Abstract
In animal models of temporal lobe epilepsy (TLE), neurosteroid sensitivity of GABAA receptors on dentate granule cells (DGCs) is diminished; the molecular mechanism underlying this phenomenon remains unclear. The current study investigated a mechanism for loss of neurosteroid sensitivity of synaptic GABAA receptors in TLE. Synaptic currents recorded from DGCs of epileptic animals (epileptic DGCs) were less frequent, larger in amplitude, and less sensitive to allopregnanolone modulation than those recorded from DGCs of control animals (control DGCs). Synaptic currents recorded from epileptic DGCs were less sensitive to diazepam and had altered sensitivity to benzodiazepine inverse agonist RO 15-4513 (ethyl-8-azido-6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5α][1,4]benzodiazepine-3-carboxylate) and furosemide than those recorded from control DGCs. Properties of synaptic currents recorded from epileptic DGCs appeared similar to those of recombinant receptors containing the α4 subunit. Expression of the α4 subunit and its colocalization with the synaptic marker GAD65 was increased in epileptic DGCs. Location of the α4 subunit in relation to symmetric (inhibitory) synapses on soma and dendrites of control and epileptic DGCs was examined with postembedding immunogold electron microscopy. The α4 immunogold labeling was present more commonly within the synapse in epileptic DGCs compared with control DGCs, in which the subunit was extrasynaptic. These studies demonstrate that, in epileptic DGCs, the neurosteroid modulation of synaptic currents is diminished and α4 subunit-containing receptors are present at synapses and participate in synaptic transmission. These changes may facilitate seizures in epileptic animals.
Keywords: GABAA receptor α4 subunit, temporal lobe epilepsy, synapse, hippocampus, dentate granule cells, mIPSCs
Introduction
Temporal lobe epilepsy (TLE) is a common form of drug-refractory epilepsy characterized by recurrent spontaneous seizures arising from limbic structures. In many women with epilepsy, seizure frequency increases at ovulation and in the period just preceding menstruation; this exacerbation of seizures has been termed catamenial epilepsy (Herzog et al., 1997). Cyclical changes in the levels of neurosteroid allopregnanolone derived from progesterone modulate seizure frequency by acting on GABAA receptors (Smith et al., 1998b; Majewska, 1992; Kokate et al., 1999; Reddy et al., 2001; Herzog and Frye, 2003). GABAA receptors are altered in TLE such that they become less sensitive to neurosteroid modulation (Mtchedlishvili et al., 2001). The mechanism underlying reduced neurosteroid sensitivity of GABAA receptor remains unclear.
The GABAA receptor is a pentamer composed of subunit proteins derived from six gene families, α, β, γ, δ, ε, and π. Each receptor is believed to be composed of 2 α, 2 β, and a γ, δ, ε, or π subunits. The neurosteroid sensitivity of GABAA receptors is strongly influenced by their subunit composition, and the δ subunit containing receptors are particularly sensitive to neurosteroids (Mihalek et al., 1999; Wohlfarth et al., 2002). The expression of the δ subunit of GABAA receptors is diminished in dentate granule cells (DGCs) of epileptic rats (epileptic DGCs), and this leads to enhanced excitability (Peng et al., 2004). In addition to the δ subunit, increased expression of one of its partnering subunits, the α4 subunit, diminishes neurosteroid sensitivity of GABAA receptors. In experimental animals, rapid withdrawal from progesterone treatment increases the expression of the α4 subunit and diminishes neurosteroid sensitivity of GABAA receptors in hippocampal neurons (Smith et al., 1998a,b).
In hippocampal DGCs, GABAA receptors containing the α1 and γ2 subunits are present at synapses, in which they mediate synaptic inhibition, and those containing the α4 and δ subunits are present in the extrasynaptic membrane, in which they mediate tonic inhibition (Nusser et al., 1995; Stell and Mody, 2002; Wei et al., 2003; Sun et al., 2004). Synaptic currents mediated by α1 and γ2 subunits are also sensitive to neurosteroid modulation (Reith and Sillar, 1997; Brussaard et al., 1999; Wohlfarth et al., 2002), as well as currents mediated by α1-containing recombinant receptors (Bianchi et al., 2002). Previous studies on epileptic animals demonstrated that the properties of GABAergic synaptic transmission on DGCs of epileptic animals are distinct from those of control animals (Buhl et al., 1996; Cohen et al., 2003; Leroy et al., 2004). However, it is not known whether the sensitivity of synaptic inhibition to physiological concentrations of neurosteroids is diminished in epileptic animals. Furthermore, the changes in synaptic GABAA receptor subunits responsible for altered synaptic transmission have not been studied. Here, we demonstrate reduced neurosteroid sensitivity of synaptic currents recorded from DGCs of epileptic rats and altered location of the α4 subunit, which is located in inhibitory synapses on DGCs of epileptic animals but in the perisynaptic region in control animals.
Materials and Methods
Induction of TLE.
Male Sprague Dawley rats (175–250 g) were housed at 22°C, on a standard light/dark schedule with access to food and water ad libitum and handled according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and Use Guidelines and a protocol approved by the University of Virginia Animal Care and Use Committee. TLE was produced in experimental animals by continuous hippocampal stimulation method developed by Lothman et al. (1989). Animals were anesthetized with ketamine (50 mg/kg) and xylazine (40 mg/kg) and implanted with a pair of bipolar stimulating electrodes in the left posterior ventral hippocampus (in mm: anteroposterior, 3.6; mediolateral, 4.0; dorsoventral, 5.0 from dura; incisor bar at +5.0). After recovering for 1 week, the left hippocampus was stimulated with 10 s trains of 50 Hz, 1 ms, 400 mA biphasic square wave current pulses delivered every 13 s for 90 min to induce status epilepticus (Lothman et al., 1989). At approximately 4–6 weeks after stimulation, animals developed spontaneous limbic seizures with motor component. For this study, seizures were documented by either direct observation of the behavioral seizures continuously or EEG recording, and epileptic animals were killed at least 24 h after the last seizure (Bertram et al., 1997).
Acquisition and analysis of miniature IPSCs from DGCs.
Animals were anesthetized with halothane before decapitation. Brains were dissected free and immersed in cold (2–4°C) artificial CSF (ACSF) saturated with 95%O2–5%CO2. The ACSF consisted of the following (in mm): 127 NaCl, 2 KCl, 1.5 CaCl2, 1.5 MgSO4, 25.7 NaHCO3, 1.1 KH2PO4, and 10 glucose (osmolarity, 300–305 mOsm). The brains were mounted on a vibratome stage (Camden Instruments, Loughborough, UK), and 300-μm-thick horizontal sections containing the ventral hippocampus were cut. Slices were maintained in continuously oxygenated ASCF at 32°C in a holding chamber for 30–45 min and then at room temperature in a recording chamber mounted on the stage of an Olympus Optical (Tokyo, Japan) BX51 microscope equipped with a 40× water-immersion objective, infrared differential interference contrast optics, and video. Patch electrodes (final resistances of 6–8 MΩ) were pulled from borosilicate glass (Sutter Instruments, Novato, CA) on a horizontal Flaming-Brown microelectrode puller (model P-97; Sutter Instruments), using a two-stage pull protocol. Electrode tips were filled with a filtered internal recording solution consisting of the following (in mm): 153.3 CsCl, 1.0 MgCl2, 10.0 HEPES, and 5.0 EGTA, pH 7.2 (with CsOH) (osmolarity, 285–295 mOsm). The electrode shank contained the following (in mm): 3 ATP Mg2+ salt and 0.1 GTP Na+ salt. Neurons were voltage clamped to −65 mV with Axopatch 200B amplifier (Molecular Devices, Palo Alto, CA). Whole-cell capacitance and series resistance were compensated by 80% at 10 μs lag. Recording was performed when series resistance after compensation was 20 MΩ or less. Access resistance was monitored with a 10 ms, −5 mV test pulse once every 2 min, and, if the series resistance increased by 25% at any time during the experiment, then the recording was terminated. Currents were filtered at 5 kHz, then digitized at 106 Hz using a Digidata 1322 digitizer, and acquired using Axoscope 8.0 software (Molecular Devices) on an IBM PC-compatible computer hard drive.
