Abstract
Background
Many women without preexisting stress urinary incontinence (SUI) who undergo vaginal surgery to correct pelvic organ prolapse will develop symptoms of SUI. A concomitant prophylactic anti-incontinence procedure may prevent SUI symptom development in women undergoing vaginal prolapse surgery.
Purpose
To present the rationale, design and methodology of a randomized controlled surgical trial (RCT), the Outcomes Following Vaginal Prolapse Repair and Mid Urethral Sling (OPUS) Trial. The primary aims of this RCT are to determine (1) whether the prevalence of post-operative urinary incontinence (UI) differs between stress continent women receiving vaginal prolapse repair with concomitant tension-free vaginal tape (TVT®; a sling procedure commonly used to treat SUI) and those with only sham incisions at 3 months post surgery (2) whether it is more cost-effective to place a TVT prophylactically than to treat the SUI symptoms postoperatively as they occur over a 12 month period after the index surgery.. The study also incorporates a patient preference trial (PPT).
Methods
Primary outcome, defined as signs (positive cough stress test), symptoms (per validated questionnaire) and/or need for treatment of SUI and its associated cost, at 3 and 12 months post-operatively. Secondary outcomes consist of group differences in lower urinary tract and prolapse symptoms, health related quality of life, measures of vaginal anatomy, and surgical complications.
Limitations
Given the invasive nature of surgical intervention trials, some individuals may be reluctant to agree with random assignment, potentially impacting result generalizability. To evaluate the magnitude and direction of non-participation bias, the PPT will enroll a sample of those who decline participation in the RCT but are otherwise eligible.
Conclusion
This sham-controlled RCT will provide important information for patients and surgeons regarding both the short- and long-term optimal treatment approach for stress continent women undergoing a vaginal surgery for prolapse. Non-participation bias will be estimated.
Keywords: Stress Urinary Incontinence, Pelvic Organ Prolapse, Surgical Intervention, Mid-urethral slings, Tension-free Vaginal Tape, Prophylactic Incontinence Procedure, Patient Preference Trial, Randomized Controlled Trial
INTRODUCTION
Pelvic floor disorders, particularly pelvic organ prolapse (POP; a condition in which the uterus, vagina, bladder and/or rectum bulge into or outside of the vagina) and urinary incontinence (UI; involuntary urinary leakage), are common in women. One in nine American women will undergo at least one surgery for POP or UI by the age of 80. More distressingly, 30% of those will undergo at least one additional surgery.(1)
Some women with advanced POP remain continent despite significant loss of anterior vaginal and pelvic organ support. The prolapse may function to kink the urethra, maintaining continence by causing urethral obstruction.(2) It is not uncommon for continent women undergoing prolapse surgery to develop de novo stress urinary incontinence (SUI; leakage with physical exertion such as coughing or exercise) postoperatively. This may result from relieving the urethral obstruction caused by prolapse, thereby unmasking a pre-existing compromised urethral function. Some have referred to this as an unmasking of occult or potential SUI.(3) Postoperative SUI may also result from compromised urethral innervation or support secondary to the surgical repair.
The Pelvic Floor Disorders Network (PFDN), an NIH-sponsored clinical trials network, recently conducted a randomized surgical trial [the Colpopexy and Urinary Reduction Efforts (CARE) trial] to address whether adding an anti-incontinence procedure at the time of an abdominal prolapse surgery reduced post-operative SUI in women without such leakage prior to surgery. In this trial, women who were randomized to a concomitant retropubic anti-incontinence procedure (Burch colposuspension) had approximately half the rate of SUI as women who underwent only abdominal sacrocolpopexy for prolapse (24% versus 44%, p<0.001) and experienced no increase in adverse outcomes.(4)
Whether similar benefits occur with the addition of an anti-incontinence procedure at the time of vaginal surgery for prolapse in stress continent women is not yet known. However, many surgeons are performing prophylactic anti-incontinence procedures, notably placement of mid-urethral slings, at the time of vaginal prolapse surgery. This practice is supported by a single small randomized controlled trial (RCT) in which 4% of women who received an anti-incontinence sling procedure and 36% of those who did not reported SUI two years after surgery (p=0.01).(5) Only 50 women were randomized, making it difficult to effectively evaluate differences in complication rates that may potentially offset any benefit in SUI reduction. However, the rate of SUI in the controls was greater than in a retrospective study which reported SUI in only 7% of women 23 months following vaginal prolapse surgery without concomitant anti-incontinence procedures (N=76).(6) The results of a transvaginal approach for prolapse repair and vaginal anti-incontinence procedure (mid-urethral sling) may differ from those procedures performed for the same indications through an abdominal approach. Such differences may lead to disparate efficacy and complication rates, thus, women undergoing a mid-urethral sling may be at lower risk of stress leakage, but at higher risk of adverse outcomes, such as urinary urgency, voiding difficulties and sling mesh erosion. Furthermore, the cost of most mid-urethral slings adds considerably to the overall cost of prolapse repair, raising the issue of cost-effectiveness of such a prophylactic approach at the time of vaginal reconstructive surgery, especially in the absence of proven efficacy.
