Abstract
Excess weight gain contributes to increased blood pressure in most patients with essential hypertension. Although the mechanisms of obesity hypertension are not fully understood, increased renal sodium reabsorption and impaired pressure natriuresis play key roles. Several mechanisms contribute to altered kidney function and hypertension in obesity, including activation of the sympathetic nervous system, which appears to be mediated in part by increased levels of the adipocyte-derived hormone leptin, stimulation of pro-opiomelanocortin neurons, and subsequent activation of central nervous system melanocortin 4 receptors.
Keywords: Adipocyte, Cytokine, Neuron, Obesity, Tumor Necrosis Factor (TNF), Adrenergic Blockade, Cardiovascular, Hypertension, Leptin, Melanocortins
Introduction
The worldwide prevalence of obesity and associated cardiometabolic diseases have increased dramatically in the past 2–3 decades, rapidly becoming major challenges to the health care systems of most industrialized countries. Current estimates indicate that >1 billion people in the world are overweight or obese (1). In the United States, at least 65% of adults are overweight, and approximately one-third of adults are obese with a body mass index (defined as kilograms of weight/m2 of height) of >30 (2). In children, the prevalence of obesity has also risen rapidly in parallel with increasing obesity in adults; a recent report indicates that 18.4% of 4-year-old children in the United States are obese, with significantly higher rates of obesity in Hispanic, black, and Native American children (3).
Associated with obesity is a cascade of metabolic and cardiovascular disorders, including hypertension, a primary mediator of obesity-induced cardiovascular disease. Population studies show that excess weight gain predicts future development of hypertension, and the relationship between body mass index and blood pressure (BP)2 appears to be nearly linear in diverse populations throughout the world (4). Some studies suggest that excess weight gain may account for 65–75% of human essential hypertension (5). Moreover, clinical studies indicate that weight loss is effective in primary prevention of hypertension and in reducing BP in most hypertensive subjects (6). Although the importance of obesity as a major cause of essential hypertension is well established, the physiological and molecular mechanisms that mediate the BP effects of excess weight gain are only beginning to be elucidated.
Excess Weight Gain Increases Renal Sodium Reabsorption and Impairs Pressure Natriuresis
Table 1 summarizes some of the changes in cardiovascular, neurohormonal, and renal function that occur in obese humans and experimental animals (4, 7, 8). Notable changes, in addition to increased BP, include increases in cardiac output and heart rate as well as activation of the sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS). Rapid weight gain also stimulates renal tubular sodium reabsorption, and obese subjects require higher than normal BP to maintain balance between intake and renal excretion of sodium, indicating impaired renal pressure natriuresis (4).
TABLE 1.
Hemodynamic, neurohumoral, and renal changes in experimental obesity caused by a high fat diet and in human obesity
| Model | Arterial pressure | Heart rate | Cardiac output | Renal sympathetic activity | Plasma renin activity | Na+ balance | Renal tubular reabsorption | GFRa | Insulin resistance |
|---|---|---|---|---|---|---|---|---|---|
| Obese rabbits (high fat diet) | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Obese dogs (high fat diet) | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Obese humans | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
a GFR, glomerular filtration rate. The GFR changes refer to the early phases of obesity before major loss of nephron function has occurred.
Three factors are especially important in increasing renal sodium reabsorption, impairing pressure natriuresis, and causing the initial rise in BP during rapid weight gain: 1) increased SNS activity, 2) activation of the RAAS, and 3) physical compression of the kidneys by fat accumulation within and around the kidneys and by increased abdominal pressure due to accumulation of excess visceral fat. These mechanisms are closely interrelated and interact to raise BP in obese subjects. For example, SNS activation and physical compression of the kidneys both cause activation of the RAAS, and pharmacological blockade of either the RAAS or the SNS attenuates obesity-induced hypertension by at least 50–60% (7, 8). When obesity is prolonged over many years, however, renal injury may exacerbate hypertension and make it more difficult to control with antihypertensive drugs (9). In this minireview, we focus on the SNS, discussing its overall importance and mechanisms of activation in obesity hypertension.