The digitized current traces were analyzed with Mini Analysis software (Synaptosoft, Leonia NJ). The detection threshold was set at five times of root mean square. After detection, decay time constants and peak amplitude were analyzed in individual miniature IPSCs (mIPSCs). The decay was analyzed in 10–90% of the peak amplitude, and 100 iterations were used for each event. The mIPSCs could be fitted with double-exponential decay time constants. The weighted decay time constant (τw) was then calculated with following equation: τw = τ1 A1 + τ2 (1 − A1), where τ1 and τ2 are fast and slow decay time constants (first and second exponential functions), and A1 is the proportion of the peak amplitude of the first exponential function. Weighted decay time constants were compared with Student's t test (Prism; GraphPad Software, Mountain View, CA).
Western blot.
For Western blot analysis, nine epileptic animals and nine age-matched controls were decapitated, the brains removed, and the hippocampi were dissected and homogenized as described previously (Smith et al., 1998a). Hippocampal proteins were subjected to electrophoresis on 10% SDS polyacrylamide gels and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA) (Towbin et al., 1979). The α4 subunit of the GABAA receptor was detected with a rabbit antibody against a peptide (amino acids, 1–14) of rat α4 as a 67 kDa band. Membranes were incubated with a 1:1000 dilution of the antibody for 16 h at 4°C on a shaker and then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) at a dilution of 1:25,000 for 1 h at room temperature. Then, the membranes were incubated with an enhanced chemiluminescence (ECL; Pierce, Rockford, IL) system and detected with a CCD camera and Bio-Rad Fluor-S MultiImager driven by Quantity One-4.0.1 (Bio-Rad). The results were standardized to the control protein (β-actin, 42 kDa) and were then expressed as a ratio of the average optical density of control values. No signal was detected when α4 antiserum was omitted.
Immunohistochemistry.
Eight epileptic and eight age-matched control animals were perfused and processed in parallel. The procedures of tissue preparation for immunohistochemistry have been described in detail previously (Sun et al., 2004). Briefly, animals were anesthetized with an overdose of pentobarbitone sodium and perfused through the ascending aorta with 50–100 ml of 0.9% NaCl, followed by 350–450 ml of 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4. For the gephyrin immunohistochemistry, two epileptic animals and two control were perfused with 2% paraformaldehyde. The brains were removed and postfixed in the same fixative for 2 h at 4°C. After overnight incubation in 25% sucrose in 0.1 m PB for cryoprotection, the brains were separated into two hemispheres, and the right hemisphere was blocked at 2 mm posterior to optic tract on a coronal matrix. The left hemisphere was not studied because electrodes were implanted into the left hippocampus. The brains were then frozen by immersion in −70°C isopentane and blocked. The posterior block was sectioned horizontally at 40 μm. Sections of posterior block were collected from 1 mm above the posterior commissure (horizontal section, bregma −4.1 mm) to just beneath the anterior commissure (bregma −6.82 mm). Sections were put into eight vials sequentially, and six to eight ventral sections from each animal were used for double labeling for the α4 subunit (5 μg/ml) and presynaptic marker GAD65 (1.5 μg/ml, clone GAD-6, MAB351R; Chemicon, Temecula, CA) or postsynaptic marker gephyrin (2.5 g/ml, clone 45; BD Biosciences, Franklin Lakes, NJ).
Sections were processed for free-floating immunohistochemistry, as described previously in detail (Sun et al., 2004). After 3 washes in 0.1 m PB, sections were incubated with blocking solution containing 5% normal goat serum (NGS) and 2% bovine serum albumin (BSA) (Jackson ImmunoResearch) and 0.3% Triton X-100 in 0.1 m PBS, pH 7.4, for 1 h. Then, tissue sections were incubated with a mixture of primary antibodies (rabbit anti-α4 subunit of GABAA receptor and mouse anti-GAD65 or gephyrin) at 4°C for 72 h on a shaker.
Subsequently, the sections were incubated in goat anti-rabbit or goat anti-mouse secondary antibodies conjugated with Alexa Fluor 488 or 594 (5 μg/ml; Invitrogen, Carlsbad, CA), respectively, for 1 h on a shaker at room temperature in darkness. All antibodies were diluted with a solution containing 1% NGS and 1% BSA in 0.1 m PBS. Sections were rinsed six times for 10 min each after incubation with primary and secondary antibodies. Sections were then mounted on slides with Gel/Mount (Biomeda, Foster City, CA) and air dried. Each coverslip was sealed with clear nail polish, and slides were stored at −20°C. All chemicals were obtained from Sigma (St. Louis, MO) except when noted.
Sections were studied on a Nikon (Melville, NY) PCM2000 confocal microscope system as described previously in detail (Sun et al., 2004). Confocal laser scanning microscopy was performed at the W. M. Keck Center for Cellular Imaging at the University of Virginia. A Nikon TE-200 inverted epifluorescence microscope (Nikon) was equipped with an argon and a helium–neon laser. Confocal and camera-based image acquisition and processing were driven by SimplePCI software (version 4.0.6; Compix, Cranberry Township, PA). Argon–ion and helium–neon lasers were used to visualize the fluorochromes Fluoro-Jade B (or Alexa Fluor 488) and Alexa Fluor 594, respectively. The intensity of each laser was optimized for the sections of each animal and each combination of antibodies. The parameters were kept constant for each animal. Controls included dual-channel recording of sections without antigen labeling and single antigen labeling to detect nonspecific staining and bleedthrough phenomena.
The dentate granule cell layer of hippocampus was scanned and captured with a high-magnification objective lens (100×, numerical aperture 1.4). The immunoreactive area of a randomly chosen region (70 × 70 μm) of the upper blade of the dentate granule cell layer was quantified using MetaMorph 6.03 software (Universal Imaging, Downingtown, PA). The same threshold was applied to all images acquired from the sections of one brain in each experiment. The ratio of colocalization area of GABAA receptor subunit α4 and GAD65 versus total GABAA receptor α4 was compared between epileptic and control animals using Prism 4.0 software (GraphPad Software). Unless otherwise specified, all values are reported as mean ± SEM, and unpaired two-tailed t test was used for comparisons.
Postembedding electron microscopy.
Six epileptic and six age-matched control animals were anesthetized with an overdose of pentobarbitone sodium, and the brain was perfused with 100–150 ml of Tyrode's solution (composition: 137 mm NaCl, 2 mm KCl, 0.9 mm CaCl2, 1.2 mm MgCl2, 11.9 mm NaHCO3, 0.4 mm NaH2PO4, 5.5 mm glucose, 281 mOsm, pH 7.4) via the left ventricle (Heck et al., 2002) until the effluent was clear. A fixative consisting of 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 m PB was then perfused. Brains were removed from skulls and postfixed in 4% paraformaldehyde for 2 h at 4°C, blocked, and then cut in PB with a vibratome at a thickness of 50 μm. Sections were treated with 0.1% NaBH4 at room temperature for 30 min, rinsed three times for 5 min each in PB, and osmicated for 30 min with 1% osmium tetroxide in PB at room temperature. After being rinsed three times for 5 min each with PB, they were dehydrated with 50, 70, and 90% ethanol for 5 min each, and 100% ethanol three times for 5 min each. After treating with acetone three times for 10 min each, a 1:1 mixture of acetone and Epon for 2–4 h or overnight, and Epon for 2–4 h or overnight sequentially, sections were flattened between two sheets of Aclar film-embedded in Epon resin (Electron Microscopy Sciences, Fort Washington, PA). After resin polymerization, small sections including the molecular layer, granule cell layer, and part of the hilus were dissected from the ventral hippocampus and mounted on capsules.
