Abstract
Thyroglobulin (Tg, precursor for thyroid hormone synthesis) is a large secreted glycoprotein composed of upstream regions I-II-III, followed by the ∼570 residue cholinesterase-like (ChEL) domain. ChEL has two identified functions: 1) homodimerization, and 2) binding to I-II-III that facilitates I-II-III oxidative maturation required for intracellular protein transport. Like its homologs in the acetylcholinesterase (AChE) family, ChEL possesses two carboxyl-terminal α-helices. We find that a Tg-AChE chimera (swapping AChE in place of ChEL) allows for dimerization with monomeric AChE, proving exposure of the carboxyl-terminal helices within the larger context of Tg. Further, we establish that perturbing trans-helical interaction blocks homodimerization of the Tg ChEL domain. Additionally, ChEL can associate with neuroligins (a related family of cholinesterase-like proteins), demonstrating potential for Tg cross-dimerization between non-identical partners. Indeed, when mutant rdw-Tg (Tg-G2298R, defective for protein secretion) is co-expressed with wild-type Tg, the two proteins cross-dimerize and secretion of rdw-Tg is partially restored. Moreover, we find that AChE and soluble neuroligins also can bind to the upstream Tg regions I-II-III; however, they cannot rescue secretion, because they cannot facilitate oxidative maturation of I-II-III. These data suggest that specific properties of distinct Tg ChEL mutants may result in distinct patterns of Tg monomer folding, cross-dimerization with wild-type Tg, and variable secretion behavior in heterozygous patients.
Keywords: Acetylcholinesterase, Endoplasmic Reticulum, Protein Folding, Secretion, Thyroid, Dimerization, Four-helix Bundle, Intramolecular Chaperone, Neuroligin, Oxidative Folding
Introduction
Thyroid hormone synthesis requires secretion of thyroglobulin (Tg)2 to the apical luminal cavity of thyroid follicles in the thyroid gland (1). After secretion, Tg iodination (by thyroid peroxidase (2)) leads to a coupling reaction between di-iodotyrosyl residues 5 and 130 to form thyroxine within the amino-terminal portion of the Tg polypeptide (3, 4). Hormonogenic iodination depends not upon the specificity of the peroxidase (5) but upon the sequence and structure of Tg (6, 7). No other thyroidal proteins are known to effectively substitute for Tg in this role. The Tg primary sequence (∼2,750 amino acids after signal peptide cleavage) encodes three disulfide-rich regions comprising 80% of the overall Tg polypeptide, called I-II-III (8, 9), followed by ∼570 residues of cholinesterase-like (ChEL) domain (10) with homology to acetylcholinesterase (AChE) (see Fig. 1A) (11–13). Although I-II-III contains the conserved amino-terminal hormonogenic site, I-II-III by itself is incompetent for hormonogenesis, because its export from the endoplasmic reticulum (ER), leading to secretion, requires ChEL (10, 14).
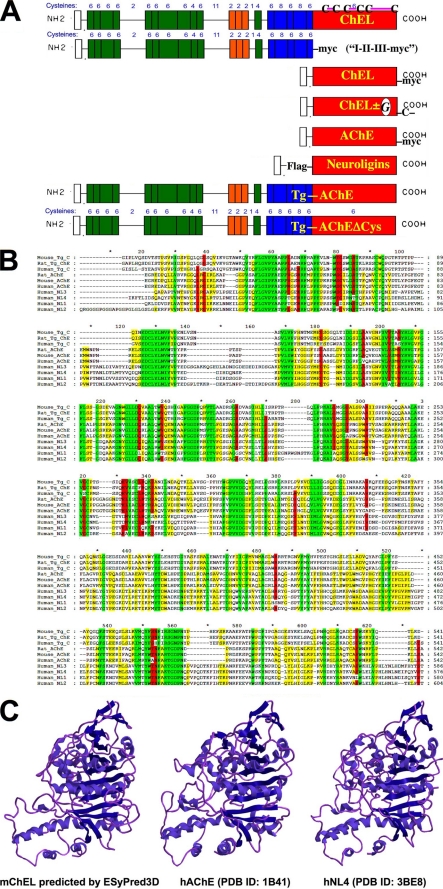
FIGURE 1.
Homologs of the Tg ChEL domain. A, constructs used in this study, in relation to the domain structure of Tg shown schematically. Top to bottom: the concluding ChEL domain of wild-type Tg (uppermost) contains six Cys residues, and based on conservation of structure with AChE, the mature mouse Tg ChEL domain is predicted to have three disulfide bonds engaging Cys2243–Cys2260, Cys2421–Cys2432, and Cys2570–Cys2694. Not shown in the schematic are engineered Tg variants, including wild-type Tg bearing a carboxyl-terminal Myc epitope tag, and rdw-Tg (Tg-G2298R) bearing carboxyl-terminal GFP (see Fig. 5). A Myc-tagged Tg construct lacking the ChEL domain is called “I-II-III-Myc.” Next shown is secretory ChEL bearing a carboxyl-terminal Myc tag. Thereafter, secretory ChEL bearing an extra unpaired carboxyl-terminal Cys residue for covalent dimerization (ChEL-CD) was engineered to either include an Asn-linked glycosylation site (called “ChELG-CD”) or lack that site. Next, AChE bearing a carboxyl-terminal Myc tag is shown; note that AChE already contains a naturally occurring seventh (extra, unpaired) Cys residue at its carboxyl terminus. Next, secretory (FLAG-tagged) neuroligins 1, 2, 3, or 4 are indicated. Finally, a Tg-AChE chimera, which already contains a natural seventh (extra, unpaired) Cys residue at its carboxyl terminus (14), is shown; an identical Tg-AChE chimera lacking the carboxyl-terminal Cys residue (called Tg-AChEΔCys) was also prepared. B, multiple sequence alignment with mouse Tg, listed in order of greatest homology, of the primary structures derived from rat Tg, human Tg, rat AChE, mouse AChE, human AChE, and human neuroligins (NL) 3, 4, 1, and 2, respectively. Areas of perfect homology are colored green; 80 and 60% conservation are colored red and yellow, respectively. C, a comparison of the known structure of human AChE (PDB ID: 1B41) and human NL4 (PDB ID: 3BE8) with the three-dimensional structure of mouse ChEL predicted by the ESyPred3D structural prediction program.
The ChEL domain is a commonly affected site of mutation in human congenital hypothyroidism with deficient Tg, including the recently described A2215D (15, 16), R2223H (17), G2300D, R2317Q (18), G2355V, and G2356R and the skipping of exon 45 (which normally encodes 36 amino acids), as well as the Q2638stop mutant (19). Additionally, the homozygous Tg-G2300R mutation in the ChEL domain (equivalent to residue 2298 of mature mouse Tg) is responsible for congenital hypothyroidism in rdw/rdw rats (20, 21), whereas the Tg-L2263P point mutation is responsible for congenital hypothyroidism in the cog/cog mouse (22). Such mutations, when transplanted into AChE, inhibit enzyme activity (14), strongly suggesting that they structurally perturb local folding within this domain.
The present state of knowledge highlights the role of the ChEL domain in two critical activities for Tg: 1) homodimerization of Tg within the ER (23, 24) requires ChEL (25) and 2) ChEL is linked to a rate-limiting process of Tg-oxidative folding within the ER (26, 27). Specifically, although upstream regions I-II-III possess 116 of the 122 Tg cysteine residues (10), ChEL binds to I-II-III, functioning as a molecular chaperone to facilitate I-II-III oxidative maturation (10). Indeed, ChEL is not only contiguous with I-II-III in Tg, but the ChEL domain remains associated with I-II-III throughout the secretory pathway and after secretion (10).
