Abstract
Structural data of integrin αIIbβ3 have been interpreted as supporting a model in which: 1) the receptor exists primarily in a “bent,” low affinity conformation on unactivated platelets and 2) activation induces an extended, high affinity conformation prior to, or following, ligand binding. Previous studies found that “clasping” the αIIb head domain to the β3 tail decreased fibrinogen binding. To study the role of αIIb extension about the genu, we introduced a disulfide “clamp” between the αIIb thigh and calf-1 domains. Clamped αIIbβ3 had markedly reduced ability to bind the large soluble ligands fibrinogen and PAC-1 when activated with monoclonal antibody (mAb) PT25-2 but not when activated by Mn2+ or by coexpressing the clamped αIIb with a β3 subunit containing the activating mutation N339S. The clamp had little effect on the binding of the snake venom kistrin (Mr 7,500) or αIIbβ3-mediated adhesion to immobilized fibrinogen, but it did diminish the enhanced binding of mAb AP5 in the presence of kistrin. Collectively, our studies support a role for αIIb extension about the genu in the binding of ligands of 340,000 and 900,000 Mr with mAb-induced activation but indicate that it is not an absolute requirement. Our data are consistent with αIIb extension resulting in increased access to the ligand-binding site and/or facilitating the conformational change(s) in β3 that affect the intrinsic affinity of the binding pocket for ligand.
Keywords: Blood, Integrin, Platelet, Receptor Regulation, Receptor Structure-Function, Thrombosis, GPIIb/IIIa
Introduction
The platelet αIIbβ3 receptor plays an important role in both hemostasis and thrombosis (1). Ligand binding to αIIbβ3 is controlled by an activation process that affects the conformation of the receptor and ligand binding, in turn, can also affect the conformation of the receptor (2). Several different conformations of αIIbβ3 have been identified based on inferences from biochemical analyses (3), studies employing monoclonal antibodies (4–7) and electron microscopy (8–10), comparison of the crystal structures of the liganded αIIbβ3 headpiece (11) and the unliganded complete ectodomain (12), and analysis of the unliganded and liganded ectodomain of the related αVβ3 receptor (13, 14). Receptor extension about the regions encompassing the thigh, genu, and calf-1 domains of αIIb and the plexin-semaphorin-integrin (PSI),2 integrin epidermal growth factor-1 (IEGF-1), and IEGF-2 domains of β3 or comparable regions of other integrin receptors has been proposed to play an important role in receptor activation (12, 15–18), but there is uncertainty about whether this conformational change occurs prior to or after ligand binding (19–21). Thus, “cross-clasping” the αIIb headpiece β-propeller domain to the β3 IEGF-4 domain in the tail region via a newly engineered disulfide bond prevented the binding of fibrinogen induced by activating mAb in concert with the activating divalent cation Mn2+, and a similar effect was observed with cross-clasped αVβ3 (16). In both cases the loss of ligand binding could be rescued by reducing the cross-clasped receptors with dithiothreitol (DTT). In contrast, the binding of a fragment of fibronectin (Mr = ∼50 kDa) to the ectodomain of αVβ3 activated by Mn2+ was not associated with receptor extension as judged by electron microscopy (22).
To understand better the role of αIIb extension around the αIIb genu region in ligand binding, we selectively clamped the αIIb thigh domain to the αIIb calf-1 domain with a newly engineered disulfide bond created by mutating αIIb Arg597 and Tyr645 to cysteine residues. We directly confirmed the formation of the disulfide bond by mass spectrometry and tested the interaction of both large and small ligands to clamped αIIbβ3, as well as both soluble and immobilized ligands. We also tested the effects of multiple αIIbβ3 activation mechanisms, including Mn2+ and coexpression of normal and mutant αIIb with a mutant β3 subunit that when coexpressed with normal αIIb results in spontaneous fibrinogen binding (N339S) (23). Our data support an important role for receptor extension around the genu for the binding of large soluble ligands induced by some, but not all activation mechanisms. In contrast, restricting αIIb extension about the genu had only a modest effect on αIIbβ3-mediated adhesion to immobilized fibrinogen and no discernable effect on the binding of small soluble ligands. These data provide new insight into the contribution of αIIb extension around the genu to high affinity ligand binding.