In order to estimate the efficacy, adverse outcomes and cost-effectiveness of performing a prophylactic mid-urethral sling procedure in stress continent women undergoing vaginal surgery for prolapse, the Outcomes Following Vaginal Prolapse Repair and Mid Urethral Sling (OPUS) Trial was designed. The purpose of this paper is to describe the design of the trial, highlighting key design features including the patient preference trial (PPT) and the cost-effectiveness analysis (CEA).
Methods
Design Overview
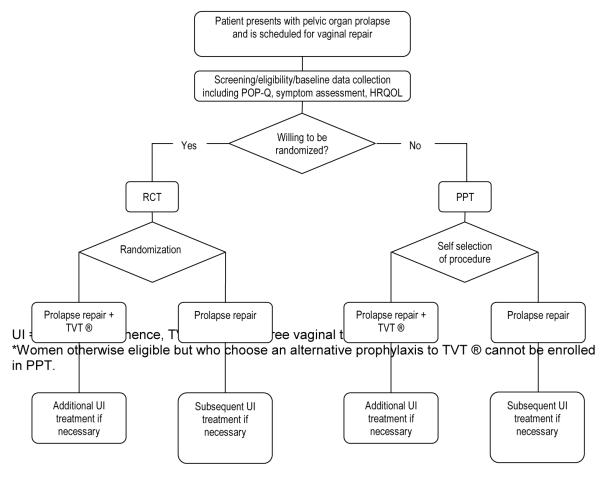
Figure 1 depicts the OPUS trial design. There are two primary hypotheses, one short-term and one long-term. The short-term primary hypothesis is that among stress continent women undergoing vaginal prolapse surgery, those receiving a concomitant tension-free vaginal tape (TVT®) procedure will have lower prevalence of and less treatment for bothersome UI during the first three months following surgery compared with those who do not receive a TVT procedure.
Figure 1.
Study Schema*
The long-term primary hypothesis is that the overall health care cost and quality of life are similar in the two groups at 12 months after the index surgery. The primary study aims are to: 1) test whether the prevalence of post-operative urinary incontinence differs between stress continent women undergoing vaginal prolapse repair with concomitant TVT and those undergoing vaginal prolapse repair with only sham incisions at 3 months post index surgery, 2) evaluate whether prevalence of bothersome UI at 12 months following index surgery differs between the two groups and 3) compare the cost-effectiveness of performing concomitant TVT® at the time of vaginal prolapse repair for all stress continent women versus expectant treatment of UI if it occurs postoperatively (i.e., a “wait and see” approach) over the 12 months following index surgery. Secondary aims are to evaluate, at three, six and 12 months post surgery, group differences in postoperative anterior vaginal prolapse, lower urinary tract symptoms, health related quality of life (HRQOL), and surgical complications.
The primary study design is a randomized, single-blind, sham-controlled surgical intervention trial. Both the control and experimental groups will undergo vaginal prolapse repair while the experimental group will receive concomitant TVT®. Assignment to study arms will be randomly allocated in a 1:1 ratio. A systematic sample of those declining participation in the RCT (three subjects for every five enrolled in the RCT), who are otherwise eligible for the study, will be offered participation in the PPT. The PPT will therefore also have two groups: those who undergo a TVT® at the time of prolapse surgery and those who do not. The PPT will be used to quantify sample selection bias due to RCT non-participation. Potentially important differences in demographics, clinical characteristics and outcomes between the RCT and PPT samples will be examined, stratified by surgeon and treatment received. Inclusion and exclusion criteria for the PPT are otherwise identical to those of the RCT, except for willingness to be randomized. In order to minimize selection bias due to the availability of the PPT itself, the PPT will only be offered to the subject once she has explicitly declined participation in the RCT. This study is registered at www.clinicaltrials.gov.
Study Population
Subjects will consist of women being considered for an apical and/or anterior vaginal prolapse repair via a vaginal approach without subjective complaints of SUI. Participants must have vaginal bulge symptoms defined by positive responses to a validated instrument, the Pelvic Floor Distress Inventory (PFDI) (7) and anterior vaginal prolapse with point Aa at −1 cm or greater (i.e., loss of anterior vaginal support implying the urethra is not supported behind the pubic bone) as determined by the Pelvic Organ Prolapse Quantification (POP-Q) system (8), a validated tool designed to assess the degree of vaginal prolapse. In addition, the participant must not experience clinically significant symptoms of SUI per responses to the PFDI (7), must be a candidate for surgical intervention, and must be able and willing to complete written informed consent. The complete list of inclusion and exclusion criteria is summarized in Table 1.
Table 1.
