Abstract
A mouse model of recurrent herpes simplex type 2 (HSV-2) would improve our understanding of the immunobiology of recurrent disease and provide a useful model for evaluating antiviral treatments. We developed a model to evaluate recurrent vaginal HSV-2 shedding using high dose Acyclovir (ACV) therapy begun at 3 days post infection (dpi). Treatment with 150 mg/kg of ACV for 10 days increased survival to 80% following vaginal challenge with HSV-2 strain 186 and to 100% after challenge with strain MS. We then evaluated recurrent vaginal HSV-2 shedding in surviving mice. Although infectious virus was not detected in vaginal samples after 21 dpi, viral DNA was detectable by PCR in 80% of mice (47/59) on at least one day, while no animal was positive for virus on every day. ACV therapy administered from day 21–31 significantly reduced recurrent virus shedding during this period from 7.3% (8/109 swabs) to 0.8% (1/126 swabs) (P=0.013). Lastly, ACV-rescued HSV-2 infected mice treated with cyclophosphamide at 35 and 38 dpi rapidly succumbed, indicating that this model can be used to study immune control of the persistent infection. Thus, this model provides an inexpensive model for evaluating therapeutic strategies and immune control of persistent HSV.
Keywords: herpes simplex virus, recurrent HSV shedding, genital herpes, animal model
1. Introduction
Herpes simplex virus (HSV) infected individuals exhibit spontaneous viral shedding both in the presence and absence of clinically evident recurrent disease. The persistence of viral shedding in the absence of a recognized recurrence is believed to be the primary route of genital HSV transmission between partners (Mertz et al., 1992). Multiple studies have shown that genital shedding of HSV type 2 (HSV-2) is detectable on 2–8% of patient days by culture in both men and women (reviewed in (Sacks et al., 2004); (Wald, 2004)). These numbers increase to as much as 28% of patient days when viral shedding is detected using PCR (Wald et al., 2000); (Wald et al., 1997). Other factors, such as immunosuppressive therapy or HIV infection, can increase the frequency of HSV-2 shedding (Magaret et al., 2009); (Mayaud et al., 2008). With the continued high incidence of genital HSV-2 infections despite the availability of potent antiviral drugs, new strategies are clearly needed to prevent genital HSV infection and transmission.
The process that leads to genital shedding of HSV-2 originates in the sensory ganglia where the virus reactivates from a latent state established during the initial (primary) infection. Reactivation of HSV is thought to be caused by a variety of stimuli, including physical trauma to the nerves, trauma to the skin, UV radiation, fever, and emotional stress. After reactivation, the virus descends within the nerve and can produce symptoms or replicate without producing symptoms (or at least recognized symptoms). During recurrences, a localized immune response, in particular the CD8+ T cell response quickly controls replication (Zhu et al., 2007).
A murine model of recurrent genital HSV shedding would be exceptionally useful for characterizing the immune response that controls recurrent virus infection and for evaluating the ability of novel antiviral treatments to limit or prevent virus shedding. However, a mouse model of persistent HSV-2 infection is currently not available since, in mice, genital HSV-2 infections are lethal. HSV-infected mice do not develop herpetic lesions, as in humans, but instead experience erythema and hair loss near the site of infection, chronic staining of the fur with urine or feces, hind limb paralysis, and death usually by 14 days post infection (dpi) (Overall et al., 1975). Other non-lethal animal models of genital HSV-2 infection such as guinea pigs have been useful (Stanberry, 1991); however, the development of a model of genital recurrences in mice is desirable due to the availability of both immunological reagents and transgenic/knockout mice which can be used for evaluating the immunological control of reactivation and recurrent shedding.
A number of early studies demonstrated that treatment of HSV-2 infected mice with acyclovir (ACV), famciclovir (FCV) and valaciclovir (VACV), improves survival of infected mice and that persistent HSV-2 infection in ganglia occurs (Kern, 1982); (Kern et al., 1983), (reviewed in (Efstathiou et al., 1999); (Thackray and Field, 1996); (Thackray and Field, 2000). Cessation of VACV in the HSV-2 ear infection model resulted in transient reappearance of infectious virus, either in the neural system or skin tissue whereas infectious virus was only observed in the neural tissues following cessation of FCV (Thackray and Field, 1996). Recurrence of infectious virus was even further pronounced when mice were subjected to immunosuppression during the VACV treatment period (Field et al., 1995); (Thackray and Field, 1997). A more recent study has shown that mice receiving antiviral therapy with VACV and a monoclonal antibody to gD2 can survive primary genital HSV-2 disease and occasionally exhibit symptoms indicative of recurrent genital disease several weeks after inoculation, and that in vivo depletion of T cells with monoclonal antibodies caused an increase in the incidence of the recurrence of herpes symptoms (Parr et al., 2005). These studies indicate that HSV-2 can establish a latent or persistent infection in mice and can undergo spontaneous reactivation which then can result in the recurrence of infectious virus.
Here, we examine whether mice that are rescued from a lethal genital HSV-2 infection with ACV therapy establish a persistent HSV infection that can spontaneously reactivate and produce vaginal virus replication and shedding. To further validate this model and explore the effects of antiviral therapy, we evaluated the effects of an anti-herpes drug, ACV, to reduce recurrent virus shedding. The murine model of persistent HSV shedding presented here should aid in the evaluation of the immune mechanisms that control reactivation and/or shedding and, therefore, assist in the development of novel strategies for preventing HSV transmission.
2. Materials and Methods
2.1 Animals
Female Swiss-Webster mice weighing 18–21 g were obtained from Harlan-Sprague (Indianapolis, IN). Animals were housed in AAALAC approved facilities. All procedures and protocols were approved by the Cincinnati Children’s Hospital Research Foundation Animal Care and Use Committee.
2.2 Virus
HSV-2 strain 186 (originally obtained from Dr. Lawrence Stanberry, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) and HSV-2 strain MS (ATCC-VR540) were grown in low passage primary rabbit kidney cells and titered on Vero cell monolayers as previously described (Bourne et al., 2000).
2.3 HSV-2 Infection of Mice
To increase susceptibility, animals were treated at 7 and 1 days prior to challenge with 3 mg medroxyprogresterone administered by subcutaneous injection (Parr et al., 1994). The vagina was pre-swabbed with a wet and a dry calcium alginate tipped swab immediately prior to instillation of either 1×104 pfu (strain 186) or 5×103 pfu (strain MS) HSV-2. Infected mice were monitored daily for symptoms of genital disease (erythema, hair loss, hind limb paralysis, etc.) and mortality. Animals developing debilitating symptoms of disease or paralysis were sacrificed and considered to have died the following day in all survival analyses. Infection was verified in all animals by the presence of replicating virus in vaginal swabs collected at 2 dpi. Vaginal swabs were collected on the days indicated using sterile calcium alginate tipped swabs, and stored in 500 µl BME (GibcoInvitrogen) containing 2% FBS (Hyclone, Thermo Fisher Scientific) at −80°C until analysis (Bourne et al., 2000).
2.4 Acyclovir (ACV) and Cyclophosphamide Treatments
All animals received ACV treatments twice daily (total 100–150 mg/kg/day) by intraperitoneal injection beginning 3 dpi for 10 days at the dose indicated based on the average weight of all animals on the day of challenge. The stock ACV solution (APP Pharmaceuticals, LLC) was diluted in saline and administered in 250 µl volumes per injection. Additional treatments with ACV were administered in the same manner. Cyclophosphamide (CY, 150 mg/kg, Sigma) was diluted in saline as necessary, and administered by intraperitoneal injection based on each individual animal’s weight. Depletion of immune cells was determined by flow cytometry after staining with anti-mouse CD4 and CD8 antibodies (PharMingen).
