Abstract
δ subunit-containing γ-aminobutyric acid, type A (GABAA)receptors are expressed extrasynaptically and mediate tonic inhibition. In cerebellar granule cells, they often form receptors together with α1 and/or α6 subunits. We were interested in determining the architecture of receptors containing both subunits. We predefined the subunit arrangement of several different GABAA receptor pentamers by concatenation. These receptors composed of α1, α6, β3, and δ subunits were expressed in Xenopus oocytes. Currents elicited in response to GABA were determined in the presence and absence of 3α,21-dihydroxy-5α-pregnan-20-one (THDOC) or ethanol, or currents were elicited by 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol (THIP). Several subunit configurations formed active channels. We therefore conclude that δ can assume multiple positions in a receptor pentamer made up of α1, α6, β3, and δ subunits. The different receptors differ in their functional properties. Functional expression of one receptor type was only evident in the combined presence of the neurosteroid THDOC with the channel agonist GABA. Most, but not all, receptors active with GABA/THDOC responded to THIP. None of the receptors was modulated by ethanol concentrations up to 30 mm. Several observations point to a preferred position of δ subunits between two α subunits in α1α6β3δ receptors. This property is shared by α1β3δ and α6β3δ receptors, but there are differences in the additionally expressed isoforms.
Keywords: GABA Receptors, Neurobiology, Neuroscience, Neurotransmitter Receptors, Receptor Structure-Function, Neurosteroids, Subunit Concatenation
Introduction
GABAA2 receptors are the most prominent inhibitory neurotransmitter receptors in the mammalian brain. They are part of the family of Cys loop ligand-gated ion channels together with nicotinic acetylcholine, glycine, and serotonin type-3 receptors. GABAA receptors are composed of combinations of five subunits selected from α (subunits 1–6), β (subunits 1–4), γ (subunits 1–3), δ, ϵ, θ, and π (Refs. 1–4). The five subunits are arranged around a central Cl− selective channel (1). Subunit composition and relative positioning of the subunits confer specific physiological and pharmacological properties to GABAA receptors (5–7).
Synaptic receptors mediate phasic inhibition, whereas extrasynaptic receptors mediate tonic inhibition (8–10). The δ subunit is part of the extrasynaptically located GABAA receptors, which have been shown to be operational in many regions of the brain (for review, see Ref. 10), among them cerebellar granule cells (11). The δ subunit is co-assembled either with α4 or α6 subunits (12, 13) or with α1 subunits, at least in hippocampal interneurons (14).
Several studies suggest that δ and γ2 subunits do not coexist in the same receptor (15–17). Therefore, δ has generally been considered as a substitute of the γ2 subunit. δ subunit-containing receptors and their sensitivity to neurosteroids have been functionally characterized (18–20). Interestingly, δ subunit-containing GABAA receptors have been implicated in altered seizure susceptibility and altered states of anxiety during ovarian cycle (21) and in postpartum depression (22).
α6 subunits are exclusively expressed in cerebellar granule cells (6). By immunogold staining, this subunit has been shown to be concentrated at Golgi synapses and at mossy fiber synapses and at a lower density in the extrasynaptic membrane (23). The δ subunit has been found exclusively in extrasynaptic locations, in the soma and on dendritic membranes (24). Thus, α6 and δ subunits may co-localize in extrasynaptic membranes. α6 knock-out mice display a strongly reduced expression of δ (12), suggesting an association of the two subunits. Immunoprecipitation experiments provided direct evidence for this (25). In whole cerebellum, GABAA receptor subtypes have been quantified using sequential immunoaffinity adsorption (26). α6 and δ subunits may be associated with the α1 subunit. The receptors composed of α1, α6, βx (x = 2,3), and δ subunits have been estimated to constitute 6% of all receptors in rat cerebellum and 10% in mouse cerebellum, respectively. As the α6 subunit is exclusively expressed in granule cells, the percentage of α1α6βxδ receptors is substantially higher in these cells. Evidence for the simultaneous presence of α1 and α6 in the same receptor pentamer in recombinant systems has also been provided by functional experiments at least for γ2-containing receptors (27). Their relative position in the pentamer has been shown to strongly affect the pharmacological properties in the case of the α1α6β2γ2 receptor (7).
To enable an approach to establish the number and molecular location of pharmacologically important drug binding sites of α1α6β3δ GABAA receptors, it is important to understand their architecture. It is unfortunately not possible to determine membrane protein architecture directly in neurons. Thus, model systems have to be used. We combined subunit concatenation with functional expression in Xenopus oocytes. Thus, we covalently linked α1, α6, β3, and δ subunits to force a defined arrangement of different subunits in a pentamer (28) and characterized the concatenated receptor in detail. From our work with α1β3δ (29) and α6β3δ GABAA receptors (30), we anticipated that positional roles of subunits were not as well defined as in α1β2γ2 receptors (31, 32). We indeed provide evidence for the facts that the δ subunit can assume different positions in the receptor pentamer and that neurosteroids strongly enhance currents elicited by GABA in most receptor forms and are co-agonists at one of the receptors. Ethanol, at concentrations below 30 mm, fails to modulate all functional receptors.