Sympathetic Activation Contributes to Obesity Hypertension
Several observations indicate that increased SNS activity contributes to obesity hypertension (4, 8). 1) SNS activity, especially renal sympathetic nerve activity (RSNA), is increased in obese subjects; 2) pharmacological blockade of adrenergic activity lowers BP to a greater extent in obese compared with lean subjects; and 3) denervation of the kidneys markedly attenuates sodium retention and hypertension in obese experimental animals.
Administration of α- and β-adrenergic blockers or clonidine, a drug that stimulates central α2-receptors and reduces SNS activity, prevents most of the rise in BP in dogs fed a high fat diet (8). In obese hypertensive patients, combined α- and β-adrenergic blockade for 1 month reduced ambulatory BP significantly more than in lean essential hypertensive patients (10). These findings suggest that increased adrenergic activity contributes to the development and maintenance of obesity hypertension in experimental animals and humans (4, 8).
The renal sympathetic nerves mediate most, if not all, of the chronic effects of SNS activation on BP in obesity. Bilateral renal denervation greatly attenuates sodium retention and hypertension in obese dogs fed a high fat diet. Thus, obesity increases renal sodium reabsorption and causes hypertension in large part by increasing RSNA (4, 7, 8).
Differential Activation of Tissue SNS Activity in Obesity and Possible Role of Visceral Adiposity
Obesity does not cause mass activation of the SNS. Instead, increased SNS activity in various tissues is modest and appears to be differentially controlled in obesity. For example, cardiac SNS activity is normal or reduced in obese compared with lean subjects, and increased heart rate is due mainly to decreased parasympathetic activity rather than increased SNS activity (11). In contrast, SNS activity is increased in the kidneys and skeletal muscles of obese hypertensive subjects (7, 8). However, obesity-induced increases in SNS activity are mild and not sufficient to cause vasoconstriction of the kidneys or other organs, although they do stimulate renin secretion and increase renal sodium reabsorption (4, 7, 8).
SNS responses in obesity may also depend on ethnicity and other factors such as fat distribution (8). In Pima Indians, who have a high prevalence of obesity but a relatively low prevalence of hypertension, muscle SNS activity is lower than in whites and does not track well with adiposity. Black men have higher muscle SNS activity, and hypertension is more prevalent than in white men with comparable levels of obesity. Adiposity is also associated with sympathetic hyperactivity in young overweight black women. Factors such as differences in fat distribution may contribute to some of these variations in SNS responses. For reasons that are still unclear, abdominal obesity elicits much greater SNS activation than does subcutaneous or lower body obesity (12).
The mechanisms that link visceral obesity and SNS activation in humans have not been widely studied, and in most cases, muscle SNS activity has been measured rather than renal SNS activity, the primary pathway by which the SNS causes chronic hypertension (4, 8). Because of the heterogeneity in the control of autonomic outflow to different organs, measurements of muscle SNS activity may not reflect RSNA. Thus, additional studies are needed to assess the factors that influence the relationships among visceral obesity, RSNA, and hypertension in different populations.
Mechanisms of SNS Activation in Obesity Hypertension: Role of Leptin
Several mediators of SNS activation in obesity have been suggested, including 1) hyperinsulinemia; 2) angiotensin II; 3) impaired baroreceptor reflexes; 4) activation of chemoreceptor-mediated reflexes associated with sleep apnea; and 5) cytokines released from adipocytes (i.e.“adipokines”) such as leptin, tumor necrosis factor-α, and interleukin-6. These mechanisms have been reviewed previously, and there is scant evidence to support cause and effect relationships for most of these factors and obesity-induced SNS activation (8).
A promising mediator of obesity-induced SNS activation is leptin, a peptide hormone secreted by adipocytes in proportion to the degree of adiposity. Leptin crosses the blood-brain barrier (BBB) via a saturable receptor-mediated transport system (13). The short form of the leptin receptor (LRa), highly expressed in brain microvessels, is believed to be one of the important transporters (13). However, other factors modulate leptin transport across the BBB, and in obesity, the efficiency of leptin uptake by the brain is reduced (13). High levels of triglycerides, which inhibit BBB leptin transport, may at least partially explain this reduced efficiency of leptin transport in obesity, but the mechanisms involved are still uncertain (13).
