Abstract
Five basic tastes (bitter, sweet, umami, salty, and sour) are detected in the four taste areas where taste buds reside. Although molecular mechanisms for detecting bitter, sweet, and umami have been well clarified, those for sour and salty remain poorly understood. Several channels including acid-sensing ion channels have been proposed as candidate sour receptors, but they do not encompass all sour-sensing abilities in vivo. We recently reported a novel candidate for sour sensing, the polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 channel complex. This channel is not a traditional ligand-gated channel and is gated open only after removal of an acid stimulus, called an off response. Here we show that off responses upon acid stimulus are clearly observed in native taste cells from circumvallate, but not fungiform papillae, of glutamate decarboxylase 67-green fluorescent protein (GAD67-GFP) knock-in mice, from which Type III taste cells can be visualized, using Ca2+ imaging and patch clamp methods. Off responses were detected in most cells where PKD2L1 immunoreactivity was observed. Interestingly, the pH threshold for acid-evoked intracellular Ca2+ increase was around 5.0, a value much higher than that observed in HEK293 cells expressing the PKD2L1-PKD1L3 complex. Thus, PKD2L1-PKD1L3-mediated acid-evoked off responses occurred both in HEK293 cells and in native taste cells, suggesting the involvement of the PKD2L1-PKD1L3 complex in acid sensing in vivo.
Keywords: Calcium Channels, Calcium Imaging, Ion Channels, Membrane Function, Receptors, TRP Channels, Sour Sensation
Introduction
We sense five basic tastes (bitter, sweet, umami, salty, and sour) on all regions of the tongue where taste buds exist. Taste buds are composed of four distinct morphological types of cells: basal, Type I, Type II, and Type III taste cells. Basal cells are believed to be stem cells or precursors of the other cellular elements of the taste bud, whereas Type I taste cells function similarly to glial cells (1, 2). Type II taste cells possess receptors for detecting bitter, sweet, and umami (1, 3), and their receptors are coupled to different G proteins (G protein-coupled receptors) (4–7). Although a receptor for salty taste is still not clear, a recent study showed that ENaC worked as the sodium taste receptor and sodium taste was mediated by a population of taste cells that are separate from those mediating sweet, umami, bitter, and sour taste (8). On the other hand, only Type III taste cells, which form morphologically synaptic contacts with nerve fibers (9), express several proteins involved in neuronal activity, such as neural cell adhesion molecule (NCAM), serotonin (5-HT), synaptosomal-associated protein 25 (SNAP-25) (1), and glutamate decarboxylase 67 (GAD67)2 (10), and are thought to detect sour stimulus (1, 3, 11). However, the molecular identity of receptors sensing sour taste is still controversial, although acid-sensing ion channels (ASICs) (12), hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (13), and two-pore domain potassium (K2P) channels (14) have been proposed as candidate sour receptors. We recently reported a new candidate receptor for sour sensing, the polycystic kidney disease-2-like 1 (PKD2L1)/PKD1L3 channel complex (15), with PKD2L1 reportedly expressed in Type III taste cells of mice (1). The PKD2L1-PKD1L3 complex functions as a non-selective cation channel with a linear current-voltage (I-V) relationship and relatively high Ca2+ permeability. The apparent activation threshold for the complex by acid was around pH 3.0 in HEK293 cells expressing mouse PKD2L1-PKD1L3 (16). Interestingly, the PKD2L1-PKD1L3 complex is gated open after the removal of acid stimulus, but not during acid application, a phenomenon called an “off response” (16). There are four taste areas where taste buds reside in mammals: circumvallate papillae, foliate papillae, fungiform papillae, and palate (17). Although PKD2L1 mRNA was reported to be expressed in all four of these regions, PKD1L3 mRNA was expressed only in the circumvallate and foliate papillae in mice (15). The fact that only the PKD2L1-PKD1L3 channel complex, but not each subunit alone, is properly translocated to the plasma membrane and makes a functional channel (15) suggests that PKD2L1-PKD1L3-mediated sour sensation occurs in the circumvallate and foliate papillae. Because our previous study investigated PKD2L1-PKD1L3 channel complex function only in HEK293 cells heterologously expressing the proteins, we examined whether the PKD2L1-PKD1L3 complex works as a channel with an off response property in native circumvallate taste cells.
