Abstract
The epithelial-derived cytokine thymic stromal lymphopoietin (TSLP) has important roles in the initiation of allergic airway inflammation and activation of dendritic cells. We have shown that the human TSLP gene is regulated in an NFκB-dependent manner; however the factors that negatively regulate TSLP expression are not known. In this paper we demonstrate that 9-cis retinoic acid (9-cisRA) is a negative regulator of TSLP expression in airway epithelial cells. This inhibition is manifested as a block in the IL-1β-mediated recruitment of NFκB to the human TSLP promoter. 9-cisRA-mediated inhibition is not restricted to TSLP gene expression, but rather reflected a general inhibition of NFκB activation as other NFκB-regulated-genes were also inhibited in a similar manner by 9-cisRA treatment. Taken as a whole, these data demonstrate that inhibition of IL-1β-dependent genes by active RXR involves antagonism of NFκB signaling.
INTRODUCTION
Thymic stromal lymphopoietin (TSLP) is an IL-7-like cytokine implicated in airway inflammatory diseases such as asthma. For example, mice that express a lung-specific TSLP transgene develop a spontaneous airway inflammatory disease with characteristic features found in human asthma, and human asthmatics display elevated TSLP levels in the lung(1,2). In addition, mice that lack the TSLPR fail to develop inflammation in an antigen-driven model of asthma(1). TSLP is expressed primarily by epithelial cells, and is induced in airway epithelial cells exposed to proinflammatory mediators, including IL-1β, TNF-α, and selected TLR agonists, and activation of NFκB is a critical regulator for inflammation-induced expression of TSLP(3).
Nuclear receptor (NR)s are members of a superfamily of ligand-dependent transcription factors that regulate diverse aspects of reproduction, development, homeostasis and immune responses by both positively and negatively regulating gene expression(4–6). Responses to retinoic acid and its isomers are mediated by 2 members of this family, the retinoic acid receptors (RAR), and the retinoid X receptors (RXR), for which 9-cis retinoic acid (9-cisRA) acts as a high-affinity ligand(7). There are three RXR genes, coding for RXRα, -β, and -γ, which are obligate heterodimerization partners for many members of the nuclear receptor family, including RAR(5). In vivo studies using knockout animals showed that disruption of RXRα lead to embryonic lethality, while deficiencies in RXRβ or γ were less severe(8–11). Studies using conditional knockouts showed that keratinocyte-selective ablation of RXRα and RXRβ (referred to as RXRαβep−/− mice) triggered an inflammatory response similar to human atopic dermatitis (AD). Interestingly, TSLP expression was rapidly induced in keratinocytes of RXRαβep−/− mice(12). This study supported previous work showing that TSLP is important in the initiation of skin inflammation, and suggested that RXRα and β are involved in regulating TSLP expression in the skin.
In this study, we have used IL-1β signaling as a model system to investigate mechanisms by which different members of nuclear receptor superfamily repress TSLP gene expression. The RXR agonist 9-cisRA was found to repress IL-1β-mediated TSLP gene expression through inhibition of NFκB, not through direct binding to the TSLP gene promoter. These findings demonstrate that inhibition of NFκB-dependent genes by RXR involves direct antagonism of NFκB signaling.
MATERIALS AND METHODS
Cells and chemicals
The 16HBEo¯ cell line was a gift from Dieter C. Gruenert (California Pacific Medical Research Institute, San Francisco, CA) and were grown in bronchial/tracheal epithelial cell basal medium (Lonza, MD). The HEK293 cell line was grown in DMEM with 10% FCS, and penicillin, and streptomycin (100 U/ml). Recombinant human IL-1β was purchased from R&D System (Minneapolis, MN). Antibodies to normal rabbit IgG, NFκB p50 (sc-114), NFκB p65 (sc-109), RXRα (D-20) and RXR (ΔN 197) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FLAG M2 mAb, RXR agonist 9-cisRA, LXR agonist GW3965, PPARα agonist GW7647, PPARδ agonist GW0742, PPARγ agonist GW1929 and Dexamethasone were purchased from Sigma-Aldrich (St. Louis, MO). Anti-HA mAb was purchased from Roche (Penzberg, Germany).
