Abstract
Ca2+ sensitivity of smooth muscle (SM) contraction is determined by CPI-17, an inhibitor protein for myosin light chain phosphatase (MLCP). CPI-17 is highly expressed in mature SM cells, but the expression level varies under pathological conditions. Here, we determined the expression of CPI-17 in embryonic SM tissues and arterial neointimal lesions using immunohistochemistry. As seen in adult animals, the predominant expression of CPI-17 was detected at SM tissues on mouse embryonic sections, whereas MLCP was ubiquitously expressed. Compared with SM α-actin, CPI-17 expression doubled in arterial SM from embryonic day E10 to E14. Like SM α-actin and other SM marker proteins, CPI-17 was expressed in embryonic heart, and the expression was down-regulated at E17. In adult rat, CPI-17 expression level was reduced to 30 % in the neointima of injured rat aorta, compared with the SM layers, whereas the expression of MLCP was unchanged in both regions. Unlike other SM proteins, CPI-17 was detected at non-SM organs in the mouse embryo, such as embryonic neurons and epithelium. Thus, CPI-17 expression is reversibly controlled in response to the phenotype transition of SM cells that restricts the signal to differentiated SM cells and particular cell types.
Keywords: smooth muscle contraction, smooth muscle development, vascular biology, vascular injury, CPI-17, myosin light chain phosphatase, myocardin, PKC, ROCK
Introduction
Vascular smooth muscle (SM) contraction is triggered by the phosphorylation of myosin light chain (MLC), which is controlled through two major signaling pathways: the activation of MLC kinase (MLCK) and the inhibition of MLC phosphatase (MLCP). The inhibition of MLCP occurs upon G-protein activation in response to agonist stimuli. MLCP activity is increased in response to nitric oxide production causing myosin dephosphorylation and SM relaxation. Thus, MLCP activity plays an important role in the regulation of both SM contraction and relaxation. MLCP is a heterotrimeric enzyme, consisting of a delta isoform of protein phosphatase-1 catalytic subunit (PP1∂), a myosin targeting subunit (MYPT1), and an accessory M21 subunit (Hartshorne et al. 2004). MYPT1 tethers PP1∂ to myosin filaments and determines the substrate specificity (Hartshorne et al. 2004). MLCP activity is suppressed via the bi-phasic phosphorylation of MYPT1 and an inhibitor protein for MLCP, CPI-17 (Dimopoulos et al. 2007). CPI-17, a 17-kDa cytosolic protein, potently and selectively inhibits MLCP in SM when it is phosphorylated at Thr38 (Eto et al. 1995; Eto et al. 2004). The phosphorylation of CPI-17 occurs in response to agonist stimulation, and it reversibly declines in response to NO/cGMP (Etter et al. 2001; Dimopoulos et al. 2007), suggesting a critical role of CPI-17 in the regulation of MLC phosphorylation and SM contraction.
In adult animals, MYPT1 is ubiquitously expressed (Okubo et al. 1994), whereas CPI-17 is predominantly expressed at SM tissues and brain in adult rat (Woodsome et al. 2001; Eto et al. 2002). Among SM tissues, CPI-17 expression is higher in tonic muscles, such as arteries, but lower in phasic muscles, such as ileum and urinary bladder. Importantly, the expression level of CPI-17 in each tissue correlates with the extent of PKC-induced contraction (Woodsome et al. 2001). Furthermore, arterial SM from adult farm chicken expresses undetectable level of endogenous CPI-17 and produces a negligible level of the force in response to G-protein activation (Kitazawa et al. 2004). Thus, the expression level of CPI-17 is a critical determinant of the extent of the myosin phosphorylation and SM contraction induced through G-protein-mediated signals.
Accumulating evidence shows that up- and down-regulation of CPI-17 occurs under pathological conditions, such as inflammation, asthma, diabetes, and hypoxia, resulting in abnormal SM contraction (Ohama et al. 2003; Dakshinamurti et al. 2005; Sakai et al. 2005; Chang et al. 2006). It is known that these pathological as well as physiological factors, such as an increase in blood flow, affect the differentiation state of SM cells causing the reversible transition between a contractile and proliferative phenotype (Owens et al. 2004). A group of transcriptional cofactors, myocardin and other family members, Myocardin Related Transcription Factors (MRTFs), play an important role in orchestrating the gene expression of SM marker proteins, including SM α-actin, SM myosin heavy chain (MHC), SM22 and calponin, through serum-response transcription factor (Wang and Olson. 2004). However, it is not yet known whether the phenotype transition of SM cells alters the expression of CPI-17. In the present study, we examined CPI-17 expression at three different stages during mouse embryo development and at de-differentiated neointimal lesions at the injured site of rat aorta. The results revealed SM differentiation-dependent regulation of CPI-17 expression, as well as the novel expression of CPI-17 in embryonic epithelium. This provides evidence that the regulation of MLCP activity is programmed at each locus through the transcription of CPI-17.
Materials and Methods
Mouse embryo tissue sections
Sagittal sections of paraffin embedded mouse whole embryo at embryonic day 10, 14, and 17 (E10, 14, 17) were purchased from Zyagen (Zyagen, San Diego, CA, USA).