Inclusion and Exclusion Criteria*
| Inclusion criteria: |
|
|
|
| Exclusion criteria: |
|
PFDI= Pelvic Floor Distress Inventory, POPQ=Pelvic Organ Prolapse Quantification
Urodynamic studies are not required as a part of this trial.
Pre-intervention assessments
All women presenting to the participating PFDN clinical centers with the complaint of prolapse, as defined by the inclusion criteria, will be screened for the subjective complaint of SUI using three SUI items from the PFDI.(9) Eligible individuals will be offered RCT participation. A systematic sample of those who decline the RCT will be offered participation in the PPT.
The following baseline data will be collected: demographics, POP-Q (8), cough stress test at bladder volume of 300ml, post void residual volume (PVR), general health and urogynecological history, and HRQOL. A series of instruments will be used to measure HRQOL: the Medical Outcome Study Short-Form-36 Health Survey (SF-36) (10), EuroQol (EQ-5D) (11), PFDI (7), Pelvic Floor Impact Questionnaire (PFIQ) (7), Incontinence Severity Index (ISI) (12), Pelvic Organ Prolapse/Urinary Incontinence Sexual Functioning Questionnaire Short Form (PISQ-12) (13), PFDN Adaptations Index (14), an analogue pain scale adapted for suprapubic pain (15), and the Patient Global Impression of Improvement (PGI-I) Scale.(16) The telephone assessment of HRQOL will be performed by female interviewers masked to group assignment at a centralized Quality of Life (QOL) Interviewing Center. Medical data will be obtained by clinical sites using standardized data collection forms. Surgeons will be blinded to the cough stress test results.
Randomization and Masking
Randomization will be stratified by surgeon and category of planned surgery, including colpocleisis (a type of vaginal closure), apical support procedure and/or anterior repair procedure. The PFDN Data Coordinating Center at the University of Michigan will set up a random block design. Randomization will be assigned in the operating room to minimize surgeon and subject bias. While it is impossible for surgeons to be masked to the randomization, subjects and research staff will be masked during the entire one-year follow-up period. Dictated operative notes will describe the actual procedure(s) performed; however, the hand-written chart note will indicate the surgery by stating “transvaginal procedure as per OPUS trial protocol, see the dictated operative report.”
Precautions will be made to minimize unmasking the TVT® procedure. Since TVT® requires two one-centimeter suprapubic incisions, the control group will receive comparable sham incisions. Skin closure will be identical for all subjects. Intraoperative data collection will be conducted by the study surgeon rather than other research staff. Lastly, all subjects, regardless of group, will have post-operative indwelling urethral catheters until voiding trials are performed.
Study intervention
The primary intervention, or surgical technique, will be the TVT® (Gynecare TVT®, Ethicon, Johnson & Johnson) anti-incontinence procedure. Prolapse procedures will be recorded but not controlled by study protocol. Participating surgeons are required to have performed at least 20 TVT® procedures prior to enrolling subjects in OPUS. In order to minimize the risk of altering the urethrovesical angle and the likelihood of postoperative SUI, the anterior repair will be conducted using only one incision proximal to the bladder neck level and separate from TVT® incisions. Allowable techniques for anterior vaginal wall prolapse repair include anterior colporrhaphy (plication of vesicovaginal fibromuscular tissue), vaginal paravaginal repair, colpocleisis, and use of allograft, xenograft, or synthetic graft material. Plication of periurethral fibromuscular tissue (“Kelly” or suburethral plication) or any sutures near the urethra or bladder neck will not be allowed. Details of the anterior repair will be recorded.
Post-intervention assessments
A voiding trial will take place on post-operative day 1. A successful voiding trial is defined as a post residual volume (PVR) of ≤150cc (combined with a minimum void of at least 150cc) documented by an ultrasound measurement, a catheterized residual, or the calculated difference between volume instilled and volume voided. Subjects with unsatisfactory voiding trials will continue to receive catheterization per clinical site care standards and have PVRs checked and recorded at least twice weekly until demonstration of successful voiding. Subjects will be interviewed by the study coordinator, by phone, or in person at follow-up visits, at two and five weeks post-surgery. Follow-up interviews will document urinary retention and the need for intermittent or continuous self catheterization.
The primary short-term endpoint, treatment for and/or signs and/or symptoms of bothersome UI, will be assessed at three months after the index surgery via a clinic visit and HRQOL interview. The research nurse at each clinical site will conduct an updated directed medical history, a physical examination including POP-Q (8), and testing including cough stress test, urine screening for infection, and PVR measurement (by ultrasound or catheter). The primary long-term endpoint will be obtained in the same manner at 12 months after the index surgery to determine whether prevalence of bothersome UI symptoms differs between the two arms, while allowing for further incontinence treatment in either group. The QOL Interviewing Center will assess HRQOL at 3, 6 and 12 months; study coordinators will collect follow-up health care utilization data and a medical history at 3, 6, 9 and 12 months.