Transfection assay
16HBEo¯ cells (3 × 105) were seeded into 6-well plates and transfected 24 h later with Mirus transfection reagent (Mirus Bio Coporation, WI). Each well was transfected with 1 µg of reporter plasmid and 1 µg of a β-galactosidase plasmid (pRSV-β-Gal). After transfection, cells were cultured for 19 h then treated with 1 ng/ml IL-1β in the absence or presence of nuclear receptor agonists. Cells were harvested 5 h after stimulation, lysed in 100 µl of lysis buffer (Promega, WI), and luciferase activity measured. Relative luciferase activity was given as the ratio of relative light units to relative β-Galactosidase units. In each experiment, samples were analyzed in triplicate, and each experiment was repeated in at least three independent experiments.
Real-time quantitative PCR
Total RNA and cDNA synthesis was prepared as previous described(3). The primers used were as follows: hTSLP (5′-TAGCAATCGGCCACATTGCC-3′ and 5′-CTGAGTTTCCGAATAGCCTG-3′), hGAPDH (5′-ATGGCACCGTCAAGGCTGAG-3′ and 5′-GCTAAGCAGTTGGTGGTGCA-3′). Real-time PCR reaction was carried out using Plantinum SYBR Green qPCR Super Mix-UDG with ROX (Invitrogen). Amplification was performed on ABI 7700 Sequence Detector (Applied Biosystems, Foster City, CA). The levels of TSLP mRNA were normalized with GAPDH mRNA as previously described(3).
EMSA
Nuclear extracts were prepared as previously described(13). The sequences of double strand oligonucleotides used as probes were as follows: NFκB consensus motif, 5′-AGAGGATCTGTACAGGATGTTCTAGAT-3′; hTSLP NFκB motif, 5′-CTGCTAGGGAAACTCCATTATTAC-3′.
Coimmunoprecipitation (Co-IP) and Western blot analysis
HA-tagged RXR and FLAG-tagged p65 were transiently cotransfected into HEK293 cells using Mirus transfection reagent. Cells were cultured for 24 h then treated with either or both 1 ng/ml IL-1β and 1µM 9-cisRA for 30 min. After centrifugation, Cell lysates were immunoprecipitated with anti-FLAG or anti-HA mAbs, and resolved on 10% SDS-PAGE and transferred to membrane. The membranes were incubated with anti-FLAG or anti-HA mAbs, and visualized with western blotting luminal reagent (Santa Cruz).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed as previously described(14). 2.5 µg of anti-NFκB (sc-114, Santa Cruz), anti-RXR (sc-553, Santa Cruz) or normal rabbit IgG (Upstate Biotechnology, Lake Placid, NY) antibodies were used in immunoprecipitation experiments. Purified ChIP DNA was measured by real-time quantitative PCR using Plantinum SYBR Green qPCR Super Mix-UDG with ROX. PCR condition was 95°C for 10 min, followed by 40 cycles consisting of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s. The level of ChIP DNA was normalized with that of input DNA. In each experiment, samples were analyzed in triplicate. The primers used were as follows: hTSLP/NFκB (5′-GAGGGTCCAGAGCAATACAC-3′ and 5′-CCTCTCTGATATCCCTTCCA-3′), hTSLP/RXR (5′-CACTAGCCACTTCTCCTTAC-3′ and 5′-CCAAAGAACACCCTTCTGCT-3′), hiNOS/NFκB (5′-CCTGTAGCAGTGACGTCTGT-3′ and 5′-CTCAATGAGTGATGCTCTGG), and hDef-2/NFκB (5′-CTCACTCCATTCACACACTG-3′ and 5′-CACCAGGTAAGTGGCTGAAT).