As best as is currently known, all Tg mutants causing congenital hypothyroidism are defective for intracellular transport (28). However, until now, no studies have explored the severity of impairment of mutant Tg protein co-expressed in the presence of wild-type Tg, which is the situation found in simple heterozygous patients who are vastly more common than newborns with congenital hypothyroidism from homozygous or compound heterozygous Tg mutations (29). The potential for interaction of non-identical Tg partners in trans is a complex problem; for ChEL mutants this requires investigation of both dimerization and chaperone/escort functions. Notably, ChEL is part of a large family of cholinesterase-like proteins such as the neuroligins, which subserve cell adhesion and neuronal interaction functions (30, 31). In this report we have studied non-identical cholinesterase-like domains, either from structural relatives or from ChEL mutants, to examine interaction in trans with wild-type Tg domains and to examine the extent to which they can substitute for ChEL function in Tg secretion. Such studies shed new light on the folding, dimerization, and export of mutant Tg protein in the presence of co-expressed wild-type Tg.
EXPERIMENTAL PROCEDURES
Materials
Lipofectamine 2000, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, penicillin, and streptomycin were from Invitrogen (Carlsbad, CA); Zysorbin was from Zymed Laboratories Inc.; complete protease inhibitor mixture was from Roche Applied Science (Indianapolis, IN); brefeldin A, N-ethylmaleimide, and protein A-agarose were from Sigma; protein G-agarose was from EMD Chemicals (Gibbstown, NJ); dithiobis(succinimidyl propionate) was from ThermoFisher Scientific, Inc. (Waltham, MA); peptide-N-glycosidase F from New England Biolabs (Beverly, MA); and Trans35S-Label was from MP Biomedicals (Irvine, CA). Chicken polyclonal anti-Myc and rabbit polyclonal anti-FLAG were from Immunology Consultants, Inc. (Newberg, OR); rabbit anti-chicken IgY was from Jackson ImmunoResearch (West Grove, PA). Rabbit polyclonal anti-Tg (containing antibodies against epitopes at both N- and C-terminal regions of the protein) has been previously described (32).
Mutagenesis of Mouse Tg, Human AChE, and Human Neuroligin cDNAs
A cDNA encoding the “T-form” of hAChE (matching the exon 4 to exon 6 splice form (33)) was employed as template for further mutagenesis. AChE-Myc was constructed using the QuikChange site-directed mutagenesis kit (Stratagene) with the following primer and its complement (5′-CGGTGCTCAGATCTGGAACAGAAGCTCATCAGTGAAGAGGACCTCTAGGCGGCCGCTTCC-3′). The Tg-AChEΔCys mutation was introduced with the same kit using Tg-AChE as template (14) and the following primer and its complement (5′-CCAGTTCGACCACTACAGCAAGCAGGATCGCAGCTCAGATCTGTGATAGTCGACTCTAG-3′). Tg-GFP, Tg-Myc, Tg-AChE, truncated Tg regions I-II-III and I-II-III-Myc, and secretory ChEL, ChEL-Myc, ChEL-HA, ChEL-CD, and ChELG-CD constructs have been previously described (10, 14, 25). Each final product was confirmed by direct DNA sequencing. The rdw mutation encoding Tg-G2298R (34) was introduced to create rdw-Tg-GFP.
The cDNAs encoding epitope-tagged neuroligin 1, 2, 3, and 4 truncation constructs (devoid of splice inserts) have been described previously (35); each were truncated before the O-glycosylation attachment site and transmembrane domain rendering them secretable proteins, and each construct is composed of a cleavable pre-trypsin signal peptide followed by the FLAG octapeptide (DYKDDDDK), a linker peptide of 4–8 residues, followed by the neuroligin sequence beginning with the first amino acid of the mature protein. The precise truncation points were made by introducing stop codons at NL1–639, NL2–616, NL3–640, and NL4–620 generating the proteins NL1–638, NL2–615, NL3–639, and NL4–619, respectively.
Sucrose Velocity Gradient Centrifugation Assay of ChEL Dimerization
Transiently transfected 293 cells expressing ChEL-CD and ChELG-CD (25) were pulse-labeled with 35S-amino acids for 30 min and chased in complete media for 5 h at 37 °C. Chase media were collected and treated with a protease inhibitor mixture, and 0.5 ml of the treated media was overlaid atop 11.5-ml 5 to 10% linear gradients of sucrose in 0.3 m NaCl, 0.05 m Tris, pH 7.5. The gradients were spun at 187,000 × g (33,000 rpm) in an SW41 rotor for 18 h at 15 °C. At the conclusion of the velocity gradient centrifugation, 400-μl fractions were collected sequentially by puncture from the bottom of each tube, and each was diluted to 1 ml in 0.3 m NaCl, 0.05 m Tris, pH 7.5. Each fraction was immunoprecipitated with anti-Tg and analyzed by non-reducing SDS-PAGE and fluorography.
Cell Culture and Transfection
HEK293 cells (simply called 293 cells) were cultured in DMEM with 10% fetal bovine serum in 6-well plates at 37 °C in a humidified 5% CO2 incubator. Plasmids were transiently transfected using Lipofectamine 2000 transfection reagent, following the manufacturer's instructions.
Metabolic Labeling and Immunoprecipitation
Transfected 293 cells were starved for 30 min in Met/Cys-free DMEM, then pulse-labeled with 180 μCi/ml 35S-amino acids. The cells were then washed with an excess of cold Met/Cys and chased in complete DMEM plus serum. At each time point, cells were lysed in buffer containing 1% Nonidet P-40, 20 mm N-ethylmaleimide, 0.1% SDS, 0.1 m NaCl, 2 mm EDTA, 25 mm Tris, pH 7.4, and a protease inhibitor mixture. For immunoprecipitation, anti-Tg antibody was incubated with cell or media samples overnight at 4 °C, and the immunoprecipitate was recovered with protein A-agarose. For co-immunoprecipitation studies, lysates prepared in the same lysis buffer lacking SDS were incubated overnight at 4 °C with immunoprecipitating antibodies and protein A- or protein G-agarose. Immunoprecipitates (or co-precipitates) were washed three times before boiling in SDS sample buffer with or without reducing agent, resolved by SDS-PAGE, and analyzed by fluorography or phosphorimaging.
Enzymatic Activity of Tg AChE Chimeras
For measurement of the catalytic activity of recombinant Tg-AChE and Tg-AChEΔCys, 293 cells expressing each construct to be tested were lysed under native conditions (300 μl of 100 mm NaCl, 1% Nonidet P-40, 2 mm EDTA, 25 mm Tris, pH 7.5). One-sixtieth of each cell lysates was then used directly for colorimetric acetylthiocholinesterase activity as described before (36). Activity measurements for each construct were performed at least three times with mean ± S.D. values calculated. Parallel samples of cell lysate from the identical transfections were used for immunoblotting with anti-Tg to examine recombinant protein expression level normalized to cell protein. The specific activity was calculated as enzymatic activity per unit of immunoreactive protein.
