Abstract
Acid-sensing ion channels (ASICs) are sodium channels gated by extracellular protons. The recent crystallization of ASIC1a identified potential binding sites for Cl− in the extracellular domain that are highly conserved between ASIC isoforms. However, the significance of Cl− binding is unknown. We investigated the effect of Cl− substitution on heterologously expressed ASIC1a current and H+-gated currents from hippocampal neurons recorded by whole-cell patch clamp. Replacement of extracellular Cl− with the impermeable and inert anion methanesulfonate (MeSO3−) caused ASIC1a currents to desensitize at a faster rate and attenuated tachyphylaxis. However, peak current amplitude, pH sensitivity, and selectivity were unchanged. Other anions, including Br−, I−, and thiocyanate, also altered the kinetics of desensitization and tachyphylaxis. Mutation of the residues that form the Cl−-binding site in ASIC1a abolished the modulatory effects of anions. The results of anion substitution on native ASIC channels in hippocampal neurons mirrored those in heterologously expressed ASIC1a and altered acid-induced neuronal death. Anion modulation of ASICs provides new insight into channel gating and may prove important in pathological brain conditions associated with changes in pH and Cl−.
Keywords: Acid-sensing Ion Channels (ASIC), Biophysics, Cell Surface Protein, Ion Channels, Neuron, Chloride, Protons
Introduction
Acid-sensing ion channels (ASICs)3 are H+-gated members of the DEG/ENaC ion channel family. In mammals, ASICs include four genes (ASIC1, -2, -3, and -4) that encode for six subunits (ASIC1 and -2 both have alternative splice transcripts as follows: ASIC1a, -1b, -2a, and -2b) (1, 2). Functional ASIC channels consist of a complex of three subunits (3), and they are principally expressed in neurons in the central nervous system and in peripheral sensory nerves. In the brain, the isoform ASIC1a is the best studied, and evidence suggests it plays a role in learning and memory. Genetic or pharmacological perturbation of ASIC1a affects spatial memory, eye-blink conditioning, and it seems to be particularly important for fear-related learning and behaviors (4–6). ASIC1a also has important functions during pathological conditions, including stroke, seizures, depression, and brain tumors (7–10).
For several reasons, ASIC channels are ideally positioned to sense changes in brain interstitium. First, the structure of ASICs is unique for ion channels in that ∼70% of the entire protein consists of a single large extracellular loop. Second, ASIC1a homomeric channels are profoundly sensitive to subtle pH changes; the threshold of activation is ∼7.0, and half-maximal activation occurs at pH ∼6.8 (11), which is well within the range that occurs in the brain interstitium during ischemia, seizures, or spreading depression (12, 13). In fact, loss of ASIC1a abolishes currents in central nervous system neurons evoked by extracellular pH changes in the range between 7.2 and 6.0 (6). Third, multiple other chemicals that are released by metabolically stressed brain cells can potentiate ASICs. For example, ASIC currents are increased by physiological concentrations of lactate, ATP, or arachidonic acid (14–16), all of which are released into the interstitium during brain ischemia (17–19).
The recently resolved ASIC1a crystal structure revealed the surprising finding that three Cl− ions were bound to the channel complex in the extracellular domain (3). These sites are coordinated by two nearby residues (Arg-310 and Glu-314) on an α-helix of one subunit, a residue (Lys-212) from an adjacent subunit, and are almost completely conserved between all H+-gated ASIC isoforms. Extracellular anions are known to modulate a wide variety of ion channels, including the ASIC-related epithelial sodium channel ENaC (20–23). However, the significance of Cl− binding to ASIC channels is unknown. Here, we investigated the effect of extracellular Cl− and other anions on heterologously expressed ASIC1a as well as native ASICs in mammalian central nervous system neurons.
EXPERIMENTAL PROCEDURES
Heterologous Expression of cDNA in CHO Cells
Mouse ASIC1a was cloned as described previously (11). The mutations ASIC1aK211A, ASIC1aR309A, and ASIC1aE313A (the residues are numbered per the mouse ASIC1a sequence) were generated by site-directed mutagenesis using the QuikChange kit (Stratagene, La Jolla, CA) and sequenced at the University of Iowa DNA core. Chinese hamster ovarian (CHO) cells plated at ∼10% confluence were transfected with ASIC cDNAs (0.36 μg/1.5 ml) using Transfast transfection reagent (Promega, Madison, WI) in 35-mm dishes according to the manufacturer's recommendations. DsRed (Express-C1, Clontech) cDNA (1.64 μg/1.5 ml) and green fluorescent protein (pGreen Lantern, Invitrogen) cDNA (0.33 μg/1.5 ml) were cotransfected to facilitate detection of expressing cells by epifluorescence. Cells were cultured in F12 nutrient medium (Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C, 5% CO2 and were studied 48–72 h after transfection.