Results and Discussion
RXR inhibits IL-1β-induced TSLP expression
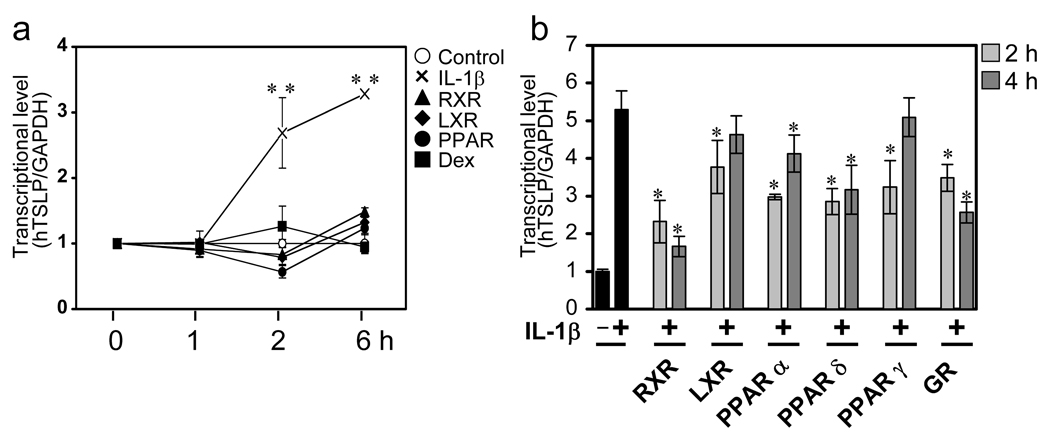
The increased TSLP expression in the skin of RXRαβep−/− mice suggested the possibility that TSLP gene expression was directly regulated by nuclear receptors. To test the effect of nuclear receptor agonists on TSLP gene expression, the human bronchial epithelial cell line 16HBEo¯ was stimulated with agonists for RXR (9-cisRA), LXR (GW3965), PPARγ(GW1929), GR (dexamethasone), as well as the inflammatory cytokine IL-1β. As previously shown, IL-1β treatment lead to an increase in TSLP mRNA levels(3). However, basal TSLP mRNA levels were not affected by treatment with RXR, LXR, PPARγ, or GR agonists (Fig. 1a). Next, we determined the effect on TSLP gene expression of co-treatment with IL-1β and these NR agonists, including three distinct classes of PPAR agonists (PPARα, PPARγ, PPARδ). 16HBEo¯ cells were stimulated with IL-1β in the absence or presence of nuclear receptor agonists for 2 and 4 hours, and TSLP gene expression was measured. Co-treatment with IL-1β plus each nuclear receptor agonist affected TSLP gene expression to varying degrees. Interestingly, treatment with 9-cisRA had the most dramatic effect, significantly reducing TSLP mRNA level at both time points (Fig. 1b).
Figure 1. Selective nuclear receptor agonists inhibit induction of human TSLP mRNA in response to IL-1β.
(a) IL-1β but not nuclear receptor agonists induce expression of TSLP mRNA. 16HBEo¯ cells were stimulated with 1µM RXR (9-cisRA), LXR (GW3965), PPARγ (GW1929), and GR (dexamethasone) or 1 ng/ml IL-1β for the indicated time course, respectively. ** p < 0.01 comparing TSLP mRNA levels in IL-1β-treated cells as compared to NR agonists alone.
(b) RXR agonist, 9-cisRA, suppress IL-1β induced TSLP mRNA expression. Cells were treated with IL-1β in the absence or presence of 1µM RXR, LXR, PPARα, PPARδ, PPARγ, and GR agonists for the indicated time course, respectively. Cells were harvested and measured for mRNA level by real-time quantitative PCR. TSLP mRNA levels were normalized to GAPDH. Data are the mean ± SD of triplicate data points from a representative experiment. *p < 0.05, statistically significant difference compared with IL-1β treatment.