Clinical Outcomes
The primary outcome is defined at three months by a positive cough stress test and/or bothersome UI (at least moderately bothersome positive responses to any of the first four items in Table 2) and/or need for UI treatment. Secondary outcome measures will assess the degree to which the study intervention influences adverse events at three and 12 months. These secondary outcomes include POP, urinary tract infections, serious adverse events, and HRQOL. In addition, a cost-effectiveness analysis will be conducted at 12 months to determine whether there is a significant difference between prophylactic UI treatment at the time of the index surgery versus UI treatment, if needed, after the index surgery.
Table 2.
Primary HRQOL Outcome measures*
| PFDI Items for Primary & Secondary Aims Outcome Measurement |
| Stress Urinary Incontinence Items |
|
| Urge Urinary Incontinence Items |
|
| After each question: |
| Bothersome? (Yes/No) |
| If yes, how bothersome? (Not at all/Somewhat/Moderately/Quite a Bit) |
Where the primary outcome is defined as any one of the following: 1) a positive answer to any of the stress and/or urge urinary incontinence items above also characterized as being at least moderately bothersome, 2) the occurrence of a positive stress test at the lower of at least 300ml or maximum bladder capacity with or without prolapse reduction using the swab technique, or 3) the need/desire for treatment including sling, suspension surgery, collagen injections, supervised pelvic muscle therapy, medication, pessary, etc.
Economic Evaluation and Analyses
The cost-effectiveness analysis of performing a concomitant TVT® at the time of vaginal prolapse repair versus prolapse surgery alone will be conducted from a societal perspective. Both direct medical and non-medical costs and indirect costs (i.e., productivity loss) will be estimated. Information on resource use will be collected using each clinical site’s billing records and subject self-report data. Direct medical costs include incremental costs of the index surgery associated with TVT® and subsequent use of urologic/urogynecologic-related medical services during the 12 month follow-up period. The Medicare reimbursement rate (17) will be used to assign unit cost for each type of medical service. Average wholesale prices recorded in the Drug Topics Red Book® (18) will be used as medication unit costs. Direct non-medical costs include expenditures associated with incontinence care, such as absorbent pads, laundry and skin care products, and transportation for care of relevant urologic/gynecologic conditions or complications subsequent to the index surgery. Indirect costs will be estimated by data on days of work loss, household productivity loss, and reduced efficiency while at work due to complications of the index surgery and symptoms and treatment of UI or other urologic/gynecologic conditions.
Patient-level quality-adjusted life years (QALYs) will be calculated assuming linear changes in each subject’s utility scores over time between every two assessments and calculating the area under the curve over the 12 month period.(19) To assess incremental costs associated with each additional QALY gained, incremental cost-effectiveness ratios (ICER) will be calculated as the differential mean cost divided by the differential mean QALYs between the two arms. The base case analysis will be conducted using data on subjects with complete cost information and QALY measures based on the EQ-5D.(11) Sensitivity analysis will be performed to include the information of subjects with incomplete data using multiple imputation,(20) and QALY measures based on the PFIQ (7) UI scale to shed light on the cost-effectiveness of the concomitant TVT® procedure when condition-specific utility scores are used. The non-parametric bootstrapping resampling technique (21) will be used to derive the ICER 95% confidence interval.
Sample Size
The primary outcome, defined by positive cough stress test and/or bothersome UI and/or need for UI treatment, will be ascertained at the three month follow-up visit. The investigators believe that a difference in primary outcome less than 15% will not change clinical practice. However, they also agree that the sample size should be large enough so that an observed difference in failure of 10% should be statistically significant. Thus, an observed difference of 10% or more would lead to a statistically significant result; otherwise equivalence will be claimed. To allow for inclusion of urge incontinence as a part of our primary outcome, we assume that the group with superior results will meet the primary outcome (fail) 20% of the time while the other group will fail at least 35%. With 150 subjects per group, there will be 80% power to differentiate the two groups with respect to the primary outcome of 20% and 35% using a two-tailed test with 5% level of significance. Furthermore, the observed difference must be less than 10% in order to claim that there is NO statistically significant difference in outcome rates. In the intent-to-treat analysis, dropouts will be treated as failures. Approximately 350 subjects will be randomized to allow for approximately 15% loss to follow up during the 12 month period.
The PPT sample size will be limited to three subjects per every five enrolled in the RCT, per clinical site, up to a total of 115 per arm. A sample size of 100 will provide 80% power to identify a difference of 15% (i.e., 20% versus 35%) when comparing the primary outcome of comparable PPT and RCT arms using a two-tailed t-test with 5% significance level. Detection of a difference greater than this level would indicate a meaningful difference in the primary outcome between comparable PPT and RCT arms. An addition of 100 subjects to the risk factor analysis would reduce the standard error of a prediction by more than 20%. To allow for approximately 15% loss to follow-up over 12 months, up to 115 subjects may be enrolled into each PPT arm. Since enrollment into the PPT will terminate when enrollment into the RCT is completed, a smaller number may be enrolled into the PPT if the majority of eligible subjects accrue to the RCT or if one of the arms of the PPT is not selected by participants.