Culture of Rat Hippocampal Neurons
Primary cultures of hippocampal neurons from E18 rat embryos were prepared as described previously (24). Briefly, neurons were plated on poly-l-lysine-coated glass coverslips at a density of 30,000/cm2 and were maintained in serum-free NS21 media in a humidified incubator (5% CO2 at 37 °C). Experiments were performed on neurons at 10–15 days in vitro.
Electrophysiology
Whole-cell patch clamp recordings (at −70 mV) from CHO cells and hippocampal neurons were performed at room temperature with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and were acquired and analyzed with PULSE/PULSEFIT 8.70 (HEKA Electronics, Lambrecht, Germany) and IGOR Pro 6.01 (WaveMetrics, Lake Oswego, OR) software. Currents were filtered at 5 kHz and sampled at 2 or 0.2 kHz. Micropipettes (2–4 megohms) were filled with internal solution (in mm) as follows: 100 KCl, 10 EGTA, 40 HEPES, and 5 MgCl2, pH 7.4, with KOH. Standard external solutions contained (in mm) the following: 120 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 10 MES; pH was adjusted with tetramethylammonium hydroxide, and osmolarity was adjusted with tetramethylammonium chloride. The total Cl− in each pH solution was (in mm) as follows: 148 pH 5.0, 143 pH 6.0, 140 pH 6.5, 138 pH 6.8, 137 pH 7.0, 135 pH 7.2, 134 pH 7.4, and 131 pH 8.0. In other anion solutions, we substituted NaCH3SO3, NaSCN, NaBr, or NaI for NaCl and omitted tetramethylammonium chloride for a total Cl− concentration of 11 mm. In some experiments, as stated we used Cl−-free external solutions (in mm) as follows: 120 NaCH3SO3, 5 KOH, 2 Ca(OH)2, 10 HEPES, 10 MES; pH was adjusted with methanesulfonic acid or tetramethylammonium hydroxide. Extracellular solutions were changed within 20 ms by using a computer-driven solenoid valve system (25). To compensate for ASIC1a current tachyphylaxis in pH dose response and recovery from desensitization experiments, we bracketed each test pH application with maximal stimulating pulses (pH 8 to 5), and we then used the following formula to normalize the data: Itest/(Imax, 1 + Imax, 2)/2. Kinetics of desensitization were fit to single exponential equations and time constants (τ) as reported. pH-dependent steady-state desensitization and the curves plotting the effect of anion concentration substitution on the desensitization kinetics were fit to the Hill equation with IGOR Pro 6.01. Data are means ± S.E. Statistical significance was assessed using unpaired Student's t test.
Neuronal Death Assay
Rat hippocampal neurons were exposed to pH 7.4 or 5.0 solutions consisting of NaCl, NaCH3SO3, or NaSCN (see external solutions described above) for 15 min at room temperature, followed by two washes with PBS+ (1 mm phosphate-buffered saline, 1 mm MgCl2, 1 mm CaCl2, pH 7.4). Cells were then incubated in 1 mm phosphate-buffered saline containing 1 μm ethidium homodimer-1 (Molecular Probes, Eugene, OR) for 30 min at room temperature to label dead cells and then fixed in 3% paraformaldehyde at 4 °C for 30 min. Samples were washed three times with PBS+, mounted on slides, and imaged using a Zeiss AXIO ImagerA1 fluorescent microscope. Two to three frames per coverslip were imaged using an AxioCam MRc5 camera under phase or fluorescence (Zeiss filter set 43) at ×10 magnification. Total and dead cells from three different neuronal preparations were counted in phase and fluorescent images, respectively, by a blinded individual, and summed data were reported as the percentage of dead cells in each experimental condition. Statistical difference was assessed using unpaired Student's t test.
RESULTS
Extracellular Cl− Modulates the Desensitization Kinetics of ASIC1a
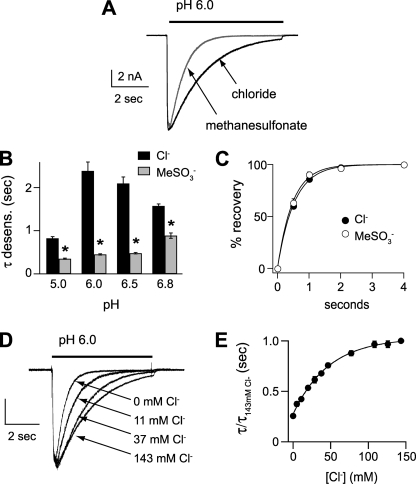
To begin to test the functional effect of extracellular Cl−, we first studied currents recorded in CHO cells expressing ASIC1a. Fig. 1A demonstrates that rapid extracellular solution changes from pH 8 to more acidic pH values evoked rapidly activating and desensitizing inward currents. Replacement of Cl− with the generally impermeable and inert anion, methanesulfonate (MeSO3−), had no effect on peak current amplitude (Fig. 1A). Cl− substitution also had no effect on the pH dose response of activation (Fig. 1B) or steady-state desensitization (Fig. 1C). Additionally, replacement of extracellular NaCl with NaMeSO3 did not alter the reversal potential of the current-voltage relationship, indicating it did not affect the relative Na+ selectivity of the channel (Fig. 1, D and E). However, closer inspection of the currents revealed that those evoked in MeSO3− desensitized at a faster rate than those in Cl− (Fig. 2A). Fitting the desensitizing phase of the currents to a single exponential equation and plotting the time constants revealed that the currents desensitized faster in MeSO3− at all pH solutions tested (Fig. 2B). The rate of recovery from desensitization was not different in the two different anion solutions (Fig. 2C).