2.5 Detection of Virus in Tissues
Tissue samples were homogenized in BME containing 2% FBS at 2% w/v (for dorsal root ganglia) or 5% w/v (spinal cords, other tissues). To detect replicating virus present in the tissues, Vero cell monolayers were inoculated with 200 µl of homogenate for 1 hour at 37°C prior to overlaying with BME containing 2% FBS and 0.75% methyl cellulose. After 72 hours, monolayers were fixed in methanol and stained with Giemsa stain in order to visualize plaques.
2.6 HSV-2 PCR
The PCR analysis was performed as previously described (Bernstein et al., 2009). Briefly, vaginal swabs and dorsal root ganglia (DRG) were isolated from infected mice using sterile swabs and dissection tools pre-treated with DNA Away (Molecular BioProducts) and stored at −80°C. DRG tissue was homogenized on ice in 500 µl of 2% FBS BME. DNA was isolated from 200 µl of vaginal swab media and DRG homogenate using QIAamp DNA Mini Kit (Qiagen #51306) according to manufacturer’s protocol. The gB gene was amplified by PCR using two sets of primers (Jerome et al., 2002). The primer sequences were: gB External Forward, 5’- CCACCGGCGCTACTTCATCT -3’ and gB External Reverse, 5’- CGGATGACCGTGTCGATGTC -3’ to generate a 264 bp product, and gB Internal Forward, 5’- CCGTCAGCACCTTCATCGA -3’, and gB Internal Reverse, 5’- CGCTGGACCTCCGTGTAGTC -3’ to generate a 124 bp product. Each PCR reaction contained 50 ng purified DNA, 100 pmol each primer, Promega Master Mix (Promega) in a total volume of 25 µl. PCR was performed using a Biorad iCycler and the temperature cycling profile began with an initial 95°C for 1 min followed by annealing at 61°C for 1 min, and elongation at 72°C for 1 min 30 sec for 35 cycles. A HSV-2 viral DNA control, HSV-2 Quantitated Viral DNA (Advanced Biotechnologies Inc, Columbia MD), which harbors the entire HSV-2 genome, was used as a positive control for amplification and specificity. To determine the limit of detection of genome copy number, the HSV-2 DNA was serially diluted into uninfected tissue DNA. The amount of virus shed during recurrences was small and required nested PCR to detect on most days. Nested PCR was performed by first using the gB External primers, followed by second round PCR with 2 µl of the product formed as the template for amplification using the gB Internal primers. The results of the second round PCR reaction were analyzed by gel electrophoresis for the presence of a 124 bp product. The specificity of the PCR reaction was verified in select samples by Southern Blot analysis of first round PCR products.
2.7 Southern Blot
PCR reaction products were separated by gel electrophoresis and transferred to a nylon membrane overnight by capillary action using 20X SSC buffer. DNA was cross linked to the membrane by exposure to 96,000 µJ/cm 2 for 1 minute. A Digoxigenin (DIG)-labeled probe was creating using the PCR DIG Probe Synthesis Kit (Roche Scientific, Switzerland) according to the manufacturer’s protocol. Hybridization was performed at 47°C in DIG Easy Hyb Buffer, followed by blocking/washing with DIG Wash and Block Buffer Kit, and detection with DIG Luminescent Detection Kit, all according to the manufacturer’s directions (Roche Scientific, Switzerland).
2.8 Statistics
All statistical analyses shown were performed using the two-sided Fischer’s exact test.
3. Results
3.1 Effect of ACV Treatments during Primary Infection
We first wanted to establish an ACV regimen that would allow virus to infect the DRG, the site of latency, but not be lethal to the mouse. To ensure efficient spread of virus from the vaginal site to the nervous system, variables such as mouse strain, age of mouse, virus strain, and dose and timing of ACV treatment were considered. Since susceptibility of mice to HSV-2 infection decreases with age, 6 week old mice were used. Medroxyprogesterone was used to increase susceptibility to vaginal HSV-2 infection (Parr et al, 1994). A number of studies indicated that ACV treatment early post infection greatly diminishes latent infection in ganglia whereas treatment after 96 or 120 hpi had no effect (reviewed in (Efstathiou et al., 1999); (Kern, 1982); (Kern et al., 1983); (Sawtell et al., 2001)). Therefore, we initiated ACV therapy at 3 dpi to allow adequate time for infection of the DRG. Plaque assays were used to measure HSV-2 replication in the neurons and eventual clearance of infectious virus from the DRG whereas latent virus in the DRG was measured by the presence of detectable viral DNA in the absence of infectious virus.
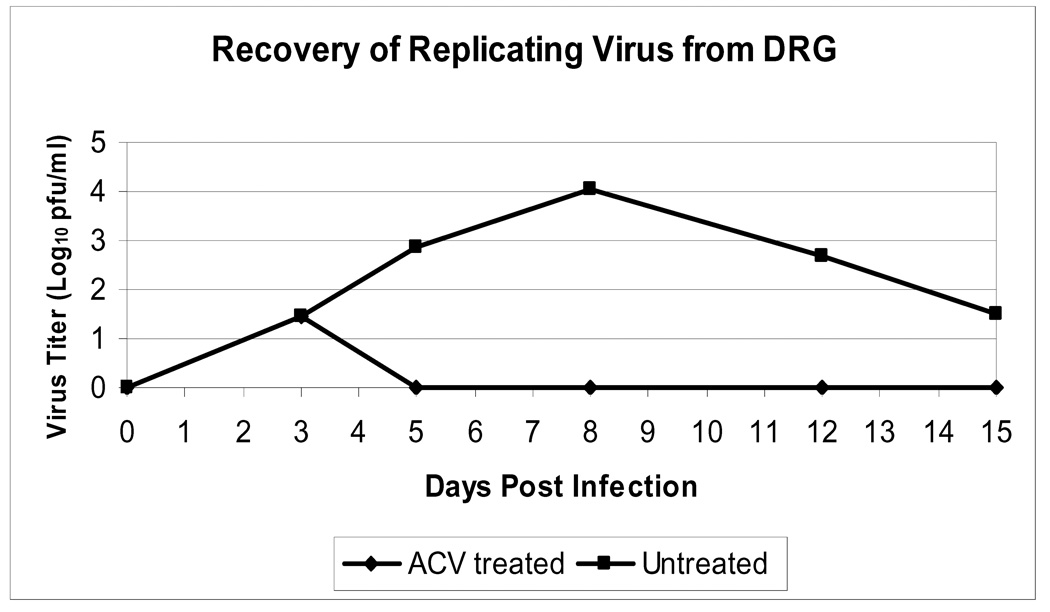
To evaluate the effects of ACV treatment on HSV-2 replication in the DRG, female Swiss-Webster mice were vaginally infected with HSV-2 (strain 186) and treated at 3 dpi with 100 mg/kg/day ACV (intraperitoneal (IP) BID) for 10 days (N=15) or placebo (N=13). Figure 1 shows the levels of replicating virus present in the DRG of untreated and ACV-treated mice during primary infection with HSV-2, as determined by plaque assay. Viral replication was detected in the DRG of all mice by 3 dpi and peaked in untreated mice at 8 dpi. In mice receiving ACV, no replicating virus was detected in the ganglia by 5 dpi. Thus, the administration of ACV beginning 3 dpi allows HSV to reach the DRG, but severely limits viral replication.
Figure 1. Effect of ACV treatment on levels of replicating HSV-2 in the dorsal root ganglia.