Leptin binds to its receptors in various regions of the central nervous system (CNS), including the hypothalamus and brainstem, where it activates neural pathways that decrease appetite and increase SNS activity and energy expenditure (Fig. 1) (8, 14). Evidence that leptin is a powerful controller of energy balance comes from studies in mice and humans that demonstrate that missense mutations of the leptin gene or leptin receptor (LR) cause extreme early-onset obesity (15). However, mutations of the leptin gene in humans are rare, and there is little evidence that LR mutations contribute significantly to excess weight gain in most obese people. Yet, obesity may cause “resistance” to the anorexic effects of leptin, perhaps analogous to obesity-induced resistance to the metabolic effects of insulin (16). To the extent that resistance to the anorexic effects of leptin occurs in obese subjects, impaired feedback regulation of appetite could perpetuate or amplify weight gain.
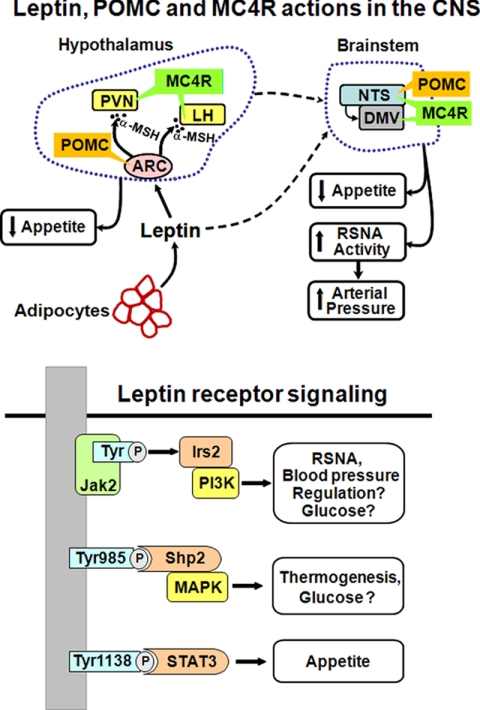
FIGURE 1.
Leptin-melanocortin activation in distinct areas of the brain and through multiple intracellular signaling pathways may differentially regulate appetite, energy expenditure, and arterial pressure. LH, lateral hypothalamus; DMV, dorsal motor nucleus of the vagus; α-MSH, α-melanocyte-stimulating hormone.
Acute and Chronic Effects of Leptin on BP
Acute injections of leptin have little effect on BP despite SNS activation perhaps due in part to counterbalancing vasodilator effects of nitric oxide, which is stimulated by leptin (17). Also, leptin-mediated increases in SNS activity may not be great enough to cause marked peripheral vasoconstriction and acutely raise BP.
However, chronic increases in plasma leptin, comparable with those found in severe obesity, can raise BP. Leptin-mediated increases in BP occur over a period of several days and are completely abolished by α- and β-adrenergic blockade, indicating that they are mediated by SNS activation (8, 17). The fact that the rise in BP occurs slowly during hyperleptinemia suggests that there is not a massive increase in SNS activity capable of causing marked peripheral vasoconstriction; instead, increased adrenergic activity raises BP through slowly acting mechanisms, including its renal effects.
The hypertensive effects of leptin in lean animals are modest but occur despite decreased food intake and weight loss that would otherwise tend to lower BP (8, 17). Moreover, the chronic hypertensive effects of leptin are greatly exacerbated by reduced NO synthesis, as often occurs in obese subjects with endothelial dysfunction (8, 17). Thus, increased leptin, comparable with that found in obese subjects, can raise BP by adrenergic activation especially when NO synthesis is impaired.
Further support for a possible role of leptin in linking obesity and hypertension comes from the observation that leptin-deficient mice are not hypertensive despite severe obesity, insulin resistance, and dyslipidemia (18). Similar results have been found in obese children with leptin gene mutations who have normal BP despite early-onset morbid obesity and many other features of the metabolic syndrome, including insulin resistance and dyslipidemia (19). These children had decreased rather than increased SNS activity as well as postural hypotension and attenuated RAAS responses to upright posture (19). Thus, the functional effects of leptin may be important in linking obesity with SNS activation and hypertension in humans as well as in rodents.