Statistical Analysis
The primary short-term RCT analysis is a comparison of the rates of our composite primary outcome, consisting of a positive cough stress test and/or bothersome UI symptoms and/or need for UI treatment, between groups at three months post index surgery (prevention aim). Intent-to-treat analysis will compare the primary outcome between groups using a conditional logistic model where group assignment will be included as an indicator variable. Prior to unmasking the study, the data will be examined for outliers. In addition, the distribution of the continuous outcome measures will be examined to determine whether transformations are needed. Demographic variables will be compared between treatment groups at baseline. If differences between groups are found, the baseline variable will be included as covariates in models fitted to the outcome measures. Stratification variables will also be included in all models of the outcome measures. Dropouts will be considered as failures unless there is clear evidence that the reason for withdrawal or loss to follow-up was unrelated to the trial (e.g., accidental death). Sensitivity analyses will be performed to assess the impact of subject withdrawal on the analytical conclusions. All models will be adjusted for the randomization stratification variables. In addition, we will estimate the difference in rates of our primary outcome and its confidence interval.
Twelve-month data will be analyzed in a similar manner except that the definition of the primary outcome will not include need for additional UI treatment. Primary outcome rates at 12 months will include subjects lost to follow-up as they will be considered to have failed. A cost-effectiveness analysis will be performed to compare the two treatment groups.
In addition, subject-level clinical and outcomes data will be compared between participants in the RCT and PPT. Significant differences in key baseline variables and outcomes will be quantified as measures of non-participation bias. Data from the RCT and PPT will be combined in the evaluation of factors associated with the primary outcome. Dichotomous outcomes will be analyzed in a manner similar to that of the primary endpoint. Continuous outcome measures will be fitted into a general linear model using the same primary outcome covariates. Measures with skewed distributions may be transformed prior to analysis.
Discussion
Surgery for prolapse has been associated with postoperative de novo SUI in 13% to 80% of women.(22-24) Due to lack of evidence-based treatment recommendations, different strategies have been employed to manage this problem.(25) Some clinicians perform prophylactic anti-incontinence procedures for all subjectively stress-continent patients undergoing prolapse surgery. Others do not perform prophylactic anti-incontinence procedures, treating only those who develop bothersome SUI postoperatively. Another approach is preoperative stress testing with prolapse reduction, in attempt to unmask occult SUI, thus guiding the selection of patients who might benefit the most from a prophylactic anti-incontinence procedure. In the CARE trial, Burch colposuspension performed in stress continent women undergoing abdominal sacral colpopexy significantly reduced postoperative SUI without increasing the rates of irritative or obstructive voiding symptoms.(4) Such benefit occurred whether or not preoperative testing suggested occult SUI; although women who leaked during pre-operative testing were more likely to leak post-operatively. While important, these results do not provide guidance on how to decrease postoperative SUI following vaginal, as opposed to abdominal, prolapse repair in stress continent women.
The risk-benefit ratio of performing concomitant surgery for SUI prevention during vaginal prolapse surgery is unknown. There may be less benefit from adding a sling procedure during vaginal, as opposed to abdominal, prolapse surgery because orientation of the vaginal apex and anterior vagina differ following the two surgical approaches. Patient populations and postoperative risks of those receiving vaginal versus abdominal prolapse procedures may also differ. The literature suggests that, compared with Burch colposuspension, slings are associated with greater risk of urinary tract infections, voiding dysfunction or difficulty, and urinary urgency symptoms.(26) These risks may be mitigated by the type of sling used. For example, the TVT®, a synthetic midurethral sling, seems to have higher efficacy with fewer side effects than proximal urethral slings.(27) Results of the trial by Meschia et al. suggested that prophylactic TVT® results in less de novo SUI after surgery when compared with prolapse surgery alone.(5) However, the small sample size in this trial makes it susceptible to selection bias. In addition, women who did not demonstrate SUI upon prolapse reduction pre-operatively were excluded. Thus, the effect of prophylactic TVT® on women without occult SUI in preoperative testing is not known. The OPUS trial will directly compare the safety, efficacy and cost-effectiveness of prophylactic TVT® at the time of vaginal prolapse surgery to expectant treatment within 12 months of index surgery. In addition, the value of preoperative cough stress testing will be assessed in all subjects, with and without prolapse reduction, with results masked to the surgical team.