EXPERIMENTAL PROCEDURES
Animals
The generation of GAD67-GFP (Δneo) mice has been described (18), and these heterozygous mice, used in the present study, were termed GAD67-GFP knock-in mice. These mice specifically express GFP in Type III taste cells, facilitating their identification (3). The mice were maintained with a genetic background of C57BL/6 at our animal facility. Adult mice (>8 weeks) were killed by exposure to 100% CO2 followed by cervical dislocation to minimize distress. All procedures involving the care and use of animals were approved by The Institutional Animal Care and Use Committee of the National Institutes of Natural Sciences and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (27).
Isolated Taste Cells
Lingual epithelium containing taste papillae was removed from the tongue by injecting a mix solution containing 1 mg/ml collagenase A (Roche Applied Science), 2.5 mg/ml Dispase II (Roche Applied Science), 0.25 mg/ml Elastase (Worthington), and 0.5 mg/ml DNase I (Worthington), directly under the epithelium surrounding the taste papillae. The peeled epithelium was bathed in the mix solution for 2 min and 30 s and then in Ca2+-, Mg2+-free solution for 14 min at room temperature. The isolated taste cells were then drawn into fire-polished glass micropipettes with gentle suction. Isolated taste cells were transferred to a shallow recording chamber having a glass coverslip coated with Cell-Tak (BD Biosciences). Ca2+-, Mg2+-free Tyrode's solution contained 135 mm NaCl, 5 mm KCl, 5 mm NaHCO3, 10 mm Hepes, 10 mm glucose, 10 mm sodium pyruvate, 2 mm EGTA, 2 mm BAPTA (pH 7.4). Because cell conditions deteriorate rapidly following isolation, we performed Ca2+ imaging and patch clamp experiments within a few hours of isolation.
Ca2+ Imaging and Electrophysiological Analysis of Taste Cells
Whole-cell patch clamp recordings were sampled at 10 kHz and filtered at 5 kHz for analysis using an Axopatch 200B amplifier equipped with pCLAMP software (Molecular Devices, Sunnyvale, CA). The standard bath solution used for fura-2-mediated Ca2+ imaging and whole-cell recordings contained 135 mm NaCl, 5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, 5 mm NaHCO3 10 mm sodium pyruvate, and 10 mm glucose (pH 7.4, adjusted with NaOH). The pipette solution contained 140 mm KCl, 5 mm EGTA, and 10 mm HEPES (pH 7.4, adjusted with KOH). Bath solutions of different pHs contained 135 mm NaCl, 5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 5 mm HEPES, 5 mm MES, 5 mm citric acid, 5 mm NaHCO3, 10 mm sodium pyruvate, and 10 mm glucose (pH adjusted with NaOH). To confirm Type III taste cell viability, 50 mm KCl solution consisting of 90 mm NaCl, 50 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, 5 mm NaHCO3 10 mm sodium pyruvate, and 10 mm glucose (pH 7.4, adjusted with NaOH) was used. In the Ca2+-free bath solution, CaCl2 was removed, and EGTA was added. KCl was replaced with CsCl for voltage ramp-pulse application to obtain current-voltage relationship to inhibit voltage-gated K+ channel activation (19).
Ca2+ Imaging and Transient Transfection of Genes to HEK293 Cells
HEK293 cells were transfected with mouse PKD1L3 cDNA and/or mouse PKD2L1 cDNA using Lipofectamine Plus reagents (Invitrogen). The pDsRed (Clontech) plasmid was used as a PKD expression marker for Ca2+ imaging. For immunostaining, the pGreen Lantern 1 (Invitrogen) plasmid was used instead of pDsRed. HEK293 cells were transfected with the indicated cDNAs and incubated for 30–36 h before the experiments were begun.
Immunocytochemistry
Isolated taste cells and transfected HEK293 cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, blocked with Block Ace, and incubated with anti-PKD2L1 antiserum (1/500 dilution) (a generous gift from H. Matsunami) (15) followed by incubation with Alexa Fluor 594-conjugated anti-rabbit IgG.