EXPERIMENTAL PROCEDURES
Construction of Concatenated δ Subunit-containing cDNAs
We thank Dr. Lüddens for the cDNA coding for the rat δ subunit. The approach used for subunit concatenation of GABAA receptors has been described previously (28, 31–34). We made use of the constructs detailed in Kaur et al. (29) and Baur et al. (30). Two adjacent non-concatenated subunits α and β are designated as αβ or α/β, and concatenated subunits are designated as α-β. Subunit sequences must be read counterclockwise. We prepared the triple subunit constructs α1-10-β3-26-α6 and α6-11-β3-23-α1 and the pentameric construct β3-26-α6-11-δ-26-β3-23-α1. For the design of the linkers, we applied the rule that the sum of the predicted C-terminal protrusion of a preceding subunit and the artificial linker has to be minimally 23 residues in length. Shorter linkers do not result in receptor expression (31). The linkers were as described previously (29, 30).
Expression in Xenopus Oocytes
Capped cRNAs were synthesized (Ambion, Austin, TX) from the linearized vectors containing different non-concatenated and concatenated subunits. A poly(A) tail of about 400 residues was added to each transcript using yeast poly(A) polymerase (USB Corp., Cleveland, OH) to stabilize them. The concentration of the cRNA was quantified on a formaldehyde agarose gel using Radiant Red stain (Bio-Rad) for visualization of the RNA with known concentrations of RNA ladder (Invitrogen) as standard on the same gel. The cRNAs were dissolved in water and stored at −80 °C. Isolation of oocytes from the frogs, culturing of the oocytes, injection of cRNA, and defolliculation were done as described earlier (35). cRNA coding for each dual and triple subunit concatemer was injected either alone or in different combinations in oocytes, resulting in a total of nine different concatenated receptors. Oocytes were injected with 50 nl of RNA solution containing each construct at 50 nm. Keeping the amount of injected RNA constant is crucial for the concatenation approach (36). The combination of α1, α6, β3, and δ subunits was expressed at a ratio of 10:10:10:50 nm. The injected oocytes were incubated in modified Barth's solution (35) at 18 °C for about 72 h for the determination of Imax and for at least 24 h before the measurements for the detailed characterization of the functional receptors.
Two-electrode Voltage Clamp Measurements
All measurements were done in medium containing 90 mm NaCl, 1 mm MgCl2, 1 mm KCl, 1 mm CaCl2, and 5 mm HEPES, pH 7.4, at a holding potential of −80 mV. For the determination of maximal current amplitudes, 1 mm GABA (Fluka) was applied in the absence and presence of 1 μm THDOC (Sigma) for 20 s. THDOC was prepared as a 10 mm stock solution in dimethyl sulfoxide (DMSO) and was dissolved in external solution resulting in a maximal final DMSO concentration of 0.5%. The perfusion solution (6 ml/min) was applied through a glass capillary with an inner diameter of 1.35 mm, the mouth of which was placed about 0.4 mm from the surface of the oocyte (5). Non-concatenated and concatenated receptors containing the δ subunit showed a pronounced decrease in response to GABA with time. This decrease amounted to about 50% and did not recover. The experiments were performed after the measured currents became constant. Concentration-response curves for GABA were fitted with the equation I(c) = Imax/(1 + (EC50/c)n), where c is the concentration of GABA, EC50 is the concentration of GABA eliciting half-maximal current amplitude, Imax is the maximal current amplitude, I is the current amplitude, and n is the Hill coefficient. Two component curves were fitted with the equation I(c) = Ia/(1 + EC50a/c) + Ib/(1 + EC50b/c), where Ia and Ib are the maximal current amplitudes of component a and b, and EC50a and EC50b are the concentrations of GABA eliciting half-maximal current amplitudes in component a and b, respectively. The individual were curves were standardized to the sum of Ia and Ib and subsequently averaged.
The current responses to 1 μm THDOC, 1 μm THDOC + 1 mm GABA, and 100 μm THIP were determined on independent oocytes. The current amplitudes were averaged from oocytes from at least two batches. It should be noted that there is an up to 4-fold variation between two batches of oocytes in the mean current amplitude expressed from δ subunit-containing receptors.
Data are given as mean ± S.E. for the Imax values for GABA with and without THDOC and as mean ± S.D. for analysis of properties of receptors using GABA. The perfusion system was cleaned between two experiments by washing with 100% DMSO after application of THDOC experiments to avoid contamination.