The immunohistochemistry procedure was performed by floating the grids with the tissue face down in drops of solutions on silicone rubber grid pads. Ultrathin sections collected on Formvar-coated nickel grids were treated following a modified previously described postembedding immunogold procedure to label profiles for GABAA receptor (Heck et al., 2002). Briefly, the grids were incubated with 4% 1,4-phenylenediamine dihydrochloride in Tris-buffered saline with 0.1% Triton X-100 (TBST), pH 7.4, for 1–2 min and then rinsed with TBST for 5 min. The grids containing ultrathin sections were incubated with a rabbit anti-GABAA receptor subunit α4 at a concentration of 40 μg/ml overnight (gift from Dr. Werner Sieghart, Medical University of Vienna, Vienna, Austria). They were then rinsed with TBST (two times for 5 min, pH 7.4, and 5 min in TBST, pH 8.2) and incubated with a 1:30 dilution of goat anti-rabbit IgG conjugated to gold particles (GAR15; Ted Pella, Redding, CA) in TBST at pH 8.2 for 1 h at room temperature. After this, sections were rinsed in TBST, pH 7.4, twice, rinsed with distilled water twice, and then fixed in 2% glutaraldehyde (EM grade; Electron Microscopy Sciences) diluted in distilled water for 10 min. Some sections were counterstained with uranyl acetate in methanol and 2% lead citrate. Grids were examined on Jeol (Peabody, MA) JEM 1010 microscope, and images were captured by a 16 megapixel SIA-12C (sia-cam.com) digital camera coupled with MaxIm DL CCD software (Diffraction, Ottawa, Ontario, Canada).
Quantitative analysis was performed on randomly selected grids of α4 subunit-labeled synaptic profiles within the granule cell layer and inner molecular layer, respectively. Only symmetric synapses were captured and analyzed in this study, and symmetric synapses were identified using the following criteria. (1) The apposed plasma membranes of the two neuronal components were parallel to each other but separated by a cleft. (2) There was an associated density in the adjacent cytoplasm; one of the components contained a concentration of synaptic vesicles (usually pleomorphic) close to the synapse, and the other one lacked a pronounced postsynaptic density. The distribution of the α4 subunit in symmetric synapses in control and epileptic DGCs was studied quantitatively using the methods of Zhang et al. (2007); however, synapses were considered labeled with α4 immune particles only if two or more gold particles were present at or around a synapse. The localization of synaptic ends was defined at the points where presynaptic and postsynaptic membrane were no longer parallel and the synaptic cleft disappeared. The distribution of α4 immunogold particles was classified as one of the following (as illustrated in Fig. 7E): (1) within the central third (label 1), (2) within the outer third (label 2), (3) within 40 nm of the ends of synapses that were considered perisynaptic (label 3), and (4) extrasynaptic, located 40–100 nm from the end of synapses along the adjacent membrane (label 4). Gold particles within the center third and outer third were classified as synaptic labeling, whereas those within perisynaptic and extrasynaptic were classified as extrasynaptic labeling. The number of synapses with synaptic α4 labeling in control and epileptic animals was analyzed using the χ2 test.
Figure 7.

Postembedding immunogold electron microscopy of the α4 subunit of the GABAA receptor in the hippocampal DGC layer from epileptic and control animals. The α4-immunoreactive gold particles were found close to or at the end of symmetric axosomatic synapses in the hippocampal DGC layer from control animals (A, C). However, some α4 subunit-immunoreactive particles were also found at the symmetric synapses made of terminals (T) and granule cell soma in hippocampal DGC layer from epileptic animals (B, D). Arrowheads in A–D indicate the center of synapses. Scale bar: A–D, 100 nm. E, A representation of the classification of subdivisions of the synaptic and extrasynaptic regions: central third (1), outer third (2), perisynaptic (3), and extrasynaptic (4) used for quantitative analysis in the present study. F, The number of immunogold particles found in center and outer third of synapses in epileptic DGCs was approximately twice as that found in controls. However, there were fewer immunogold particles in perisynaptic and extrasynaptic regions in epileptic DGCs. G, Immunogold particles in regions 1–4 expressed as a percentage fraction of total number of gold particles in symmetric synapses in control and epileptic DGCs. *p < 0.05, χ2 test.
The contrast and brightness of the digital images was adjusted for final production with PhotoShop 6.0 (Adobe Systems, San Jose, CA).
Results
Altered inhibitory synaptic transmission in DGCs of epileptic rats
To compare the effect of allopregnanolone on synaptic GABAA receptors in epileptic DGCs with that in control DGCs, we first characterized the mIPSCs recorded from these two groups of animals by blocking all action potentials and ionotropic glutamate receptors (Fig. 1A). Synaptic receptors are activated by the release of the contents of a single GABA-containing vesicle from a presynaptic terminal, which generates an mIPSC. The mean of median amplitudes of mIPSCs recorded from DGCs in TLE (121.9 ± 7.6 pA; n = 8 cells, 3 animals) was 105% larger than that in control cells (59.29 ± 4.1 pA; n = 15 cells, 4 animals; p < 0.0001). The frequency of mIPSCs recorded from epileptic DGCs (0.72 ± 0.09 Hz; n = 25 cells, 4 animals) was 74.4% of that recorded from control DGCs recorded from age-matched controls (2.04 ± 0.2 Hz; n = 32 cells, 7 animals; p < 0.001). Finally, the mean weighted decay time constant of mIPSCs was 24.4 ± 2.9 ms in control DGCs (n = 15) and 21.3 ± 1.1 ms in epileptic DGCs (n = 8) (Fig. 1B, p > 0.05). We then tested whether synaptic receptors in epileptic DGCs were less sensitive to neurosteroid allopregnanolone.
Figure 1.
Kinetic properties of mIPSCs were significantly altered in epileptic DGCs. A, Typical recordings of mIPSCs from a control DGC (top 3 traces) and an epileptic DGC (bottom 3 traces). B, Cumulative fraction plots of interevent intervals of mIPSCs recorded from a control (solid line) and an epileptic (dotted line) DGC, demonstrating reduced frequency of mIPSCs in epileptic DGC. C, Averaged mIPSCs recorded from control (black trace) and epileptic (gray trace) DGCs. In epileptic DGCs, the amplitude of mIPSCs was increased. D, Cumulative fraction plots of amplitudes of mIPSCs recorded from a control (solid line) and an epileptic (dotted line) DGC, demonstrating increased amplitude of mIPSCs in epileptic DGC.