FIGURE 1.
Extracellular Cl− does not affect pH sensitivity or selectivity of ASIC1a. A, representative currents evoked by stepping from pH 8 to the indicated pH test solutions in CHO cells transfected with ASIC1a. Solutions consisted of high (131–148 mm) or low (11 mm) Cl− (Cl− was replaced with MeSO3−). Plot at right is the mean current density evoked by pH 5 for groups of cells (n = 42–49). B, pH dose-response curves for activation in the above solutions. Data are acquired by stepping from pH 8 to the indicated test solutions and are normalized to the peak currents evoked by pH 5 (n ≥ 10). A similar result was obtained in solutions containing 0 mm Cl− (data not shown). Note the asymmetry of the curves; thus they did not fit well to the Hill equation. C, pH dose-response curves for steady-state desensitization in the above solutions. Data are acquired by varying the conditioning pH and then stepping to pH 5 test solutions and are normalized to the currents evoked by stepping from pH 8 to pH 5 (n ≥ 5). Lines are fits of the Hill equation. D, overlay of currents evoked by pH 6 during steps to various membrane potentials in extracellular NaCl or NaMeSO3 solutions. The internal solution was KCl. E, current versus voltage curves for the data in D. Data obtained in MeSO3− lie on top of those obtained in Cl−. The reversal potential for both curves is ∼50 mV, indicating Na+ selective permeability over K+ (n = 4).
FIGURE 2.
Extracellular Cl− dose-dependently modulates the desensitization kinetics of ASIC1a. A, superimposed pH 6-evoked currents in Cl− or MeSO3− solutions. B, mean time constants of desensitization (τ) as measured from single exponential fits to the falling phase of the currents evoked by the indicated pH solutions containing high or 0 mm Cl−. (*, p < 0.01 versus currents evoked in Cl−; n ≥ 10.) C, recovery from desensitization in Cl− or MeSO3− solutions. Current was completely desensitized with a 7-s pulse to pH 6. Cells were then exposed to pH 8 solution for the indicated times before they were stimulated again with pH 6. Recovery is percentage of current evoked by the second pH 6 pulse compared with the first. To compensate for tachyphylaxis, data were normalized to currents recorded at 4 s of recovery. Lines are fits of single exponentials (n ≥ 6). D, superimposed and normalized currents evoked by pH 6 solutions containing varying mixtures of Cl−/MeSO3− solutions, demonstrating the effect of Cl− concentration on the desensitization rate. Vertical scale bar shows the following: 1 nA for 143 mm Cl−, 0.6 nA for 37 mm Cl−, and 0.5 nA for 11 mm and 0 mm Cl−. E, mean time constants of desensitization of currents recorded in solutions of varying Cl− concentration as in D, normalized to the time constant of desensitization of current recorded in 143 mm Cl− (n ≥ 4). Line is fit of the Hill equation.
To begin to test if these effects were due to Cl− binding to the channel, we studied the effect of varying Cl− concentrations on the desensitization kinetics of ASIC1a current. Fig. 2, D and E, shows that as the Cl− concentration is increased, the rate of desensitization slows. A plot of this relationship reveals that at higher doses of Cl− the curve begins to plateau (Fig. 2E), suggesting that Cl− binding is saturating. The half-maximal concentration of Cl that modulated the desensitization rate was 49 mm, and the Hill coefficient was 0.95. These data suggest that extracellular Cl− is binding to the channel (or other associated protein or lipid) at a low affinity site and show that Cl− can modulate ASIC1a through a broad concentration range, including those associated with pathological conditions in the brain.
As others have shown (26), ASIC1a homomeric channels displayed significant rundown (tachyphylaxis) to repeated applications of acidic solution. Sequential activation of the channels with pH 5 solutions in 1-min intervals caused current amplitudes to progressively decrease (Fig. 3). However, the rate of tachyphylaxis was significantly attenuated when currents were recorded in MeSO3− compared with Cl−. The changes in the rate of tachyphylaxis and in desensitization kinetics were both dependent upon the anion in the test solution and not the conditioning solution and were immediately reversible upon switching anions (data not shown). To summarize, these results show that extracellular Cl− modulates the kinetics of both desensitization and tachyphylaxis of ASIC1a in a concentration-dependent manner, but it does not affect the pH sensitivity of activation or steady-state desensitization nor Na+ selectivity.