Activation of RXR agonist inhibits NFκB signaling
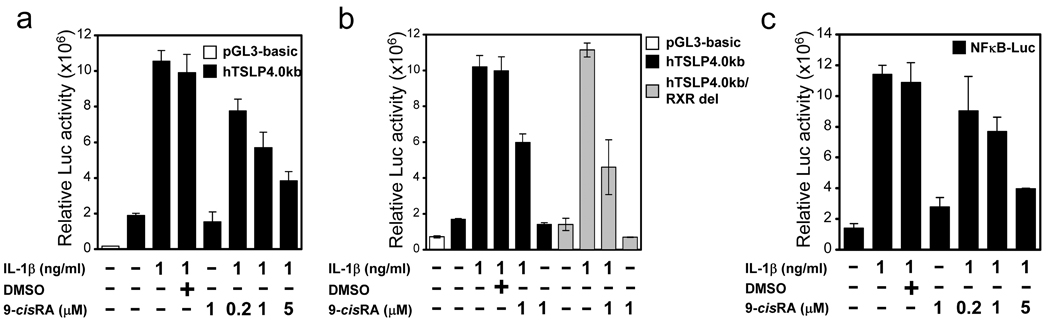
We next investigated the mechanism by which RXR inhibits TSLP expression, using reporter plasmids containing the human TSLP gene promoter(3). 16HBEo¯ cells were transfected with a luciferase reporter plasmid containing 4 Kb of the human TSLP promoter, which contains the IL-1β-responsive NFκB site(3), and stimulated with IL-1β in the absence or presence of 9-cisRA. IL-1β treatment led to a 5-fold increase in TSLP promoter activity, while treatment with 9-cisRA alone had no effect. However, in a dose dependent fashion, 9-cisRA was capable of reducing the IL-1β-mediated activation of the human TSLP promoter (Fig. 2a). Li et al(12,15) identified a putative RXR binding site in the human TSLP gene promoter at position −3912 ~ −3900 (relative to the start of transcription). To determine whether this site was involved in the 9-cisRA-dependent repression of TSLP expression, site-directed mutagenesis was used to eliminate it in the human TSLP gene reporter. No difference was found in the ability of 9-cisRA to inhibit IL-1β-mediated activation of the mutated reporter (Fig. 2b). These data demonstrate that this site is not required for RXR-mediated inhibition of IL-1β-induced TSLP gene expression. As we had previously shown that activation of the human TSLP promoter was NFκB-dependent, this result led us to determine whether NFκB signaling was affected by 9-cisRA treatment. To test this hypothesis, we evaluated the effect of 9-cisRA on the IL-1β-mediated activation of an NFκB reporter plasmid (pNFκB-Luc;(16)). As shown in Fig. 2c, 9-cisRA effectively inhibited the IL-1β-mediated activation of this reporter in a dose dependent fashion. These results demonstrate that the effect of RXR on TSLP gene expression is mediated through inhibition of NFκB, not by direct action on the TSLP gene promoter.
Figure 2. 9-cisRA inhibition of NFκB activation.