Aorta injury model
Aorta injury was performed in Sprague Dawley rats (7 to 9 w) using a balloon catheter introduced through the carotid artery. Vessels were harvested 14 days later, as described previously (Jin et al. 2007). The animal protocol was approved by ICUC at University of Virginia.
Immunohistochemistry of sections of mouse embryo and aorta
All processes were done at room temperature. Deparaffinization and rehydration were performed by successive immersion for 10 min in each of the following solutions: xylene, ethanol (100%, 90%, 80%, 70%), and distilled water. After a 5-min permeabilization 0.1% Triton X-100 in PBS and then 1-h blocking with 3% BSA/PBS, sections were incubated for 2 h with primary antibody (1:100 in BSA/PBS). The primary antibodies used were rabbit anti-CPI-17 (Woodsome et al. 2001), mouse anti-SM α-actin (Sigma-Aldrich Inc., Saint Louis, MO, USA), mouse anti-MYPT1 (Babco, Richmond, CA, USA). Mouse embryo sections were incubated for 1 h with alkaline phosphatase (AP)-conjugated secondary antibody for rabbit or mouse IgG at 1:500 dilution. Dual color stain of mouse embryo section was carried out for 1 h with a mixture of the secondary antibody conjugated with AP (1:100) and cy5 (1:500). Rat aorta sections were stained for 1 h with a mixture of Cy3- and Cy5-conjugated secondary antibodies (1:500). Specimen AP was visualized with Vector Red AP Substrate Kit I that develop visible red color as well as red fluorescence (Vector laboratories Inc., Burlingame, CA, USA). Bright field color images of mouse whole embryo with red stain of AP substrate kit (Fig. 1) were taken using LeicaMZFLIII (Leica Microsystems Inc., Bannockburn, IL, USA) with a color CCD camera. Fluorescence images were captured using Olympus IX70 (Olympus America Inc., NY, USA) equipped with CoolSNAP ES2 CCD camera (Roper Sci), CARV II spinning desk confocal unit (BD Biosciences) and Cy3/Cy5 and GFP/Texas Red filter sets from Chroma. (Fig. 2–4). Image capturing, densitometry of fluorescence intensity, and digital image processing were carried out using IPLab software (BD Biosciences). Pseudo colors, green for SM α–actin, and red for CPI-17 and MYPT1, were given for all the fluorescence images. There was no noticeable auto-fluorescence under Cy3, Texas Red and Cy5 filters, except for the signal derived from red blood cells in arterial lumen, which was used as a marker to find arteries. The staining without primary antibody gave no detectable fluorescence signal. Representative images are shown in the figures. 3,3’-diaminobenzidine (DAB)/Hematoxylin double stain was performed using DAB substrate kit and VECTOR Hematoxylin (VECTOR laboratories Inc., Burlingame, CA, USA). Color images were captured using Nikon TS100 inverted microscope with Coolpix 995 digital camera.
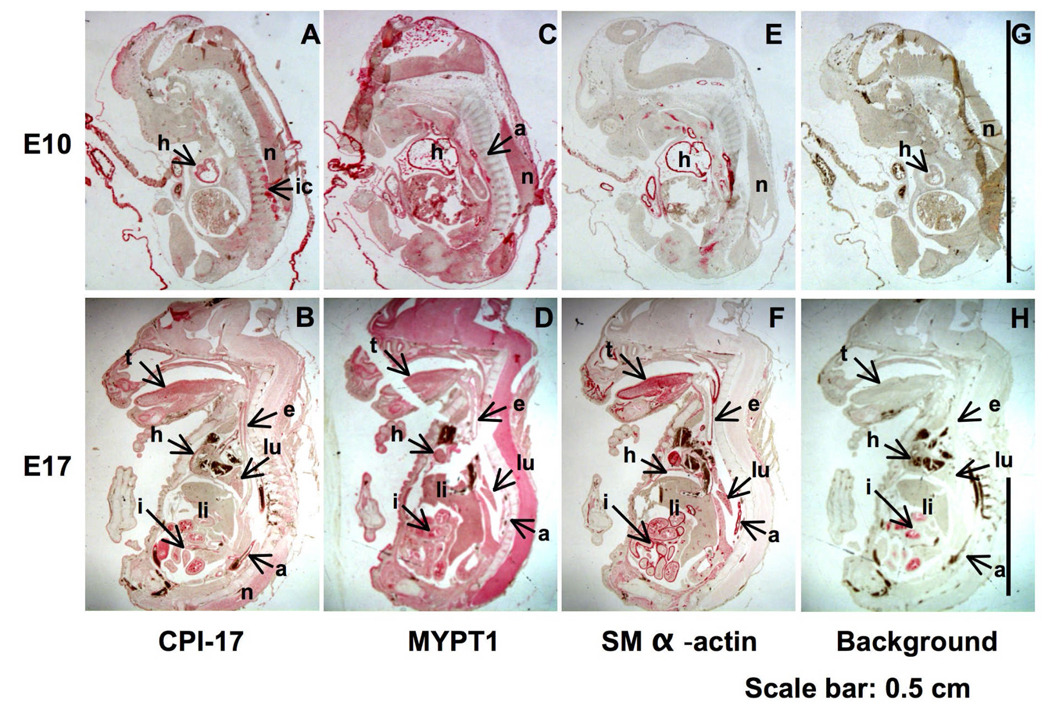
Fig. 1. Immunohistochemistry showing the presence of CPI-17, MYPT1, and SM α–actin in tissue sections of mouse embryos.