Dorsal root ganglia (DRG) were harvested from HSV-2-infected mice at 0, 3, 5, 8, 12, and 15 dpi and the viral titer in tissue homogenates was quantified by plaque assay. For each day, the average viral titer ***(N=3, except the untreated group on day 15 (N=1) is indicated. ACV-treated animals received 100 mg/kg IP BID for 10 days beginning 3 dpi.
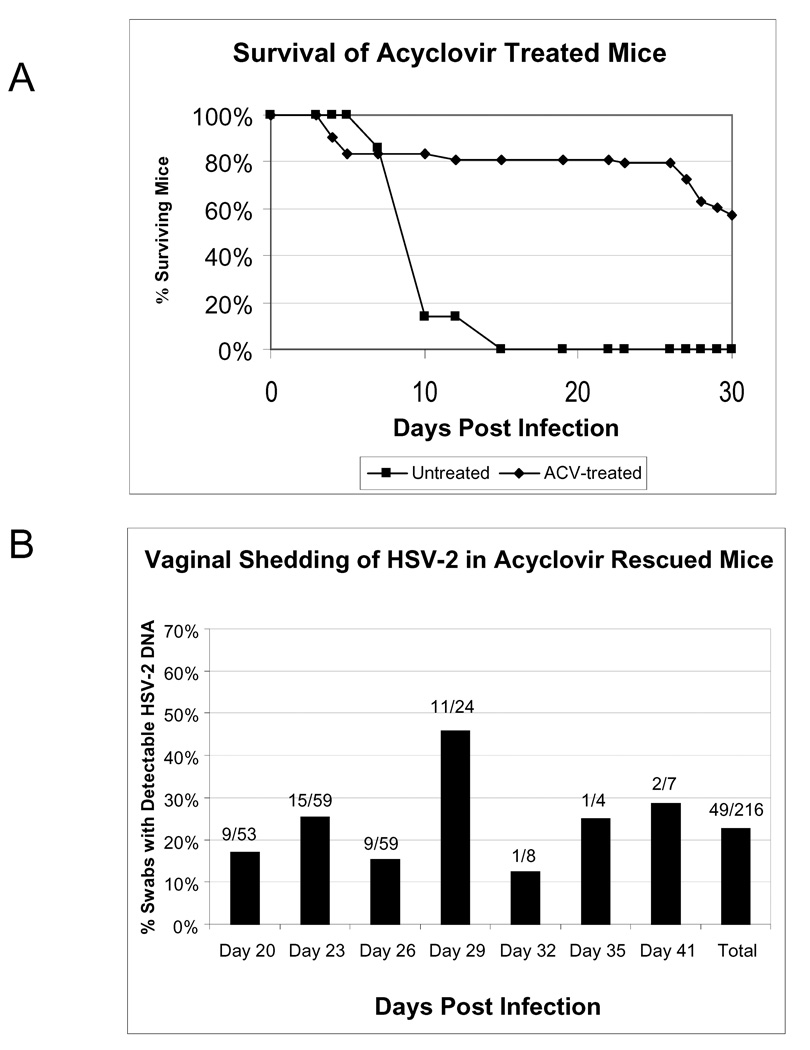
Since this regimen of ACV treatment appeared to be highly potent at inhibiting viral replication in the DRG, we asked whether it could rescue mice from a lethal HSV-2 infection. Mice were vaginally infected with HSV-2 strain 186, and beginning on 3 dpi, treated mice (N=72) received 100 mg/kg/day ACV IP BID for 10 days. As a control for lethal HSV-2 infection, untreated mice were included (N=7). Infection of all animals was verified by the presence of replicating virus in vaginal swabs collected at 2 dpi (data not shown). Figure 2A shows the survival of ACV-treated mice compared to untreated animals. None of the untreated mice survived past 15 dpi (mean day of death = 9.3 dpi), while 80% of ACV treated animals survived past 20 dpi, and 58% survived to 30 dpi. During the primary infection (days 1–10), 100 % of the untreated mice developed symptoms of HSV disease (hair loss and erythema combined or HLE) compared to approximately 70% of the ACV-treated mice, which often exhibited less severe symptoms or even a delay in symptoms, with 8% of the treated mice (6/72) not showing detectable symptoms until after 20 dpi. Hind limb paralysis was detected in only one of the ACV-treated mice (1.72 or 1.4%) but was observed commonly in untreated mice (6/7 or 86%).
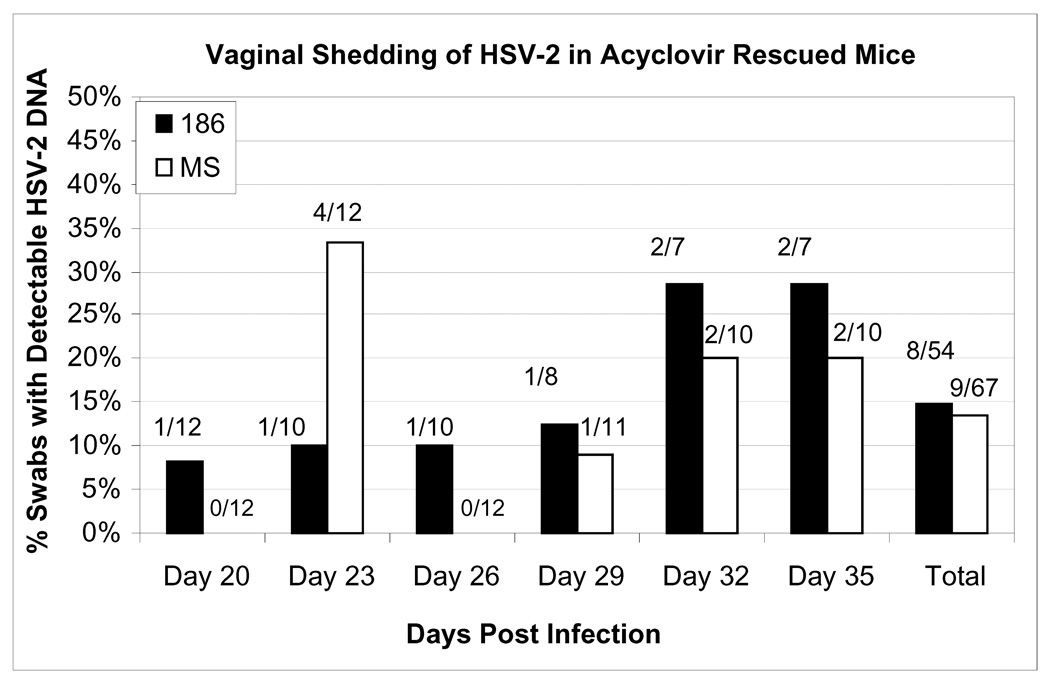
Figure 2. Survival and vaginal HSV-2 shedding in ACV rescued mice.
HSV-2-infected mice were administered ACV at 100 mg/kg IP BID for 10 days beginning 3 dpi. Animals were monitored daily for mortality and vaginal swabs were collected from surviving animals at 20, 23, 26, 29, 32, and 41 dpi. (A) Survival of ACV-treated and untreated mice. (B) Nested PCR was used to determine the percentage of swabs containing HSV-2 DNA on each day. The fractions above each bar indicate the number of positive swabs over the total from each day. Results shown are combined data from two independent experiments (ACV treated, N=72; untreated, N=7).