RESULTS
Preparation and Characterization of Concatenated δ Subunit-containing GABAA Receptors
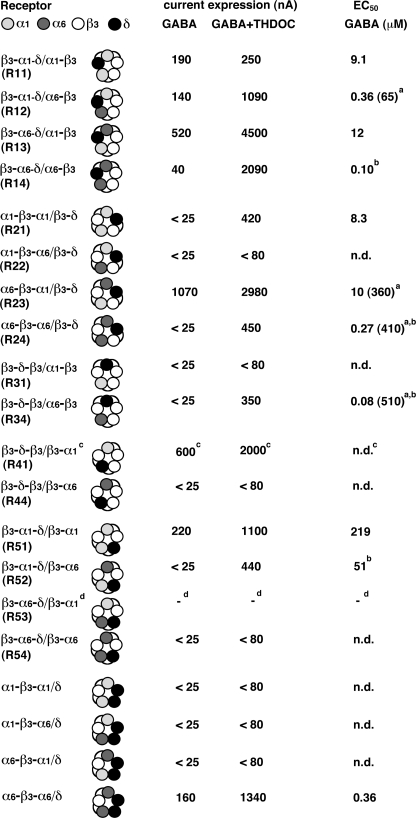
The subunit arrangement of α1β2 and of α1β2γ2 GABAA receptors has been determined to be β2β2α1β2α1 and γ2β2α2β2α1, respectively, counterclockwise when viewed from the synaptic cleft (Fig. 1) (31, 32, 34). We assume that in α1α6β3δ receptors, α6 is located in the position of one of the α1 subunits. We forced the δ subunit either into the position corresponding to the γ subunit (R52 and R53 in Fig. 1) or into the position corresponding to one of the two β subunits (R12, R13, R22, R23) in αβγ receptors. This was achieved by subunit concatenation. Fig. 1 also shows for comparison receptor pentamers made up of triple and dual subunit constructs, exclusively containing α1 (R11, R21, R31, R41, and R51) or exclusively containing α6 (R14, R24, R34, R44, and R54). Two triple subunit constructs were prepared additionally to the previously described constructs (29, 30) to form the six different GABAA receptor pentamers containing α1 and α6 (Fig. 1). For reasons mentioned below, R53 was prepared in its pentameric concatenate, P53. As in previous work with α6β3δ GABAA receptors (30), we had observed a functional receptor containing two adjacent δ subunits together with α6-β3-α6; we also prepared the two corresponding receptors α1-β3-α6/δ and α6-β3-α1/δ (see Fig. 3).
FIGURE 1.
Pentameric subunit arrangement of concatenated α1β2γ2, α1β2, α1β3δ, α6β3δ, and α1α6β3δ GABAA receptors composed of dual and triple subunit constructs. The subunit arrangement of α1β2γ2 and α1β2 is unique and has been established before (31, 32). In receptors composed of α1, β3, and δ subunits (R11, R21, R31, R41, and R51 (described in Ref. 29)); α6, β3, and δ subunits (R14, R24, R34, R44, and R54 (described in Ref. 30)), and α1α6β3δ receptors (R12, R13, R22, R23, R52 and R53 (described in this study)), subunit assembly is promiscuous. The investigated receptors with two δ subunits are also shown.
FIGURE 3.
Structure and functional expression in the Xenopus oocytes of the GABAA receptors investigated. The code for the subunits is given at the top line of the table (read the subunit sequence of concatenated receptors anti-clockwise). The figure shows subunit composition and current amplitude (nA) evoked by 1 mm GABA in the absence and presence of 1 μm THDOC, the EC50 for GABA, and the current elicited by 100 μm THIP of non-concatenated and concatenated receptors. Please note the considerable variability of current expression by δ subunit-containing receptors (see “Experimental Procedures”). Unless indicated otherwise, oocytes were injected with 50 nl of water containing 50 nm RNA coding for each subunit. At this high concentration, some of the multisubunit constructs resulted in the expression of small currents, for reasons that are poorly understood. Therefore, we consider here a current amplitude of >25 nA in the absence and >80 nA in the presence of THDOC as evidence for functional expression of a receptor type. α1/α6/β3/δ receptors (line 1) were formed by injection of 10 nm RNA each coding for α1, α6, and β3 and 50 nm RNA coding for δ. The concatenated β3-α1 subunit was injected at 50, 25, and 10 nm as indicated. Mean ± S.E. for each subunit combination is shown. n, number of oocytes from at least two batches; −, not analyzed. Footnotes a and b, a better fit was obtained if two components reflecting two sites for GABA were assumed. For R12, the respective EC50 values were 0.36 ± 0.06 and 65 ± 24 μm, the first component accounting for about 72% and the second accounting for about 28%; for R23, the respective EC50 values were 10.4 ± 4.6 and 357 ± 126 μm, the two components amounting to 24 and 76%. Footnote c, the experiments were carried out in the presence of 1 μm THDOC.
We characterized the response of concatenated receptors to THDOC, THIP, and ethanol, drugs thought to affect preferentially δ subunit-containing receptors. In addition, these receptors were compared with non-concatenated receptors.
Properties of Non-concatenated Receptors
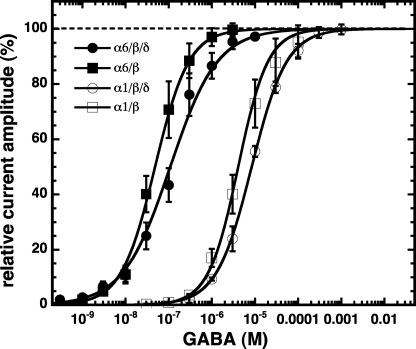
Non-concatenated α1/β3, α1/β3/δ, α6/β3, and α6/β3/δ receptors were expressed in Xenopus oocytes and characterized by electro-physiological techniques. Fig. 2 shows the concentration-response curves for GABA obtained from these receptors. They were characterized by an EC50 and a Hill coefficient of 4.6 ± 1.8 μm and 1.1 ± 0.2, 8.5 ± 0.8 μm and 1.1 ± 0.1, 0.05 ± 0.02 μm and 1.2 ± 0.2, and 0.11 ± 0.04 μm and 0.9 ± 0.1, respectively. The non-concatenated receptor α1/α6/β3/δ was also studied (Fig. 3). This combination resulted in the expression of a large current amplitude, as did some concatenated receptors containing both α1 and α6 subunits. This subunit combination might additionally result in α1/β3, α1/β3/δ, α6/β3, and α6/β3/δ receptors.
FIGURE 2.