Definition of Percentage of Ratio for Determination of Changes in Cytosolic Ca2+ Concentration
We calculated changes in the fluorescence emission ratio at 340 and 380 nm (340/380, percentage). Values (ratios) from each experiment were normalized with respect to the response values induced by ionomycin. The ratio was defined using the following formula: change in ratio (percentage) = (maximum off response ratio values) − (ratio values just before the off response occurred)/(maximum ionomycin ratio values) − (ratio values just before the off response occurred). When the difference in the numerator was a negative value, a zero value was used.
Statistical Analysis
Statistical significance for pH threshold measurement was evaluated by analysis of variance followed by Bonferroni-type multiple t test. Evaluation of the off response current sizes between circumvallate and fungiform papillae was done with Student's t test.
RESULTS
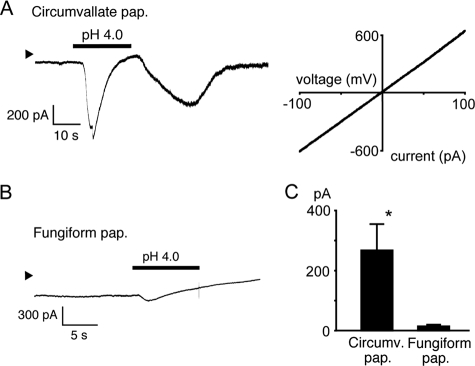
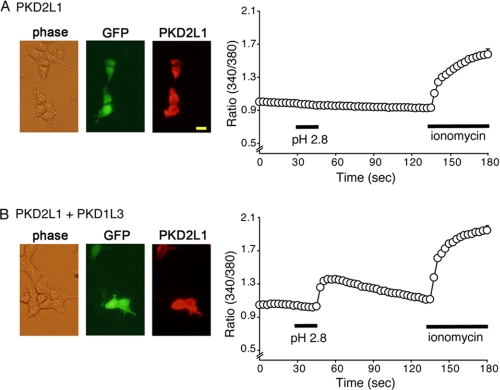
To effectively isolate the sour-sensing cells that presumably express the PKD2L1-PKD1L3 complexes, we used taste cells from GAD67-GFP knock-in mice because GAD67 is expressed in Type III taste cells (10). We stained the circumvallate taste cells with an anti-PKD2L1 antibody after measuring changes in cytosolic free Ca2+ concentrations ([Ca2+]i) upon acid stimulus. As shown in Fig. 1, A and B, a GAD67-GFP- and PKD2L1-positive taste cell showed a small transient during and a large [Ca2+]i increase after acid stimulus (pH 4.0), which corresponds to the on and off responses, respectively. Cell viability was confirmed by their response to 50 mm extracellular KCl. Cells without PKD2L1 expression did not respond to acid stimulus (Fig. 1B). Among GAD67-GFP-positive cells, most of the KCl-responsive cells also responded to acid stimulus (80%, 24 out of 30 cells), and 92.8% (116 out of 125 cells) expressed PKD2L1 protein. Similar on and off responses were observed in 10 circumvallate taste cells (Fig. 1C, left), all of which were GAD67-GFP-positive. On the other hand, such clear on and off responses were not observed in the absence of extracellular Ca2+ (Fig. 1D, left), indicating that the observed on and off responses are caused by Ca2+ influx. Some gradual [Ca2+]i increase was, however, observed during acid exposure even in the absence of extracellular Ca2+, which suggests the involvement of Ca2+ release from internal Ca2+ stores. Next, we observed two different inward currents during (230.5 ± 117.8 pA, n = 6) and after (267.7 ± 83.7 pA, n = 6) acid (pH 4.0) stimulus in GAD67-GFP-positive taste cells held at −60 mV (Fig. 2A, left, and C), indicating the existence of acid-activated channels, the latter of which could be activated PKD2L1-PKD1L3. The off currents had a linear current-voltage relationship (Fig. 2A, right), as reported for HEK293 cells expressing the PKD2L1-PKD1L3 complex (15). We then examined acid responses in Type III taste cells from fungiform papillae. Acid (pH 4.0) caused gradual [Ca2+]i increases without clear off responses after sharp [Ca2+]i rises (on response) in the presence of extracellular Ca2+ (Fig. 1C, right). The clear on responses disappeared in the absence of extracellular Ca2+, although gradual [Ca2+]i increases were still observed (Fig. 1D, right), suggesting that the on responses were made by Ca2+ influx. Consistent with the Ca2+-imaging results, no clear inward currents were observed upon washout of the pH 4.0 solution (p < 0.05 versus circumvallate taste cells), although some inward currents were recorded during acid stimulus (Fig. 2, B and C). These data suggest that off responses are detected in GAD67-GFP-positive taste cells from circumvallate, but not fungiform papillae, consistent with the notion that acid-evoked off responses are produced by the PKD2L1-PKD1L3 complex. To check PKD2L1 protein expression in GAD67-GFP-positive taste cells, we stained the GFP-positive cells with an anti-PKD2L1 antibody. As shown in supplemental Fig. 1, GAD67-GFP-positive taste cells from both circumvallate and fungiform papillae expressed PKD2L1 protein. In addition, HEK293 cells transfected with PKD2L1 cDNA alone did not show any [Ca2+]i increases upon acid (pH 2.8) stimulus, whereas cells transfected with both PKD2L1 and PKD1L3 cDNAs showed off [Ca2+]i increases in response to acid stimulus (Fig. 3), as reported previously (16). Both cell types were confirmed to express PKD2L1 proteins with the antibody. Therefore, loss of acid-evoked off responses in fungiform taste cells could be due to the lack of the PKD2L1 partner protein, PKD1L3 in these cells, which is consistent with earlier studies (15).