No symptoms were observed in 14 of the treated mice (19%), even though these mice were positive for vaginal HSV-2 at 2 dpi. After 14 dpi, the erythema was no longer observed in the treated mice whereas the hair loss was maintained in the majority of mice, although some mice with hair loss at day 26 returned to having normal coat appearance by day 33. One treated animal developed hair loss at day 33, then exhibited bloating and weak activity at day 39 and died at day 40. A second treated animal exhibited hair loss out to day 26, appeared normal by day 33, showed weak activity levels at day 37, and was dead at day 39. Two mice did not exhibit symptoms until day 38, then developed wetness due to urinary incontinence and bloating, followed by death within one day. In general, variable levels of symptoms were observed in the treatment group over the time course of the study, with some developing late symptoms or appearing normal after symptoms were observed. Some mice sporadically succumbed after 25 days. We noted that animals with ruffled fur, wetness, and/or abdominal bloating usually succumbed to the infection within 24–48 hours. In animals with bloating, we observed that the intestines were enlarged without areas of obvious bleeding. These animals were also evaluated for virus replication and no replicating virus was detected in the DRG, spinal cord, or brains of these mice (data not shown).
To evaluate long term HSV-2 infection of the DRG in the ACV-treated mice, 16 mice were sacrificed at day 27 (N=3), day 35 (N=4), day 41 (N=7), and day 42 (N=2) and the DRG examined for infectious virus (by plaque assay) and viral DNA (by PCR analysis). In this study, replicating virus was not detected in the DRG, but all DRG samples were positive for HSV DNA by first round PCR (~102 copies viral genome/50 ng total DNA, data not shown), indicating that latency was established within the ganglia of ACV-treated animals.
3.2 Vaginal HSV-2 Shedding in ACV Rescued Mice
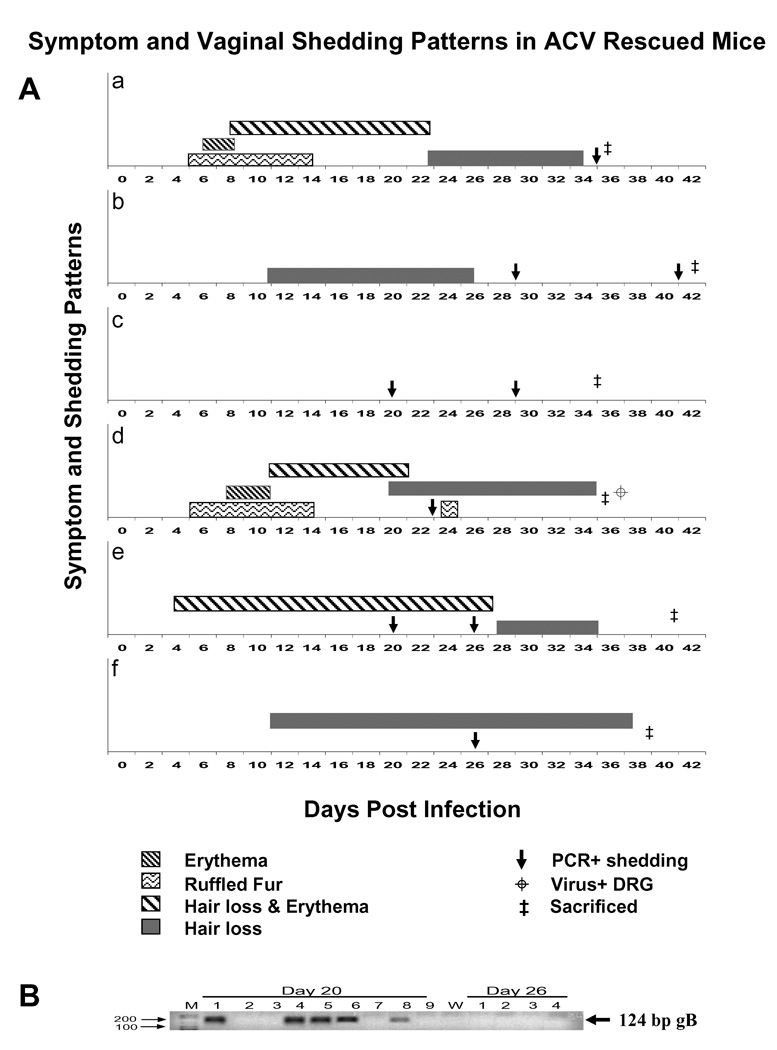
To determine whether mice rescued from a primary lethal HSV-2 infection would exhibit recurrent vaginal viral shedding, vaginal swabs were collected from the surviving mice in the study described above from days 20 – 41 and analyzed by PCR for the presence of HSV-2 DNA. Swabs were collected every 3 days when possible and a total of 216 swab samples were analyzed. Figure 2B shows the number and percentage of swabs positive for viral DNA by nested PCR on each day. Viral DNA was detected in 23% of swabs (49/216) between 20 and 41 dpi. No replicating virus was detected in these swabs by plaque assay (data not shown), and, in virtually all cases, nested PCR was required in order to detect the presence of HSV-2 DNA, indicating that a very small amount of virus is shed during each episode. An example of the nested PCR results is shown in Figure 3B. Southern blot analysis was performed to confirm the specificity of the viral sequence which was amplified (data not shown). Overall, 80% of treated mice (47/59) shed virus on at least one day, while no animal shed virus on every day. At least 24% of treated mice (14/59) shed virus on multiple days during the 3-week period, and a similar number (24%) did not shed detectable virus on any of the days sampled. Viral shedding was detected in animals that experienced symptoms of primary infection or delayed symptoms as well as those without visible evidence of primary infection. No significant differences were observed in the frequency of shedding between symptomatic and asymptomatic animals. Representative shedding patterns are shown in Figure 3A. As shown in this figure, the detectable virus shedding varied between animals and did not correlate with detectable disease symptoms. Whereas at least 70% of the treated mice exhibited symptoms of infection with expected kinetics (see panels a, d, and e), some mice did not exhibit detectable symptoms of infection (panel c) yet were both positive for vaginal virus at 2 dpi, positive for viral genome in the DRG (by PCR), and shed at least once or twice after 20 dpi. Treated mice also exhibited delayed symptoms of infection (panels b, f), with hair loss typically observed around 10 dpi. The mice in panels b and e represent mice which returned to normal appearance until the time of sacrifice, but shed virus on several days. Some mice only exhibited hair loss (panels b, f) and in most cases, as shown in panel f, the hair loss did not return to normal coat appearance. Lastly, some mice (panels a, d, and e) showed typical ruffled fur and more prolonged HLE out to days 20–27, followed by hair loss, and were positive for viral shedding only on one or two days, even though HLE was more prolonged in some of these mice. Importantly, the mouse in panel d is only one of the three mice, out of a total of 102 mice from four separate studies, which were positive for low levels of replicating virus (< 5 pfu) in the DRG. This mouse demonstrates that although multiple symptoms were observed over the course of the infection, shedding was only detected at 23 dpi.
Figure 3. Symptom and Vaginal shedding patterns in ACV rescued mice.
(A) Symptom and shedding patterns from 0–41 dpi observed in 6 selected mice, infected vaginally with HSV-2 strain 186 are shown in panels a–f. The symbols as shown in the figure depict a PCR+ vaginal sample, erythema, ruffled fur, hair loss and erythema, hair loss, time of sacrifice, and virus+ DRG (replicating virus). All mice were positive for replicating virus at 2 dpi and positive for viral DNA in DRG at time of sacrifice (not shown in figure). (B) Results from a typical PCR analysis is depicted for 20 and 26 dpi. The 124 bp gB product is shown by an arrow.