(a) 9-cisRA-mediated, dose-dependent inhibition of a luciferase reporter containing the human TSLP promoter. (b) 9-cisRA inhibition of a luciferase reporter containing human TSLP promoter with putative RARE site deleted. (c) Dose-dependent inhibition by 9-cisRA of a luciferase reporter containing multiple NFκB binding sites. For each set of experiments, 16HBEo¯ cells were transiently transfected with the indicated luciferase constructs, and 19 hr after transfection cells were incubated for 5 h in 1 ng/ml IL-1β in the absence or presence of 9-cisRA, at the indicated concentration. At that time cells were harvested and lysates prepared for determination of luciferase activity. Luciferase activity in the whole cell lysate was normalized to β-galactosidase activity. Data are the mean ± SD of triplicate data points from a representative experiment.
RXR activation inhibits DNA binding by NFκB
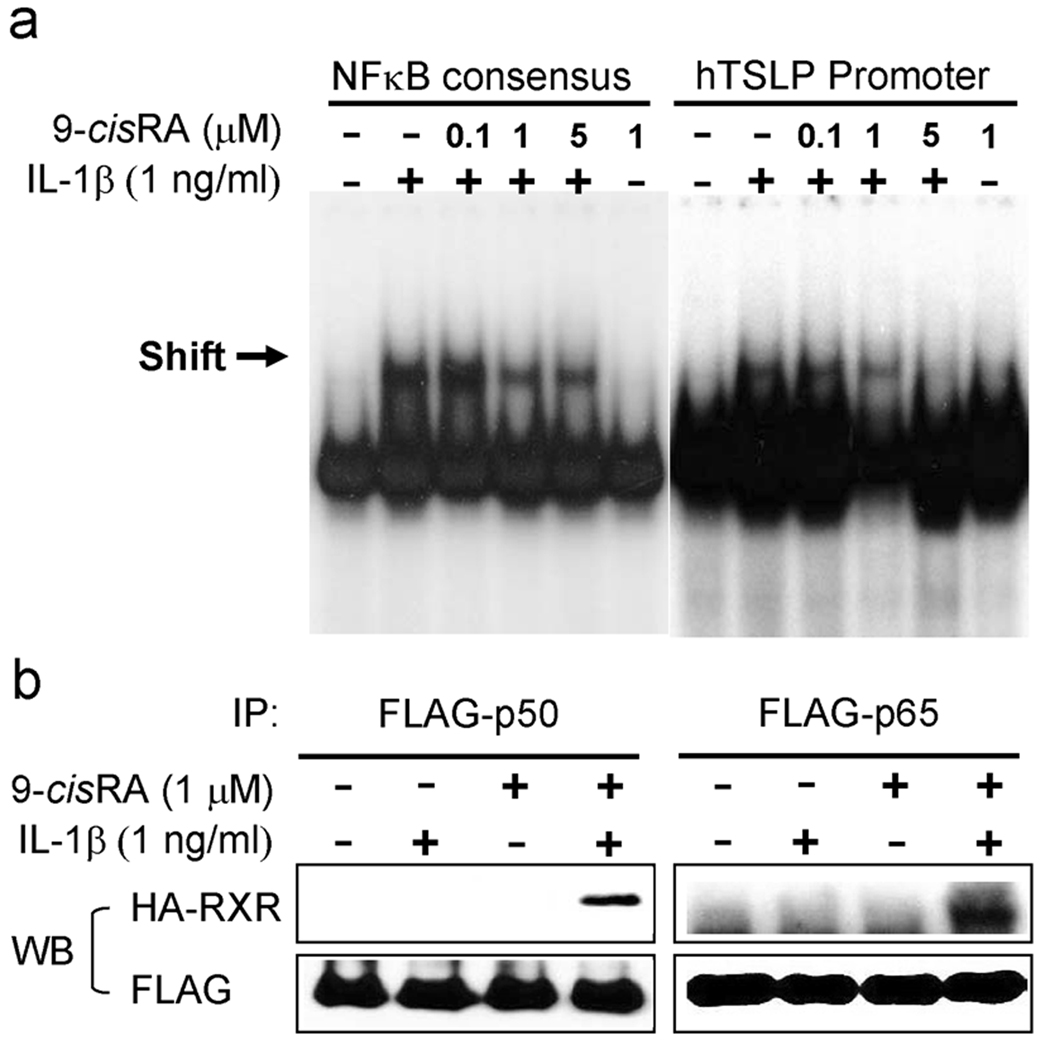
We next analyzed whether the binding of NFκB to the human TSLP promoter was inhibited by the effect of 9-cisRA. 16HBEo¯ cells were treated with IL-1β in the absence or presence of 9-cisRA, and analyzed for NFκB transactivation by EMSA, using oligonucleotide probes corresponding to either the NFκB consensus or to the NFκB site in the TSLP promoter(3). Binding of NFκB to both probes was markedly increased in nuclear extracts from IL-1β-treated cells. In contrast, nuclear extracts from cells co-treated with IL-1β and increasing amounts of 9-cisRA showed reduced NFκB DNA binding activity to each probe that correlated with increasing concentration of 9-cisRA (Fig. 3a). Similar results were obtained using extract from a second human lung epithelial cell line (A549) treated in the same fashion (data not shown). These results demonstrate that IL-1β-mediated NFκB binding to the human TSLP gene promoter is abrogated by co-treatment with the RXR agonist 9-cisRA.
Figure 3. 9-cisRA inhibits DNA-binding activity of NFκB in response to IL-1β.
(a) 16HBEo¯ cells were stimulated with 1 ng/ml IL-1β in the absence or presence of 9-cisRA as indicated concentration. Nuclear extracts were incubated with labeled oligonucleotide probes containing the NFκB consensus or the NFκB binding site in the TSLP promoter, and subjected to EMSA. Arrows indicate specific binding activity. (b) Interaction of NFκB with RXR in vitro. 16HBEo¯ cells were transfected with the HA-tagged RXR and FLAG-tagged p65 or p50, and stimulated with either or both 1 ng/ml IL-1β and 1µM 9-cisRA for 30 min. Co-IP assay were performed using anti-FLAG antibodies and blotted with anti-HA or anti-FLAG antibodies.