FIGURE 3.
Extracellular Cl− facilitates tachyphylaxis of ASIC1a. A, representative sequential currents evoked by pH 5 in either 148 or 0 mm Cl− (MeSO3−) solutions. Cells were bathed in pH 8 solutions for 1 min between pH 5 applications. B, mean current amplitudes of successive pH 5 applications normalized to the first pH 5 current amplitude (n ≥ 6). Lines are fits of single exponentials.
Other Anions Modulate ASIC1a
We asked if other anions can modulate ASIC1a or is this a unique property of Cl−? Interestingly, currents evoked in extracellular thiocyanate (SCN−) displayed very fast desensitization and, in fact, were faster than those evoked in MeSO3− (τ = 355 ± 15 ms versus 450 ± 15 ms) (Fig. 4, A and B). Currents evoked in Br− and I− desensitized slower than those in MeSO3− but faster than those in Cl− (Fig. 4B). We also noted that currents evoked in I− and SCN− tended to be of smaller amplitude than those evoked in Cl− (Fig. 4C). Moreover, I− and SCN− attenuated tachyphylaxis of ASIC1a to a greater extent than MeSO3−, whereas tachyphylaxis in Br− was similar to that in Cl− (Fig. 4D). The pH activation dose response was similar in all anions tested (data not shown).
FIGURE 4.
Other anions modulate ASIC1a. A, superimposed pH 6-evoked currents in either Cl− or thiocyanate (SCN−). B, mean time constants of desensitization (desen.) of pH 6-evoked currents recorded in MeSO3− (0 mm Cl−), Cl−, Br−, I−, or SCN− (n ≥ 8; *, p < 0.01 versus MeSO3−; †, p < 0.05 versus Cl−; ‡, p < 0.01 versus Cl−). C, mean current density of pH 5-evoked currents in indicated anion solutions (n ≥ 8; *, p < 0.01 versus Cl−). D, tachyphylaxis of successive pH 5-evoked current amplitudes normalized to the first pH 5 current amplitude in indicated anion solutions (n ≥ 4). Dashed lines are fits of data from Fig. 3B. E, superimposed and normalized currents evoked by pH 6 solutions containing varying mixtures of Cl−/SCN− solutions. Vertical scale bar shows the following: 1 nA for 0 and 16 mm, 0.7 nA for 60 mm, and 0.4 nA for 120 mm SCN−. F, mean time constants of desensitization of currents recorded as in E normalized to the time constant of desensitization of current recorded in 143 mm Cl− (n ≥ 4). The x scale shows both the Cl− and the SCN− concentrations per solutions. Line is fit of the Hill equation. The dashed line is the fit of data from Fig. 2E of Cl−/MeSO3− solution mixtures.
Because SCN− is a large anion with weak hydration, it often binds to proteins and modulates ion channels more potently than smaller, more hydrophilic anions such as Cl− (21). Thus, we were surprised that currents evoked in SCN− desensitized even faster and had even less tachyphylaxis than those evoked in MeSO3−. To test the binding capacity of SCN− relative to Cl−, we measured current desensitization rates in various mixtures of SCN− and Cl− solutions. Fig. 4, E and F, shows that currents desensitized faster with increasing SCN−:Cl− concentration ratios. Moreover, the nonlinear shape of the dose-response relationship shows that small concentrations of SCN− generate large changes in the desensitization kinetics, suggesting that SCN− binds more avidly than Cl−. Thus, it appears that other anions, besides Cl−, can bind to ASIC1a; however, binding does not appear to be sufficient to reproduce the functional effects seen with Cl−.
Mutations of Cl−-binding Sites Abolished the Effect of Anions
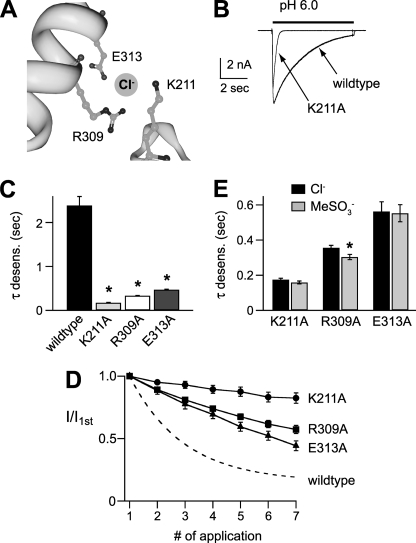
To directly test the hypothesis that Cl− modulates ASIC1a by binding to the extracellular domain, we mutated the amino acids involved in Cl− binding in the cASIC1a crystal structure (Fig. 5A) (3). The mutant channels K211A, R309A, and E313A each generated functional channels with large currents, and their pH activation dose responses were similar to those of wild type ASIC1a (data not shown). However, each of the mutations markedly increased the rate of desensitization (Fig. 5, B and C) and slowed the rate of tachyphylaxis (Fig. 5D). These results mimicked the effect of replacing extracellular Cl− with MeSO3− on wild type ASIC1a, suggesting that mutation of any one of these amino acids abolished the effect of Cl−. To directly test this possibility, we compared the desensitization rate of the mutant channels in Cl− and MeSO3−. Fig. 5E demonstrates that anion substitution had minimal effect on the mutations (compare with wild type channels in Fig. 2B) and suggests that Cl− modulation of ASIC1a is dependent upon the coordinated binding of Cl− to these three amino acids.