Dithiobis(succinimidyl Propionate) Cross-linking
Cells were incubated in phosphate-buffered saline containing 2 mm dithiobis(succinimidyl propionate) for 30 min at room temperature. Cells were then washed in phosphate-buffered saline containing 20 mm N-ethylmaleimide and then lysed in buffer containing both 20 mm N-ethylmaleimide and 50 mm glycine to quench residual cross-linker. The lysates were then analyzed by immunoprecipitation and SDS-PAGE under reducing conditions.
RESULTS
Similarity of the Tg ChEL Domain to Other AChE-family Members
The Tg ChEL domain has 6 Cys residues, which engage in 3 disulfide bonds shown schematically in full-length Tg at the top of Fig. 1A. Related constructs employed or engineered for this study include secretory ChEL (including modifications such as carboxyl-terminal Myc tag, extra unpaired Cys residue, or N-linked glycosylation site), AChE (bearing a carboxyl-terminal Myc tag), FLAG-tagged neuroligins 1, 2, 3, or 4, and a Tg-AChE chimera (bearing or lacking a carboxyl-terminal Cys residue) (Fig. 1A). Amino acid sequence alignment of the first ∼500 residues of the ChEL domains of mouse, rat, and human Tg with rat, mouse, and human AChE and human neuroligins 1–4 reveals areas of identity (Fig. 1B, green) as well as 80 and 60% conservation, respectively (red and yellow). Comparison of the three-dimensional structure of AChE from crystal structure coordinates, that of a representative neuroligin and that predicted for ChEL by ESyPred3D (Fig. 1C) (37), supports a shared overall protein architecture between these AChE paralogs.
The Carboxyl-terminal ChEL Domain in Tg Homodimerization, and Cross-dimerization of Non-identical Partners
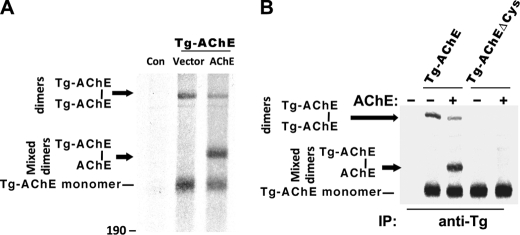
We recently hypothesized that Tg homodimerization may be initiated by noncovalent interactions involving two predicted α-helices at the carboxyl terminus of each ChEL domain, with the potential for Tg to form a 4-helix bundle similar to that reported in the dimerization of AChE (25). However, upstream regions of Tg, comprising >2000 amino acids, might potentially interfere with accessibility of the α-helices in the ChEL domain. We therefore examined a full-length Tg chimera in which catalytically active AChE replaced ChEL in Tg (14), to determine whether, in the context of Tg, these carboxyl-terminal α-helices were available for dimerization with co-transfected monomeric AChE. Formation of an AChE intermolecular disulfide bond engaging an unpaired carboxyl-terminal cysteine, neighboring the α-helices, is a natural reporter of such dimerization (38). Notably, Tg-AChE expressed alone already formed covalent homodimers intracellularly (Fig. 2A). When co-expressed with AChE, more Tg-AChE monomers were consumed (i.e. fewer Tg-AChE monomers were recovered), involving cross-dimerization (i.e. Tg-AChE–AChE) that appeared to compete with Tg-AChE homodimerization (Fig. 2A). The ability to form covalent homodimers or heterodimers was eliminated when expressing the Tg-AChEΔCys chimera that deleted the unpaired cysteine at the carboxyl terminus (Fig. 2B), proving that the carboxyl-terminal segment must be accessible for the apposition of monomer partners even when conjoined to the large upstream Tg regions I-II-III.
FIGURE 2.
Availability of AChE dimerization helices within the context of a Tg-AChE chimera. A, 293 cells were transiently co-transfected with empty vector + vector (“Con”) or with plasmids expressing Tg-AChE (14) plus either vector or human AChE. The cells were metabolically labeled with 35S-amino acids and chased for 5 h in the presence of 5 μg/ml brefeldin A. The cell lysates were immunoprecipitated with anti-Tg and analyzed by non-reducing SDS-4%-PAGE. Monomeric Tg-AChE migrated with an apparent molecular mass of ∼300 kDa; the covalent Tg-AChE homodimer migrated at ∼600 kDa; the position of mixed Tg-AChE–AChE covalent dimers is shown. B, the Tg-AChE chimera construct was mutagenized to substitute its extra unpaired Cys residue with Ser near the carboxyl terminus to create Tg-AChEΔCys. Pulse-labeling and chase of co-transfected 293 cells included either Tg-AChE or Tg-AChEΔCys with either empty vector (−) or a plasmid to co-express AChE (+), as indicated. Samples were analyzed as in panel A.
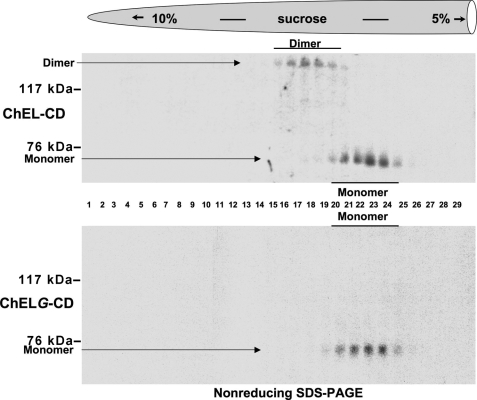
We introduced an extra, unpaired Cys residue at the tail of the ChEL domain to create a similar opportunity for covalent dimerization of ChEL monomers (called “ChEL-CD”), while also engineering an N-linked glycosylation site into the first of the two dimerization helices (called “ChELG-CD”) that blocks formation of the intermolecular covalent bond (Fig. 1A) (25). However, such an assay cannot exclude non-covalent dimerization of ChELG-CD. To obtain stronger evidence of the α-helical contribution to homodimerization of the Tg ChEL domain, we examined the migration of ChEL-CD and ChELG-CD by centrifugation on sucrose velocity gradients. Secretion of ChEL-CD was recovered as a combination of dimers and monomers, and upon analysis of gradient fractions by non-reducing SDS-PAGE, there was perfect correspondence between the presence of an intermolecular disulfide bond and recovery in a distinct peak of faster sedimentation (Fig. 3, upper gradient). By contrast, ChELG-CD was not only blocked in formation of the covalent inter-subunit linkage but was also blocked in faster sedimentation in the sucrose-velocity gradient (Fig. 3, lower). These data directly implicate the carboxyl-terminal α-helices in ChEL dimerization, strongly supporting a 4-helix bundle dimerization mechanism similar to that used by AChE and other cholinesterase-like family members (39, 40).
FIGURE 3.
Tail-to-tail ChEL homodimerization. Upper gradient: 293 cells transiently expressing secretory ChEL-CD were pulse-labeled with 35S-amino acids and chased, and the secretion was resolved on 5–10% linear sucrose velocity gradients as described under “Experimental Procedures.” Fractions collected from the bottom (left side) were immunoprecipitated with anti-Tg antiserum and analyzed by non-reducing SDS-PAGE and fluorography. Monomers sedimented between fractions 20 and 25; dimers sedimented between fractions 15 and 20. Monomeric ChEL-CD migrated by SDS-PAGE with an apparent molecular mass of ∼70 kDa; covalent ChEL-CD dimer migrated with an apparent molecular mass of ∼140 kDa. Lower gradient: 293 cells transiently expressing the secretory ChELG-CD mutant were analyzed as above. None of the ChELG-CD contained an intermolecular covalent bond; all molecules migrated to the monomer peak in the gradient.