GABA concentration dependence of non-concatenated receptors expressed in Xenopus oocytes. Averaged GABA concentration-response curves of α1/β3, α1/β3/δ, α6/β3, and α6/β3/δ receptors are shown. Individual curves were first normalized to the observed maximal current amplitude and subsequently averaged. Mean ± S.D. of experiments carried out with 3–4 oocytes from two batches for each subunit combination is shown.
α1α6β3δ GABAA Receptors Containing δ in the Position of β between the Two α Subunits
Concatenated receptors β3-α1-δ/α6-β3 (R12) and β3-α6-δ/α1-β3 (R13) (Fig. 3) were expressed in Xenopus oocytes. We estimated receptor expression with 1 mm GABA in the absence and presence of the neurosteroid THDOC. Both concatenated receptors resulted in the absence of THDOC in currents >100 nA. THDOC enhanced the current in both receptors about 8-fold. None of the functional receptors was directly activated by 1 μm THDOC alone (Fig. 3).
To characterize these receptors further, concentration-response experiments with GABA as the agonist were performed. Fig. 4A shows current traces from β3-α6-δ/α1-β3 (R13). Averaged GABA concentration-response curves for the concatenated β3-α1-δ/α6-β3 (R12) and β3-α6-δ/α1-β3 (R13) are illustrated in Fig. 4B. In the case of R13, the curve was well fitted with an EC50 of 11.9 ± 1.6 μm and a Hill coefficient of 1.0 ± 0.1, whereas in the case of R12, a better fit was obtained when two components with Hill coefficients of 1.0 each were assumed. The respective EC50 values were 0.36 ± 0.06 and 65 ± 24 μm, the first component accounting for about 72% and the second for about 28%. These values should be compared with that of non-concatenated α1α6β3δ receptor of 2.0 ± 0.9 μm (Fig. 3) despite the fact that it is not clear in the latter case if all subunits are incorporated in all receptors formed (see above).
FIGURE 4.
GABA concentration dependence of concatenated receptors. A, current traces from a GABA concentration-response curve obtained from a Xenopus oocyte expressing β3-α6-δ/α1-β3 (R13) receptors. The bars indicate the time period of GABA perfusion. GABA concentrations are indicated above the bars. B, averaged GABA concentration-response curves of β3-α1-δ/α6-β3 (R12), β3-α6-δ/α1-β3 (R13), α6-β3-α1/β3-δ (R23), and β3-α1-δ/β3-α6 (R52) receptors. The curves for β3-α6-δ/α1-β3 (R13) and β3-α1-δ/β3-α6 (R52) receptors are shown as fitted with a single component, and those for β3-α1-δ/α6-β3 (R12) and α6-β3-α1/β3-δ (R23) receptors are shown as fitted with a two components. Individual curves were first normalized to the observed maximal current amplitude and subsequently averaged. Mean ± S.D. of experiments carried out with 3–4 oocytes from two batches for each subunit combination is shown.
α1α6β3δ GABAA Receptors Containing δ in the Position of β between γ and α Subunits
Concatenated receptors α1-β3-α6/β3-δ (R22) and α6-β3-α1/β3-δ (R23) (Fig. 3) were expressed in Xenopus oocytes. Both receptors displayed a strikingly different behavior. Although R23 expressed >1000 nA current, both in the absence and in the presence of THDOC, in both conditions, R22 expressed very small currents amounting to <70 nA.
An averaged GABA concentration-response curve for the concatenated α6-β3-α1/β3-δ (R23) receptors is illustrated in Fig. 4B. The curve was fitted assuming two components with an EC50 of 10.4 ± 4.6 and 357 ± 126 μm, respectively, the two components amounting to 24 and 76%, respectively.
α1α6β3δ GABAA Receptors Containing δ in the Position of γ
Concatenated receptors β3-α1-δ/β3-α6 (R52) and β3-α6-δ/β3-α1 (R53) (Fig. 3) were expressed in Xenopus oocytes. R52 remained practically silent with GABA alone, but on co-application of GABA and THDOC, it produced currents amounting to >400 nA. R53 contains β3-α1, which, at relatively high amounts of injected RNA used here, for unknown reasons, results in currents itself (29) (Fig. 3). Therefore, we constructed the pentamer β3-α6-δ-β3-α1 (P53). As this receptor did not result in functional expression, we assume that R53 is not formed.
An averaged GABA concentration-response curve for the concatenated β3-α1-δ/β3-α6 (R52) is illustrated in Fig. 4B. As the current elicited by GABA alone was very small, the curves were obtained by simultaneous application of varying concentrations of GABA supplied with 1 μm THDOC. The EC50 for GABA amounted to 51 ± 15 μm (Fig. 3).
Current Response to THIP
With the exception of β3-α1-δ/β3-α6 (R52), 100 μm THIP was able to open all the channels that responded with current to application of GABA alone (Fig. 3), but the extent varied largely in different channels. Although THIP was more potent than 1 mm GABA at β3-α1-δ/α6-β3 (R12), it was less potent at β3-α6-δ/α1-β3 (R13) and α6-β3-α1/β3-δ (R23). In all three cases, GABA supplied with 1 μm THDOC elicited more current than 100 μm THIP.