Sagittal sections of mouse embryo at E10 and E17 were stained with anti-CPI-17 (A, B), MYPT1 (C, D), and SM α–actin (E, F) or without primary antibody as background (G, H). Red color was developed for 7 min (CPI-17) and for 6 min (SM α–actin and MYPT1). a, aorta; e, esophagus; h, heart; i, intestine; ic, intercostal muscle; li, liver; lu, lung; n, neuronal cells; t, tongue
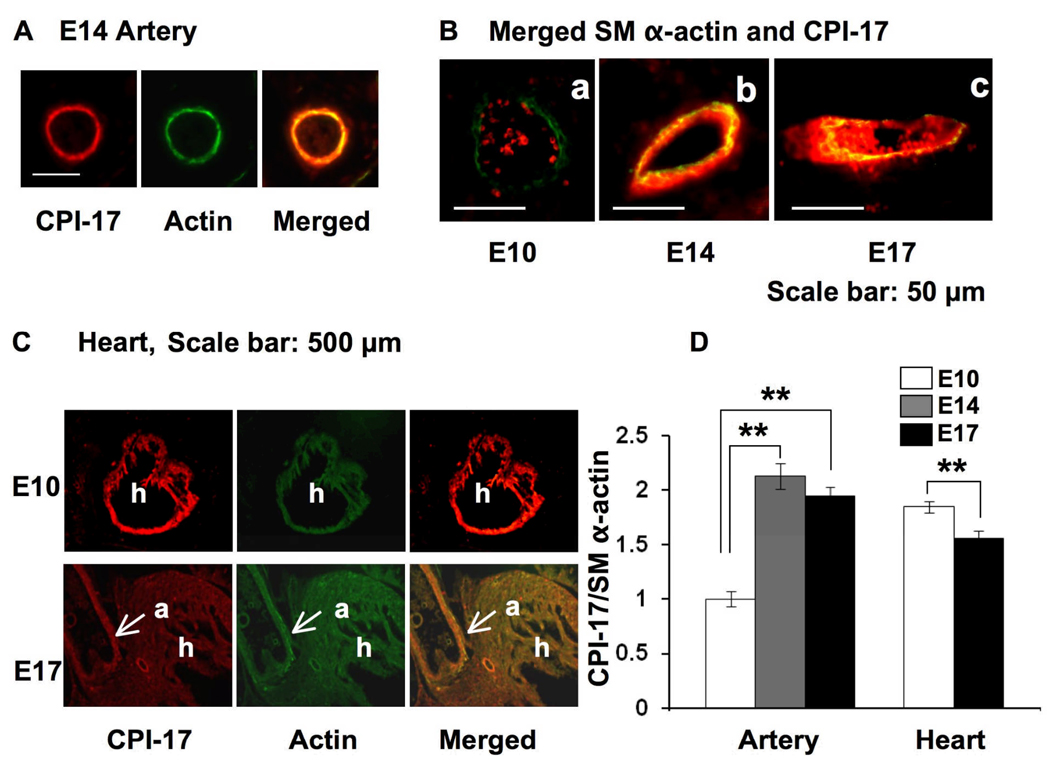
Fig. 2. Immunofluorescence of CPI-17 and SM α–actin in artery and heart in sagittal sections of mouse embryo.
All the samples were stained under identical conditions including a 3.5-min color development. (A) Dual color Images for the presence of CPI-17 (red) and SM α–actin (green) in artery at E14. (B) Merged images of CPI-17 and SM α–actin in artery. The dots in the lumen are auto-fluorescence from red cells. (C) The expression of CPI-17 and SM α–actin in embryonic heart at E10 and 17. a, aorta; h, heart (D) The ratio of CPI-17 expression to SM α–actin in arterial SM and cardiac muscle. Mean values ± SEM were obtained from the staining intensity of CPI-17 normalized to that of SM α–actin at randomly-picked regions of 10 arteries in the slice of E10, 14, 17 and 10 different spots in cardiac muscle of the E10 and 17. ** indicates statistical significance (p<0.01).