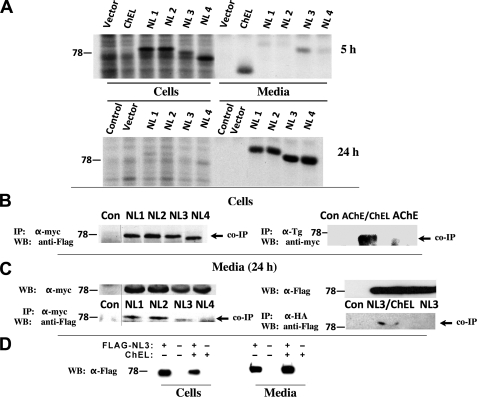
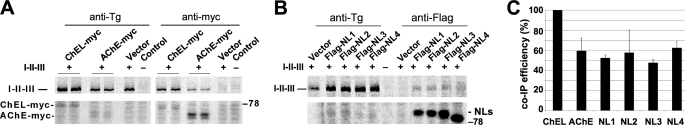
Such a dimerization mechanism might render it possible to obtain cross-dimerization of related cholinesterase-like proteins (Fig. 1, B and C). We therefore extended our analysis to include secretory versions of human neuroligins (NLs) 1, 2, 3, and 4. FLAG-tagged NL1, NL2, NL3, and NL4 were each secreted slowly but efficiently, with release from cells primarily between 5 and 24 h after synthesis (Fig. 4A, lower panel). Within cells that co-expressed secretory ChEL-Myc and FLAG-tagged neuroligins, after chemical cross-linking, ChEL could be observed to interact with each of the NL proteins as detected upon co-immunoprecipitation with anti-Myc and immunoblotting with anti-FLAG (Fig. 4B, left panel). A similar interaction was observed when secretory ChEL was co-expressed with AChE-Myc (co-precipitated with anti-Tg and immunoblotted with anti-Myc (Fig. 4B, right panel)). Even without cross-linker, from media, secreted FLAG-tagged neuroligins could be co-precipitated with ChEL-Myc (Fig. 4C, left) or with ChEL-HA (Fig. 4C, right). The data underscore the ability of non-identical cholinesterase-like partners (Fig. 1, B and C) to cross-dimerize. Cross-dimerization of non-identical partners could have important physiological consequences, as demonstrated when NL3 was co-expressed with secretory ChEL-HA, resulting in increased release of NL3 to the media (while the intracellular amount decreased), indicating enhanced secretion (Fig. 4D).
FIGURE 4.
Cross-dimerization with ChEL. A, 293 cells were transiently transfected with empty vector or plasmids to express secretory ChEL-Myc (ChEL) or secretory FLAG-NL1, -NL2, -NL3, or -NL4. Transfected cells were pulse-labeled for 30 min with 35S-amino acids and chased for either 5 h (upper panel, a time when ChEL secretion has gone to completion) or 24 h (lower panel, a time when neuroligin secretion has gone to completion). The cells were lysed, and both cells and media were immunoprecipitated with anti-Myc (for ChEL) and anti-FLAG (for neuroligins). B, the left panel shows 293 cells co-transfected with plasmids expressing secretory ChEL-Myc and either empty vector (Con) or secretory FLAG-NL1, -NL2, -NL3, or -NL4. Cell lysates were immunoprecipitated (IP) with α-Myc antibody, resolved by SDS-PAGE and immunoblotted (WB) with anti-FLAG antibody. The right panel shows 293 cells co-transfected with plasmids expressing secretory ChEL and either empty vector (Con) or AChE-Myc (AChE). The cells were lysed in the presence of 1 mm dithiobis(succinimidyl propionate) cross-linker, immunoprecipitated (IP) with α-Tg antibody, resolved by SDS-PAGE and immunoblotted (WB) with anti-Myc antibody. C, the left panel shows 293 cells co-transfected as in the left panel of B, or transfected with empty vector (Con). The right panel shows 293 cells co-transfected with plasmids expressing secretory ChEL-HA plus secretory FLAG-NL3, or secretory FLAG-NL3 alone, or transfected with empty vector (Con). Media were collected from transfected cells for 24 h; the upper left panel shows the comparable expression of secretory ChEL partner in each sample; the upper right panel shows comparable expression of the secretory NL3 partner. Media were immunoprecipitated (IP) with α-Myc (lower left) or anti-HA (lower right); the immunoprecipitates were resolved by SDS-PAGE and immunoblotted (WB) with anti-FLAG antibodies. D, cells were transfected to either singly express secretory FLAG-NL3, singly express secretory ChEL-HA, or co-express both constructs, or neither. After 24 h, the cells were lysed and the media were collected; samples were analyzed by Western blotting with anti-FLAG antibodies.
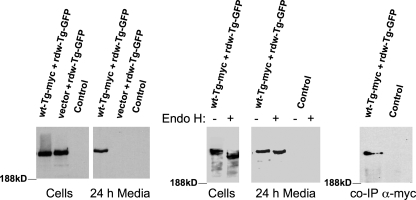
Congenital hypothyroidism with defective Tg is an autosomal recessive trait; thus heterozygous individuals in the population go largely undetected. Because mutant Tg ChEL is more similar to wild-type Tg ChEL than is AChE or neuroligins, it seemed plausible that cross-dimerization between mutant and wild-type Tg partners also might take place. To test the potential for mutant rdw-Tg to be rescued by wild-type Tg, the two proteins were co-expressed in 293 cells and incubated for 24 h to examine secretion. To distinguish these proteins unequivocally, rdw-Tg was tagged with GFP at the carboxyl terminus. When expressed along with an empty vector, rdw-Tg-GFP was recovered in cells but was undetectable in the medium. However, when co-expressed with wild-type Tg-Myc, rdw-Tg-GFP began to be recovered in the medium (Fig. 5, left panels). To confirm that this reflected authentic secretion, rdw-Tg-GFP was subjected to endoglycosidase H (endo H) digestion to assess modification of any of the N-linked glycans of Tg by Golgi glycosylation enzymes. Intracellular rdw-Tg-GFP was endo H-sensitive, indicating failure to reach the Golgi complex, whereas all of the rdw-Tg-GFP molecules recovered from the medium had some of their many N-linked glycans acquire endo H resistance (Fig. 5, middle panel), indicating normal intracellular trafficking through the secretory pathway. Moreover, immunoprecipitation of wt-Tg-Myc from the medium co-precipitated rdw-Tg-GFP (Fig. 5, right panel). The data clearly indicate potential for Tg cross-dimerization, as might occur in states of heterozygosity.
FIGURE 5.
Secretion rescue of mutant rdw-Tg by co-expressed wild-type Tg. Side-by-side, identical wells of 293 cells were co-transfected with plasmids encoding empty vector plus wild-type Tg-Myc (wt-Tg-Myc) and rdw-Tg-GFP such that total plasmid DNA and rdw-Tg-GFP DNA was held constant in all samples (wt:rdw plasmid ratio 6:1); Control lanes were transfected only with empty vector. At 48 h post-transfection, the medium was changed, and cells were cultured for an additional 24 h in complete growth medium. The cells were lysed, and 3.33% of each cell lysate and 1% of each collection of medium were resolved by SDS-PAGE and analyzed by immunoblotting with anti-GFP antibody. Left panels: direct immunoblotting of lysates and media. Middle panels: endoglycosidase H (Endo H) digestion of the samples indicated prior to immunoblotting. Note that the small mobility shift observed after endo H digestion of the medium is distinct from the larger shift seen for intracellular Tg, indicating Golgi sugar modification of Asn-linked glycans on all secreted rdw-Tg-GFP molecules (although some sugar chains on each molecule remain endo H-sensitive). Right panel: media from co-transfected or control cells were immunoprecipitated with α-Myc antibody, and co-precipitated rdw-Tg-GFP (co-IP) was analyzed by immunoblotting as in the other panels.