We next investigated whether active RXR undergoes direct physical interaction with NFκB. We next hypothesized that associations of NFκB with RXR may lead to the inhibition of NFκB by 9-cisRA. Co-immunoprecipitation using antibodies directed against epitope-tagged NFκB p65 and RXR showed an interaction between NFκB p65 and RXR induced by treatment with IL-1β and 9-cisRA, but not with IL-1β or 9-cisRA alone (Fig. 3b). Taken together, these results indicate that inhibition of NFκB activation by 9-cisRA occurs by direct physical interaction between RXR and NFκB.
9-cisRA inhibits NFκB binding to the human TSLP gene promoter in vivo
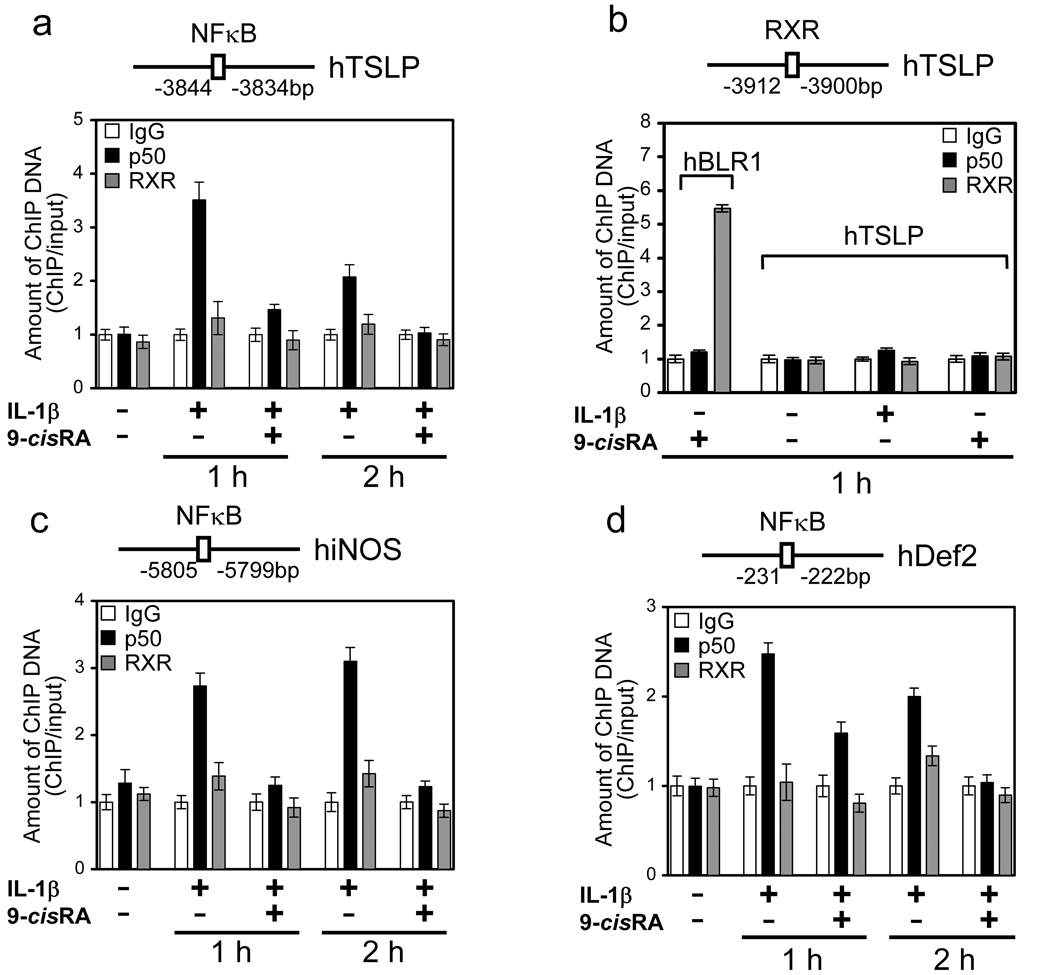
We next investigated whether NFκB is recruited to the TSLP promoter following IL-1β treatment, and whether this recruitment is affected by co-treatment with 9-cisRA. Chromatin immunoprecipitation (ChIP) assays were performed, using 16HBEo¯ cells stimulated with IL-1β in the absence or presence of RXR agonist 9-cisRA. The chromatin fraction was isolated, and binding to the human TSLP promoter was determined following immunoprecipitation using antibodies against NFκB (anti-p50), RXRα, or isotype control. As expected, NFκB was recruited to the TSLP promoter in response to IL-1β. However, recruitment of NFκB was inhibited at both time points in the presence of the RXR agonist 9-cisRA (Fig. 4a). These results show that RXR acts to repress IL-1β-mediated induction of the TSLP gene by preventing recruitment of NFκB to its promoter. Recently, Li et al.(15) identified a putative RXR binding sites in the human and mouse TSLP gene promoters, and suggested that RXR may be involved in directly repressing transcription of the TSLP gene through a RXR/RAR heterodimers. To determine whether RXR is recruited to the RARE in the human TSLP gene promoter (−3912 ~ −3900), we examined recruitment of RXR in the absence or presence of 9-cisRA. RXR binding to the putative RARE in the TSLP promoter was not detected (Fig. 4b). However, lack of RXR binding was not due to the inability to analyze RXR binding by ChIP, as binding to the human BLR1 promoter was seen (Fig. 4b). This result indicates that inhibition of NFκB activation by 9-cisRA occurs at the level of NFκB, and not at the TSLP promoter. To extend these findings, we examined the effect of 9-cisRA treatment on the binding of NFκB to the IL-1β-inducible genes iNOS and Def 2(17,18). ChIP experiments revealed that NFκB was recruited to the promoters of each of these genes in response to IL-1β at 1 and 2 hours, but treatment with the RXR agonist 9-cisRA significantly inhibited this recruitment (Fig. 4c, d). These results suggest that RXR agonist inhibits inflammatory responses by transrepression of NFκB target genes.