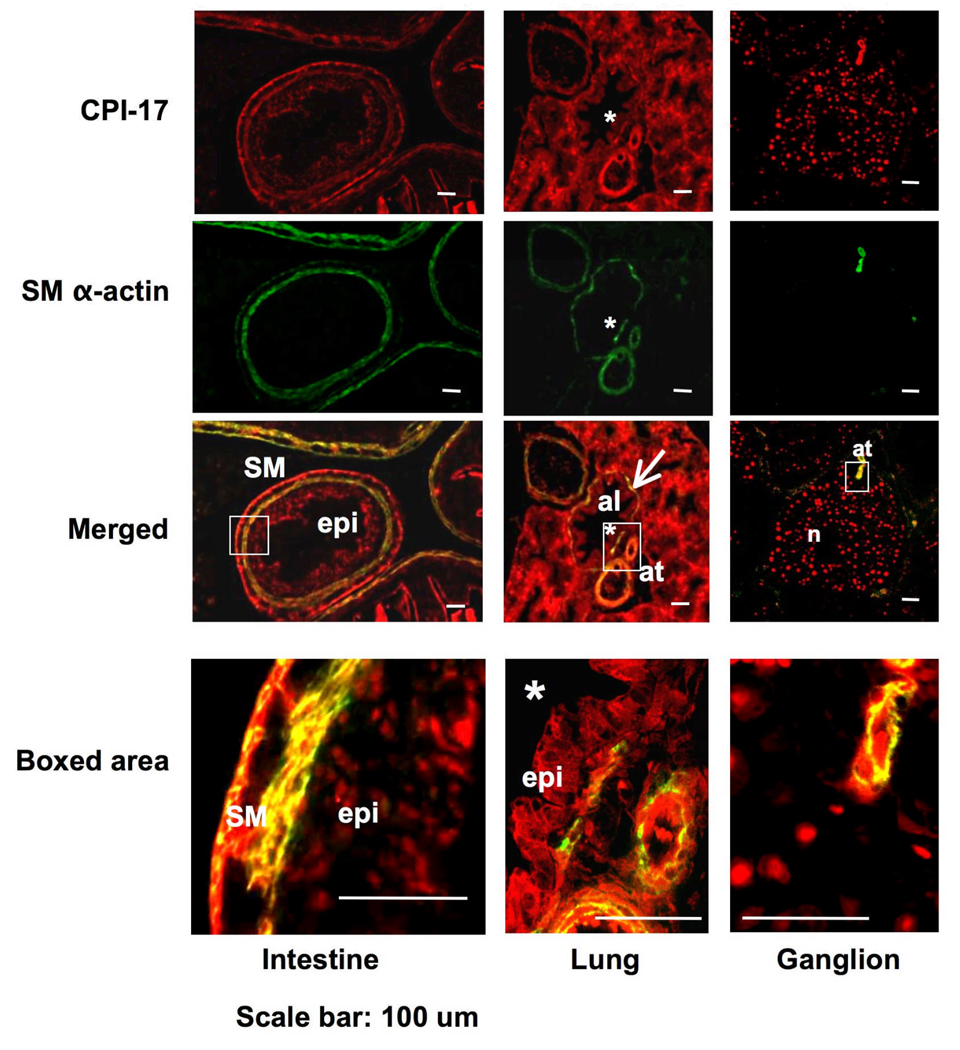
Fig. 4. Immunofluorescence staining for the presence of CPI-17 in non-arterial embryonic organs.
An E17 embryo section was subjected to dual color immunofluorescence for the expression of CPI-17 (red) and SM α–actin (green). Confocal images focused on intestine (left), lung (center), and neuronal area (right) are shown. Color was developed for 7 min. Bottom panels show images with higher magnifications at the boxed area. SM, SM layer; epi, epithelial cells; *, alveolus lumen; at, artery; n, neuronal cells; b, precursor bone cells.
Statistical Analysis
Student's t-test analysis assuming an equal distribution was carried out using Excel software to compare to two groups. P values < 0.01 were regarded as statistically significant and indicated with ** in the figures.
Results
Expression of CPI-17 in mouse embryo
Fig 1 shows immunostaining for CPI-17 (Fig. 1 A and B), MYPT1 (C, D), and SM α–actin (E, F) in mouse embryonic slices at E10 and 17. In E10 slice, CPI-17 signal (red) was exclusively detected at heart (h), intercostal muscle (ic), and neuronal tissues (n) (Fig. 1, panel A). The expression of CPI-17 was more prominent in E17 embryonic SM tissues such as aorta (a), tongue (t) esophagus (e), intestine (i) lung (lu), heart (h), as well as neuronal tissues (n) (Panel B). There is no significant background except for intestine in E17 (G, H), which is probably derived from endogenous alkaline phosphatases. On the other hand, MYPT1 existed in all organs examined in the E10 and 17 slices, indicating the ubiquitous expression of MLCP (Fig. 1 C, D). Overall, the expression pattern of CPI-17 was similar to that of SM α–actin (E, F) and SM MHC (data not shown). However, there were significant differences between the expression of CPI-17 and SM α–actin at multiple loci (also see Fig. 4), such as neuronal tissues (n), where the expression of SM α–actin was negligible.