ChEL Interactions with Upstream Tg Regions I-II-III
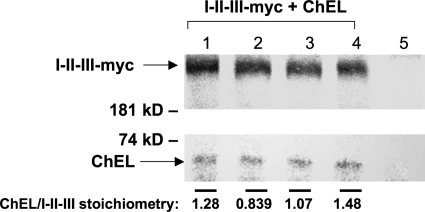
The Tg coding sequence links each I-II-III directly to its own contiguous ChEL polypeptide. However, because Tg homodimerizes, there is more than one potential stoichiometry and orientation of ChEL interaction with I-II-III. We proceeded to test the stoichiometry when the two are expressed as separate secretory proteins. After co-transfection of I-II-III-Myc and secretory ChEL, 293 cells were radiolabeled continuously at fixed specific radioactivity with [35S]cysteine in complete medium for 2 days to approach steady state. The medium was then collected and immunoprecipitated with anti-Myc to recover I-II-III-Myc and co-precipitated ChEL. The relative band intensities were quantified from scanned SDS-PAGE fluorograms (Fig. 6). An equimolar ChEL:region I-II-III binding stoichiometry binding should be seen as an intensity ratio of 6:116, reflecting precisely the number of Cys residues in each polypeptide. Indeed, from quadruplicate samples, the molar ratio measured was 1.17 ± 0.28, i.e. an equimolar binding stoichiometry (Fig. 6).
FIGURE 6.
Equimolar ChEL:I-II-III binding stoichiometry. 293 cells were co-transfected to express I-II-III-Myc and secretory ChEL. In quadruplicate, transfected cells were metabolically labeled in complete growth medium (containing serum) plus 1 mCi/ml pure [35S]cysteine at fixed specific radioactivity. One day later, the medium was removed and replaced with fresh labeling medium for a further day before collection of secretion with analysis by immunoprecipitation with anti-Myc antibody, SDS-PAGE, fluorography, and scanning densitometry of the relevant bands. The stoichiometry of co-precipitated ChEL:I-II-III was quantified by correcting for relative isotope abundance. (6:116 reflects the ratio of Cys residues present in each binding partner. For purposes of easy visualization we increased exposure of the ChEL bands shown in the figure.) From these measurements, the average molar ratio was 1.17 ± 0.28, i.e. an equimolar binding stoichiometry. Lane 5 shows results from untransfected control cells. Results shown here were repeated and confirmed in two independent experiments (not shown).
ChEL and structurally similar cholinesterase-like family members exhibit potential for cross-dimerization, raising the question of whether such family members could also substitute for ChEL in I-II-III binding. Indeed, AChE-Myc was also found able to associate with I-II-III (Fig. 7A), and this was similarly true for FLAG-NL1, -NL2, -NL3, or -NL4 (Fig. 7B). However, the efficiency of I-II-III interaction, as measured by co-precipitation, was weaker, i.e. only approximately half of that observed for ChEL itself (Fig. 7C).
FIGURE 7.
AChE and secretory neuroligin association with Tg regions I-II-III. A, 293 cells were co-transfected with plasmids expressing I-II-III (or a negative control marked with “−”) and either secretory ChEL-Myc or AChE-Myc. The cells were pulse-labeled with 35S-amino acids and chased for 6 h in the presence of brefeldin A (to block all protein export). At the conclusion of the experiment, the cells were lysed in a non-denaturing lysis buffer containing 1% Nonidet P-40 but lacking SDS, and then immunoprecipitated with either anti-Tg or anti-Myc antibodies for analysis by SDS-PAGE and fluorography. Anti-Tg antibodies directly immunoprecipitated I-II-III; anti-Myc antibodies could recover I-II-III only if co-immunoprecipitated with a Myc-tagged binding partner. Note (from Fig. 6) that the relative isotope abundance of Cys residues in one ChEL versus one I-II-III is a ratio of 6:116, explaining the relative intensities of ChEL and I-II-III. B, 293 cells were co-transfected with plasmids expressing I-II-III and either empty vector or secretory FLAG-NL1, -NL2, -NL3, or -NL4. At the 6-h chase time after pulse labeling, cell lysates were divided in half and immunoprecipitated with anti-Tg (first five lanes) or anti-FLAG (last five lanes) and analyzed by SDS-PAGE and fluorography. Anti-Tg antibodies directly immunoprecipitated I-II-III; anti-FLAG antibodies could recover I-II-III only if co-immunoprecipitated with a FLAG-tagged binding partner. C, quantitation of efficiency of co-immunoprecipitation of I-II-III with AChE or secretory neuroligins relative to co-precipitation obtained with ChEL (n = 3 experiments).
ChEL Is Unique in Its Ability to Rescue I-II-III Secretion
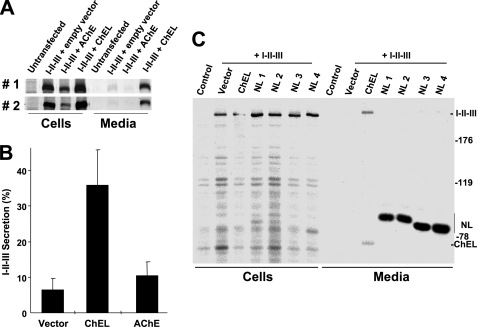
The ability of structurally related cholinesterase-like family members (Fig. 1, B and C) to physically interact with regions I-II-III raised the question of whether such interactions could rescue the secretion of region I-II-III lacking their own ChEL domain (10). In side-by-side comparisons, AChE could not promote significantly more I-II-III secretion than was observed with empty vector alone, and indeed, intracellular recovery of I-II-III appeared diminished (Fig. 8, A and B). Likewise, the secretory neuroligins, despite excellent expression, could not promote I-II-III secretion (Fig. 8C). The data suggest that sequence-specific differences among cholinesterase-like family members alter their ability to rescue I-II-III secretion.
FIGURE 8.
ChEL is unique in its ability to promote I-II-III secretion. A, in two independent transfections, 293 cells were co-transfected with the plasmids indicated at the top. The cells were pulse-labeled with 35S-amino acids for 30 min and chased for 4 h in complete medium. The cells were lysed and the media collected; both were immunoprecipitated with anti-Tg and analyzed by reducing SDS-PAGE and fluorography. B, from three experiments like those shown in panel A, secretion efficiency of I-II-III in the presence of empty vector, secretory ChEL, or AChE was quantified. C, 293 cells were co-transfected with plasmid expressing I-II-III and secretory FLAG-NL1, -NL2, -NL3, or -NL4 as indicated. Co-transfection with secretory ChEL was included as a positive control. The cells were pulse-labeled with 35S-amino acids for 30 min and chased for 4 h in complete medium. The cells were lysed, and the medium was collected; both were immunoprecipitated with a combination of anti-Tg plus anti-FLAG and analyzed by reducing SDS-PAGE and fluorography. As shown, secretory neuroligins could not rescue I-II-III secretion.