Loss of high-affinity response to allopregnanolone
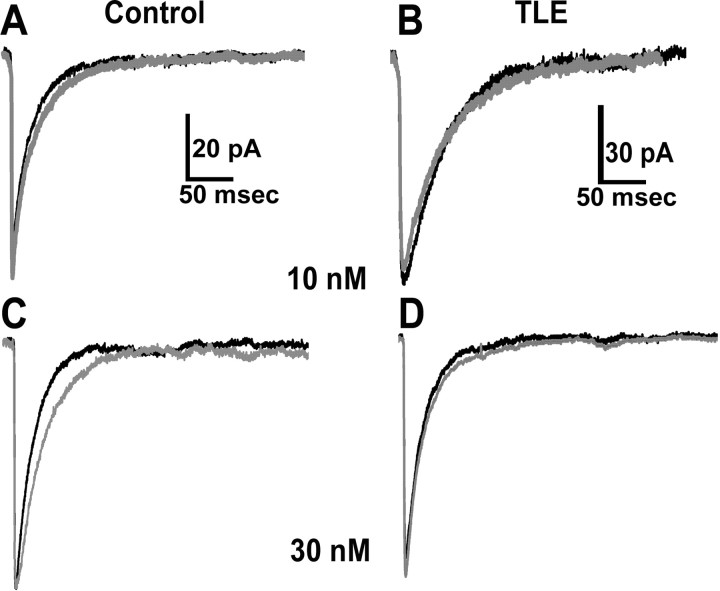
Allopregnanolone (10 nm) was bath applied to DGCs in slices from control and epileptic animals. In control DGCs (n = 7 cells from 3 animals), 10 nm allopregnanolone significantly prolonged the decay time constant of mIPSCs (Fig. 2A, Table 1), whereas in epileptic DGCs (n = 5 cells from 3 animals), it did not prolong the decay of mIPSCs (Fig. 2B, Table 1). A similar effect was observed when the effect of 30 nm allopregnanolone was studied. It caused prolongation of mIPSC decay recorded from control (n = 12 cells from 3 animals) (Fig. 2C, Table 1), but it did not change the decay of mIPSCs recorded from epileptic DGCs (n = 5 cells from 3 animals) (Fig. 2D, Table 1). Effect of 10 and 30 nm allopregnanolone on mIPSC amplitude was studied by comparing means and cumulative frequency plots of amplitudes before and after application of the drug in control and epileptic DGCs. Allopregnanolone (10 or 30 nm) did not enhance mIPSC amplitude in control or epileptic DGCs (Fig. 2, Table 1).
Figure 2.
Allopregnanolone (10 and 30 nm) prolonged decay of mIPSCs recorded from control DGCs (A, C) but did not alter decay of mIPSCs recorded from epileptic DGCs (B, D). In this and subsequent figures, the black trace of averaged mIPSCs represent recordings in ACSF, and gray trace represents recordings after bath application of drug. Note that allopregnanolone did not alter amplitude of mIPSCs recorded from control or epileptic DGCs (see Table 1).
Table 1.
Drug modulation of mIPSCs recorded from control and epileptic DGCs
| Control | TLE | ||||
|---|---|---|---|---|---|
| Allopregnanolone | 10 nm | Baseline (n = 7) | Drug | Baseline (n = 5) | Drug |
| τ | 21.7 ± 2.4 | 27.4 ± 2.0** | 21.9 ± 1.7 | 20.9 ± 0.8 | |
| ampl | 39.95 ± 2.7 | 39.33 ± 7.7 | 57.12 ± 8.01 | 58.42 ± 13.6 | |
| 30 nm | (n = 12) | (n = 5) | |||
| τ | 22.1 ± 3.3 | 40.9 ± 3.5** | 19.1 ± 1.9 | 20.0 ± 3.19 | |
| ampl | 33.82 ± 1.4 | 38.55 ± 1.2 | 47.41 ± 2.4 | 54.66 ± 8.6 | |
| 100 nm | (n = 7) | (n = 4) | |||
| τ | 27.8 ± 4.7 | 46.1 ± 2.2** | 35.6 ± 1.5 | 42.1 ± 2.2* | |
| ampl | 37.37 ± 3.8 | 51.43 ± 5.9** | 65.3 ± 4.2 | 90.5 ± 11.1** | |
| Diazepam | 30 nm | Naive (n = 6) | TLE (n = 6) | ||
| τ | 13.9 ± 2.2 | 22.4 ± 2.3** | 24.9 ± 1.4 | 22.1 ± 1.06 | |
| ampl | 62.06 ± 12.5 | 92.32 ± 13.8** | 92.08 ± 2.3 | 90.83 ± 1.6 | |
| RO15-4513 | 300 nm | Naive (n = 7) | TLE (n = 8) | ||
| τ | 23.9 ± 1.0 | 22.5 ± 1.3 | 23.7 ± 1.8 | 29.4 ± 1.9* | |
| ampl | 43.2 ± 1.1 | 37.5 ± 0.6* | 61.83 ± 1.8 | 85.50 ± 1.2** | |
| Furosemide | 100 μm | Naive (n = 8) | TLE (n = 6) | ||
| τ | 24.3 ± 1.8 | 27.4 ± 1.6 | 38.1 ± 1.3 | 33.4 ± 1.1 | |
| ampl | 55.8 ± 0.5 | 61.2 ± 0.7 | 59.1 ± 2.3 | 38.2 ± 1.5** | |
*p < 0.05;
**p < 0.001.
There are two binding sites for neurosteroids on GABAA receptors: low concentrations of neurosteroids such as allopregnanolone promote GABA binding to the receptors, whereas high concentrations of the drug directly activate the receptors (Hosie et al., 2006). We tested whether sensitivity to a higher concentration allopregnanolone was preserved in mIPSCs recorded from epileptic DGCs. A higher concentration of allopregnanolone (100 nm) significantly prolonged decay time constants of mIPSCs recorded from both control (n = 7 cells from 3 animals) and epileptic (n = 5 cells from 3 animals) DGCs. Allopregnanolone increased the amplitude of mIPSCs in both control and epileptic DGCs (Table 1).
Switch from classical benzodiazepine pharmacology to a unique profile
Altered decay and reduced sensitivity of synaptic currents to allopregnanolone further suggested that GABAA receptors on postsynaptic membrane had been altered. Increased expression of the α4 subunit has been demonstrated previously to reduce allopregnanolone sensitivity of GABAA receptors in hippocampal neurons (Smith et al., 1998a,b). The α4 subunit-containing receptors have unique benzodiazepine pharmacology. They are insensitive to the classical, broadly active benzodiazepine, diazepam. However, the imidazobenzodiazepine, RO 15-4513 (ethyl-8-azido-6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5α][1,4]benzodiazepine-3-carboxylate), is a partial agonist at the benzodiazepine-insensitive, α4 and γ2 subunit-containing receptors, and it acts as an inverse agonist at receptors with classical benzodiazepine pharmacology (α1βxγ2, α2βxγ2, and α5βxγ2) (Wafford et al., 1996). Therefore, we compared the actions of diazepam and RO 15-4513 on mIPSCs recorded from control and epileptic animals.
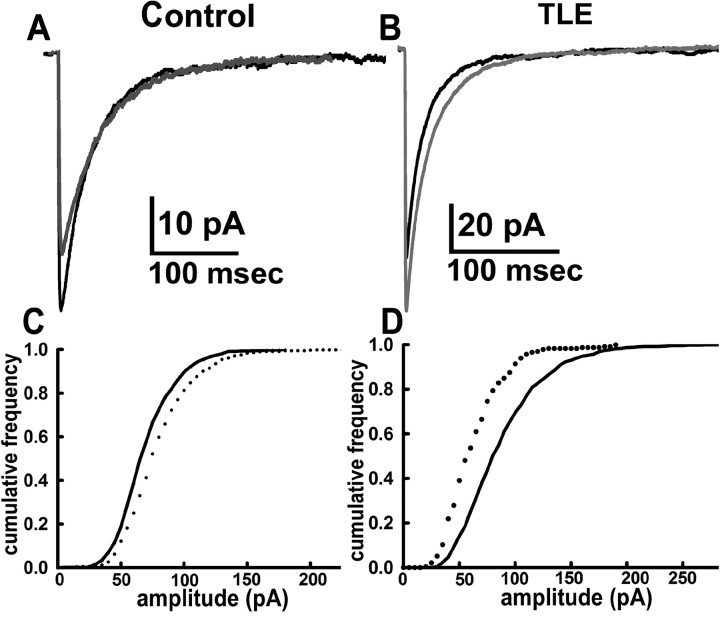
In control DGCs, mIPSCs have a classical response to the benzodiazepine diazepam and are strongly potentiated. In control DGCs, 30 nm diazepam prolonged the mIPSC decay time constants (n = 6 cells from 3 animals) (Table 1). The amplitude of mIPSCs was increased by 48% (Fig. 3A,C, Table 1). In contrast, in epileptic DGCs (n = 6 cells from 3 animals), diazepam (30 nm) did not prolong the decay and had no effect on amplitude (Fig. 3B,D, Table 1).