Expression of CPI-17 in arterial SM and cardiac muscle during embryonic development
The expression levels of CPI-17 at embryonic heart and artery were determined by semi-quantitative immunofluorescence (Fig. 2). SM α–actin was used as a control, because it is expressed at the early stage during vascular development and the expression is insensitive to environmental factors (Aikawa et al. 1993; Owens et al. 2004). The dual-color stain of mouse embryonic slices showed co-expression of CPI-17 (red) and SM α–actin (green) in arterial SM cells (Fig. 2A, and B) and cardiac muscle (panel C). The relative expression level of CPI-17 appeared to be higher in the arterial SM at E14, compared to E10 (Fig. 2B). The staining intensity of CPI-17 varied in each artery, although it did not depend on the location or the size of artery. Fig. 2D shows the relative expression of CPI-17 normalized with the level of SM α–actin. The ratio of CPI-17 to SM α-actin doubled in arterial SM from E10 to E14 and sustained at E17. SM marker proteins are expressed in embryonic cardiac muscle due to the early activity of myocardin (Wang and Olson. 2004). Indeed, both CPI-17 and SM α–actin were detected in cardiac muscle at E10 and E17 (Fig. 2C). In the E10 slice, the ratio of CPI-17 expression to SM α–actin was 2-fold higher in heart compared to arteries. However, unlike arterial SM, the relative expression of CPI-17 significantly decreased from E10 to E17 (Fig. 2D).
Expression of CPI-17 and MLCP in the neointimal region in an aorta injury model
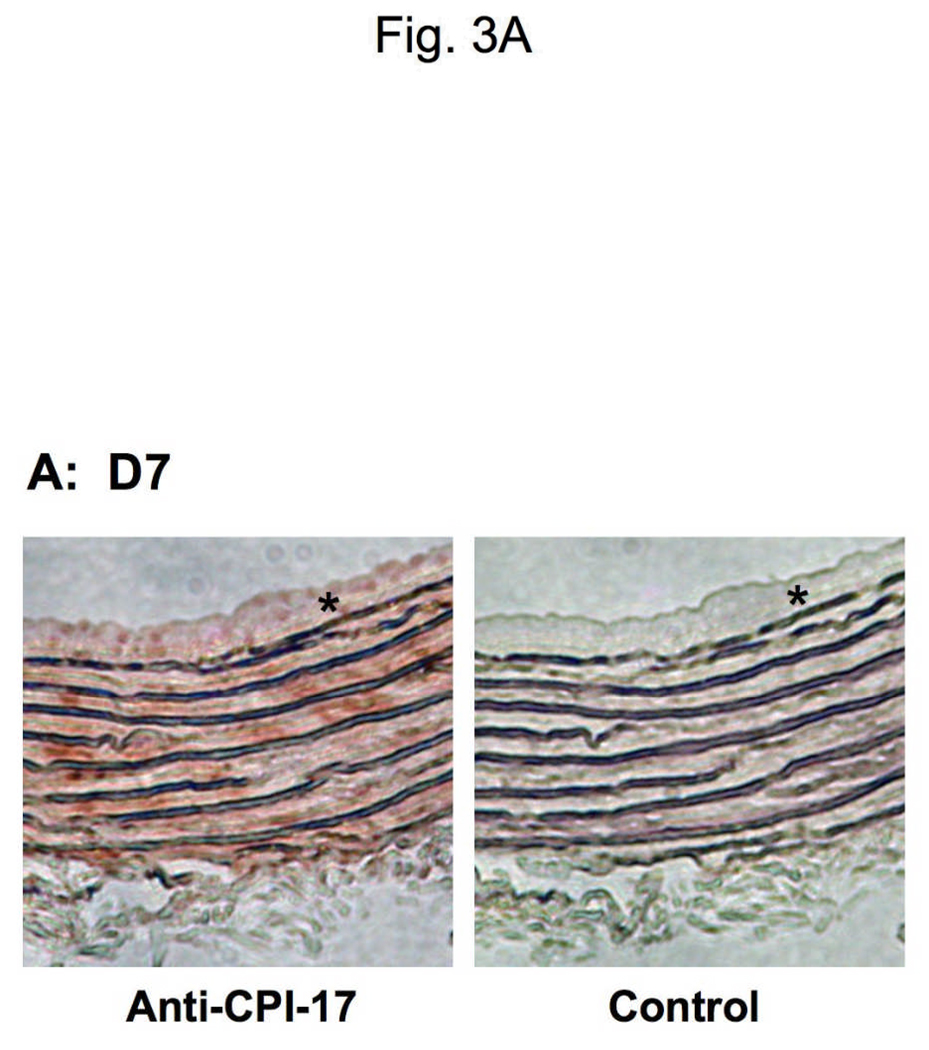
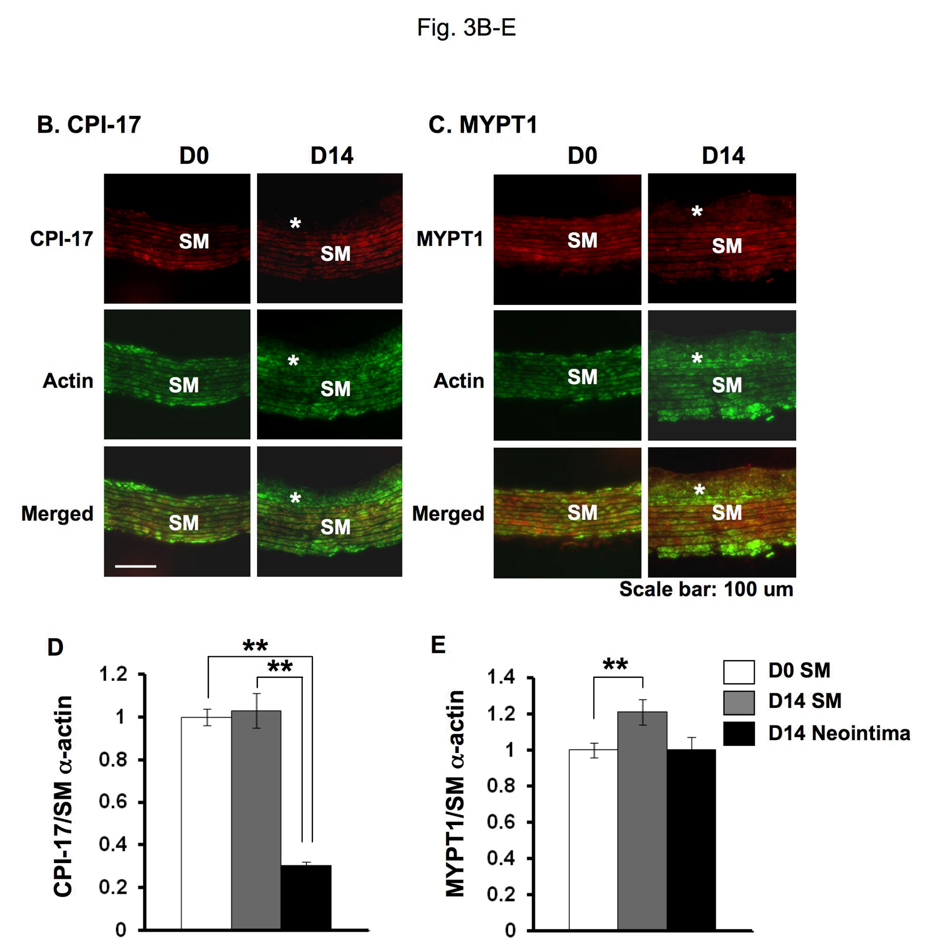
The neointimal formation of adult rat aorta was initiated by removing the endothelium using a wire catheter. Sections were subjected to immunohistochemistry with DAB/hematoxylin stain (Fig. 3 and Supplemental figure S1). After endothelium removal SM cells outgrew from the SM layers between elastin sheets to the lumen, where the cells are disoriented (Fig. 3A). The neointimal lesions were visible at day 7 (D7) and thickened at day 14 (D14) after the surgery (Jin et al. 2007). The conventional immunohistochemistry