Modulation by Ethanol
Fig. 5 summarizes the effect of different concentrations of ethanol (7.5–45 mm) on the functional receptors. 45 mm ethanol was also tested with a preincubation with ethanol for 30 s. A GABA concentration was used that elicited about 20% of the maximal current amplitude in each receptor (EC20). None of the concatenated receptors showed any modulation to 30 mm ethanol. Experiments at β3-α1-δ/α6-β3 (R12), β3-α6-δ/α1-β3 (R13), and α6-β3-α1/β3-δ (R23) receptors were carried out in the absence of 1 μm THDOC, and experiments at β3-α1-δ/β3-α6 (R52) receptors were carried out in the presence of 1 μm THDOC.
FIGURE 5.
Lack of an effect by physiological concentrations of ethanol. GABAA receptors were expressed in Xenopus oocytes. The receptors were activated by a concentration of GABA eliciting EC20 followed by applications of the same concentration of GABA in combination with subsequently 7.5, 15, 30, and 45 mm ethanol. The relative current amplitude of the responses in the presence of ethanol as compared with GABA alone is given. The data in the right column were obtained with 45 mm ethanol including a 30-s preapplication of ethanol before the combined application of GABA with ethanol. Footnote a, the experiments were carried out in the presence of 1 μm THDOC.
Comparison of α1β3δ, α6β3δ, and α1α6β3δ Receptors
Fig. 6 compares α1β3δ, α6β3δ, and α1α6β3δ receptors in terms of functional expression in Xenopus oocytes and EC50 for GABA. To denominate subunit positions, we allude to the major adult isoform that has been shown to be arranged γ2β2α1β2α1. Irrespective of the type of α subunit in the two α positions, δ can assume the position of the β subunit between the two α subunits (R11-R14). The corresponding EC50 values for GABA range between 0.1 μm for two α6 and near 10 μm for two α1 reasonably close to non-concatenated α1β3δ and α6β3δ receptors, respectively. Irrespective of the type of α subunit in the two α positions, δ can assume the position of the β subunit between γ and α subunits (R21-R24), provided the two α subunits are identical, producing receptors with the expected EC50. In the case of mixed α subunits, if α1 neighbors the δ subunit, there is little channel activity observed; if α6 neighbors the δ subunit, channels with an unusually high EC50 for GABA of about 150 μm form. We next discuss channels in which the δ subunit replaces one of the α subunits. Interestingly, if δ occupies the position between the two β subunits (R31, R34) in the major GABAA receptor isoform, α6 is tolerated in the other α position, but α1 is not. The functional receptor with an α6 subunit has an EC50 that compares well with the corresponding non-concatenated receptor. Conversely, if δ occupies the α position normally neighboring the γ subunit (R41, R44), α1 is tolerated in the other α position, but α6 is not, but for reasons discussed in Kaur et al. (29), the receptor with α1 may not be formed. Finally, we consider the case when δ replaces the γ subunit (R51-R54). A functional receptor only results if δ is neighbored by an α1 subunit, irrespective of the second α subunit. In both cases, the corresponding EC50 is unexpectedly high. In addition we studied α1-β3-α1/δ, α1-β3-α6/δ, α6-β3-α1/δ, and α6-β3-α6/δ receptors (Figs. 3 and 6). Only α6-β3-α6/δ was able to form a functional receptor (30).
FIGURE 6.
Comparison of α1α6β3δ GABAA receptors with α1β3δ and α6β3δ receptors. The table compares currents elicited by 1 mm GABA in the absence and presence of 1 μm THDOC and the EC50 for GABA for all investigated variants of α1β3δ, α6β3δ, and α1α6β3δ GABAA receptors. Footnote a, the concentration-response curve was fitted with two components. The low affinity phase is mentioned in parentheses. Footnote b, the experiments were carried out in the presence of 1 μm THDOC. Footnote c, this receptor may not be formed for reasons discussed in Ref. 29. Footnote d, the corresponding pentameric construct did not result in expression.
DISCUSSION
We investigated the architectural role of the δ subunit in α1α6β3δ GABAA receptor pentamers. Functional expression of non-concatenated α1α6β3δ might theoretically result in a large variety of subunit arrangements. In such a case, subunit concatenation (28, 31–34) may be used to control arrangement. To construct all possible subunit arrangements clearly exceeded our work capacity. We investigated all variants of the major GABAA receptor isoform γβαβα, where the γ subunit, or one of the β subunits, was replaced by the δ subunit. If a β subunit was substituted, the γ subunit position was occupied by a β subunit, as is the case in αβ receptors.
Validity of the Approach
In the case of γ2 subunit-containing receptors, there seems to be a single pentameric subunit arrangement (31, 32). This pentameric arrangement may be produced with the help of a number of different sets of triple and dual subunit constructs. Concatenated receptors are characterized by a 2–3.5-fold higher EC50 for GABA and a current amplitude that amounts to 25–100% of that expressed by non-concatenated subunits (32). We assume, but cannot prove, that similar rules apply to δ subunit-containing receptors. As mentioned below, we cannot completely exclude that a specific linker promotes or inhibits the corresponding subunit assembly, thereby affecting expression levels.
Concatenated subunits, in some cases, produce, for poorly understood reasons, current by themselves. The presence of β3 in these constructs results in larger expression in comparison with β2-containing constructs (36). The amplitude of these currents strongly depends on the amount of RNA coding for this subunit that is injected into an oocyte (36). In the case of the γ2 subunit, we never prominently described these currents as we used relatively small amounts of RNA and therefore only observed small currents. For the expression of δ subunit-containing receptors, we used 5-fold higher concentrations of RNA and replaced the β2 subunit by β3. In these cases, we observed for some constructs, but not for others, expression of currents (Refs. 30 and 39 and this study). When such a conspicuous subunit was used for the expression of a pentameric receptor, we required that the functional properties of this receptor pentamer had to differ from those of the individual construct. Alternatively, when such functional differences were not evident, we constructed the concatenated pentamer.