Does Obesity Cause “Selective” Leptin Resistance?
The fact that most obese humans have high circulating leptin and continue to ingest excess calories is consistent with the notion that obesity is associated with resistance to the anorexic effects of leptin. Experimental studies have shown that acute injections of leptin are less effective in suppressing appetite in obese than in lean animals (16). Although the specific mechanisms of leptin resistance are still unclear, there is evidence for decreased transport of leptin across the BBM and impaired post-receptor signaling in obese compared with lean rodents (16).
To the extent that obesity induces global leptin resistance, including the SNS response to leptin, the chronic hypertensive effects of leptin would also be attenuated in obese subjects. However, obesity may induce selective leptin resistance, whereby the RSNA responses to leptin are maintained while the appetite suppressant effects of leptin are attenuated (20). This hypothesis is supported by the finding that increases in RSNA during acute infusion of leptin are preserved in obese rodents, whereas the anorexigenic effects of leptin are attenuated (14, 20).
Possible Molecular Mechanisms of the Differential Effects of Leptin on Energy Balance and BP
In rodents, there are at least six isoforms of the LR, which are termed LRa to LRf. All of the major known physiological actions of leptin, including regulation of appetite, thermogenesis, and SNS activity, are mediated by activation of the long form of the receptor, LRb (21).
LRb is a cytokine receptor that activates Jak2 (Janus tyrosine kinase 2) (21, 22). Jak2 then associates with specific C-terminal domains of the LR, resulting in transphosphorylation of Jak2 and phosphorylation of specific Tyr residues located within the C-terminal domain of LRb (Fig. 1). In the CNS, LRb-activated Jak2 phosphorylates three Tyr residues and three main signaling pathways: 1) Tyr1138, which recruits latent Stat3 (signal transducers and activators of transcription 3) to the LRb-Jak2 complex, resulting in phosphorylation and nuclear translocation of Stat3, where it regulates transcription; 2) IRS2 (insulin receptor substrate 2) phosphorylation, which activates phosphatidylinositol 3-kinase (PI3K); and 3) Tyr985 phosphorylation, which recruits the tyrosine phosphatase Shp2, which then activates the MAPK pathway.
CNS Stat3 phosphorylation clearly mediates much of the anorexigenic effects of LR activation. Neuron-specific deletion of Stat3 causes hyperphagia and greatly attenuates leptin-mediated appetite suppression (23). However, to our knowledge, there have been no studies that have assessed the role of Stat3 signaling in mediating leptin-induced increases in RSNA and BP.
The Shp2-MAPK pathway appears to mediate major part of the effects of leptin to stimulate thermogenesis and energy expenditure. Selective deletion of Shp2 in forebrain neurons causes a prominent phenotype that includes early-onset obesity, insulin resistance, and some other characteristics of the metabolic syndrome (24). Surprisingly, the mutant mice were not hyperphagic, suggesting that forebrain deletion of Shp2 may cause obesity mainly by reducing thermogenesis and metabolic rate. Consistent with this hypothesis, the body temperature of these mice was lower compared with wild-type controls, and thermogenesis was impaired (24). Whether forebrain Shp2 deletion attenuates the effects of leptin on RSNA activity and BP is unknown, however.
Activation of the IRS2-PI3K pathway may not play a major role in mediating the effects of leptin on energy balance because mice with IRS2 deleted in the entire brain have normal body weight or only mild obesity (25). However, the IRS2-PI3K pathway may be critical for leptin-mediated increases in RSNA because pharmacological blockade of PI3K almost completely abolishes the acute effects of leptin on RSNA (25). Further studies are needed, however, to assess the role of the IRS2-PI3K pathway in mediating the chronic effects of leptin on RSNA and BP. Thus, the molecular pathways by which the anorexogenic effects of leptin are attenuated while the effects on SNS activity and BP remain intact in obesity are still poorly understood and remain an important area for further investigation.