3.3 Effect of HSV-2 Strain on Survival and Vaginal Shedding
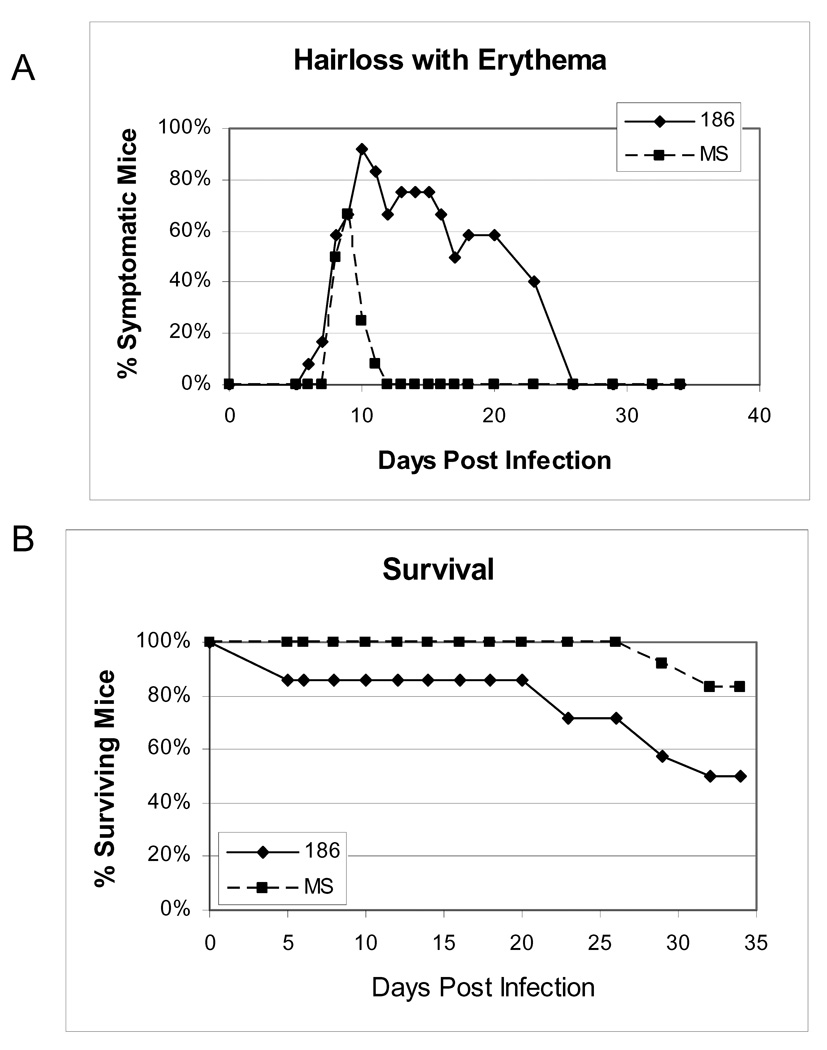
To examine whether a second strain of HSV-2, MS, which appears to be less virulent in guinea pigs (Bernstein, unpublished) would increase the survival of HSV-2 infected mice, we compared the 186 strain (N=14) to the MS strain (N=12). Uninfected mice (N=8) were included as a negative control for PCR analysis. In this and subsequent experiments, HSV-2-infected mice received an increased dose of ACV (150 mg/kg/day) for 10 days beginning 3 dpi in an effort to further enhance survival. Figure 4 shows the percentage of symptomatic and surviving mice. As predicted, mice infected with the MS strain presented with less severe and protracted primary disease symptoms compared to animals infected with the 186 strain. Even though mice infected with the MS strain received ½ log lower virus inoculum, all of the mice in both groups were infected with HSV-2 at 2 dpi (data not shown). Significantly, mice infected with the MS strain exhibited increased survival compared to mice infected with the 186 strain, with 100% of MS-infected mice surviving to 25 dpi and greater than 80% of these mice surviving to 35 dpi. Although these differences in survival and symptoms may be due to the differences in challenge dose, the virulence of the MS strain, or the increased dose of ACV treatment, these conditions enhanced the survival of the infected mice to provide an improved model to further evaluate HSV-2 vaginal shedding.
Figure 4. Comparison of symptoms and survival in mice infected with either HSV-2 strain 186 or HSV-2 strain MS.
Mice were intravaginally infected with either 1×104 pfu (strain 186, N=14) or 5×103 pfu (strain MS, N=12) HSV-2. All mice received ACV at 150 mg/kg IP BID for 10 days beginning 3 dpi. (A) The percentage of animals in each group experiencing the combined symptoms of hair loss and erythema, and (B) the percentage of surviving animals on each day are indicated.
To determine whether there is any difference in the frequency of HSV vaginal shedding episodes between mice infected with the 186 strain and mice infected with the MS strain, vaginal swabs were collected every three days from day 20–35 pi. All vaginal swabs from both groups were negative for replicating virus (data not shown). Figure 5 shows the percentage of swabs positive for HSV-2 DNA by nested PCR on each day. There was no significant difference in the frequency of viral shedding observed between 186-infected mice (15% of days) and MS-infected mice (13% of days). In both groups, 58% of the mice (7/12) shed virus at some time during this time period. For the 186 strain, 50% of the mice (6/12) shed the virus on only one day whereas the MS strain was shed only once by 42% of the mice (5/12). Two mice shed the MS strain on two days compared to one mouse which shed the 186 strain. Thus, shedding was similar between both strains of virus, although symptoms and survival were significantly different.
Figure 5. Comparison of vaginal HSV shedding in mice infected with either HSV-2 strain 186 or HSV-2 strain MS.
Vaginal swabs were collected from surviving animals at 20, 23, 26, 29, 32, and 35 dpi, and the presence of HSV-2 DNA was determined by nested PCR. The fractions above each bar indicate the number of positive swabs over the total from each day.
The infection of the DRGs and spinal cords in the surviving mice by both the MS and 186 strains was also analyzed for replicating virus or the presence of viral DNA. All of the DRGs from both the surviving 186 and MS-infected mice were positive for viral DNA by first round PCR, indicating that both strains were able to reach this site even though the ACV treatment dose was increased to 150 mg/kg. Interestingly, detectable replicating virus was detected in one of the DRG isolated at 35 dpi from the 186-infected group (1 pfu detected by plaque assay). This mouse exhibited detectable HLE to 20 dpi and then only hair loss afterwards. This mouse shed virus only at 23 dpi. Two of the DRG isolated at 35 dpi from the MS-infected group had detectable replicating virus (5–10 pfu), but unlike the 186-infected mouse, showed no signs of obvious HSV disease after 15 dpi. Both of these mice shed virus only once at days 23 or 29. Viral DNA was detected by first round PCR in 6/7 spinal cords isolated from the 186-infected mice and in 4/10 spinal cords isolated from the MS-infected mice. Replicating virus was not detected in any of the spinal cord samples. No positive PCR signal was found in any of the uninfected mouse specimens.