Several δ Subunit-containing Receptors Show Activity
Four of the six studied receptors responded with current amplitudes >130 nA to the exposure of 1 mm GABA, namely β3-α1-δ/α6-β3 (R12), β3-α6-δ/α1-β3 (R13), α6-β3-α1/β3-δ (R23), and β3-α6-δ/β3-α1 (R53). As the latter arrangement fails to produce current upon concatenation of all five subunits, we consider the current observed a consequence of the poorly understood ability of the construct β3-α1 to produce currents by itself. In the simultaneous presence of 1 μm of the neurosteroid THDOC with GABA, β3-α1-δ/β3-α6 (R52) receptors resulted in the expression of currents >400 nA (Fig. 3). This shows that in the absence of neurosteroid, one of the α1α6β3δ GABA receptors may remain almost silent and therefore contribute little to inhibition. However, in the presence of THDOC, this silent receptor is activated and thereby could exert a profound inhibitory influence on the neuronal activity. The property of β3-α1-δ/β3-α6 (R52) receptors to be uncovered by neurosteroids is shared by α1-β3-α1/β3-δ (R21) (29), β3-α6-δ/α6-β3 (R14), α6-β3-α6/β3-δ (R24), and β3-δ-β3/α6-β3 (R34) receptors (30). 1 μm THDOC alone elicited currents <10 nA in all receptors.
The EC50 for GABA of the dominating component of β3-α1-δ/α6-β3 (R12) and the EC50 of the single component of β3-α6-δ/α1-β3 (R13) receptors were with about 0.4 and 12 μm in the range of non-concatenated α1/β3, α1/β3/δ, α6/β3, and α6/β3/δ receptors. The EC50 for GABA α6-β3-α1/β3-δ (R23) and β3-α1-δ/β3-α6 (R52) receptors was about 360 μm (major phase) and 50 μm, respectively. As it is not known which subunits are really assembled in the case of non-concatenated α1/α6/β3/δ receptors, it is difficult to judge these values. It can only be speculated that ambient extrasynaptic GABA concentrations might be too low to elicit significant current in these receptors. However, it cannot be excluded that a posttranslational modification in neurons increases the potency of these receptors.
THIP elicits larger currents than GABA in α6β3δ receptors (30, 37), indicating that GABA acts as a partial agonist at these receptors. It was interesting to see how α1α6β3δ receptors performed in this respect. Of all the functional receptors, only β3-α1-δ/α6-β3 (R12) showed larger current responses to 100 μm THIP than to 1 mm GABA. Nevertheless, with the notable exception of β3-α1-δ/β3-α6 (R52), activation by THIP was also observed in the other functional receptors, but amounting to only about 7–35% of the response observed after application of 1 mm GABA + 1 μm THDOC.
Contribution of the δ Subunit to an Agonist Site
Previous work has indicated the presence of an agonist site at the β/δ subunit interface, namely in α1-β3-α1/β3-δ (29) and α6-β3-α6/β3-δ receptors (30). Therefore, it was expected that α6-β3-α1/β3-δ (R23) receptors also show such evidence. Indeed the GABA concentration-response curve was clearly biphasic, indicative of such a site (Fig. 4). Unexpectedly, β3-α1-δ/α6-β3 (R12) receptors also resulted in a biphasic curve, despite the fact that this receptor lacks a β/δ subunit interface. Without further experimentation, we cannot explain this phenomenon.
Assembly of Receptors following Injection of Individual Dual and Triple Subunit Constructs
Most of the dual and triple subunit constructs, when injected alone, did not result in current expression, with the exception of concatenated subunits β3-α1, α6-β3 and β3-α1-δ (Fig. 3). It is not clear whether these constructs are able to form tetramers or hexamers or whether one of the subunits is hanging out, not being incorporated in the pentamer (28). β3-α1 is part of β3-α6-δ/β3-α1 (R53). To avoid misinterpretations, we constructed the corresponding pentamer β3-α6-δ-β3-α1 (P53). As P53 does not result in functional expression, we interpret the current expression by R53 as an artifact. α6-β3 and β3-α1-δ are part of the β3-α1-δ/α6-β3 (R12) receptor. As the functional properties of currents mediated by α6-β3 or β3-α1-δ differ from that mediated by β3-α1-δ/α6-β3 (R12) in respect to current amplitude, relative currents elicited by 1 mm GABA, 1 mm GABA + 1 μm THDOC, and 100 μm THIP, as well as the EC50, we assume that R12 is really formed. β3-α1-δ is also part of β3-α1-δ/β3-α6 (R52). Despite the fact that β3-α1-δ/β3-α6 (R52) results in much larger current amplitudes, it cannot fully be excluded that at least part of this current is caused by β3-α1-δ.