Figure 3.
Diazepam (30 nm) strongly potentiated amplitude and decay of mIPSCs recorded from control DGC (A), whereas it had no effect on mIPSCs recorded from an epileptic DGC (B). Cumulative fraction plots of distribution of mIPSC amplitudes derived from six control and six epileptic DGCs before (black line) and after (dotted lines) application of drug indicate that diazepam caused a leftward shift in control (C) but not in epileptic (D) DGCs.
We tested whether the response to RO 15-4513 had also changed. In control DGCs, 300 nm RO 15-4513 inhibited mIPSCs, whereas it potentiated them in epileptic DGCs. In control DGCs (n = 7 cells from 3 animals), RO 15-4513 diminished mIPSC amplitude by 15% without any effect on decay (Fig. 4A,C, Table 1), whereas in epileptic DGCs, it potentiated amplitude of mIPSCs by 38% and prolonged decay (n = 8 cells from 3 animals) (Fig. 4B,D, Table 1).
Figure 4.
Averaged trace of mIPSCs recorded from a control (A) and an epileptic (B) DGC before and after bath application of 300 nm RO 15-4513. RO 15-4513 did not alter decay time constants, and the median amplitude decreased slightly in control DGC (A), whereas in an epileptic DGC (B), it significantly prolonged slow decay time constant and increased peak amplitude. Cumulative fraction plots of mIPSC amplitude data from seven control DGCs (C) and eight epileptic DGCs (D) before (black line) and after (dotted line) application of 300 nm RO 15-4513; the drug reduced mIPSC amplitude in control DGCs but increased it in epileptic DGCs.
Furosemide inhibits mIPSCs in epileptic DGCs in TLE
Loss of diazepam sensitivity and inversion of response to RO 15-4513 in epileptic DGCs could be explained by the expression of α4 subunit-containing receptors at inhibitory synapses; therefore, we further tested pharmacology of mIPSCs for the presence of receptors containing this subunit. Furosemide inhibits α4 subunit-containing GABAA receptors with high affinity (Wafford et al., 1996). In control DGCs, furosemide (100 μm) did not have any inhibitory effect on mIPSCs. There was a slight, but statistically insignificant, increase of the amplitude of mIPSCs. Decay of mIPSCs was not affected (n = 8 cells from 3 animals) (Fig. 5A,C). This concentration of furosemide inhibited mIPSCs recorded from epileptic DGCs (n = 7 cells from 3 animals) as demonstrated by a 21% reduction in amplitude and faster decay (Fig. 5C,D, Table 1).
Figure 5.
Averaged mIPSC traces demonstrate that furosemide (100 μm) had no effect on mIPSCs recorded from control DGCs (A), but it inhibited mIPSCs recorded from epileptic DGCs (B). Cumulative fraction plot of mIPSC amplitudes recorded from eight control (C) and seven epileptic (D) DGCs before (black line) and after (dotted line) drug application.
Distribution and expression of the α4 subunit
Altered pharmacological properties of mIPSCs suggested the presence of α4 subunit-containing receptors at GABAergic synapses in epileptic DGCs. Previous studies have suggested that the expression of α4 subunit mRNA and protein is increased in DGCs in other animal models of TLE. Therefore, we tested whether α4 subunit expressions was increased in DGCs in our model by semiquantitative immunohistochemistry and Western blot analysis.
In control animals, the α4 immunoreactivity (IR) was distributed diffusely over the dentate gyrus, with higher staining intensity over the dentate granule cell layer than the molecular layer. In contrast, IR in epileptic animals for the α4 subunit appeared more intense in the granule cell layer; however, molecular layer intensity was similar. This was confirmed by comparing optical density of α4-IR in images obtained from hippocampal sections taken from epileptic animals and age-matched controls, which were processed in parallel from perfusion to acquisition of images on confocal laser microscope (for details, see Materials and Methods). The IR was more intense in the granule cell layer of TLE (117.2 ± 3.77; n = 30 sections from 6 animals) (supplemental figure B,D,E, available at www.jneurosci.org as supplemental material) than that in control (106.3 ± 3.27; n = 27 sections from 6 animals; t test, p < 0.05) (supplemental figure A,C,E, available at www.jneurosci.org as supplemental material). No difference was found in the molecular layer between epileptic (80.5 ± 3.44; n = 30) and control (82.3 ± 3.56; n = 30; Student's t tests, p > 0.05) animals (supplemental figure E, available at www.jneurosci.org as supplemental material). When the data from two regions were combined, no significant difference was found in dentate gyrus from epileptic animals (98.82 ± 3.481; n = 60 sections from 6 animals) and control (95.98 ± 3.049; n = 60 sections from 6 animals). To further confirm the relative changes in subunit expression in the hippocampus, a Western blot analysis of α4 subunit expression in the hippocampus of control and epileptic animals was performed. Rabbit antibody directed against the α4 subunit of GABAA receptor revealed a 67 kDa band on PVDF membrane (supplemental figure F, available at www.jneurosci.org as supplemental material). The data were corrected with β-actin as an intrinsic control. The α4 subunit protein levels in the hippocampi of the epileptic animals (0.53 ± 0.05; n = 9) and age-matched controls (0.57 ± 0.09; n = 9) were similar (Student's t test, p > 0.05).
Increased colocalization of the α4 subunit with presynaptic marker GAD65 in epileptic animals
These studies demonstrated a modest increase in α4 subunit expression in the dentate granule cell layer. Electrophysiological studies suggested the participation of α4 subunit-containing receptors in synaptic transmission; therefore, we investigated whether this subunit was present at inhibitory synapses at epileptic DGCs. The synaptic and extrasynaptic localization of GABAA receptors has been indirectly inferred by using a combination of double-label immunohistochemistry and high-resolution confocal laser microscopy in the past (Sun et al., 2004). We used this method to initially test whether the α4 subunit-containing receptors were present at inhibitory synapses.
In high-resolution images of hippocampal sections from control animals, the α4-IR was in the form of small diffusely distributed clusters (Fig. 6A,D,G,J). The distribution of GAD65 was clustered as shown in Figure 6, B and E. In sections from control animals, the GAD65 clusters rarely colocalized with the α4 subunit-IR. In sections from epileptic animals, there were some clusters of α4-IR colocalized with GAD65 in addition to diffusely distributed small clusters. Merged images demonstrated some instances of α4 subunit clusters colocalization with GAD65 clusters (Fig. 6C,F). Quantitative analysis demonstrated that the colocalization area of GAD65 and α4-IR represented as a fraction of total α4-IR area was more in sections from epileptic animals (3.37 ± 0.3656; n = 31 sections, from 6 animals) than that in controls (2.24 ± 0.1716; n = 27, from 5 control animals; Student's t test, p < 0.05). Colocalization of α4-IR with another marker of GABAergic synapses, gephyrin, was also examined. Gephyrin-immunoreactive clusters were present surrounding the cell soma of control and epileptic DGCs (Fig. 6H,K). In control DGCs, the α4-immunoreactive clusters occasionally colocalized with that of gephyrin-IR (Fig. 6I), whereas in epileptic DGCs, such colocalization was more (Fig. 6L).