3.4 Effect of Subsequent ACV Therapy on Survival and Recurrent Vaginal Shedding
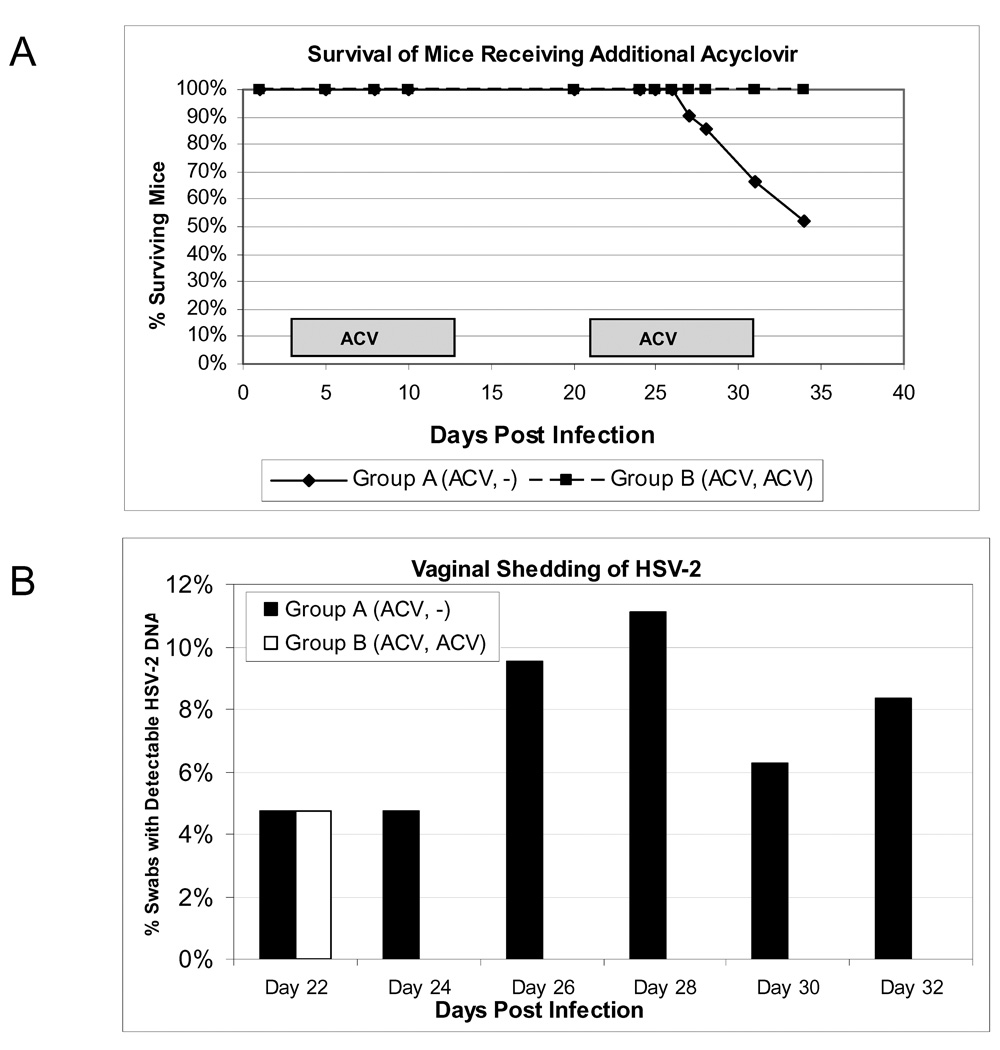
To further validate the model and determine the effects of antiviral therapy on recurrent vaginal virus shedding, mice (N=42) were inoculated with 5×103 pfu of MS and then received 150 mg/kg ACV IP BID for 10 days beginning at 3 dpi. The mice were followed daily for symptoms and survival. At 20 dpi, 100% of the mice had survived and were then divided into two groups. One group (N=21) was treated with a second round of 150 mg/kg ACV IP BID from 21– 31 dpi and compared to the second group (N=21) which did not receive a second round of ACV treatment.
Vaginal swabs were collected beginning at 22 dpi and evaluated for HSV shedding by nested PCR. Figure 6A shows the survival of HSV-2 infected mice receiving either only initial ACV therapy or those treated with ACV again on days 21–31. At 26 dpi, 100% survival was observed for both groups. However, mice receiving a second round of ACV treatment showed increased survival compared to mice receiving only the first treatment. By 35 dpi, 100% survival was observed for mice which received the second round of ACV (21/21) compared to 52% of the mice (11/21) in the group which did not receive the additional ACV therapy (p=0.005). This suggests that low level virus replication was occurring during this period, although all DRG samples evaluated from the surviving mice at 105 dpi from both groups were negative for replicating virus (see Figure 7B) but positive for viral DNA by first round PCR (data not shown). In addition, the spinal cords and brains isolated from both groups were negative by plaque assay. Taken together, these data most likely indicates that, in some animals, the immune responses that developed, even after 3 weeks following infection, were unable to clear infectious virus or were unable to control reactivated virus which contributed to their eventual death.
Figure 6. Effect of a second ACV treatment on survival and vaginal HSV-2 shedding.
All mice were intravaginally infected with 5×103 pfu HSV-2 strain MS and given ACV at 150 mg/kg IP BID for 10 days beginning 3 dpi. From 21 to 31 days post infection, Group B (ACV, ACV) (N=21) received an additional course of ACV at 150 mg/kg BID while Group A (ACV, −) (N=21) remained untreated on these days. (A). Animals were monitored daily for mortality. (B) Vaginal swabs were collected from surviving animals at 22, 24, 26, 28, 30, and 32 dpi, and the percentage of swabs containing HSV-2 DNA was determined by nested PCR. The P=0.0133 is depicted for the difference in shedding between groups A and B. The ACV treatment times are indicated by boxes.
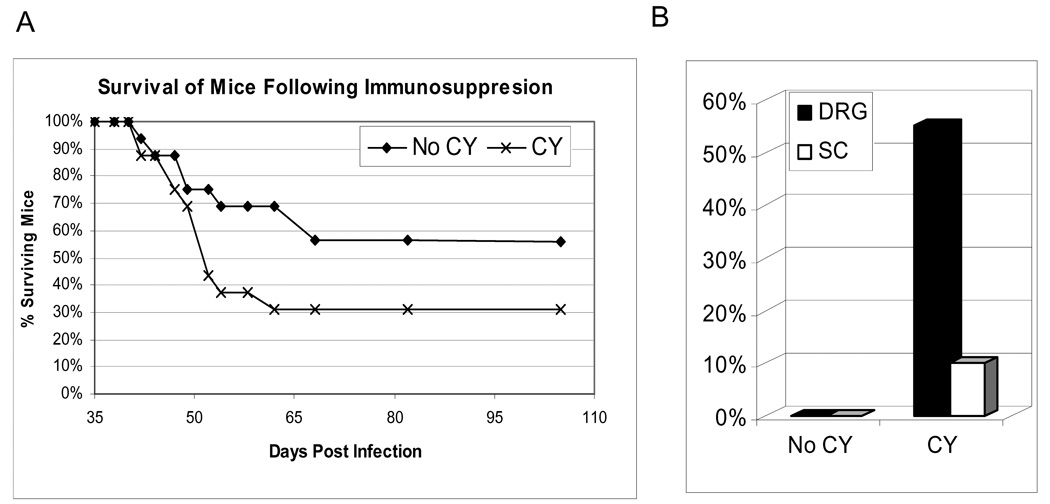
Figure 7. Effect of immunosuppresion on survival of HSV-2 infected mice and the levels of replicating virus in neural tissues.
HSV-infected mice were immunosuppressed with 150 mg/kg of cyclophosphamide at 35 and 38 dpi (CY), or remained untreated (No CY). (A) The combined survival of untreated mice and those receiving immunosuppression are shown. (B) Dorsal root ganglia (DRG) and spinal cords (SC) were harvested from sickly mice requiring euthanization and healthy mice at experiment termination (105 dpi). Replicating virus was detected in tissue homogenates by plaque assay, and the percentages of animals with detectable viral replication in each tissue are shown.
We next evaluated the impact of this second round of ACV therapy on vaginal shedding. Figure 6B shows the percentage of vaginal swabs positive for HSV-2 DNA by nested PCR in these mice. Mice receiving ACV at 21–31 dpi exhibited a significant reduction in the frequency of viral shedding (1/126 swabs, or 0.8%) compared to mice receiving no treatment (8/109 swabs, or 7.3%, p=0.013) during therapy. These data indicate that viral replication is required for vaginal shedding of HSV-2 in mice, and therefore, infectious viral particles are likely present (at undetectable levels) during episodes of asymptomatic shedding.