Modulation by Ethanol
Controversial observations have been made on the modulation of GABAA receptors by lower concentrations of ethanol. Although one group reported positive allosteric modulation (38), a consortium of several groups was unable to confirm this (39). We have previously not found any ethanol effects on concatenated α1β3δ (29) and α6β3δ (30) receptors. In principle, the evasive ethanol-sensitive receptor could be composed of α1, α6, β3, and δ subunits. Fig. 5 summarizes the effects of co-application of 7.5, 15, 30, or 45 mm ethanol and a 30-s preapplication of 45 mm ethanol before the addition of GABA on the functional receptors. All measurements were carried out at a GABA concentration eliciting EC20. However, we observed that none of the four receptors β3-α1-δ/α6-β3 (R12), β3-α6-δ/α1-β3 (R13), α6-β3-α1/β3-δ (R23), and β3-α1-δ/β3-α6 (R52) was modulated by 30 mm ethanol, a concentration corresponding to 1.38‰ (w/v). β3-α1-δ/α6-β3 (R12), β3-α6-δ/α1-β3 (R13), and α6-β3-α1/β3-δ (R23) were analyzed in the absence, and β3-α1-δ/β3-α6 (R52) was analyzed in the presence of 1 μm THDOC.
So far α1 subunit-containing receptors were only tested at 30 mm ethanol (29). In this context, we reinvestigated these receptors under the identical conditions as described above. None of the investigated receptors, β3-α1-δ/α1-β3, α1-β3-α1/β3-δ, and β3-α1-δ/β3-α1, showed any significant modulation (not shown).
Relative Abundance of the Receptors
From the present experiments, it is difficult to conclude on relative abundance of the four expressing receptors. Subunit concatenation may affect expression levels. The β3-α6-δ/α1-β3 (R13) receptor with the δ subunit between the two α subunits produces the largest current amplitudes. Nevertheless, active, non-concatenated α1α6β3δ receptors probably constitute a mixture of β3-α1-δ/α6-β3 (R12), β3-α6-δ/α1-β3 (R13), α6-β3-α1/β3-δ (R23), and β3-α1-δ/β3-α6 (R52), where R52 is only active in the presence of neurosteroids. Taken together, our findings reveal a unique assembly profile for the δ subunit in α1α6β3δ receptors that resembles that of the ϵ subunit (40) and the δ subunit in α1β3δ and α6β3δ receptors in that these subunits can assume multiple positions in a receptor.
Comparison with α1β3δ and α6β3δ Receptors
The findings described here on α1α6β3δ GABAA receptors should be compared with findings made with α1β3δ receptors (29) and α6β3δ receptors (30). All four receptors with δ between the two α subunits are formed, namely β3-α1-δ/α1-β3 (R11), β3-α1-δ/α6-β3 (R12), β3-α6-δ/α1-β3 (R13), and β3-α6-δ/α6-β3 (R14). If δ occupies the α position normally neighboring the γ subunit, receptors may not be formed irrespective of the nature of the α subunit. Formation of receptors with δ in one of the three other positions will depend on the relative positions of α1 and α6 (Fig. 6 and see “Results” for details). We conclude that there is no simple rule to predict assembly properties, but considering also the respective EC50, receptors where the δ subunit replaces one of the β subunits (R11-R14 and R21, R23, and R24, α6-β3-α6/δ) may be most prominent receptor forms. Please note that additional subunit arrangements of α1β3δ, α6β3δ, and α1α6β3δ may exist that were not tested here.
Summary
In conclusion, we have shown that GABAA receptors containing the α1, α6, β3, and δ subunits exhibit the ability to promiscuously assemble into different arrangements, at least after expression in Xenopus oocytes. Further we show that one of the δ subunit-containing receptors remains relatively silent in the absence of neurosteroid. The architecture of α1α6β3δ GABAA receptors in situ in cerebellar granule cells needs to be established. Furthermore, the mechanisms promoting the assembly of a defined receptor will have to be understood. It is intriguing to hypothesize that a neuron directs assembly of different receptor isoforms from the same set of subunits, depending on the actual functional needs. This would add to the plasticity of the neuronal system.
Acknowledgment
We thank Dr. V. Niggli for carefully reading the manuscript.
This work was supported by Swiss National Science Foundation Grant 3100A0-105272/2.
- GABA
- γ-aminobutyric acid
- GABAA
- γ-aminobutyric acid, type A
- THDOC
- 3α, 21-Dihydroxy-5α-pregnan-20-one
- THIP
- 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol
- DMSO
- dimethyl sulfoxide.