FIGURE 5.
Residues in the extracellular domain are necessary for Cl− modulation of mASIC1a. A, Cl−-binding site in chicken ASIC1a (Protein Data Bank code 2QTS) crystal structure. The site is coordinated by Arg-309 and Glu-313 from helix 4 of one subunit, and Lys-211 from an adjacent subunit. Residues are numbered per the mouse ASIC1a sequence. Image was created using the RCSB-Protein Workshop Viewer at the RCSB Protein Data Bank website. B, superimposed currents evoked by pH 6 in cells expressing wild type ASIC1a or mutant ASIC1aK211A. C, mean time constants of desensitization of pH 6-evoked currents recorded in cells expressing wild type or indicated ASIC1a mutants (n ≥ 8; *, p < 0.01 versus wild type). D, tachyphylaxis of successive pH 5-evoked current amplitudes normalized to the first pH 5 current amplitude recorded in cells expressing the indicated ASIC1a mutant (n ≥ 6). Dashed line is fit of data from Fig. 3B for wild type ASIC1a. E, mean time constants of desensitization of pH 6-evoked currents recorded in cells expressing the indicated ASIC1a mutant in either Cl− or MeSO3− solutions (n ≥ 4; *, p < 0.01 versus Cl−). Cl− data are the same as in C.
Extracellular Anions Modulate Native ASICs in Hippocampal Neurons
We tested if anions can also modulate native ASIC channels. We studied hippocampal neurons because it is well characterized that ASIC1a is the major ASIC subunit that underlies H+-gated currents in these cells (27, 28). Compared with SCN−, Cl− markedly slowed the rate of desensitization (Fig. 6, A and B), increased the current amplitude (Fig. 6C), had no effect on pH activation dose response (Fig. 6D), and increased the rate of tachyphylaxis of H+-gated currents in dissociated hippocampal neurons (Fig. 6E). The results mirror the effect of anion substitution on heterologously expressed ASIC1a and show that extracellular Cl− can modulate ASIC channels in the brain.
FIGURE 6.
Extracellular Cl− modulates H+-gated currents in hippocampal neurons. A, superimposed pH 6-evoked currents recorded from a rat hippocampal neuron in either Cl− or SCN− solutions. B, mean time constants of desensitization of currents evoked by the indicated pH in either Cl− or SCN− solutions (n ≥ 9; *, p < 0.01 versus Cl−). C, mean current density of pH 5-evoked currents in either Cl− or SCN− solutions (n ≥ 9; *, p < 0.05 versus Cl−). D, pH dose-response curves for activation in either Cl− or SCN solutions normalized to the peak currents evoked by pH 5 (n ≥ 8). E, tachyphylaxis of successive pH 5-evoked current amplitudes normalized to the first pH 5 current amplitude recorded in either Cl− or SCN− solutions (n = 6).
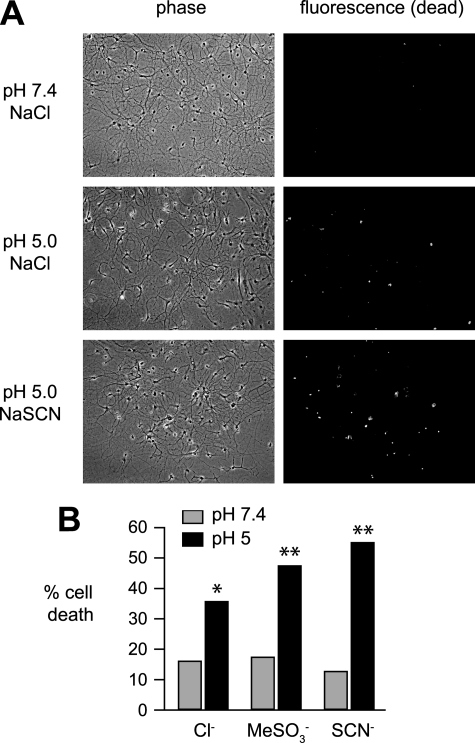
Activation of ASICs during cerebral ischemia contributes to cell death in an in vivo stroke model (8), and prolonged exposure of cultured cortical neurons to extracellular acidosis induces ASIC1a-dependent cell death (8, 29, 30). To begin to understand the physiological/pathophysiological implications of anion substitution on neuronal function, we tested if anions could affect acid-induced neuronal death. Following incubation in various pH solutions, dead neurons were labeled with ethidium homodimer-1 (Fig. 7). Compared with pH 7.4, exposure of hippocampal neurons to pH 5.0 NaCl solution caused a significant percentage of neurons to die. Exposure to NaMeSO3 or NaSCN did not increase cell death compared with NaCl at neutral pH; however, acidic NaMeSO3 and NaSCN solutions both induced a greater percentage of cell death than the respective NaCl solution. This finding suggests that anion modulation of native neuronal ASIC channels can have pathological implications in brain disease states associated with acidosis.