FIGURE 1.
Acid-evoked off responses in PKD2L1-expressing circumvallate taste cells, but not in fungiform taste cells, in mice. A, phase (left), GFP signal (green, middle), and PKD2L1 immunoreactivity (red, right) images in the same circumvallate taste cell preparation from a GAD67-GFP knock-in mouse. The bar indicates 100 μm. B, a representative trace for change in cytosolic Ca2+ concentration ([Ca2+]i) (340/380 nm ratio) of the cell positive for both GFP and PKD2L1 shown in A. Pseudo-color images at time points a and b indicated in the left trace are also shown. C, traces of [Ca2+]i changes (mean ± S.E., left and right) in response to acid (pH 4.0) and 50 mm KCl by circumvallate (n = 10, left) and fungiform (n = 5, right) taste cells in the presence of extracellular Ca2+ (Ca2+(+)). pap., papillae. D, traces of [Ca2+]i changes (mean ± S.E., left and right) in response to acid (pH 4.0) by circumvallate (n = 8, left) and fungiform (n = 5, right) taste cells in the absence of extracellular Ca2+ (Ca2+(-)).
FIGURE 2.
Representative acid-evoked current responses in circumvallate and fungiform taste cells. Membrane currents were recorded at a holding potential of −60 mV. Arrowheads indicate 0 current level. A, a representative membrane current in response to pH 4.0 stimulus in a circumvallate taste cell (left). A current-voltage relationship is shown in the right. pap., papillae. B, a representative membrane current in response to pH 4.0 stimulus in a fungiform taste cell. C, off currents in circumvallate taste cells (267.7 ± 83.7 pA, n = 6) were significantly larger than those in fungiform taste cells (14.6 ± 1.2 pA, n = 4, *, p < 0.05). Data are shown as mean ± S.E.
FIGURE 3.
PKD2L1 expression and [Ca2+]i changes in response to acid stimulus in HEK293 cells expressing PKD2L1 alone (A) and cells expressing PKD2L1 and PKD1L3 (B). A, HEK293 cells expressing PKD2L1 alone (GFP-positive) showed no [Ca2+]i increase in response to acid stimulus (pH2.8, n = 55). Data are shown as mean ± S.E. The bar indicates 25 μm. B, HEK293 cells expressing PKD2L1 and PKD1L3 (GFP-positive) showed clear [Ca2+]i increase after acid stimulus (pH 2.8, n = 28). Data are shown as mean ± S.E.