Figure 6.
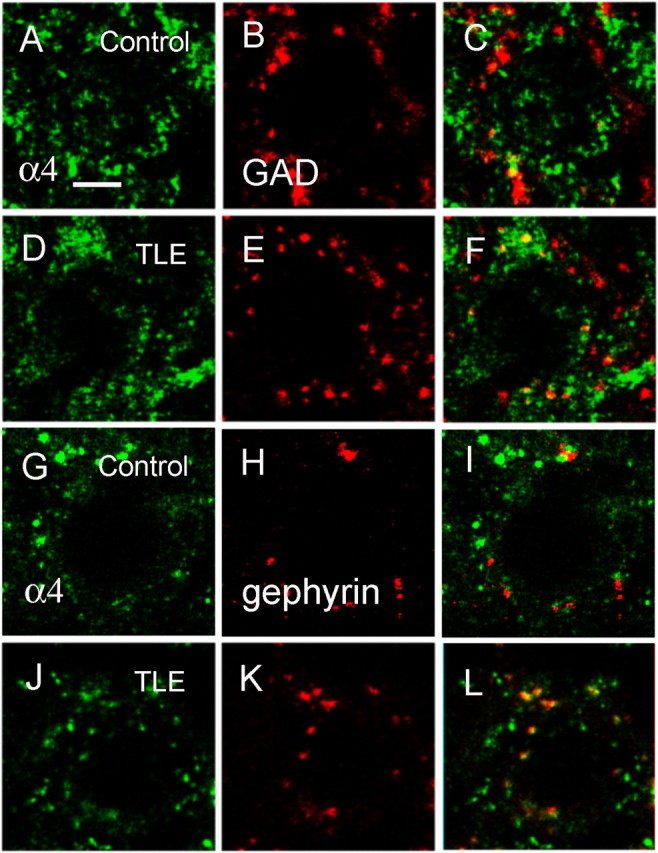
There were more clusters of the α4 subunit of the GABAA receptor colocalized with synaptic makers (GAD65 and gephyrin) in epileptic DGCs than in controls. The DGCs were double labeled with the α4 subunit of the GABAA receptor (A, D, G, J) and the presynaptic marker GAD65 (B, E) or the postsynaptic marker gephyrin (H, K). The subunit was visualized by secondary antibody conjugated with Alexa Fluor 488 (green), and synaptic markers were visualized with Alexa Fluor 594 (red). C, F, I, and L are merged images of the subunit and synaptic markers. Scale bar, 5 μm.
Electrophysiological and immunohistochemical studies suggested that some α4 subunits might have relocated from perisynaptic or extrasynaptic sites to synapses in epileptic animals. However, confocal laser microscopy cannot fully resolve synapses. To directly evaluate inhibitory synapses, the subcellular location of α4 subunit was examined with postembedding immunogold electron microscopy of symmetric synapses in the perisomatic region and dendrites of control and epileptic DGCs.
α4 subunits on symmetric synapses in the perisomatic region of DGCs of epileptic animals
The α4 subunit labeling was found in the extrasynaptic membrane around symmetric synapses (Fig. 7A), or close to the edge of the apposition between presynaptic terminal and postsynaptic membrane (Fig. 7C). Symmetric synapses rarely contained the α4 subunit in the center third of the synaptic profile. The α4 subunit immunogold-labeled particles were also found in cytoplasm, which perhaps represent subunit protein in traffic. A similar distribution of α4 subunit in perisynaptic region of symmetric synapses was recently described in mice (Liang et al., 2006; Zhang et al., 2007). In epileptic animals, many more α4 subunit-labeled immunogold particles were in the symmetric GABAergic synapses (Fig. 7B,D). A few α4 subunit immunogold particles were located on the outer third of the synapses (Fig. 7D).
The distribution of α4 subunit in perisomatic symmetric synapses in control and epileptic DGCs was studied quantitatively using methods of Zhang et al. (2007), but synapses were considered labeled with α4 immune particles only if two gold particles were present at or around a synapse. In control DGCs from three animals, 56 of 61 (92.8%) synapses were labeled with α4 subunit gold particles, and, in epileptic DGCs from three animals, 51 of 58 (88%) were labeled. In control DGCs, there were a total of 189 gold particles in 56 perisomatic synapses, whereas in epileptic DGCs, there were 204 gold particles in 51 synapses. The number of gold particles per synapse in control and epileptic animals was similar (control, 3.38 ± 0.27 vs epileptic, 4.04 ± 0.31; unpaired t test, p > 0.05).
In control DGCs, 12.9% of gold particles were in the central synapse, 16 0.9% in the outer third synaptic membrane, 24.9% perisynaptic, and 45.5% extrasynaptic. In epileptic DGCs, the fraction of gold particles in the center of the synapse was increased to 23.5%, and that in the outer third of the synapse was also increased to 32.8%. The fraction remained unchanged perisynaptic region (18.2%), and it was diminished in extrasynaptic region to 25.5% (Fig. 7F,G). Thus, compared with control, a larger faction of gold particles was present within synapses in perisomatic symmetric synapses on epileptic DGCs.
α4 subunit distribution on symmetric synapses of proximal dendrites of the dentate granule cells in the inner molecular layer
Synapses on proximal dendrites of control and epileptic DGCs were also studied by postembedding electron microscopy. Symmetric synapses were more sparsely distributed in the molecular layer and interspersed with asymmetric synapses. In control DGCs from three animals, 59 of 68 (87%) synapses were labeled with α4 subunit gold particles, and, in epileptic DGCs from three animals, 62 of 73 (85%) were labeled. In control DGCs, there were a total of 165 gold particles in 59 dendritic synapses, whereas in epileptic DGCs, there were 261 gold particles in 62 synapses. There were more gold particles per synapse in epileptic DGCs (2.81 ± 0.21 vs 4.21 ± 0.32; unpaired Student's t test, p < 0.05).
In control DGCs, α4 subunit immunogold particles were commonly present in the extrasynaptic membrane, 26.7% in perisynaptic region and 35.8% extrasynaptic region. Within the synapse, 6.4% of the particles were in the central synapse and 31.5% in the outer third (Fig. 8A,C). In epileptic DGCs, the distribution was changed with more gold particles in the central (20.3%) and outer third of the synapse (43.3%) (Fig. 8B,D). The fraction of perisynaptic (14.6%) and extrasynaptic (21.4%) gold particles was diminished in epileptic DGCs (Fig. 8E,F). Thus, compared with control DGCs, a larger faction of gold particles was present within synapses, and a smaller fraction was present in the extrasynaptic and perisynaptic regions in proximal dendritic symmetric synapses on epileptic DGCs.
Figure 8.