3.5 Immunosuppression of HSV-2 Infected Mice
To determine if immunosuppression would affect the frequency of viral shedding or survival in persistently HSV-2 infected mice, cyclophosphamide (CY, 150 mg/kg), was given IP at 35 and 38 dpi. In uninfected adult mice, this dose of CY resulted in a 70% reduction in total leukocytes and 57% reduction in T cell numbers within two days of the second injection (data not shown). As seen in Figure 7A, HSV-2-infected mice receiving CY experienced accelerated and increased mortality (11/16) compared to untreated, immunocompetent HSV-2-infected animals (7/16), p = 0.285.
To explore whether CY-induced immunosuppression in this study resulted in increased virus replication in the persistently infected animals, the brain, spinal cord, DRG, liver and spleen were harvested from moribund and surviving mice at 105 dpi. As shown in Figure 7B, CY treated animals 36% (5/14) had detectable virus at the time of sacrifice in neural tissue (4 in the DRG, 1 in the spinal cord), while no replicating virus was detected in any of the tissues from non-CY treated mice (0/13 mice, p=0.040). No significant difference in the frequency of vaginal viral shedding was observed from 38–54 dpi between immunocompetent (28% shedding) and CY-immunosuppressed (20% shedding) mice (data not shown). These data indicate that this model can be used to explore the mechanisms that control persistent HSV-2 infection in the DRG and spinal cords of HSV-2 infected mice.
4. Discussion
An animal model of genital HSV shedding which accurately mimics human disease and allows for in depth immunological studies would be highly beneficial to understanding the pathogenesis and immunobiology of genital herpes recurrences. Models of spontaneous HSV-2 reactivation and shedding exist in guinea pigs (Stanberry, 1991) while the rabbit has been used to study HSV-1 (Centifanto-Fitzgerald et al., 1987); (Kang et al., 2003). However, sophisticated immunological reagents are not available for these animals as opposed to the mouse where there are a plethora of reagents available for studying mouse immunology, including transgenic and knockout mouse strains.
In the studies presented here, we show that mice treated with ACV can survive an HSV-2 infection and surviving mice will establish a persistent infection and intermittently shed HSV-2 from the vaginal mucosa. Significantly, we have shown for the first time that mice surviving primary genital HSV-2 infection, like HSV-infected humans, undergo periods of asymptomatic viral shedding. We observed intermittent viral shedding in mice with and without symptoms of primary HSV infection, and at an overall shedding frequency of 10–25%. These numbers are comparable to the frequency of vaginal shedding in HSV-infected women (regardless of whether symptoms of primary infection were observed) in which an average of 28% of daily swabs contained HSV DNA by PCR (Wald, 2004). Interestingly, HSV shedding in humans can be detected by culture, although the detection is drastically reduced (2–8% of days) compared to PCR. In ACV-rescued mice, no replicating virus was detectable by culture, and virtually all shedding events required nested PCR to detect viral DNA. These data indicate that the amount of virus present in the mouse vagina during a shedding episode is extremely low (<100 genome copies), therefore attempts to use real-time PCR to quantify the amount of virus shed were unsuccessful.
In the studies presented here, all of the DRG from infected animals were positive for viral DNA, while replicating virus was identified rarely (2.9% of DRG evaluated, 3/102) in four separate studies. Thus, it appears that the majority of mice were latently-infected, at least in the DRG using conventional definitions of latency. Similarly, replicating virus was not detected in other neural tissues, including the brain and spinal cord. However, most of the spinal cords evaluated from the ACV-rescued mice were positive for viral DNA, indicating a latent infection at this site.
While it appears that the virus shed from the vagina after the acute disease has resolved is from reactivated virus, it is also possible that the recovered virus is from low levels of persistent virus in the ganglia or other peripheral sites. In this regard, it is important to note that in humans this question has also not been resolved and recent evidence from the University of Washington suggests that perhaps virus is continually released from nerve endings (Schiffer and Corey, 2009). The intermittent nature of shedding in our model would seem to preclude persistent virus in the genital tract as an explanation for the identified virus.
In our attempt to increase the numbers of HSV-2 infected mice that survive past primary infection, we increased the dose of ACV used in our studies and we infected mice with the MS strain of HSV-2 mice. These modifications to our model resulted in a marked improvement on survival of the ACV-treated mice. While infection with the MS strain of HSV-2 resulted in reduced symptoms and mortality compared to the 186 strain, the frequency of shedding was similar between the two strains. We concluded that use of the MS strain was preferred in this model because it reduced the number of animals required for each experiment and the mice survived longer so that persistent HSV-2 infection could be analyzed. In order to further optimize this model for future studies, it may be of interest to evaluate other strains of HSV-2 to optimize the frequency of shedding and/or further enhance survival. Alternatively, it is not known whether mouse strains that are more resistant to lethal HSV infection would exhibit the same frequency of asymptomatic shedding. In our current studies, we chose to use the Swiss Webster mouse strain since we routinely use this strain for HSV-2 antiviral and vaccine studies, and hypothesized that infection of these outbred mice may more closely reflect the variability of HSV-2 infection in humans. It is tempting to speculate whether this contributes to the variability in symptoms that we observe in our studies. It is possible that ACV rescue of HSV-2 infected inbred mice, such as BALB/c or C57BL/6 strains, will lead to less variability in symptoms and shedding, as well as the opportunity to characterize certain virus specific immune responses that may control HSV-2 vaginal shedding since immunological reagents are readily available (Gill et al., 2000); (Haynes et al., 2006); (Johnson et al.); (Milligan and Bernstein, 1997); (Muller et al., 2009).
In a previous study, Parr and Parr (Parr et al., 2005) reported that BALB/c mice treated with anti-HSV antibody and VACV also survived an intravaginal strain 333 HSV-2 infection and developed a persistent infection in the DRG. Although they did not evaluate the animals for recurrent shedding, they reported that rescued animals often developed typical symptoms of primary disease including hindlimb paralysis that often lead to death after 39–160 days following vaginal inoculation. During the shorter observation period in the studies reported here, we observed that most of the rescued animals infected with the MS strain resolved most visible symptoms of the disease within 2 weeks post infection, such as erythema, but once hair loss was observed, this often remained unchanged. In a few mice, however, the fur returned to normal, and then unexpectedly, symptoms such as hair loss or HLE would reappear as late as 35 dpi. Some treated animals showed no symptoms at all during the primary infection, or showed delayed symptoms that spontaneously appeared after 20 dpi. Hindlimb paralysis, a hallmark of genital HSV infection in mice, was observed acutely in 86% of the untreated HSV-2 infected mice whereas, unlike the Parr and Parr studies described above, we observed hind limb paralysis in only one of the ACV-rescued mice and in none after recovery from the acute disease. Instead, the most common reason for euthanasia after 30 dpi in our studies was severe and debilitating abdominal bloating, a symptom that appeared to be caused by intestinal blockage. It is possible that reactivated HSV produced damage to the neurons that innervate the intestines leading to an inability of these animals to defecate.
To determine if this model would have utility as a model for evaluation of therapies for recurrent shedding and to further validate the model, we examined the effects of ACV treatment during a period of recurrent shedding. ACV treatment significantly reduced the number of days we could detect shedding (p=0.013). Reduced vaginal shedding by ACV supports the hypothesis that the viral shedding that we detect is from replicating virus and not persistent DNA, even though we could not culture virus. ACV functions as a nucleoside analog that inhibits the viral polymerase, thus preventing viral replication at the site of reactivation in the DRG or at peripheral sites, such as the vaginal mucosa. Further, this data supports the use of the model to evaluate strategies to reduce recurrent HSV-2 shedding, the most important means for transmission of genital HSV-2 infections.