Multiple CNS Sites for Leptin-mediated Effects on Energy Balance and BP
Deletion of the LR in the entire brain recapitulates all of the known metabolic phenotypes of leptin-deficient mice, including obesity, hyperphagia, decreased thermogenesis, and impaired glucose homeostasis, indicating that the CNS actions of leptin play a dominant role in mediating its metabolic actions (16). High levels of LR mRNA and protein are expressed in the forebrain, especially in the ventromedial hypothalamus, arcuate nucleus (ARC), and dorsomedial areas of the hypothalamus, as well as in vasomotor centers of the brainstem (16).
The effects of leptin on the ARC appear to be important for controlling energy balance but provide only a partial explanation of the role of leptin in body weight regulation because rescue of the LR specifically in the ARC of db/db mice only partially reverses hyperphagia (26). Also, mice lacking leptin signaling specifically in pro-opiomelanocortin (POMC) neurons are only mildly obese (27). This suggests that other neuronal populations may contribute to the effects of leptin on body weight regulation.
Supporting this idea is the finding that leptin activates c-Fos and Stat3 at several sites beyond the ARC and POMC neurons, including the paraventricular nucleus (PVN) and the nucleus solitary tract (NTS) of the brainstem (16). Although the specific functions of each of the CNS centers in mediating the various actions of leptin are largely unknown, studies in which leptin was injected into specific brain regions (e.g. ARC and NTS) suggest differential regulation of appetite and SNS activity (16). Thus, emerging evidence suggests that LR activation of different intracellular signaling cascades in different CNS centers may provide a basis for the phenomenon of selective leptin resistance and divergent regulation of appetite, energy expenditure, and BP in obesity.
Role of CNS Melanocortins in Mediating Obesity-induced SNS Activation and BP Effects of Leptin
Neurons that express POMC play a major role in regulating energy balance by reducing food intake and stimulating thermogenesis in experimental animals and humans (28, 29). Located in the ARC of the hypothalamus, these neurons send projections to the PVN and lateral hypothalamus, where they release α-melanocyte-stimulating hormone, which in turn activates melanocortin 3 and melanocortin 4 receptors (MC3/4Rs) (Fig. 1). Other regions of the brain, especially the NTS, also contain POMC neurons and MC3/4Rs, which may be important in regulating energy balance (28, 29).
Increased food intake and rapid weight gain stimulate POMC neuronal activity and MC3/4R signaling, whereas negative energy balance inactivates POMC neurons and MC3/4R signaling. The importance of this pathway in regulating energy balance and body weight is clearly demonstrated by the observation that defects of POMC neuronal function or MC3/4R signaling cause severe obesity in humans and experimental animals (15). Mutations of the MC4R have been suggested to be responsible for up to 5% of severe childhood obesity, making this one of the most common single gene disorders known (30).
Chronic Activation of the CNS POMC-MC3/4R Pathway Causes SNS Activation and Hypertension, whereas Blockade of POMC-MC3/4 Signaling Reduces BP
In addition to regulating energy balance, the CNS POMC-MC3/4R system may be essential for obesity to cause SNS activation and hypertension. Chronic pharmacological activation of CNS MC3/4Rs in rats increases BP while reducing appetite and causing weight loss, effects that would normally decrease BP (31). Conversely, blockade of CNS MC3/4Rs in rodents causes voracious feeding and rapid weight gain and reduces rather than increases BP (31). Similarly, mice with MC4R deficiency are hyperphagic and obese and have many features of the metabolic syndrome but are not hypertensive on normal or high salt diets (32).
The effects of MC3/4R antagonism to lower BP are especially pronounced in spontaneously hypertensive rats (SHRs), a genetic model of hypertension characterized by increased SNS activity (33). Chronic CNS antagonism of MC3/4Rs caused a much greater reduction in BP in SHRs than in normotensive Sprague-Dawley or Wistar-Kyoto rats despite marked increases in food intake, weight gain, and insulin resistance; the fall in BP in SHRs after MC3/4R blockade was similar to that achieved with α-and β-adrenergic blockade (33). These observations suggest that MC3/4R activation may contribute to tonic activation of the SNS and may be necessary for rapid weight gain to increase BP.