REFERENCES
- 1.Macdonald R. L., Olsen R. W. (1994) Annu. Rev. Neurosci. 17, 569–602 [DOI] [PubMed] [Google Scholar]
- 2.Rabow L. E., Russek S. J., Farb D. H. (1995) Synapse 21, 189–274 [DOI] [PubMed] [Google Scholar]
- 3.Barnard E. A., Skolnick P., Olsen R. W., Mohler H., Sieghart W., Biggio G., Braestrup C., Bateson A. N., Langer S. Z. (1998) Pharmacol. Rev. 50, 291–313 [PubMed] [Google Scholar]
- 4.Whiting P. J., Bonnert T. P., McKernan R. M., Farrar S., Le Bourdellès B., Heavens R. P., Smith D. W., Hewson L., Rigby M. R., Sirinathsinghji D. J., Thompson S. A., Wafford K. A. (1999) Ann. N.Y. Acad. Sci. 868, 645–653 [DOI] [PubMed] [Google Scholar]
- 5.Sigel E., Baur R., Trube G., Möhler H., Malherbe P. (1990) Neuron 5, 703–711 [DOI] [PubMed] [Google Scholar]
- 6.Sieghart W., Sperk G. (2002) Curr. Top. Med. Chem. 2, 795–816 [DOI] [PubMed] [Google Scholar]
- 7.Minier F., Sigel E. (2004a) Proc. Natl. Acad. Sci. U.S.A. 101, 7769–7774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stell B. M., Mody I. (2002) J. Neurosci. 22, RC223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mody I., Pearce R. A. (2004) Trends Neurosci. 27, 569–575 [DOI] [PubMed] [Google Scholar]
- 10.Farrant M., Nusser Z. (2005) Nat. Rev. Neurosci. 6, 215–229 [DOI] [PubMed] [Google Scholar]
- 11.Stell B. M., Brickley S. G., Tang C. Y., Farrant M., Mody I. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14439–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones A., Korpi E. R., McKernan R. M., Pelz R., Nusser Z., Mäkelä R., Mellor J. R., Pollard S., Bahn S., Stephenson F. A., Randall A. D., Sieghart W., Somogyi P., Smith A. J., Wisden W. (1997) J. Neurosci. 17, 1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sur C., Farrar S. J., Kerby J., Whiting P. J., Atack J. R., McKernan R. M. (1999) Mol. Pharmacol. 56, 110–115 [DOI] [PubMed] [Google Scholar]
- 14.Glykys J., Peng Z., Chandra D., Homanics G. E., Houser C. R., Mody I. (2007) Nat. Neurosci. 10, 40–48 [DOI] [PubMed] [Google Scholar]
- 15.Quirk K., Whiting P. J., Ragan C. I., McKernan R. M. (1995) Eur. J. Pharmacol. 290, 175–181 [DOI] [PubMed] [Google Scholar]
- 16.Araujo F., Ruano D., Vitorica J. (1998) Eur. J. Pharmacol. 347, 347–353 [DOI] [PubMed] [Google Scholar]
- 17.Jechlinger M., Pelz R., Tretter V., Klausberger T., Sieghart W. (1998) J. Neurosci. 18, 2449–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena N. C., Macdonald R. L. (1994) J. Neurosci. 14, 7077–7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxena N. C., Macdonald R. L. (1996) Mol. Pharmacol. 49, 567–579 [PubMed] [Google Scholar]
- 20.Zheleznova N., Sedelnikova A., Weiss D. S. (2008) Br. J. Pharmacol. 153, 1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maguire J. L., Stell B. M., Rafizadeh M., Mody I. (2005) Nat. Neurosci. 8, 797–804 [DOI] [PubMed] [Google Scholar]
- 22.Maguire J., Mody I. (2008) Neuron 59, 207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusser Z., Sieghart W., Stephenson F. A., Somogyi P. (1996) J. Neurosci. 16, 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nusser Z., Sieghart W., Somogyi P. (1998) J. Neurosci. 18, 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan Z. U., Gutiérrez A., De Blas A. L. (1996) J. Neurochem. 66, 685–691 [DOI] [PubMed] [Google Scholar]
- 26.Pöltl A., Hauer B., Fuchs K., Tretter V., Sieghart W. (2003) J. Neurochem. 87, 1444–1455 [DOI] [PubMed] [Google Scholar]
- 27.Sigel E., Baur R. (2000) J. Neurochem. 74, 2590–2596 [DOI] [PubMed] [Google Scholar]
- 28.Minier F., Sigel E. (2004b) Trends Pharmacol. Sci. 25, 499–503 [DOI] [PubMed] [Google Scholar]
- 29.Kaur K. H., Baur R., Sigel E. (2009) Biol. Chem. 284, 7889–7896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baur R., Kaur K. H., Sigel E. (2009) J. Neurochem. 111, 1172–1181 [DOI] [PubMed] [Google Scholar]
- 31.Baumann S. W., Baur R., Sigel E. (2001) J. Biol. Chem. 276, 36275–36280 [DOI] [PubMed] [Google Scholar]
- 32.Baumann S. W., Baur R., Sigel E. (2002) J. Biol. Chem. 277, 46020–46025 [DOI] [PubMed] [Google Scholar]
- 33.Baumann S. W., Baur R., Sigel E. (2003) J. Neurosci. 23, 11158–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baur R., Minier F., Sigel E. (2006) FEBS Lett. 580, 1616–1620 [DOI] [PubMed] [Google Scholar]
- 35.Sigel E. (1987) J. Physiol. 386, 73–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sigel E., Kaur K. H., Lüscher B. P., Baur R. (2009) Biochem. Soc. Trans 37, 1338–1342 [DOI] [PubMed] [Google Scholar]
- 37.Stórustovu S. I., Ebert B. (2006) J. Pharmacol. Exp. Ther. 316, 1351–1359 [DOI] [PubMed] [Google Scholar]
- 38.Wallner M., Hanchar H. J., Olsen R. W. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15218–15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borghese C. M., Stórustovu S., Ebert B., Herd M. B., Belelli D., Lambert J. J., Marshall G., Wafford K. A., Harris R. A. (2006) J. Pharmacol. Exp. Ther. 316, 1360–1368 [DOI] [PubMed] [Google Scholar]
- 40.Bollan K. A., Baur R., Hales T. G., Sigel E., Connolly C. N. (2008) Mol. Cell. Neurosci. 37, 610–621 [DOI] [PubMed] [Google Scholar]