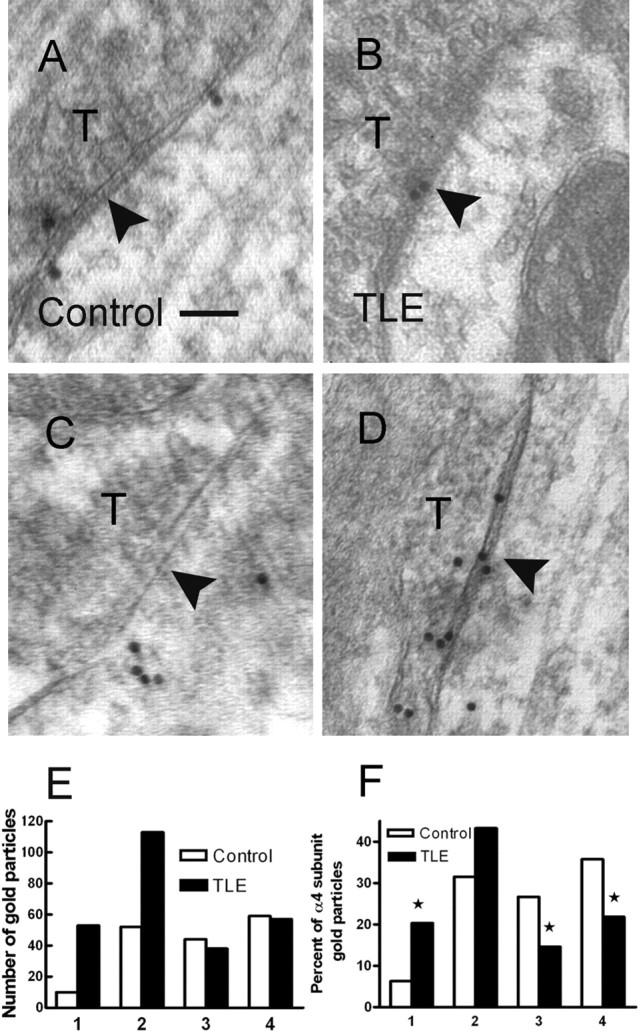
Postembedding immunogold electron microscopy of the α4 subunit of the GABAA receptor in the hippocampal inner molecular layer of control and epileptic DGCs. The α4-immunoreactive gold particles were found close to or at the end of symmetric axodendritic synapses in the hippocampal inner molecular layer from control animals (A, C). However, some α4 subunit-immunoreactive particles were also found at the symmetric synapses made of terminals (T) and granule cell dendrite shaft in hippocampal inner molecular layer from epileptic animals (B, D). Arrowheads in A–D indicate the center of synapse. Scale bar: A–D, 100 nm. E, The number of immunogold particles found in central and outer third of synapses in epileptic DGCs was approximately twice that in control DGCs. The number of immunogold particles in perisynaptic and extrasynaptic region was somewhat smaller in epileptic DGCs compared with control DGCs. F, Immunogold particles in regions 1–4 expressed as a percentage fraction of total number of gold particles in symmetric synapses in control and epileptic DGCs. *p < 0.05, χ2 test.
We combined data from perisomatic and dendritic synapses to compare the proportion of synapses with α4 immunogold labeling within central and outer third of the synapse and those with perisynaptic or extrasynaptic labeling in control and epileptic DGCs. The α4 immunogold labeling was present more commonly within the synapse in epileptic DGCs (79 of 113) compared with control DGCs (36 of 115; χ2 test, p < 0.001).
Discussion
This study demonstrated the following: (1) mIPSCs recorded from epileptic DGCs had lower frequency and larger amplitude than those recorded from control DGCs; (2) synaptic currents recorded from epileptic DGCs were less sensitive to allopregnanolone; (3) benzodiazepine and furosemide sensitivity of mIPSCs recorded from epileptic DGCs was similar to that of recombinant receptors containing the α4 subunit; and (4) the α4 immunogold labeling was present more commonly within the synapse in epileptic DGCs compared with control DGCs, in which the subunit was extrasynaptic. These electrophysiological, pharmacological, and neuroanatomical studies together demonstrate that, in epileptic DGCs, the neurosteroid modulation of synaptic currents is diminished and α4 subunit-containing receptors are present at synapses and participate in synaptic transmission. These changes may facilitate seizures in epileptic animals.
Reduced sensitivity of GABAergic synaptic transmission on DGCs to neurosteroid enhancement may contribute to the breakdown of the inhibitory mechanisms that gate the propagation of seizures into the hippocampus. DGCs are positioned at the center of a re-entrant limbic circuit of functional and anatomical connections that is activated during seizures (Lothman et al., 1991; Heinemann et al., 1992). In metabolic mapping and electrophysiological studies, DGCs inhibit entry of seizures into the hippocampus; once this inhibitory function fails, these cells actively support prolonged and self-sustaining electrographic seizures (Stringer and Lothman, 1992; Peng and Houser, 2005). The mechanisms of the breakdown of this inhibitory gate in chronic TLE remain a very active area of investigation. Allopregnanolone concentration in the extracellular space is 10–30 nm, and these concentrations increase the total chloride conductance of each synaptic event by 14–16% (calculations from data in Fig. 2, Table 1). Therefore, in control animals, physiological extracellular concentrations of neurosteroids will enhance the efficacy of inhibitory synaptic transmission on DGCs, but these molecules will be ineffective in epileptic animals. In addition to the reduced neurosteroid sensitivity, there are other mechanisms that contribute to reduce inhibition in TLE. Another well documented mechanism of diminished inhibition is the loss of ∼20–25% of somatostatin/NPY-containing GABAergic interneurons in the hilus (Sloviter, 1987; Obenaus et al., 1993; Kobayashi and Buckmaster, 2003; Sun et al., 2007).
Loss of neurosteroid sensitivity of synaptic inhibition would contribute to perimenstrual exacerbation of seizures in women. High neurosteroid concentrations during the midcycle are believed to exert an anticonvulsant action, whereas withdrawal of these steroids can result in seizure exacerbation (Reddy et al., 2001). Several mechanisms appear to contribute perimenstrual seizure exacerbation from progesterone withdrawal. Reduced neurosteroid sensitivity of synaptic GABAA receptors in epileptic rats is likely to exacerbate the effects of steroid withdrawal. Progesterone treatment and withdrawal caused the increased expression of the α4 subunit in the hippocampus, which is associated with diminished neurosteroid sensitivity of whole-cell GABAA receptor currents (Smith et al., 1998a,b). The additional effect of the midcycle progesterone peak is to diminish the expression of the δ subunit (Maguire et al., 2005), which in turn results in diminished tonic inhibition of DGCs.
The altered location of the α4 subunit into inhibitory synapses represents a novel form of plasticity of GABAA receptor-mediated inhibition in TLE. In control animals, GABAA receptors consisting of α4 and δ subunits are present in the extrasynaptic space and mediate a nonsynaptic form of inhibition, called tonic inhibition (Sur et al., 1999; Nusser and Mody, 2002; Stell and Mody, 2002; Wei et al., 2003; Sun et al., 2004; Mtchedlishvili and Kapur, 2006). Increased expression of α4 subunit mRNA and protein in DGCs was demonstrated in animal models of TLE in the past (Brooks-Kayal et al., 1998; Peng et al., 2004; Nishimura et al., 2005). These studies did not clarify whether the α4 subunit remains extrasynaptic in epileptic animals or it relocates to synapses. A recent study using postembedding immunogold labeling technique found that the α4 subunit was predominantly present in perisynaptic and extrasynaptic membrane in control and epileptic DGCs in a mouse model of TLE induced by lithium pilocarpine (Zhang et al., 2007). The reasons for the differences between the current study and that of Zhang et al. are not clear but may relate to the differences in species, animal model, and/or methodological procedures.