A recent study in humans also suggests that MC4R activation may contribute to obesity-induced hypertension. The prevalence of hypertension was markedly lower in MC4R-deficient humans compared with control subjects despite severe obesity and its associated metabolic disorders (34). Even after exclusion of patients taking antihypertensive medications, BP, heart rate, and 24-h norepinephrine excretion were significantly lower in MC4R-deficient subjects than in control subjects. Moreover, subcutaneous administration of a synthetic MC4R agonist for 7 days increased BP, similar to the responses found in rodents. Thus, in humans as well as in rodents, chronic activation of the MC4R raises BP, and the presence of a functional POMC-MC4R system appears to be necessary for obesity to increase SNS activity and arterial pressure.
Activation of the POMC-MC3/4R Pathway Mediates the Chronic Effects of Leptin on SNS Activation and BP
POMC-containing neurons co-localize with the LR or lie in close proximity to hypothalamic neurons containing the LR (28). Leptin increases POMC expression in the hypothalamus, resulting in increased production of α-melanocyte-stimulating hormone, the endogenous ligand for MC3/4Rs (28).
Evidence that activation of the POMC-MC3/4R pathway mediates a major part of the BP effects of leptin comes from the observation that LR deletion specifically in POMC neurons abolished the rise in BP, but not anorexia, associated with chronic leptin infusions (35). Also, pharmacological antagonism of MC3/4Rs completely abolished the effects of leptin to stimulate RSNA (36) and to raise BP (37).
The BP effects of leptin-POMC activation appear to be mediated mainly by activation of the MC4R rather than the MC3R because chronic leptin infusions did not increase BP in obese MC4R−/− mice or in nonobese MC4R−/− mice that were pair-fed to match the body weight of control wild-type mice; these findings indicate that MC4R deficiency, not obesity-induced leptin resistance, abolished the chronic BP effects of leptin in MC4R−/− mice (38). In contrast, pharmacological blockade of the CNS MC4R did not attenuate the effect of leptin to stimulate brown adipose tissue sympathetic nerve activity, suggesting differential control of brown adipose tissue sympathetic nerve activity and thermogenesis compared with RSNA and BP (36).
Possible Divergent Control of Cardiovascular Function and Metabolic Functions by the CNS POMC-MC3/4R Pathway
Although the CNS melanocortin system appears to be important in regulating BP, appetite, and thermogenesis, the specific actions of the MC4R located in distinct brain centers is still unclear. The MC4R is a G-protein-coupled receptor that, when stimulated in the brain, appears to exert almost all of its known physiological effects by activating adenylate cyclase and increasing cAMP formation (28). Therefore, any differential control of cardiovascular and metabolic functions through MC4R activation is probably related to different levels of activation in various areas of the brain.
POMC neurons are located in the forebrain and hindbrain, especially the ARC and the NTS. Expression of the MC4R is especially dense in the PVN, although significant expression of the MC4R is also found in the NTS and the dorsal motor nucleus of the vagus (29). The PVN is an area of the hypothalamus that is important for control of autonomic function as well as food intake. However, most studies investigating the role of the MC4R in distinct areas of the brain have been performed by acute injection of pharmacological agonists and antagonists. Although these studies provide important information, they are also limited by the specificity and efficacy of the drugs, possible spillover of the drugs to surrounding areas, and physiological relevance when concentrations of agonists greatly exceed the endogenous levels of MC4R stimulation. Also, the acute effects of MC4R activation may not reveal the mechanisms for chronic effects on BP and metabolic functions. Further studies are therefore needed to assess whether physiological activation of the MC4R in different areas of the brain may contribute to independent regulation of BP and energy balance in obesity.
In summary, excess weight gain is a major cause of human essential hypertension. Activation of the SNS plays a key role in increasing renal sodium reabsorption, impairing pressure natriuresis, and raising BP in obese subjects. SNS activation in obesity is mediated in part by increased leptin, stimulation of POMC neurons, and subsequent activation of the MC4R in the CNS. The molecular pathways by which the CNS leptin-melanocortin system differentially regulates energy balance, SNS activity, and BP in obesity are still unclear, however, and remain an important area for further investigation.
This work was supported, in whole or in part, by National Institutes of Health Grant P01 HL51971 from NHLBI. This work was also supported by the American Heart Association. This is the third article in the “Biochemistry in Medicine: Hypertension Minireview Series.” This minireview will be reprinted in the 2010 Minireview Compendium, which will be available in January, 2011.