Healthcare cost is an increasingly important consideration for new technologies, particularly surgical interventions. According to Anger and colleagues (28), the total expenditure related to UI among female Medicare beneficiaries nearly doubled between 1992 and 1998. Most of that rise in spending was attributed to an increase in the number of women treated for UI. The implications for overall societal costs would have been more substantial had outpatient medications, non-medical costs (e.g., use of incontinence pads) and productivity loss been considered. The addition of TVT® at the time of prolapse repair to prevent bothersome UI would add to the initial surgical cost, with potential yet currently unmeasured reduction in postoperative costs for the women who benefit. Therefore, it is crucial to rigorously examine the cost-effectiveness of this prophylactic anti-continence procedure among women undergoing prolapse surgery versus expectant treatment of UI after surgery. Drawing on the advantages of this randomized trial, we have incorporated a cost-effectiveness analysis component in our study design. This minimizes bias in treatment comparisons and makes the economic evaluation relatively efficient as research personnel and processes for data collection will already be in place.(29)
Another important feature of the OPUS trial is the combination of an RCT and a PPT into one overarching trial. This combination yields three important advantages over an RCT alone.(30) First, if the PPT results are comparable to those of the RCT, this will enhance the external validity of the RCT since patients who decline randomization may represent a significant percent of patients seen in clinical practice. Second, if the findings are inconsistent between the RCT and PPT, one can describe the direction and magnitude of the bias introduced through self-determination or physician selection of treatment, a major threat to the generalizability of RCT findings. Third, the additional subject information gathered by inclusion of a PPT improves the statistical power to identify factors associated with surgical outcomes.(31)
Surgical trials present unique challenges not typically encountered in studies evaluating medical therapy, including ethical considerations of using sham procedures, difficulty or impossibility of masking group assignment from subjects, treating clinicians, and research staff, and the reluctance of some individuals to accept random surgical assignment. The highest standards for interventional clinical trials support the use of placebos, including sham procedures for surgical interventions. Use of sham surgery in previous clinical trials has suggested a significant role for placebo effect. The need for a sham control group in OPUS is particularly relevant given that several key outcome measures are components of HRQOL, an endpoint that may be biased by the subject’s knowledge of group assignment.
Failure to systematically evaluate surgical interventions may give rise to proliferation of expensive and possibly ineffective therapies with both financial and health impacts.(28) According to Hornig and Miller (32;33), a sham-controlled trial of an invasive procedure can be ethically justified if: 1) there is a valuable, clinically relevant question to be answered by the research, 2) the sham control is methodologically necessary to test the study hypothesis, the risk of sham 3) has been minimized, 4) does not exceed the threshold of acceptable research risk, 5) is justified by valuable knowledge to be gained, and 6) the misleading involved in the sham is adequately disclosed and authorized by the subject during the research informed consent process. These six criteria are met in the OPUS trial. Moreover, those who develop UI will be offered incontinence treatment when necessary. The use of sham incisions in the control arm allows masking of subjects and medical and nursing staff providing clinical care. Steps will also be taken to minimize potential masking problems. Study coordinators, blind to treatment assignment, will perform all in-person evaluations and staff of an independent QOL interviewing center will conduct the HRQOL interviews without knowledge of treatment status.
Limitations
There are several limitations to our design. First, in spite of masking, subjects or research staff may become aware of group assignment. Intraoperative or postoperative complications relatively unique to the TVT® procedure, such as bladder perforation, retropubic hematoma, prolonged urinary retention or mesh erosion, may result in subject assignment unmasking. All episodes of code breaking will be tracked in order to assess its potential impact on results. In addition, knowledge of group assignment by the surgeon, who in most cases will also serve as primary caregiver regarding issues of bladder function, may influence the threshold for treatment or types of treatment offered in subjects who develop postoperative bladder symptoms. This has the potential to influence a number of primary and secondary outcome measures; however, the primary outcome in this trial is expected to largely be dependent on patient-reported bothersome symptoms and cough stress test rather than the need for subsequent UI treatment. Selection bias for the RCT will be minimized by offering the PPT only after individuals decline RCT participation. Subject demographics, pre-intervention medical status and outcomes will be compared between the RCT and PPT. Thus, the full range of outcomes, including that of those subjects who choose not to be randomized for surgery, will be captured by the PPT.
Conclusion
Findings from the OPUS trial will provide important information that will help surgeons to better counsel women on the benefits and risks of concomitant prophylactic anti-incontinence procedure at the time of vaginal surgery for prolapse, versus the expectant approach of treating postoperative UI. Analysis of factors associated with outcomes, including the value of cough stress testing with and without prolapse reduction, may guide more effective selection of patients likely to benefit the most from preventative anti-incontinence procedures. The cost-effectiveness analysis will inform policy makers of the incremental costs relative to the incremental outcomes expected from prophylactic TVT®.