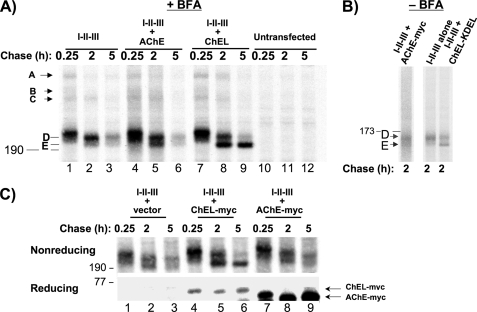
Why is association of AChE or neuroligins unproductive for Tg? Like full-length Tg, regions I-II-III go through a series of oxidative folding intermediates, including transient mixed disulfides with ER oxidoreductases (termed “A,” “B,” and “C” (41)) followed by further oxidative folding of immature “D monomers” to the mature “E isoform” (10). A major function of ChEL is as a molecular chaperone, stabilizing the oxidatively mature I-II-III, which includes formation of up to 58 potential intrachain disulfide bonds (10). To examine the relative chaperone activity of ChEL versus AChE, we examined oxidative maturation of I-II-III at different times, while retaining all folding partners within the early secretory pathway by treatment with brefeldin A. In the presence of secretory ChEL (Fig. 9A, lanes 7–9), I-II-III oxidative maturation had progressed nearly to completion at 5 h after synthesis, and the mature E band was already predominant by 2 h after synthesis. By contrast, I-II-III expressed alone became blocked at the immature D isoform (the mobility shift between lanes 1 and 2 is caused by carbohydrate trimming) and thereafter became unstable (lane 3). Co-expression of AChE offered little benefit to I-II-III: the E isoform never predominated, and I-II-III again became unstable (Fig. 9A, lanes 4–6). The story was similar in the absence of brefeldin A. For a positive control we now used secretory ChEL-KDEL (which is fully functional for association with I-II-III yet is retained intracellularly (10)). This allowed a majority of I-II-III to progress to the mature E form by 2 h of chase, whereas co-expression of AChE did not result in efficient I-II-III oxidative maturation (Fig. 9B).
FIGURE 9.
ChEL, but not AChE, promotes a stable, oxidatively mature state of regions I-II-III. A, 293 cells were transfected to express I-II-III alone (lanes 1–3) or co-express AChE (lanes 4–6) or secretory ChEL (lanes 7–9). The cells were pulse-labeled with 35S-amino acids for 30 min and chased in the presence of brefeldin A (5 μg/ml) in complete medium for the times indicated. At each chase time, the cells were lysed, immunoprecipitated with anti-Tg, and analyzed by non-reducing SDS-PAGE and fluorography. Bands A, B, and C have been described previously but are quantitatively minor (10); the positions of immature (D) and oxidatively mature (E) forms of I-II-III monomers are indicated. Untransfected cells (a negative control) are shown in lanes 10–12. B, 293 cells transfected to express I-II-III alone (middle lane) or co-express AChE-Myc (left) or secretory ChEL-KDEL (right), were pulse labeled as in A and chased (without brefeldin A) for 2 h. The cells were lysed and immunoprecipitated with anti-Tg; the immunoprecipitates were digested with PNGase F and analyzed by non-reducing SDS-PAGE and fluorography. The positions of immature (D) and mature (E) forms of I-II-III are indicated. C, 293 cells expressing I-II-III co-transfected with either empty vector (lanes 1–3) or secretory ChEL-Myc (lanes 4–6) or AChE-Myc (lanes 7–9). The cells were pulse-labeled with 35S-amino acids and chased as indicated. At each chase time, the cells were lysed, immunoprecipitated with anti-Myc, and analyzed by reducing SDS-PAGE (lower panel, the position of a 77-kDa molecular mass standard is shown on the left) to demonstrate relative production of ChEL-Myc (slower migrating) and AChE-Myc (faster migrating), or immunoprecipitated with anti-Tg and analyzed by non-reducing SDS-PAGE (upper panel, the position of a 190-kDa molecular mass standard is shown on the left) to examine the extent of oxidative maturation of Tg I-II-III. Note that, although AChE expression exceeds that of ChEL, stable, oxidatively mature I-II-III are recovered only in the presence of ChEL.
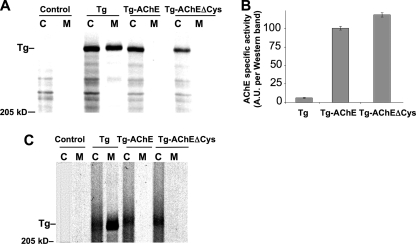
Rescue of I-II-III folding and secretion is augmented when the relative abundance of ChEL in the ER is increased (10). We wished to confirm that failure to rescue I-II-III was not caused by insufficient AChE expression. To directly compare protein production levels of AChE and secretory ChEL, we appended a carboxyl-terminal Myc tag to both proteins, and then repeated the pulse-chase experiment. We were able to achieve expression of AChE-Myc exceeding that obtained for ChEL-Myc (Fig. 9C, lower panel), yet AChE-Myc still did not support enhanced oxidative maturation of newly synthesized I-II-III, and instead led to I-II-III instability comparable to that seen in the presence of empty vector (Fig. 9C, upper panel). Moreover, we found that the Tg-AChE chimera, in which AChE is built in at a 1:1 ratio with I-II-III, could not be secreted from cells (Fig. 10A). We considered that lack of secretion might conceivably be caused by thiol-mediated ER retention (42) of those Tg-AChE molecules that exposed an extra unpaired carboxyl-terminal Cys residue rather than engaging it in an intermolecular disulfide bond (31). However, Tg-AChEΔCys (which maintains similar cholinesterase specific activity (Fig. 10B)) also could not be secreted (Fig. 10A). Indeed, neither Tg-AChE nor Tg-AChEΔCys allowed for normal oxidative maturation of I-II-III as judged by mobility upon non-reducing SDS-PAGE (Fig. 10C). Thus, the cholinesterase family members can fold as an independent unit within Tg, but information specific to the Tg ChEL domain is required for its function as an intramolecular chaperone to promote Tg oxidative maturation, stability, and secretion.
FIGURE 10.
A Tg-AChE chimera exhibiting functional cholinesterase activity is nevertheless incompetent for secretion. A, 293 cells transfected to express either wild-type Tg (as a positive control), Tg-AChE, or Tg-AChEΔCys were pulse-labeled for 30 min and chased for 3.5 h. The media were collected, and the cells were lysed and immunoprecipitated with anti-Tg before analysis by reducing SDS-PAGE and fluorography. B, specific cholinesterase activity of Tg-AChE and Tg-AChEΔCys (normalized to Western blotting with anti-Tg). C, an independent experiment similar to panel A but analyzed by non-reducing SDS-PAGE and fluorography. Despite that AChE has a smaller molecular mass than ChEL, Tg-AChE and Tg-AChEΔCys run slower (higher), because they are oxidatively immature. Untransfected cells (Control) are shown in the first two lanes of panels A and C. The position of a 205-kDa molecular mass marker is indicated.
DISCUSSION
The major hormone-forming site in Tg resides in the first 130 residues of the mature protein, yet homozygous (or compound heterozygous) mutations in the carboxyl-terminal ChEL domain result in a devastating impact on thyroid hormone formation (15–19). All evidence points to the notion that such Tg mutants exhibit impaired secretion (28). ChEL can fold as an independent domain within the full-length Tg protein, yet, ChEL is strongly implicated in the behavior of the overall Tg polypeptide within the ER. ChEL has structural similarity to AChE and the neuroligins (Fig. 1, B and C), and accumulated evidence strongly suggests that the carboxyl-terminal α-helices of the ChEL domain can allow for tail-to-tail homodimerization (Fig. 3) even in the context of full-length Tg (Fig. 2), most likely utilizing the 4-helix bundle mechanism common to AChE and neuroligins.