Inhibitory synaptic transmission in epileptic DGCs is altered in multiple animal models of TLE. A very well replicated observation is that synaptic currents recorded from DGCs of epileptic animals are sensitive to Zn2+ inhibition but not those from control animals (Buhl et al., 1996; Molnar and Nadler, 2001; Cohen et al., 2003). In addition to Zn2+, mIPSC sensitivity to zolpidem is lost in epileptic DGCs in the pilocarpine model of TLE (Cohen et al., 2003). In the lithium pilocarpine model of TLE, mIPSC sensitivity to diazepam was lost, and response to benzodiazepine competitive antagonist flumazenil changed to inverse agonist action (Leroy et al., 2004). Recombinant receptors containing the α4 and γ2 subunit are Zn2+ sensitive and are insensitive to diazepam and zolpidem, and their response to benzodiazepine inverse agonist and antagonists is altered (Knoflach et al., 1996). Therefore, altered Zn2+, zolpidem, diazepam, and flumazenil sensitivity of mIPSCs described in the past and those described in the current study can be explained by the expression of α4 subunit-containing receptors at inhibitory synapses in epileptic DGCs.
The α4 subunit likely assembles with the γ2 subunit to form GABAA receptors, which are likely unique therapeutic targets for the treatment of seizures. The pharmacological properties of α4 and γ2 subunit-containing recombinant receptors expressed in oocytes are distinct from those of α1 and γ2 subunit-containing receptors (Wafford et al., 1996), and these receptors have a lower EC50 for 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-ol and piperidine-4-sulfonic acid than α1γ2 subunit-containing receptors. Interestingly, RO 15-4513 (ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate) and RO 15-1788 (flumazenil) are partial agonists at α4 and γ2 subunit-containing receptors. It is possible that benzodiazepines with powerful agonist action at α4 and γ2 subunit-containing receptors could be useful for the treatment of seizures or catamenial exacerbation of seizures in TLE.
This study demonstrated loss of neurosteroid sensitivity of mIPSCs in epileptic animals. Alterations in neurosteroid modulation of mIPSCs from epileptic DGCs were investigated with a single high concentration of allopregnanolone (Leroy et al., 2004). A substantial reduction in sensitivity to 100 nm allopregnanolone occurred 24–48 h after lithium/pilocarpine-induced status epilepticus; however, sensitivity was restored in chronic epileptic animals (Leroy et al., 2004). The current study confirmed the finding that 100 nm allopregnanolone enhanced mIPSCs in epileptic DGCs. However, sensitivity to lower concentrations of allopregnanolone (10 and 30 nm) was not tested by Leroy et al., and we report loss of sensitivity to these concentrations. The physiological concentrations of allopregnanolone in the brain are believed to be in the 10–30 nm range (Majewska, 1992); therefore, loss of response to these concentrations is likely to be physiologically significant.
Altered location of the α4 subunit in DGCs in epileptic rats is similar to that described after chronic intermittent ethanol treatment (Liang et al., 2006). A recent study described changes in ethanol sensitivity of mIPSCs recorded from granule cells and CA1 pyramidal neurons after chronic intermittent ethanol treatment. The subunit composition of synaptic GABAA receptors was altered after treatment such that synapses contained the α4 subunit. Interestingly, chronic intermittent ethanol treatment is known to increase seizure susceptibility in experimental animals (Kokka et al., 1993). Similarly, progesterone treatment and withdrawal is associated with increased expression of α4 subunit-containing receptors, and the suppression of this subunit can suppress behavioral effects of progesterone withdrawal (Smith et al., 1998a,b). The current study found greater intensity of α4 staining in granule cell layer than in the molecular layer that was different from studies in mice (Peng et al., 2002; Glykys et al., 2007). This inconsistency likely arises from methodological differences.
In addition to the reduced drug sensitivity of mIPSCs, their amplitude was increased. Increased mIPSC amplitude has been reported in animal models of TLE and in the kindling model (Otis et al., 1994; Leroy et al., 2004). One explanation for increased amplitude of mIPSCs is that the number of GABAA receptors on the postsynaptic membrane is increased (Nusser et al., 1998). However, it is unclear whether the number of synaptic receptors on the postsynaptic membrane is the sole determinant of the mIPSC amplitude; other factors such as channel kinetics, vesicular filling, and release mechanisms may impact the mIPSC amplitude.
Reduced frequency of mIPSCs observed in the current study was similar to the reduced mIPSC frequency recorded from DGCs in kainate and this electrical stimulation model of TLE (Kobayashi and Buckmaster, 2003; Sun et al., 2007). Reduced mIPSC frequency may relate to altered mechanisms of vesicular release and/or reduction in GABAergic terminals. This reduction in mIPSC frequency is related in part to the loss of somatostatin-containing interneurons observed in TLE models and in human surgical specimens (Sloviter, 1987; Obenaus et al., 1993; Mathern et al., 1995; Buckmaster and Dudek, 1997; Sun et al., 2007).
In conclusion, the present study demonstrated reduced sensitivity of GABAergic synaptic transmission to neurosteroids, in association with altered targeting of the α4 subunit of GABAA receptors in DGCs of epileptic animals. These changes may diminish inhibition of DGCs and increase seizure susceptibility.
Footnotes
This work was supported by National Institutes of Health Grants RO1 NS 040337 and RO1 NS 044370. We thank Dr. Werner Sieghart for the gift of α4 and δ antibodies.
References
- Bertram EH, Williamson JM, Cornett JF, Spradlin S, Chen ZF. Design and construction of a long-term continuous video-EEG monitoring unit for simultaneous recording of multiple small animals. Brain Res Brain Res Protoc. 1997;2:85–97. doi: 10.1016/s1385-299x(97)00033-0. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the delta subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol (Lond) 1999;516:513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABAA receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–1616. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Heck WL, Slusarczyk A, Basaraba AM, Schweitzer L. Subcellular localization of GABA receptors in the central nervous system using post-embedding immunohistochemistry. Brain Res Brain Res Protoc. 2002;9:173–180. doi: 10.1016/s1385-299x(02)00143-5. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant γ-aminobutyric acidA receptors α4α2γ2 and α6β2γ2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5alpha-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999;288:679–684. [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABAA receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol (Lond) 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by “continuous” hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, III, Stringer JL. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37:1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar P, Nadler JV. Lack of effect of mossy fiber-released zinc on granule cell GABA(A) receptors in the pilocarpine model of epilepsy. J Neurophysiol. 2001;85:1932–1940. doi: 10.1152/jn.2001.85.5.1932. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J. Diminished allopregnanolone enhancement of GABA(A) receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol (Lond) 2001;537:453–465. doi: 10.1111/j.1469-7793.2001.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Schwarzer C, Gasser E, Kato N, Vezzani A, Sperk G. Altered expression of GABAA and GABAB receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neuroscience. 2005;134:691–704. doi: 10.1016/j.neuroscience.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JDB, Baude A, Richards JG, Sieghart W, Somogyi P. Immunocytochemical localization of the α1 and β2/3 subunits of the GABAA receptor in relation to specific GABAergic synapses in the dentate gyrus. Eur J Neurosci. 1995;7:630–646. doi: 10.1111/j.1460-9568.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of GABAA receptors underlies potentiation of hippocampal synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of γ-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Houser CR. Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci. 2005;25:7210–7220. doi: 10.1523/JNEUROSCI.0838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reith CA, Sillar KT. Pre- and postsynaptic modulation of spinal GABAergic neurotransmission by the neurosteroid, 5 beta-pregnan-3 alpha-ol-20-one. Brain Res. 1997;770:202–212. doi: 10.1016/s0006-8993(97)00809-3. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench M JM, Li X. GABAA receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-10-j0003.2002. RC223 (1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JL, Lothman EW. Bilateral maximal dentate activation is critical for the appearance of an afterdischarge in the dentate gyrus. Neuroscience. 1992;46:309–314. doi: 10.1016/0306-4522(92)90053-5. [DOI] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007;500:876–893. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human γ-aminobutyric acidA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]