FIGURE 7.
Anion substitution alters acid-induced neuronal death. A, representative phase (left) and fluorescent (right) micrographs of cultured hippocampal neurons after 15 min of exposure to pH 7.4 or 5.0 solutions containing either NaCl, NaMeSO3, or NaSCN. Dead cells were labeled with ethidium homodimer-1 staining and identified by fluorescent imaging. B, sum of the percentage of dead cells per the total number of neurons in each of the indicated anion solutions (n ≥ 355; *, p < 0.01 versus pH 7.4 NaCl; **, p < 0.01 versus pH 5.0 NaCl).
DISCUSSION
Extracellular anions, via their propensity to bind to various proteins and lipids, have been shown to affect a multitude of biological functions, including modulation of ion channel properties (21). We tested the effect of extracellular anions on ASIC1a function both in heterologous cells and in rat hippocampal neurons and found that anion substitution altered a subset of channel biophysical properties. Cl− markedly slowed the rate of desensitization and increased tachyphylaxis in a dose-dependent manner. On the other hand, anions had no effect on the pH sensitivity of activation or steady-state desensitization and did not alter Na+ selectivity. These effects were dependent upon three extracellular residues that are purported to form a Cl−-binding site.
The recent resolution of the ASIC1a crystal structure revealed that the channels consist of three subunits and the trimeric extracellular domain contains three anion-binding sites (3). These sites are located ∼10 Å below one of the residues (Asp-350) suggested to be involved in proton binding and pH activation. Thus, it would be reasonable to predict that anions might alter H+ binding and pH-dependent gating (31). However, we found that the pH sensitivity of both activation and steady-state desensitization was not altered with anion substitution or mutation of the anion-binding sites. In addition, mutation of these sites resulted in channels that generated robust currents, indicating that these sites are not required for channel assembly and trafficking. Instead, we found that anions modulated the kinetics of desensitization. Upon a rapid drop in pH, ASIC channels quickly open and then close in the continued presence of acidic pH. Desensitization rates are dependent upon the extracellular pH, the subunit composition of the channels, and involve a conformational change in the extracellular domain (11, 32, 33). Our data suggest that binding of anions to the extracellular domain has no effect on the stability of either the open or desensitized states (because steady-state desensitization was not changed), but rather anions alter the transition energy barrier between the open and desensitized states.
In addition to affecting the kinetics of desensitization, anion substitution and mutations of the anion-binding sites produced significant changes in the tachyphylaxis of ASIC1a. This characteristic of diminishing responses to repeated acid stimulations is unique for ASIC1a homomers and seems to be dependent on H+ permeating through the channel (26). It also depends upon the time that the channels remain in the open state. For example, shortening the time of acid applications or mutations that speed the rate of desensitization have both been shown to attenuate tachyphylaxis (26, 34). Similarly, our data show a strong correlation between the rate of desensitization and the degree of tachyphylaxis. ASIC1a currents generated in Cl− displayed the slowest desensitization and the most tachyphylaxis. Conversely, currents in SCN− desensitized the fastest and displayed very little tachyphylaxis. Likewise, mutation of the anion-binding sites sped up desensitization and attenuated tachyphylaxis. Interestingly, currents recorded in SCN− and those from the mutant channels also tended to have smaller amplitudes. This could also be due to the increase in desensitization rate; some channels might desensitize faster than others can open or perhaps channels can directly desensitize without opening. Thus, we speculate that alterations of tachyphylaxis and current amplitude generated in different anions both resulted from changes in the desensitization rate and highlight the importance of desensitization rate to overall ASIC current properties.