Our studies also shed some light on immune control of persistent or reactivated virus. Treatment of the ACV-rescued mice with the immunosuppressive drug cyclophosphamide accelerates the death of persistently HSV-2 infected mice. Also indicative of some level of immunological control, the immunosuppressed animals had detectable replicating virus in neural tissues while the non immunosuppressed animals did not. However, the frequency of vaginal virus shedding was not found to be significantly different between the immunocompetent and immunosuppressed mice. Further studies, however, are needed to fully evaluate shedding in immunosuppressed animals.
In summary, we have shown detectable vaginal shedding of HSV-2 in mice which survive a genital HSV-2 infection, potentially due to persistent or latent virus infection that can reactivate to produce recurrent vaginal virus shedding. This model should provide an alternative to the guinea pig model of genital herpes for the study of antivirals and vaccines aimed at preventing recurrent HSV-2 disease.
Acknowledgements
We thank Jim Ireland for technical assistance. These studies were supported by Contract N01-AI-15438 from the Virology Branch, NIAID, and NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein DI, Farley N, Bravo FJ, Earwood J, McNeal M, Fairman J, Cardin R. The adjuvant CLDC increases protection of a herpes simplex type 2 glycoprotein D vaccine in guinea pigs. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne N, Stanberry LR, Kern ER, Holan G, Matthews B, Bernstein DI. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob Agents Chemother. 2000;44:2471–2474. doi: 10.1128/aac.44.9.2471-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centifanto-Fitzgerald Y, Caldwell DR, Yates F. Herpes simplex virus: recurrent and nonrecurrent strains. Proc Soc Exp Biol Med. 1987;185:484–492. doi: 10.3181/00379727-185-42574. [DOI] [PubMed] [Google Scholar]
- Efstathiou S, Field HJ, Griffiths PD, Kern ER, Sacks SL, Sawtell NM, Stanberry LR. Herpes simplex virus latency and nucleoside analogues. Antiviral Res. 1999;41:85–100. doi: 10.1016/s0166-3542(99)00003-0. [DOI] [PubMed] [Google Scholar]
- Field HJ, Tewari D, Sutton D, Thackray AM. Comparison of efficacies of famciclovir and valaciclovir against herpes simplex virus type 1 in a murine immunosuppression model. Antimicrob Agents Chemother. 1995;39:1114–1119. doi: 10.1128/aac.39.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill TA, Morley PJ, Sweet C. Replication-defective mutants of mouse cytomegalovirus protect against wild-type virus challenge. J Med Virol. 2000;62:127–139. doi: 10.1002/1096-9071(200010)62:2<127::aid-jmv2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Haynes JR, Arrington J, Dong L, Braun RP, Payne LG. Potent protective cellular immune responses generated by a DNA vaccine encoding HSV-2 ICP27 and the E. coli heat labile enterotoxin. Vaccine. 2006;24:5016–5026. doi: 10.1016/j.vaccine.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Nelson MH, Bird MD, Chu CF, Milligan GN. Herpes simplex virus (HSV)-specific T cells activated in the absence of IFN-gamma express alternative effector functions but are not protective against genital HSV-2 infection. J Reprod Immunol. 84:8–15. doi: 10.1016/j.jri.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Seo S, Hill J, Kwon B, Lee H, Cho H, Vinay D. Changes in gene expression in latent HSV-1-infected rabbit trigeminal ganglia following epinephrine iontophoresis. Curr Eye Res. 2003;26:225–229. doi: 10.1076/ceyr.26.3.225.14894. [DOI] [PubMed] [Google Scholar]
- Kern ER. Acyclovir treatment of experimental genital herpes simplex virus infections. Am J Med. 1982;73:100–108. doi: 10.1016/0002-9343(82)90073-0. [DOI] [PubMed] [Google Scholar]
- Kern ER, Richards JT, Overall JC, Jr, Glasgow LA. Acyclovir treatment of experimental genital herpes simplex virus infections. I. Topical therapy of type 2 and type 1 infections of mice. Antiviral Res. 1983;3:253–267. doi: 10.1016/0166-3542(83)90004-9. [DOI] [PubMed] [Google Scholar]
- Magaret A, Johnston C, Wald A. Use of the designation "shedder" in mucosal detection of herpes simplex virus DNA involving repeated sampling. Sexually transmitted infections. 2009 doi: 10.1136/sti.2008.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaud P, Nagot N, Konate I, Ouedraogo A, Weiss HA, Foulongne V, Defer MC, Sawadogo A, Segondy M, Van de Perre P. Effect of HIV-1 and antiretroviral therapy on herpes simplex virus type 2: a prospective study in African women. Sexually transmitted infections. 2008;84:332–337. doi: 10.1136/sti.2008.030692. [DOI] [PubMed] [Google Scholar]
- Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med. 1992;116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Dong L, Vilalta A, Byrd B, Wilhelm KM, McClurkan CL, Margalith M, Liu C, Kaslow D, Sidney J, Sette A, Koelle DM. Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J Gen Virol. 2009;90:1153–1163. doi: 10.1099/vir.0.008771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JC, Jr, Kern ER, Schlitzer RL, Friedman SB, Glasgow LA. Genital herpesvirus hominis infection in mice. I. Development of an experimental model. Infect Immun. 1975;11:476–480. doi: 10.1128/iai.11.3.476-480.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr EL, Holliday EM, Collard MW, Parr MB. Observations on recovery from and recurrence of HSV-2 infections in adult mice that were rescued from lethal vaginal infection by antiviral therapy. Arch Virol. 2005;150:1885–1902. doi: 10.1007/s00705-005-0524-y. [DOI] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- Sacks SL, Griffiths PD, Corey L, Cohen C, Cunningham A, Dusheiko GM, Self S, Spruance S, Stanberry LR, Wald A, Whitley RJ. HSV shedding. Antiviral Res. 2004;63 Suppl 1:S19–S26. doi: 10.1016/j.antiviral.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Sawtell NM, Thompson RL, Stanberry LR, Bernstein DI. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J Infect Dis. 2001;184:964–971. doi: 10.1086/323551. [DOI] [PubMed] [Google Scholar]
- Schiffer JT, Corey L. New concepts in understanding genital herpes. Curr Infect Dis Rep. 2009;11:457–464. doi: 10.1007/s11908-009-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry LR. Evaluation of herpes simplex virus vaccines in animals: the guinea pig vaginal model. Rev Infect Dis. 1991;13 Suppl 11:S920–S923. doi: 10.1093/clind/13.supplement_11.s920. [DOI] [PubMed] [Google Scholar]
- Thackray AM, Field HJ. Comparison of effects of famciclovir and valaciclovir on pathogenesis of herpes simplex virus type 2 in a murine infection model. Antimicrob Agents Chemother. 1996;40:846–851. doi: 10.1128/aac.40.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray AM, Field HJ. The influence of cyclosporing immunosuppression on the efficacy of famciclovir or valaciclovir chemotherapy studied in a murine herpes simplex virus type 1 infection model. Antivir Chem Chemother. 1997;8:317–326. [Google Scholar]
- Thackray AM, Field HJ. Persistence of infectious herpes simplex virus type 2 in the nervous system in mice after antiviral chemotherapy. Antimicrob Agents Chemother. 2000;44:97–102. doi: 10.1128/aac.44.1.97-102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes. 2004;11 Suppl 3:130A–137A. [PubMed] [Google Scholar]
- Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, Krieger JN, Corey L. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]