Figure 4. 9-cisRA blocks recruitment of NFκB to endogenous TSLP promoter and NFκB-dependent genes in response to IL-1β.
(a, c, d) Recruitment of NFκB was inhibited at the NFκB binding site of TSLP gene promoter (a), human iNOS gene (c) and human Defensin-2 gene (d) by 9-cisRA. (b) RXR was not recruited to the RXR binding site at the TSLP promoter in spite of RXR agonist stimulation, but was recruited to BLR1 promoter. 16HBEo¯ cells were stimulated with 1 ng/ml IL-1β in the absence or presence of 1µM 9-cisRA for 1 h, and soluble chromatin preparation was immunoprecipitated with anti-NFκB, anti-RXR antibody or control normal rabbit IgG. Purified ChIP and input DNA were analyzed by real-time quantitative PCR with the primers, respectively. The amount of ChIP DNA was normalized to that of input DNA. The mean value of control antibody before stimulation was arbitrarily defined as 1. Data are the mean ± SD of triplicate data points from a representative experiment.
We previously reported that the inflammatory mediators IL-1β and TNF-α, as well as TLR stimulation, can induce TSLP expression in human airway epithelial cells via activation of NFκB. Consistent with this finding, Kato et al. showed that infection of airway epithelial cells (AECs) with rhinovirus can lead to TSLP expression through stimulation of TLR3(19). On the other hand, recent reports have suggested that the nuclear hormone receptor RXR can negatively regulate TSLP gene expression in keratinocytes(12,15). In this report we have explored whether RXR agonists can regulate TSLP expression in AECs, and found that they do through an indirect manner via inhibition of NFκB activation.
Ligand deprivation and pharmacological studies in vivo have suggested that RXR homo- and hetero-dimers are physiologically involved in epidermis development and keratinocyte differentiation(20–22). Interestingly, mice with targeted deletion of RXRα and RXRβ in the epidermis develop an inflammatory disease of the skin similar to atopic dermatitis(12). This disease development is accompanied by increased TSLP expression in the epidermis, suggesting that RXRs are involved in repressing transcription of the TSLP gene. The data presented herein support this study, and provide a mechanistic framework for RXR-mediated inhibition of TSLP gene expression. Rather than direct binding of RXR to the TSLP promoter, as suggested by Li et al (12), our data shows that RXR acts through inhibition of NFκB activation. These data are supported by work in this report showing no direct binding of RXR to the TSLP promoter, and our previous work showing that mutation of putative RXR binding sites in the human and mouse TSLP promoters had no effect on IL-1β-mediated gene induction(3). However, it remains to be determined whether RXR is functioning as a homodimer or heterodimer with other NRs.