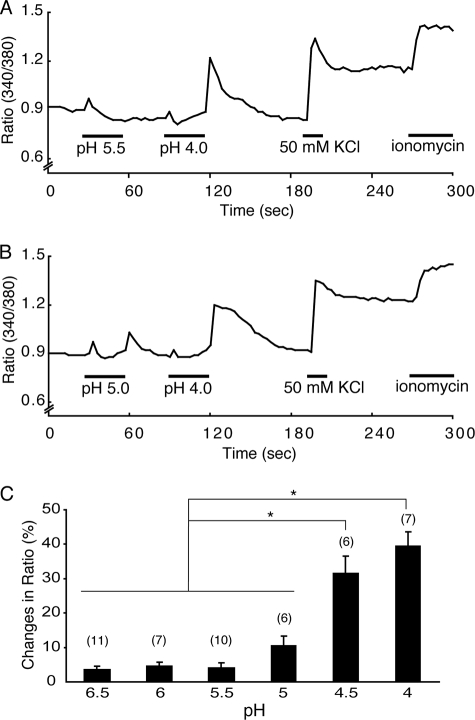
We previously reported that the pH threshold for PKD2L1-PKD1L3 complex activation is around 3.0 in HEK293 cells (16). To determine the pH threshold for acid-evoked off responses in native cells, GAD67-GFP-positive taste cells from circumvallate papillae were exposed to acids at various pH values (6.5, 6.0, 5.5, 5.0, 4.5 and 4.0) for 30 s each and then to pH 4.0 solution to confirm acid-evoked responses. A pH 5.5 solution did not cause an off [Ca2+]i increase, although a clear on [Ca2+]i increase was observed (Fig. 4A). A pH 4.0 solution evoked a large off [Ca2+]i increase in the same cell, suggesting that an acid of pH 5.5 is insufficient to gate open the off channels in circumvallate taste cells. On the other hand, a pH 5.0 solution caused a small off [Ca2+]i increase after removal followed by a larger off [Ca2+]i increase upon washout of a pH 4.0 solution (Fig. 4B). Off responses were induced by acids with a pH value of less than 5.0, and the [Ca2+]i changes (presented as the percentage of ratio normalized to the ionomycin response) increased as the pH decreased (Fig. 4C). These data indicate that the pH threshold for acid-evoked off responses in native circumvallate taste cells that is probably mediated through PKD2L1-PKD1L3 activation is around 5.0.
FIGURE 4.
pH dependence of the acid-evoked [Ca2+]i increases in the circumvallate taste cells. A and B, representative traces of [Ca2+]i changes in response to different pH solutions. pH 5.5 evoked an on response but not an off response, whereas pH 5.0 and pH 4.0 evoked both on and off responses. Cell viability was confirmed by the responses to KCl (50 mm) and ionomycin (5 μm). C, average ratio (340/380 nm) changes for off responses induced by acid solutions at various pH values (6.5, 6.0, 5.5, 5.0, 4.5, or 4.0). Percentage changes were 3.6 ± 0.9, 4.6 ± 1.1, 4.0 ± 1.5, 10.5 ± 2.8, 31.7 ± 4.8, and 39.5 ± 4.1% for pH 6.5, 6, 5.5 5, 4.5, and 4, respectively. Data are shown as mean ± S.E., and values in parentheses indicate the number of cells examined. *, p < 0.05.
DISCUSSION
We observed both on and off responses to acid stimulus in native mouse taste cells, the latter of which are consistent with our recent data obtained from HEK293 cells expressing the PKD2L1-PKD1L3 complex (16). This is the first observation of acid-evoked off responses in mouse taste cells. An RNA interference strategy could not be used to further support the involvement of endogenous PKD2L1-PKD1L3 complex in the off responses because only freshly isolated taste cells were of sufficient quality for analysis. This fact, together with a lack of reliable inhibitors, does not allow the link between the PKD2L1-PKD1L3 complex and the observed off responses in native cells to be firmly established. However, the high incidence of the off response in PKD2L1-positive Type III cells suggests that the off response property can be attributed to PKD2L1-PKD1L3 complex expression.
Using a specific anti-PKD2L1 antibody, we found that 92.8% of the GAD67-GFP-positive cells, defined as Type III cells, expressed PKD2L1 protein. In addition, almost all taste cells with acid-evoked off responses showed on responses. These results indicate that most of the Type III taste cells expressing GAD67 have an ability to sense acid, consistent with recent reports (3) that salt detection can be attributed to cells other than ones expressing gustducin, which is a Type II cell marker (20), or expressing GAD67. This notion is also in agreement with the recent report that salt can be detected in cells that are not involved in detecting the other four tastes (8). There were differences in the magnitude of on responses depending on the measurement methods used, e.g. small on responses in Ca2+ imaging experiments and relatively large inward currents (230.5 ± 117.8 pA) during acid application. However, the on responses disappeared in the absence of extracellular Ca2+ (Fig. 1D, left), suggesting that on responses are caused by Ca2+ influx. One explanation for this result is that acid-evoked on currents have relatively low Ca2+ permeability with Na+ as a main charge carrier. These transient inward Na+ currents may cause depolarization that is insufficient to activate the voltage-gated Ca2+ channels, which are expressed in Type III cells and are thought to cause large [Ca2+]i increases in response to 50 mm KCl application (10, 19). This interpretation can be supported by the notion that Na+-permeable ASIC channels could be responsible for the on responses. Indeed, ASIC1 and ASIC3 have been reported to be expressed in mouse circumvallate taste cells (21), and ASIC1a has some Ca2+ permeability (22, 23).