We now find that ChEL can also cross-dimerize with non-identical cholinesterase-like partners (11–13, 43, 44), including AChE or neuroligins 1, 2, 3, or 4 (Fig. 4, B and C). Dimerization plays an intricate role in the physical contact between the catalytic domains of cholinesterases, affecting overall protein architecture that allows members of the α/β-hydrolase fold family to associate with their heteromeric partners (45). Failure to achieve appropriate overall protein architecture of such family members is already recognized as the cause of at least 17 distinct disease entities (46). For AChE, dimers ensure their own stability by creating an intersubunit covalent bond that drives the association-dissociation equilibrium toward dimerization. However, for Tg and other members within the large cholinesterase-like family that do not utilize an intermolecular disulfide bond, stabilization of the dimer may employ ancillary mechanisms. Bi-directional stabilization (ChEL stabilizing I-II-III, and I-II-III stabilizing ChEL) appears to be such a potential mechanism (25), which may be involved in extra-allelic suppression of Tg mutations (that would be otherwise incompetent for secretion in homozygotes or compound heterozygotes). Dimerization is also critical for neuroligin function and may be important for its secretion (40), while cross-dimerization with wild-type ChEL enhances NL3 secretion efficiency (Fig. 4D).
The cross-dimerization of non-identical partners highlights a point of crucial pathophysiological significance. Specifically, most patients bearing Tg ChEL domain mutations (as well as mutations in other domains of Tg) are heterozygotes, creating intrathyroidal co-expression of wild-type and mutant Tg. As an autosomal recessive disease, it is generally assumed that Tg expression from a single allele would be sufficient for a completely normal phenotype; however, this assumption could only be tested experimentally by creating a Tg heterozygous null animal. We consider it possible that such animals might not be completely normal and might develop a substantial goiter caused by chronic thyroid stimulation throughout life. Moreover, we have considered, that in heterozygous patients bearing Tg mutations, there might be intragenic extra-allelic suppression of the mutation that operates at the level of the co-expressed proteins. In this study we found that when mutant rdw-Tg (Tg-G2298R) was co-expressed with wild-type Tg, the two proteins cross-dimerized, and secretion of rdw-Tg was partially restored (Fig. 5). This implies that, depending upon the cross-dimerization potential of the particular mutant Tg alleles expressed, different degrees of overall Tg secretory efficiency may exist in different patients heterozygous for mutant Tg, i.e. with cross-dimerization to wild-type Tg tending to suppress the disease phenotype of congenital hypothyroidism from mutant Tg. More broadly, intragenic extra-allelic suppression might help to account for a lack of phenotype in many autosomal recessive diseases involving cholinesterase family members or, potentially, other secretory proteins that oligomerize in the ER.
Cholinesterase-like proteins are implicated in mediating heterophilic protein binding (30). The Tg ChEL domain, for instance, physically associates with I-II-III with an equimolar binding stoichiometry (Fig. 6); identical to that found for neuroligin-1 and its binding partner β-neurexin (40). In this report, we found that AChE and secretory neuroligins also can bind to upstream Tg regions I-II-III (Fig. 7), mimicking interactions that may occur in cis. Evidently, conserved structural features between these AChE paralogs are sufficient to encode simple binding to the region I-II-III partner. However, neither AChE nor secretory neuroligins can promote secretion of Tg regions I-II-III (Fig. 8), because neither can facilitate formation of a stable, oxidatively mature I-II-III (Fig. 9). Moreover, we correct the finding of a previous report (14) to establish that folding of AChE to an enzymatically active conformation within the context of a Tg-hAChE chimera does not promote secretion from cells (Fig. 10). Indeed, a careful and systematic analysis of nine additional Tg-AChE chimeras, each differing by the fusion boundary between the domains, produced no secreted protein (data not shown). Further, lack of secretion could not be accounted for by thiol-mediated retention in the ER (31, 42), because Tg-AChEΔCys was also incompetent for oxidative maturation and secretion (Fig. 10). The inability to support I-II-III secretion cannot be accounted for by insufficient expression of secretory neuroligins (Fig. 8C) or AChE (Fig. 9C). Rather, the data in this study support the idea that ChEL not only binds to I-II-III but engages in additional protein-specific chaperone-like interactions that promote its oxidative maturation and stability (10). We believe that intramolecular chaperone function of the ChEL domain is likely to require three-dimensional determinants, because it is impaired by distinct single point mutations such as those found in cog or rdw mutants (10); whereas initiation of cross-dimerization may require only satisfactory exposure of the dimerization helices (Fig. 5).
In summary, we have discovered the potential for cross-dimerization between the Tg ChEL domain and cholinesterase-like relatives, with functional consequences for protein transport through the secretory pathway. At the present state of knowledge, we presume that, within each Tg monomer, the ChEL domain binds in cis to its own contiguous I-II-III to service its folding needs. However, association in trans between wild-type and mutant Tg may provide an alternative opportunity for chaperone function provided by the dimerization partner bearing wild-type ChEL. We conclude that the evolution of ChEL involves adaptation of its AChE-like structure to the specific folding and transport needs of upstream Tg regions I-II-III, and the dimeric structure of Tg subunits provides an additional potential means to suppress Tg protein defects through cross-dimerization of wild-type and mutant partners.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-DK40344 (to P. A.) and P42-ES010337 and R37-GM18360 (to P. T.). This work was also supported by the Ministero dell'Università e della Ricerca PRIN 2006069102_004 (to B. D. J.), by University of Michigan Diabetes Research and Training Center Grant NIH5P60 DK20572 for the Molecular Biology and Sequencing Cores, and by the University of Michigan Cancer Center Core laboratories (Grant P30 CA46592). This work was performed in partial fulfillment of the requirements for Ph.D. (for J. L.) in the Cellular and Molecular Biology program at the University of Michigan.
- Tg
- thyroglobulin
- ChEL
- cholinesterase-like
- ER
- endoplasmic reticulum
- DMEM
- Dulbecco's modified Eagle's medium
- GFP
- green fluorescent protein
- NL
- neuroligin
- endo H
- endoglycosidase H
- AChE
- acetylcholinesterase.