Acknowledgments
Grant support- Supported by grants from the National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health (U01 HD41249, U10 HD41250, U10 HD41261, U10 HD41267, U10 HD54136, U10 HD54214, U10 HD54215, and U10 HD54241)
Appendix
Pelvic Floor Disorders Network Members
Cleveland Clinic
Mathew D. Barber, MD, MHS, Principal Investigator
Marie Fidela R. Paraiso, MD, Co-Investigator
Mark D. Walters, MD, Co-Investigator
J. Eric Jelovsek, MD, Co-Investigator
Firouz Daneshgari, Co-Investigator
Linda McElrath, RN, Research Nurse Coordinator
Donel Murphy, RN, MSN, Research Nurse
Cheryl Williams, Research Assistant
Duke University
Anthony G. Visco, MD, Principal Investigator
Jennifer Wu, MD, Co-Investigator
Alison Weidner, MD, Co-Investigator
Cindy Amundsen, MD, Co-Investigator
Mary J. Loomis, RN, BSN, Research Coordinator
Loyola University, Chicago
Linda Brubaker, MD, MS, Principal Investigator
Kimberly Kenton, MD, MS, Investigator
MaryPat FitzGerald, MD, MS, Investigator
Elizabeth Mueller, MD, MSME, Investigator
Kathy Marchese, RN, Study Coordinator
Mary Tulke, RN, Study Coordinator
University of Alabama at Birmingham
Holly E. Richter, PhD, MD, Principal Investigator
R. Edward Varner, MD, Co-Investigator
Robert L. Holley, MD, Co-Investigator
Thomas L. Wheeler, MD, Co-Investigator
Patricia S. Goode, MD, Co-Investigator
L. Keith Lloyd, MD, Co-Investigator
Alayne D. Markland, DO, Co-Investigator
Velria Willis, RN, BSN, Research Coordinator
Nancy Saxon, BSN, Research Nurse Clinician
LaChele Ward, LPN, Research Specialist
Lisa S. Pair, CRNP
University of California, San Diego and Kaiser, San Diego
Charles W. Nager, MD, Principal Investigator
Shawn A. Menefee, MD, Co-Investigator
Emily Lukacz, MD, Co-Investigator
Karl M. Luber, MD, Co-Investigator
Michael E. Albo, MD, Co-Investigator
Margie Kahn, MD, Co-Investigator
Lysa Woodall, RN, Study Coordinator
Giselle Zazueta-Damian, Study Coordinator
University of Michigan
Morton B. Brown, PhD, Co-Investigator
Cathie Spino, PhD, Principal Investigator
John T. Wei, MD, MS, Co-Principal Investigator
Beverly Marchant, RN, BS, Project Manager
Donna DiFranco, BS, Clinical Monitor
John O.L. DeLancey, MD, Co-Investigator
Dee Fenner, MD, Co-Investigator
Nancy K. Janz, PhD, Co-Investigator
Wen Ye, PhD, Statistician
Zhen Chen, MS, Statistician
Yang Wang Casher, MS, Database Programmer
University of Texas, Southwestern
Joseph Schaffer MD - PI
Clifford Wai, MD - Co-Investigator
Marlene Corton, MD - Co-Investigator
Gary Lemack, MD - Co-Investigator
Kelly Moore - Research Coordinator
David Rahn, MD
Amanda White, MD
Shanna Atnip, NP
Margaret Hull, NP
Pam Martinez, NP
Deborah Lawson, NP
University of Utah
Ingrid Nygaard, MD, Principal Investigator
Peggy Norton, MD, Co-Investigator
Linda Freeman, RN, Research Coordinator
NIH Project Scientist
Anne M. Weber, MD, MS
Susan Meikle, MD
Footnotes
This trial is registered at clinicaltrials.gov under Registration # NCT00460434
Reference List
- (1).Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- (2).Rosenzweig BA, Pushkin S, Blumenfeld D, Bhatia NN. Prevalence of abnormal urodynamic test results in continent women with severe genitourinary prolapse. Obstet Gynecol. 1992;79(4):539–42. [PubMed] [Google Scholar]
- (3).Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynecol Obstet. 2007;97(1):31–4. doi: 10.1016/j.ijgo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- (4).Brubaker L, Cundiff GW, Fine P, Nygaard I, Richter HE, Visco AG, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006 Apr 13;354(15):1557–66. doi: 10.1056/NEJMoa054208. [DOI] [PubMed] [Google Scholar]
- (5).Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am J Obstet Gynecol. 2004;190(3):609–13. doi: 10.1016/j.ajog.2003.09.027. [DOI] [PubMed] [Google Scholar]
- (6).Roovers JPWR, van Laar JOEH, Loffeld C, Bremer GL, Mol BW, bongers MY. Does urodynamic investigation improve outcome in patients undergoing prolapse surgery? Neurourol Urodyn. 2007;26:170–5. doi: 10.1002/nau.20328. [DOI] [PubMed] [Google Scholar]
- (7).Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001 Dec;185(6):1388–95. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- (8).Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996 Jul;175(1):10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- (9).Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: The Incontinence Impact Questionnaire and urogenital Distress Inventory; Qual Life Res; Continence Program in Women (CPW) Research Group. Oct, 1994. pp. 291–306. [DOI] [PubMed] [Google Scholar]
- (10).Ware JE, Jr., Sherbourne CD. The MOS 36-Item Short Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- (11).Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: Development and testing of the D1 valuation model. Med Care. 2005;43(3):203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- (12).Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J. 2006 Oct;17(5):520–4. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- (13).Rogers RG, Kammerer-Doak D, Darrow A, Murray K, Olsen A, Barber M, et al. Sexual function after surgery for stress urinary incontinence and/or pelvic organ prolapse: a multicenter prospective study. Am J Obstet Gynecol. 2004;191(1):206–10. doi: 10.1016/j.ajog.2004.03.087. [DOI] [PubMed] [Google Scholar]
- (14).Wren PA, Janz NK, Brubaker L, Borello-France D, Bradley CS, Burgio K, et al. Development of the Measure of Adaptations for Pelvic Symptoms (MAPS): the importance of incorporating the female patient’s voice. Appl Res Qual Life. 2006;1:239–51. [Google Scholar]
- (15).Hartanto VH, DiPiazza D, Ankem MK, Baccarini C, Lobby NJ. Comparison of recovery from postoperative pain utilizing two sling techniques. Can J Urol. 2003;10(1):1759–63. [PubMed] [Google Scholar]
- (16).Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189:98–101. doi: 10.1067/mob.2003.379. [DOI] [PubMed] [Google Scholar]
- (17).Centers for Medicaid and Medicare Services Fee schedule - general information. 2007 http://www.cms.hhs.gov/FeeScheduleGenInfo.