In conclusion, 9-cisRA inhibits the induction of TSLP gene expression via RXR. This inhibition is due to a direct effect of RXR on NFκB. Since TSLP has been linked to allergic inflammatory diseases(23), these data suggest that the use of RXR agonists may be useful as a therapeutic modality in treating allergy.
ACKNOWLEDGEMENTS
We thank Theingi Aye, Weihui Shih, and Xiaocui Sun for excellent technical assistance, Drs. Jessica Hamerman and Daniel Campbell for critical discussion of manuscript prior to submission, and members of the Ziegler laboratory for helpful discussions throughout the course of this work. We thank Matt Warren for his administrative support. This work was partially supported by NIH grants AI44259, AI50864, AI68731, and AI71130 to S.F.Z.
REFERENCES CITED
- 1.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyramati D, Aye T, Campbell DJ, Ziegler SF. Thymic Stromal Lymphopoietin (TSLP) as a Key Initiator of Allergic Airway Inflammation in Mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 2.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 3.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc. Natl. Acad. Sci. U. S. A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho) physiological functions. Cell Death Differ. 2004;11 Suppl 2:S126–S143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- 6.Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu. Rev. Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- 7.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 8.Krezel W, Dupe V, Mark M, Dierich A, Kastner P, Chambon P. RXR gamma null mice are apparently normal and compound RXR alpha +/−/RXR beta −/−/RXR gamma −/− mutant mice are viable. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9010–9014. doi: 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Decimo D, Krezel W, Dierich A, Chambon P. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 10.Mascrez B, Mark M, Krezel W, Dupe V, LeMeur M, Ghyselinck NB, Chambon P. Differential contributions of AF-1 and AF-2 activities to the developmental functions of RXR alpha. Development. 2001;128:2049–2062. doi: 10.1242/dev.128.11.2049. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Chiba H, Warot X, Messaddeq N, Gerard C, Chambon P, Metzger D. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 2001;128:675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HC, Shibata H, Ogawa S, Maki K, Ikuta K. Transcriptional regulation of the mouse IL-7 receptor alpha promoter by glucocorticoid receptor. J. Immunol. 2005;174:7800–7806. doi: 10.4049/jimmunol.174.12.7800. [DOI] [PubMed] [Google Scholar]
- 14.Agata Y, Katakai T, Ye SK, Sugai M, Gonda H, Honjo T, Ikuta K, Shimizu A. Histone acetylation determines the developmentally regulated accessibility for T cell receptor gamma gene recombination. J. Exp. Med. 2001;193:873–880. doi: 10.1084/jem.193.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. U. S. A. 2007;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cron RQ, Bartz SR, Clausell A, Bort SJ, Klebanoff SJ, Lewis DB. NFAT1 enhances HIV-1 gene expression in primary human CD4 T cells. Clin Immunol. 2000;94:179–191. doi: 10.1006/clim.1999.4831. [DOI] [PubMed] [Google Scholar]
- 17.Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, Jr, Billiar TR, Geller DA. Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J. Biol. Chem. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi-Ishii Y, Nagaoka I. Modulation of human beta-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J. Immunol. 2003;170:4226–4236. doi: 10.4049/jimmunol.170.8.4226. [DOI] [PubMed] [Google Scholar]
- 19.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao JH, Feng X, Di W, Peng ZH, Li LA, Chambon P, Voorhees JJ. Identification of heparin-binding EGF-like growth factor as a target in intercellular regulation of epidermal basal cell growth by suprabasal retinoic acid receptors. EMBO J. 1999;18:1539–1548. doi: 10.1093/emboj/18.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imakado S, Bickenbach JR, Bundman DS, Rothnagel JA, Attar PS, Wang XJ, Walczak VR, Iisniewski S, Pote J, Gordon JS. Targeting expression of a dominant-negative retinoic acid receptor mutant in the epidermis of transgenic mice results in loss of barrier function. Genes Dev. 1995;9:317–329. doi: 10.1101/gad.9.3.317. [DOI] [PubMed] [Google Scholar]
- 22.Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996;10:1002–1013. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 23.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, Waal-Malefyt RR, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]