- BP
- blood pressure
- SNS
- sympathetic nervous system
- RAAS
- renin-angiotensin-aldosterone system
- RSNA
- renal sympathetic nerve activity
- BBB
- blood-brain barrier
- CNS
- central nervous system
- LR
- leptin receptor
- PI3K
- phosphatidylinositol 3-kinase
- MAPK
- mitogen-activated protein kinase
- ARC
- arcuate nucleus
- POMC
- pro-opiomelanocortin
- PVN
- paraventricular nucleus
- NTS
- nucleus solitary tract
- MC3/4R
- melanocortin 3 and melanocortin 4 receptor
- SHR
- spontaneously hypertensive rat.
REFERENCES
- 1.Yach D., Stuckler D., Brownell K. D. ( 2006) Nat. Med. 12, 62– 66 [DOI] [PubMed] [Google Scholar]
- 2.Ogden C. L., Carroll M. D., Curtin L. R., McDowell M. A., Tabak C. J., Flegal K. M. ( 2006) JAMA 295, 1549– 1555 [DOI] [PubMed] [Google Scholar]
- 3.Anderson S. E., Whitaker R. C. ( 2009) Arch. Pediatr. Adolesc. Med. 163, 344– 348 [DOI] [PubMed] [Google Scholar]
- 4.Hall J. E. ( 2003) Hypertension 41, 625– 633 [DOI] [PubMed] [Google Scholar]
- 5.Garrison R. J., Kannel W. B., Stokes J., 3rd, Castelli W. P. ( 1987) Prev. Med. 16, 234– 251 [Google Scholar]
- 6.Stevens V. J., Obarzanek E., Cook N. R., Lee I. M., Appel L. J., Smith West D., Milas N. C., Mattfeldt-Beman M., Belden L., Bragg C., Millstone M., Raczynski J., Brewer A., Singh B., Cohen J. ( 2001) Ann. Intern. Med. 134, 1– 11 [DOI] [PubMed] [Google Scholar]
- 7.Davy K. P., Hall J. E. ( 2004) Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R803– R813 [DOI] [PubMed] [Google Scholar]
- 8.Hall J. E., da Silva A. A., Brandon E., Stec D. E., Ying Z., Jones D. W. ( 2007) Comprehensive Hypertension, pp. 447– 468, Elsevier Science Publishing Co., Inc., New York [Google Scholar]
- 9.Hall J. E., Henegar J. R., Dwyer T. M., Liu J., da Silva A. A., Kuo J. J., Tallam L. ( 2004) Adv. Ren. Replace. Ther. 11, 41– 54 [DOI] [PubMed] [Google Scholar]
- 10.Wofford M. R., Hall J. E. ( 2004) Curr. Pharm. Des. 10, 3621– 3637 [DOI] [PubMed] [Google Scholar]
- 11.Esler M., Straznicky N., Eikelis N., Masuo K., Lambert G., Lambert E. ( 2006) Hypertension 48, 787– 796 [DOI] [PubMed] [Google Scholar]
- 12.Alvarez G. E., Beske S. D., Ballard T. P., Davy K. P. ( 2002) Circulation 106, 2533– 2536 [DOI] [PubMed] [Google Scholar]
- 13.Bouret S. G. ( 2008) Endocrinology 149, 875– 876 [DOI] [PubMed] [Google Scholar]
- 14.Rahmouni K., Correia M. L., Haynes W. G., Mark A. L. ( 2005) Hypertension 45, 9– 14 [DOI] [PubMed] [Google Scholar]
- 15.Farooqi S., O'Rahilly S. ( 2006) Endocr. Rev. 27, 710– 718 [DOI] [PubMed] [Google Scholar]
- 16.Morris D. L., Rui L. ( 2009) Am. J. Physiol. Endocrinol. Metab. 297, E1247– E1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva A. A., do Carmo J., Dubinion J., Hall J. E. ( 2009) Curr. Hypertens. Rep. 11, 206– 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark A. L., Shaffer R. A., Correia M. L., Morgan D. A., Sigmund C. D., Haynes W. G. ( 1999) J. Hypertens. 17, 1949– 1953 [DOI] [PubMed] [Google Scholar]