Our data support that anions modulate ASIC1a by binding to extracellular residues (K211A, R309A, and E313A) as defined by the cASIC1a crystal structure (3). Mutation of any one of the three residues disrupted anion modulation, suggesting that all three residues are necessary to coordinate Cl− binding. Although our studies do not directly measure anion binding to the channel, our Cl−:MeSO3− concentration substitution studies show dose dependence and are consistent with saturable Cl− binding (Fig. 2E). Based upon our desensitization rate data, the potency of different anions to modulate ASIC1a is SCN− < MeSO3− < I− < Br− < Cl−.
On the other hand, our data also suggest that binding alone is not sufficient to impart the modulatory effects of anions on ASIC gating. The effect of SCN− is particularly interesting. Because SCN− is a relatively hydrophobic anion, it generally binds relatively well to proteins and lipids, and it has been shown to have pronounced effects upon the properties of several ion channels (21, 23, 35). For example, SCN− generates a greater shift in voltage dependence of sodium channel activation and inactivation than Cl− (21). Therefore, we were surprised that results in SCN− mimicked those in MeSO3−, suggesting it did not bind to ASIC1a. However, our Cl−:SCN− concentration substitution data reveal that SCN− actually binds to ASIC1a with greater potency than Cl− (Fig. 4F). This suggests that SCN− might be functioning as a competitive antagonist of Cl−. It is also interesting that the rate of ASIC1a desensitization in MeSO3− (450 ± 15 ms at pH 6) was not as fast as in SCN− (355 ± 15 ms) or currents generated from the ASICK211A mutation (173 ± 8 ms). As a commonly used impermeant anion, we anticipated that MeSO3− would not interfere with Cl− binding, and thus MeSO3− substitution would mimic the effect of lack of anion binding. However, studies in the chloride channel, ClC-1, suggest that MeSO3−, although impermeant, can bind to a site outside the pore and modify the gating of the channel (23). Perhaps MeSO3− can also bind and modulate ASIC1a, although it does so less potently than Cl−. Our data suggest that various anions can modulate ASIC1a; however, their capacity to do so is not entirely dependent upon their binding affinity to the channel and that Cl− appears particularly suited to modulate ASIC1a.
Cl− could play two potential physiological and/or pathophysiological roles in ASIC function. First, in addition to sensing H+ and other metabolites, our data suggest that Cl− fluxes in the brain might modulate ASICs. Ischemia and injury are associated with major shifts of Na+ and Cl− ions into cells, which can generate significant changes in the concentration of these ions. During anoxia or spreading depression, the extracellular Cl− concentration in the brain can drop from a normal of ∼140 mm to the 50–90 mm range (36, 37). We found that changes in Cl− concentration within this range modulated ASIC1a. A reduction in extracellular Cl− associated with ischemia or hyperexcited states would increase the rate of desensitization of ASIC1a, thereby decreasing net charge conducted through the channel and decrease tachyphylaxis, both of which could have profound effects upon neuronal activity. In support of this, we found that anion substitution altered acid-induced neuronal death. We speculate that the increased death in MeSO3− and SCN− compared with Cl− was due to a decrease in ASIC1a tachyphylaxis. Alternatively, it is possible that ASICs do not sense changes in Cl− concentration, but rather Cl− serves as a necessary “cofactor” that is constitutively bound and is required for normal channel function. In a similar manner, Ca2+ functions as a cofactor for the normal gating of voltage-gated Na+ channels (38) and, interestingly, ASICs (39, 40).
Acknowledgments
We thank Kathryn Kaufman and the DNA core facility for technical assistance. We acknowledge D. P. Mohapatra and Andrew Shepherd for providing rat hippocampal neurons and assisting with the cell death assay.
This work was supported, in whole or in part, by National Institutes of Health Grant HL076419 (to C. J. B.).