showed the expression of CPI-17 at the neointimal lesions, whereas the signal at the neointimal cells was weaker, compared with SM layers (Fig. 3A left). No signal was detected in the specimen without the primary antibody (Fig. 3A, right). CPI-17 stain was more prominent in the neointima, when intense color development was carried out (Supplemental figure S1). Semi-quantitative dual-color immunofluorescence was performed to determine the expression of CPI-17 in the SM layer and the neointima (Fig. 3B and C). As reported previously, SM α–actin was expressed in the cells at the neointima as well as in those at the SM layer (Fig. 3B and C, green) (Aikawa et al. 1993). The expression levels of CPI-17 were unchanged in SM layer of the untreated D0 control and the injured D14 aorta (Fig. 3C, marked as SM). The ratio of CPI-17 expression to SM α–actin in the neointima (Fig. 3C, asterisk) declined to 30 ± 2 % of that of the SM layer at D0 and D14 (p<0.01) (Fig. 3D). In contrast, MYPT1 expression was not significantly different in the neointima and media (Fig. 3E). Thus, the expression of CPI-17 is reversibly regulated in response to SM differentiation and de-differentiation.
Fig. 3. Expression of CPI-17, MYPT1, and SM α–actin in neointimal lesions of rat adult aorta.
(A) DAB/hematoxylin dual-color stain of transverse sections of aorta at 7 days after the surgery for denuding the endothelium. Staining was carried out using with anti-CPI-17 and peroxidase-labeled secondary antibody. Right panel shows control experiments without the primary antibody. (B, C) Indirect immunohistochemistry with Cy3- and cy5-conjugated antibodies showing the presence of CPI-17 (B) or MYPT1 (C) in red with SM α–actin in green in transverse sections of aorta before (D0) and at 14 days after the surgery for denuding the endothelium (D14). Asterisk indicates the neointima lesion and SM indicates smooth muscle layers in the media. (D, E) The relative expression level of CPI-17 (D) and MYPT1 (E). Mean values ± SEM were obtained from the ratio of intensity at 20–28 different spots of SM layer and neointima of injured aorta.