- 19.Ozata M., Ozdemir I. C., Licinio J. ( 1999) J. Clin. Endocrinol. Metab. 10, 3686– 3695 [DOI] [PubMed] [Google Scholar]
- 20.Mark A. L., Correia M. L., Rahmouni K., Haynes W. G. ( 2002) J. Hypertens. 20, 1245– 1250 [DOI] [PubMed] [Google Scholar]
- 21.Münzberg H., Myers M. G., Jr. ( 2005) Nat. Neurosci. 8, 566– 570 [DOI] [PubMed] [Google Scholar]
- 22.Yang R., Barouch L. A. ( 2007) Circ. Res. 101, 545– 559 [DOI] [PubMed] [Google Scholar]
- 23.Gao Q., Wolfgang M. J., Neschen S., Morino K., Horvath T. L., Shulman G. I., Fu X. Y. ( 2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4661– 4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang E. E., Chapeau E., Hagihara K., Feng G. S. ( 2004) Proc. Natl. Acad. Sci. U.S.A. 101, 16064– 16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhury A. I., Heffron H., Smith M. A., Al-Qassab H., Xu A. W., Selman C., Simmgen M., Clements M., Claret M., Maccoll G., Bedford D. C., Hisadome K., Diakonov I., Moosajee V., Bell J. D., Speakman J. R., Batterham R. L., Barsh G. S., Ashford M. L., Withers D. J. ( 2005) J. Clin. Invest. 115, 940– 950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowalski T. J., Liu S. M., Leibel R. L., Chua S. C., Jr. ( 2001) Diabetes 50, 425– 435 [DOI] [PubMed] [Google Scholar]
- 27.Balthasar N., Dalgaard L. T., Lee C. E., Yu J., Funahashi H., Williams T., Ferreira M., Tang V., McGovern R. A., Kenny C. D., Christiansen L. M., Edelstein E., Choi B., Boss O., Aschkenasi C., Zhang C. Y., Mountjoy K., Kishi T., Elmquist J. K., Lowell B. B. ( 2005) Cell 23, 493– 505 [DOI] [PubMed] [Google Scholar]
- 28.Cone R. D. ( 2006) Endocr. Rev. 27, 736– 749 [DOI] [PubMed] [Google Scholar]
- 29.Corander M. P., Fenech M., Coll A. P. ( 2009) Circulation 120, 2260– 2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaisse C., Clement K., Durand E., Hercberg S., Guy-Grand B., Froguel P. ( 2000) J. Clin. Invest. 106, 253– 262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo J. J., Silva A. A., Hall J. E. ( 2003) Hypertension 41, 768– 774 [DOI] [PubMed] [Google Scholar]
- 32.Tallam L. S., Stec D. E., Willis M. A., da Silva A. A., Hall J. E. ( 2005) Hypertension 46, 326– 332 [DOI] [PubMed] [Google Scholar]
- 33.da Silva A. A., do Carmo J. M., Kanyicska B., Dubinion J., Brandon E., Hall J. E. ( 2008) Hypertension 51, 884– 890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenfield J. R., Miller J. W., Keogh J. M., Henning E., Satterwhite J. H., Cameron G. S., Astruc B., Mayer J. P., Brage S., See T. C., Lomas D. J., O'Rahilly S., Farooqi I. S. ( 2009) N. Engl. J. Med. 360, 44– 52 [DOI] [PubMed] [Google Scholar]
- 35.do Carmo J. M., da Silva A. A., Hall J. E. ( 2009) Hypertension 54, e102 [Google Scholar]
- 36.Haynes W. G., Morgan D. A., Djalali A., Sivitz W. I., Mark A. L. ( 1999) Hypertension 33, 542– 547 [DOI] [PubMed] [Google Scholar]
- 37.da Silva A. A., Kuo J. J., Hall J. E. ( 2004) Hypertension 43, 1312– 1317 [DOI] [PubMed] [Google Scholar]
- 38.Tallam L. S., da Silva A. A., Hall J. E. ( 2006) Hypertension 48, 58– 64 [DOI] [PubMed] [Google Scholar]