- (18).Drug Topics Red Book. Thompson Healthcare; Montvale, NJ: 2007. [Google Scholar]
- (19).Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- (20).Rubin DB. Multiple imputation for nonresponse in surveys. Wiley; New York, NY: 1987. [Google Scholar]
- (21).van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E ratios alongside a clinical trial. Health Econ. 1994;3(5):309–19. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- (22).Misrai V, Roupret M, Cour F, Chartier-Kastler E, Richard F. De novo urinary stress incontinence after laparoscopic sacral colpopexy. BJU Int. 2007 doi: 10.1111/j.1464-410X.2007.07291.x. published on line. [DOI] [PubMed] [Google Scholar]
- (23).Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000 Feb;163(2):531–4. [PubMed] [Google Scholar]
- (24).Romanzi LJ, Chaikin DC, Blaivas JG. The effect of genital prolapse on voiding. J Urol. 1999;161:581–6. [PubMed] [Google Scholar]
- (25).Haessler AL, Lin LL, Ho MH, Betson LH, Bhatia NN. Reevaluating occult incontinence. Curr Opin Obstet Gynecol. 2005 Oct;17(5):535–40. doi: 10.1097/01.gco.0000183530.03481.64. [DOI] [PubMed] [Google Scholar]
- (26).Albo ME, Richter HE, Brubaker L, Norton P, Kraus SP, Zimmern PE, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007 May 24;356(21):2143–55. doi: 10.1056/NEJMoa070416. [DOI] [PubMed] [Google Scholar]
- (27).Novara G, Ficarra V, Boscolo-Berto R, Secco S, Cavalleri S, Artibani W. Tension-free midurethral slings in the treatment of female stress urinary incontinence: A systematic review and meta-analysis of randomized controlled trials of effectiveness. Eur Urol. 2007;52:663–79. doi: 10.1016/j.eururo.2007.06.018. [DOI] [PubMed] [Google Scholar]
- (28).Anger JT, Saigal CS, Madison R, Joyce G, Litwin MS. Increasing costs of urinary incontinence among female Medicare beneficiaries. J Urol. 2006 Jul;176:247–51. doi: 10.1016/S0022-5347(06)00588-X. [DOI] [PubMed] [Google Scholar]
- (29).O’Sullivan AK, Thompson D, Drummond MF. Collection of health-economic data alongside clinical trials: Is there a future for piggyback evaluation? Value Health. 2005;8(1):67–79. doi: 10.1111/j.1524-4733.2005.03065.x. [DOI] [PubMed] [Google Scholar]
- (30).Torgerson DJ, Sibbald B. Understanding controlled trials. What is a patient preference trial? Brit Med J. 1998 Jan 31;316(7128):360. doi: 10.1136/bmj.316.7128.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cetinel B, Demirkesen O. Risk factors influencing the complication rates of tension-free vaginal tape-type procedures. Curr Opin Obstet Gynecol. 2005 Oct;17(5):530–4. doi: 10.1097/01.gco.0000178826.61013.73. [DOI] [PubMed] [Google Scholar]
- (32).Horng S, Miller FG. Is placebo surgery unethical? N Engl J Med. 2002 Jul 11;347(2):137–9. doi: 10.1056/NEJMsb021025. [DOI] [PubMed] [Google Scholar]
- (33).Horng S, Miller FG. Ethical framework for the use of sham procedures in clinical trials. Crit Care Med. 2003;31(3 Suppl):S126–S130. doi: 10.1097/01.CCM.0000054906.49187.67. [DOI] [PubMed] [Google Scholar]