- ASIC
- acid-sensing ion channel
- CHO
- Chinese hamster ovary
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Lingueglia E. (2007) J. Biol. Chem. 282, 17325–17329 [DOI] [PubMed] [Google Scholar]
- 2.Wemmie J. A., Price M. P., Welsh M. J. (2006) Trends Neurosci. 29, 578–586 [DOI] [PubMed] [Google Scholar]
- 3.Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 4.Coryell M. W., Wunsch A. M., Haenfler J. M., Allen J. E., McBride J. L., Davidson B. L., Wemmie J. A. (2008) J. Neurosci. 28, 13738–13741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wemmie J. A., Askwith C. C., Lamani E., Cassell M. D., Freeman J. H., Jr., Welsh M. J. (2003) J. Neurosci. 23, 5496–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wemmie J. A., Chen J., Askwith C. C., Hruska-Hageman A. M., Price M. P., Nolan B. C., Yoder P. G., Lamani E., Hoshi T., Freeman J. H., Jr., Welsh M. J. (2002) Neuron 34, 463–477 [DOI] [PubMed] [Google Scholar]
- 7.Coryell M. W., Wunsch A. M., Haenfler J. M., Allen J. E., Schnizler M., Ziemann A. E., Cook M. N., Dunning J. P., Price M. P., Rainier J. D., Liu Z., Light A. R., Langbehn D. R., Wemmie J. A. (2009) J. Neurosci. 29, 5381–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong Z. G., Zhu X. M., Chu X. P., Minami M., Hey J., Wei W. L., MacDonald J. F., Wemmie J. A., Price M. P., Welsh M. J., Simon R. P. (2004) Cell 118, 687–698 [DOI] [PubMed] [Google Scholar]
- 9.Ziemann A. E., Schnizler M. K., Albert G. W., Severson M. A., Howard M. A., 3rd, Welsh M. J., Wemmie J. A. (2008) Nat. Neurosci. 11, 816–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapoor N., Bartoszewski R., Qadri Y. J., Bebok Z., Bubien J. K., Fuller C. M., Benos D. J. (2009) J. Biol. Chem. 284, 24526–24541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benson C. J., Xie J., Wemmie J. A., Price M. P., Henss J. M., Welsh M. J., Snyder P. M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2338–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutch W. A., Hansen A. J. (1984) J. Cereb. Blood Flow Metab. 4, 17–27 [DOI] [PubMed] [Google Scholar]
- 13.Somjen G. G. (1984) Brain Res. 311, 186–188 [DOI] [PubMed] [Google Scholar]
- 14.Allen N. J., Attwell D. (2002) J. Physiol. 543, 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Immke D. C., McCleskey E. W. (2001) Nat. Neurosci. 4, 869–870 [DOI] [PubMed] [Google Scholar]
- 16.Naves L. A., McCleskey E. W. (2005) Braz. J. Med. Biol. Res. 38, 1561–1569 [DOI] [PubMed] [Google Scholar]
- 17.Melani A., Turchi D., Vannucchi M. G., Cipriani S., Gianfriddo M., Pedata F. (2005) Neurochem. Int. 47, 442–448 [DOI] [PubMed] [Google Scholar]
- 18.Rehncrona S., Westerberg E., Akesson B., Siesjö B. K. (1982) J. Neurochem. 38, 84–93 [DOI] [PubMed] [Google Scholar]
- 19.Schurr A., Rigor B. M. (1998) Dev. Neurosci. 20, 348–357 [DOI] [PubMed] [Google Scholar]
- 20.Collier D. M., Snyder P. M. (2009) J. Biol. Chem. 284, 29320–29325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dani J. A., Sanchez J. A., Hille B. (1983) J. Gen. Physiol. 81, 255–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao C. Y., Stanfield P. R. (1968) J. Physiol. 198, 291–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rychkov G. Y., Pusch M., Roberts M. L., Jentsch T. J., Bretag A. H. (1998) J. Gen. Physiol. 111, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Stevens B., Chang J., Milbrandt J., Barres B. A., Hell J. W. (2008) J. Neurosci. Methods 171, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson C. J., Eckert S. P., McCleskey E. W. (1999) Circ. Res. 84, 921–928 [DOI] [PubMed] [Google Scholar]
- 26.Chen X., Gründer S. (2007) J. Physiol. 579, 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Askwith C. C., Wemmie J. A., Price M. P., Rokhlina T., Welsh M. J. (2004) J. Biol. Chem. 279, 18296–18305 [DOI] [PubMed] [Google Scholar]
- 28.Baron A., Waldmann R., Lazdunski M. (2002) J. Physiol. 539, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao J., Duan B., Wang D. G., Deng X. H., Zhang G. Y., Xu L., Xu T. L. (2005) Neuron 48, 635–646 [DOI] [PubMed] [Google Scholar]
- 30.Sherwood T. W., Askwith C. C. (2009) J. Neurosci. 29, 14371–14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hille B. (1992) Ion Channels of Excitable Membranes, pp. 261–290, Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 32.Cushman K. A., Marsh-Haffner J., Adelman J. P., McCleskey E. W. (2007) J. Gen. Physiol. 129, 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesselager M., Timmermann D. B., Ahring P. K. (2004) J. Biol. Chem. 279, 11006–11015 [DOI] [PubMed] [Google Scholar]
- 34.Gitterman D. P., Wilson J., Randall A. D. (2005) J. Physiol. 562, 759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith S. S., Steinle E. D., Meyerhoff M. E., Dawson D. C. (1999) J. Gen. Physiol. 114, 799–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen A. J., Zeuthen T. (1981) Acta Physiol. Scand. 113, 437–445 [DOI] [PubMed] [Google Scholar]
- 37.Jiang C., Agulian S., Haddad G. G. (1992) J. Physiol. 448, 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong C. M., Cota G. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 6528–6531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Immke D. C., McCleskey E. W. (2003) Neuron 37, 75–84 [DOI] [PubMed] [Google Scholar]
- 40.Zhang P., Sigworth F. J., Canessa C. M. (2006) J. Gen. Physiol. 127, 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]