Expression of CPI-17 in non-arterial organs
CPI-17 was also detected in embryonic intestine, lung, ganglion (Fig. 4) and other tissues such as trachea and esophagus (data not shown). Bottom panel indicates the boxed area in the merged image. In intestine, CPI-17 was equally and prominently expressed in both external and internal layers of SM in the intestinal wall, whereas SM α–actin was present in both layers but predominantly expressed in the internal SM layer (Fig. 4 left). Furthermore, a significant amount of CPI-17 was detected in intestinal epithelium, where SM α–actin expression was negligible (Fig. 4, left, box). In the condensed embryonic lung, the expression of CPI-17 was prominent in most of cells including epithelial cells of alveoli (al) (Fig. 4, center), whereas SM α–actin was exclusively expressed in SM layers of artery (at), and bronchiole (arrow). The epithelial expression of CPI-17 at the alveolus was evident in the magnified image (Fig. 4, bottom row center). Epithelial staining of CPI-17 was also observed in trachea and esophagus (data not shown). The punctate staining pattern of CPI-17 in Fig. 4 (right) indicates the expression in neuronal cells (n, see also boxed area lower row right) and precursor bone cells (b), where the expression of SM α–actin was undetectable, whereas arterial SM layer shows both SM α–actin and CPI-17 in the same section (Fig. 4, right, at, see also boxed area lower row right). No expression of SM MHC was detected in epithelium, neuronal or bone cells (data not shown). Thus, the expression of CPI-17 is apparently distinguishable from that of SM marker proteins at particular loci.
Discussion
CPI-17 was up- and down-regulated in response to SM differentiation and de-differentiation. In contrast, the expression of MYPT1 was constant at any locus and stage, unlike SM-type MLCK, whose promoter is controlled via a myocardin signal (Yin et al. 2006). The fluctuation of CPI-17 expression causes variation in the ratio of CPI-17 expression to MLCP at each locus. (DELETED) A lack of the expression or the phosphorylation of CPI-17 via Ca2+-dependent PKC results in the slower force development of SM tissues (Kitazawa et al. 2004; Dimopoulos et al. 2007). Thus, the ratio of CPI-17 to MLCP determines the rapidity and magnitude of the agonist-induced SM contraction. It is known that heart rate and blood flow of embryo significantly increase from E10 to E14 (Keller et al. 1996). Therefore, the histological data suggest that the embryonic arterial SM cells increase the contractility through the expression of CPI-17 in response to a gain of cardiac function and an increase in blood flow. In contrast, CPI-17 is down-regulated in the proliferative phenotype of SM cells, such as cells at neointima and cultured SM cells. It is possible that mechanical stresses and/or agonist signals alter the signal into MLCP regulation upon the phenotype transition, and this will be a subject for further study.
Immunohistochemistry detected significant levels of CPI-17 expression in the embryonic heart, although CPI-17 is negligible in adult rat heart (Woodsome et al. 2001). The relative expression of CPI-17 was decreased from E10 to E17, suggesting that CPI-17 gene becomes suppressed after the maturation of cardiac muscle. It is consistent with the other SM marker proteins whose expression is turned off in adult heart (Ruzicka and Schwartz. 1988). MYPT1 was also detected in embryonic heart (Fig. 1C and D), suggesting that CPI-17 may be involved in the regulation of MLCP activity in embryonic cardiac muscle, although the roles of myosin phosphorylation and MLCP in embryonic heart are still unknown. In contrast, embryonic skeletal muscle at E10 does not express CPI-17. Wu et al reported that MYPT1 gene undergoes down-regulation upon the differentiation of myoblasts in parallel to the up-regulation of MYPT2, a close member in the MYPT1 family (Wu et al. 2003). Indeed, MYPT1 is not detected in adult skeletal muscle (Okubo et al. 1994). The phosphorylation of myosin II is required for the myotube formation of myoblasts (Wu et al. 2003). Consistent with this, the expression of MYPT1 was evident in the embryonic skeletal muscle at E10 and E17. CPI-17 seems to be unnecessary for the regulation of MLCP in myotube formation.
As seen in adult animals, CPI-17 is expressed in embryonic neuronal cells. Furthermore, this is the first report that a significant amount of CPI-17 is detected in embryonic epithelial cells. The epithelial expression of CPI-17 in embryonic lung disagrees with the results from the adult tissue (Woodsome et al. 2001). One possibility is that the condensed embryonic lung emphasizes the CPI-17 staining at epithelium, compared with inflated adult lung. Indeed, a trace amount of CPI-17 was detected in lung epithelium from adult rats (Woodsome et al. 2001). The role of CPI-17 in epithelial cells is unknown. Recently, a group of tumor cells were reported to express a high amount of CPI-17 that causes hyper-phosphorylation of merlin/NF2 oncogene product and transformation of the cells (Jin et al. 2006). These tumor cells are derived from epithelial cells. It is possible that CPI-17 functions in the regulation of epithelial cell proliferation. Our findings will provide new avenues for research studying the role of CPI-17 in the regulation of MLCP at non-SM cells.
The most important finding of the present study is that the expression of CPI-17 is reversibly regulated in arterial SM cells. It is similar to other SM marker proteins, such as SM myosin heavy chain, that is regulated upon the phenotype transition of SM via myocardin and MRTFs (Aikawa et al. 1993; Wang and Olsen 2004). However, it is unclear whether CPI-17 expression is controlled via myocardin signal. There are significant differences in the expression profiles of CPI-17 and SM α–actin. Dysregulation of CPI-17 expression can cause disorders in the regulation of SM contraction, so that the molecular basis of the gene regulation seems to deserve further investigation.