REFERENCES
- 1.Di Jeso B., Arvan P. (2004) in The Thyroid (Braverman L. E., Utiger R. eds) 9th Ed., pp. 77–95, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 2.Taurog A. (1999) Biochimie 81, 557–562 [DOI] [PubMed] [Google Scholar]
- 3.Marriq C., Lejeune P. J., Venot N., Vinet L. (1991) Mol. Cell. Endocrinol. 81, 155–164 [DOI] [PubMed] [Google Scholar]
- 4.Dunn A. D., Corsi C. M., Myers H. E., Dunn J. T. (1998) J. Biol. Chem. 273, 25223–25229 [DOI] [PubMed] [Google Scholar]
- 5.Lamas L., Taurog A. (1977) Endocrinology 100, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 6.Turakulov I., Saatov T., Babaev T. A., Rasuleva G., Makhmudov V. (1976) Biokhimiia 41, 1004–1007 [PubMed] [Google Scholar]
- 7.Xiao S., Dorris M. L., Rawitch A. B., Taurog A. (1996) Arch. Biochem. Biophys. 334, 284–294 [DOI] [PubMed] [Google Scholar]
- 8.Veneziani B. M., Giallauria F., Gentile F. (1999) Biochimie 81, 517–525 [DOI] [PubMed] [Google Scholar]
- 9.van de Graaf S. A., Ris-Stalpers C., Pauws E., Mendive F. M., Targovnik H. M., de Vijlder J. J. (2001) J. Endocrinol. 170, 307–321 [DOI] [PubMed] [Google Scholar]
- 10.Lee J., Di Jeso B., Arvan P. (2008) J. Clin. Invest. 118, 2950–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. (1986) Nature 319, 407–409 [DOI] [PubMed] [Google Scholar]
- 12.Swillens S., Ludgate M., Mercken L., Dumont J. E., Vassart G. (1986) Biochem. Biophys. Res. Commun. 137, 142–148 [DOI] [PubMed] [Google Scholar]
- 13.Mori N., Itoh N., Salvaterra P. M. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 2813–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park Y. N., Arvan P. (2004) J. Biol. Chem. 279, 17085–17089 [DOI] [PubMed] [Google Scholar]
- 15.Caputo M., Rivolta C. M., Esperante S. A., Gruneiro-Papendieck L., Chiesa A., Pellizas C. G., Gonzalez-Sarmiento R., Targovnik H. M. (2007) Clin. Endocrinol. 67, 351–357 [DOI] [PubMed] [Google Scholar]
- 16.Pardo V., Rubio I. G., Knobel M., Aguiar-Oliveira M. H., Santos M. M., Gomes S. A., Oliveira C. R., Targovnik H. M., Medeiros-Neto G. (2008) Thyroid 18, 783–786 [DOI] [PubMed] [Google Scholar]
- 17.Caron P., Moya C. M., Malet D., Gutnisky V. J., Chabardes B., Rivolta C. M., Targovnik H. M. (2003) J. Clin. Endocrinol. Metab. 88, 3546–3553 [DOI] [PubMed] [Google Scholar]
- 18.Kitanaka S., Takeda A., Sato U., Miki Y., Hishinuma A., Ieiri T., Igarashi T. (2006) J. Hum. Genet. 51, 379–382 [DOI] [PubMed] [Google Scholar]
- 19.Rivolta C. M., Targovnik H. M. (2006) Clin. Chim. Acta 374, 8–24 [DOI] [PubMed] [Google Scholar]
- 20.Kim P. S., Ding M., Menon S., Jung C. G., Cheng J. M., Miyamoto T., Li B., Furudate S., Agui T. (2000) Mol. Endocrinol. 14, 1944–1953 [DOI] [PubMed] [Google Scholar]
- 21.Hishinuma A., Furudate S., Oh-Ishi M., Nagakubo N., Namatame T., Ieiri T. (2000) Endocrinology 141, 4050–4055 [DOI] [PubMed] [Google Scholar]
- 22.Kim P. S., Hossain S. A., Park Y. N., Lee I., Yoo S. E., Arvan P. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 9909–9913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim P. S., Arvan P. (1991) J. Biol. Chem. 266, 12412–12418 [PubMed] [Google Scholar]
- 24.Di Jeso B., Pereira R., Consiglio E., Formisano S., Satrustegui J., Sandoval I. V. (1998) Eur. J. Biochem. 252, 583–590 [DOI] [PubMed] [Google Scholar]
- 25.Lee J., Wang X., Di Jeso B., Arvan P. (2009) J. Biol. Chem. 284, 12752–12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Jeso B., Ulianich L., Pacifico F., Leonardi A., Vito P., Consiglio E., Formisano S., Arvan P. (2003) Biochem. J. 370, 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arvan P., Kim P. S., Kuliawat R., Prabakaran D., Muresan Z., Yoo S. E., Abu Hossain S. (1997) Thyroid 7, 89–105 [DOI] [PubMed] [Google Scholar]
- 28.Vono-Toniolo J., Rivolta C. M., Targovnik H. M., Medeiros-Neto G., Kopp P. (2005) Thyroid 15, 1021–1033 [DOI] [PubMed] [Google Scholar]
- 29.Targovnik H. M., Esperante S. A., Rivolta C. M. (2010) Mol. Cell. Endocrinol., in press [DOI] [PubMed] [Google Scholar]
- 30.Gilbert M. M., Auld V. J. (2005) Front. Biosci. 10, 2177–2192 [DOI] [PubMed] [Google Scholar]
- 31.De Jaco A., Comoletti D., King C. C., Taylor P. (2008) Chem. Biol. Interact. 175, 349–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim P. S., Kwon O. Y., Arvan P. (1996) J. Cell Biol. 133, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massoulié J., Bon S., Perrier N., Falasca C. (2005) Chem. Biol. Interact. 157–158, 3–14 [DOI] [PubMed] [Google Scholar]
- 34.Menon S., Lee J., Abplanalp W. A., Yoo S. E., Agui T., Furudate S., Kim P. S., Arvan P. (2007) J. Biol. Chem. 282, 6183–6191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comoletti D., Flynn R. E., Boucard A. A., Demeler B., Schirf V., Shi J., Jennings L. L., Newlin H. R., Südhof T. C., Taylor P. (2006) Biochemistry 45, 12816–12827 [DOI] [PubMed] [Google Scholar]
- 36.Ellman G. L., Courtney D., Andres V., Jr., Feather-Stone R. M. (1961) Biochem. Pharmacol. 7, 88–95 [DOI] [PubMed] [Google Scholar]
- 37.Lambert C., Léonard N., De Bolle X., Depiereux E. (2002) Bioinformatics 18, 1250–1256 [DOI] [PubMed] [Google Scholar]
- 38.Kerem A., Kronman C., Bar-Nun S., Shafferman A., Velan B. (1993) J. Biol. Chem. 268, 180–184 [PubMed] [Google Scholar]
- 39.Morel N., Leroy J., Ayon A., Massoulié J., Bon S. (2001) J. Biol. Chem. 276, 37379–37389 [DOI] [PubMed] [Google Scholar]
- 40.Comoletti D., Grishaev A., Whitten A. E., Taylor P., Trewhella J. (2008) Chem. Biol. Interact. 175, 150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Jeso B., Park Y. N., Ulianich L., Treglia A. S., Urbanas M. L., High S., Arvan P. (2005) Mol. Cell. Biol. 25, 9793–9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anelli T., Alessio M., Bachi A., Bergamelli L., Bertoli G., Camerini S., Mezghrani A., Ruffato E., Simmen T., Sitia R. (2003) EMBO J. 22, 5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shafferman A., Kronman C., Flashner Y., Leitner M., Grosfeld H., Ordentlich A., Gozes Y., Cohen S., Ariel N., Barak D., Harel M., Silman I., Sussman J. L., Velan B. (1992) J. Biol. Chem. 267, 17640–17648 [PubMed] [Google Scholar]
- 44.Srivatsan M. (2006) Bioinformation 1, 281–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massoulié J., Perrier N., Noureddine H., Liang D., Bon S. (2008) Chem. Biol. Interact. 175, 30–44 [DOI] [PubMed] [Google Scholar]
- 46.Renault L., Nègre V., Hotelier T., Cousin X., Marchot P., Chatonnet A. (2005) Chem. Biol. Interact. 157–158, 339–343 [DOI] [PubMed] [Google Scholar]