The pH threshold for acid-evoked [Ca2+]i increases differed between HEK293 cells expressing PKD2L1-PKD1L3 (around 3.0) (16) and native mouse circumvallate taste cells (around 5.0) (Fig. 4). This difference might indicate that molecules other than PKD2L1-PKD1L3 are responsible for the off responses in native taste cells, which we think is unlikely, because the cell types showing off responses match well with those expressing the PKD2L1-PKD1L3 complex and the linear I-V relation of the acid-evoked currents is similar between HEK293 cells expressing PKD2L1-PKD1L3 (16) and circumvallate taste cells (Figs. 1 and 2). Other proteins or regulation systems could be involved in the sour sensation together with PKD2L1-PKD1L3 in native taste cells, but not in HEK293 cells. For example, capsaicin dose dependence is different between native mouse sensory neurons and HEK293 cells expressing mouse transient receptor potential vanilloid 1 (TRPV1) because the FAF1 protein that binds to TRPV1 in sensory neurons was reported to reduce the capsaicin sensitivity in native sensory neurons (24). A previous report showed that the detection threshold for citric acid is 0.03 mm (corresponding to pH 4.5) in humans (25), which is a value similar to that observed in mice in this study. Thus, PKD2L1-PKD1L3 complex-mediated off responses could work in vivo upon mild acidification in concert with on response molecules.
What is the physiological significance of the acid-evoked off responses that are probably mediated by PKD2L1-PKD1L3 in sour sensation? Because sour taste sensation is important for preventing animals from eating harmful foods, there could be plural channels that can be activated during and following acid application. Alternatively, off responses could have specific roles in sour sensation. The existence of multiple acid receptors might make determining whether only one molecule is involved in sour sensation difficult. Recently, the genes for both PKD2L1-PKD1L3 and ASICs were reported to be deficient in two sour ageusia patients (26), supporting the importance of PKD2L1-PKD1L3 in sour sensation in humans and suggesting that PKD2L1-PKD1L3 channels and ASICs can work in concert for detecting acid. Thus, PKD2L1-PKD1L3 channel complex would be a promising target for research about sour sensation.
Supplementary Material
Acknowledgments
We thank R. Yoshida (Kyushu University) and H. Inada (Harvard University) for technical assistance and discussion. We thank H. Matsunami (Duke University) for providing an anti-PKD2L1 antibody and Y. Ishimaru (University of Tokyo) for technical advice about immunocytochemical analysis.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan (to M. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- GAD67
- glutamate decarboxylase 67
- ASIC
- acid-sensing ion channel
- PKD
- polycystic kidney disease
- GFP
- green fluorescent protein
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Kataoka S., Yang R., Ishimaru Y., Matsunami H., Sévigny J., Kinnamon J. C., Finger T. E. ( 2008) Chem. Senses 33, 243– 254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roper S. D. ( 1989) Annu. Rev. Neurosci. 12, 329– 353 [DOI] [PubMed] [Google Scholar]
- 3.Yoshida R., Miyauchi A., Yasuo T., Jyotaki M., Murata Y., Yasumatsu K., Shigemura N., Yanagawa Y., Obata K., Ueno H., Margolskee R. F., Ninomiya Y. ( 2009) J. Physiol. 587, 4425– 4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrashekar J., Mueller K. L., Hoon M. A., Adler E., Feng L., Guo W., Zuker C. S., Ryba N. J. ( 2000) Cell 100, 703– 711 [DOI] [PubMed] [Google Scholar]