Acknowledgements
We thank Dr. Marion J. Siegman for critical reading and valuable discussions, and Dr. Shiu-Ying Ho for the technical support. This work was supported by grants from NHLBI HL83261, PA C.U.R.E. grant (to M.E.), NHLBI HL48807 (to M.E. and A.V.S.), and Korea Research Foundation (KRF-2007-357-E00004) funded by Korean Government (MOEHRD) (to J.I.K)
References
- Aikawa M, Sivam PN, Kuro-o M, Kimura K, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res. 1993;73:1000–1012. doi: 10.1161/01.res.73.6.1000. [DOI] [PubMed] [Google Scholar]
- Chang S, Hypolite JA, DiSanto ME, Changolkar A, Wein AJ, Chacko S. Increased basal phosphorylation of detrusor smooth muscle myosin in alloxan-induced diabetic rabbit is mediated by upregulation of Rho-kinase beta and CPI-17. Am J Physiol Renal Physiol. 2006;290:F650–F656. doi: 10.1152/ajprenal.00235.2005. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti S, Mellow L, Stephens NL. Regulation of pulmonary arterial myosin phosphatase activity in neonatal circulatory transition and in hypoxic pulmonary hypertension: a role for CPI-17. Pediatr Pulmonol. 2005;40:398–407. doi: 10.1002/ppul.20290. [DOI] [PubMed] [Google Scholar]
- Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res. 2007;100:121–129. doi: 10.1161/01.RES.0000253902.90489.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto M, Bock R, Brautigan DL, Linden DJ. Cerebellar long-term synaptic depression requires PKC-mediated activation of CPI-17, a myosin/moesin phosphatase inhibitor. Neuron. 2002;36:1145–1158. doi: 10.1016/s0896-6273(02)01107-8. [DOI] [PubMed] [Google Scholar]
- Eto M, Kitazawa T, Brautigan DL. Phosphoprotein inhibitor CPI-17 specificity depends on allosteric regulation of protein phosphathase-1 by regulatory subunits. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8888–8893. doi: 10.1073/pnas.0307812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem. 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- Etter EF, Eto M, Wardle RL, Brautigan DL, Murphy RA. Activation of Myosin Light Chain Phosphatase in Intact Arterial Smooth Muscle during Nitric Oxide-induced Relaxation. Journal of Biological Chemistry. 2001;276:34681–34685. doi: 10.1074/jbc.M104737200. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ, Ito M, Erdodi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004;279:37211–37214. doi: 10.1074/jbc.R400018200. [DOI] [PubMed] [Google Scholar]
- Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–579. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- Jin L, Kern MJ, Otey CA, Wamhoff BR, Somlyo AV. Angiotensin II, focal adhesion kinase, and PRX1 enhance smooth muscle expression of lipoma preferred partner and its newly identified binding partner palladin to promote cell migration. Circ Res. 2007;100:817–825. doi: 10.1161/01.RES.0000261351.54147.de. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Dedicated Myosin Light Chain Kinases with Diverse Cellular Functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- Keller BB, MacLennan MJ, Tinney JP, Yoshigi M. In vivo assessment of embryonic cardiovascular dimensions and function in day-10.5 to -14.5 mouse embryos. Circ Res. 1996;79:247–255. doi: 10.1161/01.res.79.2.247. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Polzin AN, Eto M. CPI-17-deficient smooth muscle of chicken. J Physiol. 2004;557:515–528. doi: 10.1113/jphysiol.2004.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem. 2003;278:48794–48804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]
- Okubo S, Ito M, Takashiba Y, Ichikawa K, Miyahara M, Shimizu H, Konishi T, Shima H, Nagao M, Hartshorne DJ, et al. A regulatory subunit of smooth muscle myosin bound phosphatase. Biochem Biophys Res Commun. 1994;200:429–434. doi: 10.1006/bbrc.1994.1467. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Ruzicka DL, Schwartz RJ. Sequential activation of alpha-actin genes during avian cardiogenesis: vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J Cell Biol. 1988;107:2575–2586. doi: 10.1083/jcb.107.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Chiba Y, Hirano T, Misawa M. Possible involvement of CPI-17 in augmented bronchial smooth muscle contraction in antigen-induced airway hyper-responsive rats. Mol Pharmacol. 2005;68:145–151. doi: 10.1124/mol.104.004325. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Wang D, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Current Opinion in Genetics & Development. 2004;14:558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Erdodi F, Muranyi A, Nullmeyer KD, Lynch RM, Hartshorne DJ. Myosin phosphatase and myosin phosphorylation in differentiating C2C12 cells. J Muscle Res Cell Motil. 2003;24:499–511. doi: 10.1023/b:jure.0000009810.36038.53. [DOI] [PubMed] [Google Scholar]
- Yin F, Hoggatt AM, Zhou J, Herring BP. 130-kDa smooth muscle myosin light chain kinase is transcribed from a CArG-dependent, internal promoter within the mouse mylk gene. Am J Physiol Cell Physiol. 2006;290:C1599–C1609. doi: 10.1152/ajpcell.00289.2005. [DOI] [PubMed] [Google Scholar]