12:517–25. doi: 10.1006/smim.2000.0274. [DOI] [PubMed] [Google Scholar]
- 14.Speak AO, Cerundolo V, Platt FM. CD1d presentation of glycolipids. Immunol Cell Biol. 2008;86:588–97. doi: 10.1038/icb.2008.42. [DOI] [PubMed] [Google Scholar]
- 15.Prigozy TI, Naidenko O, Qasba P, et al. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–7. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 16.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 17.Motohashi S, Nakayama T. Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci. 2008;99:638–45. doi: 10.1111/j.1349-7006.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silk JD, Salio M, Brown J, Jones EY, Cerundolo V. Structural and functional aspects of lipid binding by CD1 molecules. Annu Rev Cell Dev Biol. 2008;24:369–95. doi: 10.1146/annurev.cellbio.24.110707.175359. [DOI] [PubMed] [Google Scholar]
- 19.Finlay BB, Hancock RE. Can innate immunity be enhanced to treat microbial infections? Nat Rev Microbiol. 2004;2:497–504. doi: 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]
- 20.Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991;352:70–3. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- 21.Di A, Brown ME, Deriy LV, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–44. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 22.Teichgraber V, Ulrich M, Endlich N, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14:382–91. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb RA, Dosanjh A. Mutant cystic fibrosis transmembrane conductance regulator inhibits acidification and apoptosis in C127 cells: possible relevance to cystic fibrosis. Proc Natl Acad Sci USA. 1996;93:3587–91. doi: 10.1073/pnas.93.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson GA, Hill WG, Weisz OA. Evidence against the acidification hypothesis in cystic fibrosis. Am J Physiol Cell Physiol. 2000;279:C1088–99. doi: 10.1152/ajpcell.2000.279.4.C1088. [DOI] [PubMed] [Google Scholar]
- 25.Haggie PM, Verkman AS. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J Biol Chem. 2007;282:31422–8. doi: 10.1074/jbc.M705296200. [DOI] [PubMed] [Google Scholar]
- 26.Lukacs GL, Chang XB, Kartner N, Rotstein OD, Riordan JR, Grinstein S. The cystic fibrosis transmembrane regulator is present and functional in endosomes. Role as a determinant of endosomal pH. J Biol Chem. 1992;267:14568–72. [PubMed] [Google Scholar]
- 27.Seksek O, Biwersi J, Verkman AS. Evidence against defective trans-Golgi acidification in cystic fibrosis. J Biol Chem. 1996;271:15542–8. doi: 10.1074/jbc.271.26.15542. [DOI] [PubMed] [Google Scholar]
- 28.Barriere H, Bagdany M, Bossard F, Okiyoneda T, Wojewodka G, Gruenert D, Radzioch D, Lukacs GL. Revisiting the role of cystic fibrosis transmembrane conductance regulator and counter-ion permeability in the pH regulation of endocytic organelles. Mol Biol Cell. 2009;20:3125–41. doi: 10.1091/mbc.E09-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haggie PM, Verkman AS. Unimpaired lysosomal acidification in respiratory epithelial cells in cystic fibrosis. J Biol Chem. 2009;284:7681–6. doi: 10.1074/jbc.M809161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirschfeld AF, Bettinger JA, Victor RE, et al. Prevalence of Toll-like receptor signalling defects in apparently healthy children who developed invasive pneumococcal infection. Clin Immunol. 2007;122:271–8. doi: 10.1016/j.clim.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 32.Yu KO, Im JS, Molano A, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci USA. 2005;102:3383–8. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishi T, Forgac M. The vacuolar (H+)-ATPases – nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 34.Huss M, Wieczorek H. Inhibitors of V-ATPases: old and new players. J Exp Biol. 2009;212:341–6. doi: 10.1242/jeb.024067. [DOI] [PubMed] [Google Scholar]
- 35.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–8. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62:360–7. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura K, Nakamura H, Trapnell BC, Chu CS, Dalemans W, Pavirani A, Lecocq JP, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991;19:5417–23. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Tertilt C, Krause A, Quadri LE, Crystal RG, Worgall S. Influence of the cystic fibrosis transmembrane conductance regulator on expression of lipid metabolism-related genes in dendritic cells. Respir Res. 2009;10:26. doi: 10.1186/1465-9921-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haggie PM, Verkman AS. Defective organellar acidification as a cause of cystic fibrosis lung disease: reexamination of a recurring hypothesis. Am J Physiol Lung Cell Mol Physiol. 2009;296:L859–67. doi: 10.1152/ajplung.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]