- 5.Chaudhari N., Landin A. M., Roper S. D. ( 2000) Nat. Neurosci. 3, 113– 119 [DOI] [PubMed] [Google Scholar]
- 6.Nelson G., Chandrashekar J., Hoon M. A., Feng L., Zhao G., Ryba N. J., Zuker C. S. ( 2002) Nature 416, 199– 202 [DOI] [PubMed] [Google Scholar]
- 7.Nelson G., Hoon M. A., Chandrashekar J., Zhang Y., Ryba N. J., Zuker C. S. ( 2001) Cell 106, 381– 390 [DOI] [PubMed] [Google Scholar]
- 8.Chandrashekar J., Kuhn C., Oka Y., Yarmolinsky D. A., Hummler E., Ryba N. J., Zuker C. S. ( 2010) Nature 464, 297– 301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang R., Crowley H. H., Rock M. E., Kinnamon J. C. ( 2000) J. Comp. Neurol. 424, 205– 215 [DOI] [PubMed] [Google Scholar]
- 10.DeFazio R. A., Dvoryanchikov G., Maruyama Y., Kim J. W., Pereira E., Roper S. D., Chaudhari N. ( 2006) J. Neurosci. 26, 3971– 3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang A. L., Chen X., Hoon M. A., Chandrashekar J., Guo W., Tränkner D., Ryba N. J., Zuker C. S. ( 2006) Nature 442, 934– 938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ugawa S., Minami Y., Guo W., Saishin Y., Takatsuji K., Yamamoto T., Tohyama M., Shimada S. ( 1998) Nature 395, 555– 556 [DOI] [PubMed] [Google Scholar]
- 13.Stevens D. R., Seifert R., Bufe B., Müller F., Kremmer E., Gauss R., Meyerhof W., Kaupp U. B., Lindemann B. ( 2001) Nature 413, 631– 635 [DOI] [PubMed] [Google Scholar]
- 14.Richter T. A., Dvoryanchikov G. A., Chaudhari N., Roper S. D. ( 2004) J. Neurophysiol. 92, 1928– 1936 [DOI] [PubMed] [Google Scholar]
- 15.Ishimaru Y., Inada H., Kubota M., Zhuang H., Tominaga M., Matsunami H. ( 2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12569– 12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inada H., Kawabata F., Ishimaru Y., Fushiki T., Matsunami H., Tominaga M. ( 2008) EMBO Rep. 9, 690– 697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herness M. S., Gilbertson T. A. ( 1999) Annu. Rev. Physiol. 61, 873– 900 [DOI] [PubMed] [Google Scholar]
- 18.Tamamaki N., Yanagawa Y., Tomioka R., Miyazaki J., Obata K., Kaneko T. ( 2003) J. Comp. Neurol. 467, 60– 79 [DOI] [PubMed] [Google Scholar]
- 19.Medler K. F., Margolskee R. F., Kinnamon S. C. ( 2003) J. Neurosci. 23, 2608– 2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang R., Tabata S., Crowley H. H., Margolskee R. F., Kinnamon J. C. ( 2000) J Comp. Neurol. 425, 139– 151 [DOI] [PubMed] [Google Scholar]
- 21.Richter T. A., Dvoryanchikov G. A., Roper S. D., Chaudhari N. ( 2004) J. Neurosci. 24, 4088– 4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutherland S. P., Benson C. J., Adelman J. P., McCleskey E. W. ( 2001) Proc. Natl. Acad. Sci. U.S.A. 98, 711– 716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. ( 1997) Nature 386, 173– 177 [DOI] [PubMed] [Google Scholar]
- 24.Kim S., Kang C., Shin C. Y., Hwang S. W., Yang Y. D., Shim W. S., Park M. Y., Kim E., Kim M., Kim B. M., Cho H., Shin Y., Oh U. ( 2006) J. Neurosci. 26, 2403– 2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens J. C. ( 1997) Physiol. Behav. 62, 1137– 1143 [DOI] [PubMed] [Google Scholar]
- 26.Huque T., Cowart B. J., Dankulich-Nagrudny L., Pribitkin E. A., Bayley D. L., Spielman A. I., Feldman R. S., Mackler S. A., Brand J. G. ( 2009) PLoS One 4, e7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institutes of Health ( 1985) Guide for the Care and Use of Laboratory Animals, Publication Number 85-23, National Institutes of Health, Bethesda, MD [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.