EXPERIMENTAL PROCEDURES
Monoclonal Antibodies and Ligands
mAbs 7E3 (24) (anti-αIIbβ3 and αVβ3) and 10E5 (25) (anti-αIIbβ3) were produced at the National Cell Culture Center (Minneapolis, MN). The ligand-induced binding site mAb AP5 (5) (anti-β3) and the αIIbβ3-activating mAb PT25-2 (27) (anti-αIIb) were generously provided by Dr. Peter Newman (Blood Center of Southeastern Wisconsin) and Shigenori Honda (Osaka University, Osaka, Japan), respectively. The anti-αVβ3 mAb LM609 (28) was a gift of Dr. David Cheresh (University of California, San Diego), and the αIIb-specific mAb PMI-1 (29) was a gift of Dr. Mark Ginsberg (University of California, San Diego). FITC-PAC-1 (30) was purchased from BD Biosciences (San Jose, CA). The disintegrin kistrin (rhodostomin) from the venom of Agkistrodon rhodostoma (31) was the gift of Dr. Tur-Fu Huang (Taiwan University) and was labeled for binding studies using Alexa488 carboxylic acid, 2,3,5,6-tetrafluorophenyl ester (Invitrogen). Fluorescent labeling of antibodies 10E5, AP5, and PT25-2 with Alexa488 was carried out according to the manufacturer's instructions (Alexa Fluor 488 mAb labeling kit; Invitrogen). Alexa488-fibrinogen was obtained from Invitrogen.
Molecular Modeling, Target Residue Selection, and Site- directed Mutagenesis
To “clamp” αIIb in a bent position, we engineered a new disulfide bond between the αIIb thigh domain and the αIIb calf-1 domain. An energy optimized model of the complete extracellular domain of αIIbβ3 was built to identify candidate regions of the molecule that might selectively restrict αIIb extension around the genu. As previously described (32), the conformation of the αIIb chain of the bent, inactive, unliganded αIIbβ3 was generated with MODELLER 8v2 (33) using the coordinates of the β propeller from the crystal structure of αIIbβ3 (residues 1–453; Protein Data Bank entry 1TY6 (11)) and the coordinates of αV for the remainder of the sequence (Protein Data Bank entry 1U8C (34)) Most of the coordinates of the β3 chain of the bent, inactive, unliganded αIIbβ3 conformation were extracted from the αVβ3 complex (Protein Data Bank entry 1U8C), whereas the missing domains, IEGF-1 (435–475) and IEGF-2 (486–522), were constructed with MODELLER 8v2 using the crystal structures of the β2 IEGF-1 (Protein Data Bank entry 1YUK (35)) and the αVβ3 IEGF-3 (Protein Data Bank entry 1U8C) domains, respectively, as templates. A model of the extended conformation of αIIbβ3 based on changes around the αIIb genu and β3 IEGF-1 and 2 domains was generated from our energy-minimized model of the complete extracellular unliganded bent conformation of αIIbβ3 by a 26° rotation of the αIIb 601–960 and β3 480–690 regions around the Cα-N bond of αIIb residue 600, followed by a 22° rotation of the αIIb 599–960 and β3 480–690 regions around the Cα-Cα axis of αIIb residues 607–608.
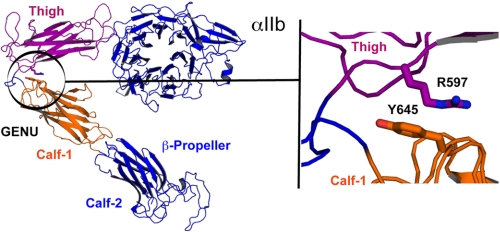
Our energy-minimized model of the bent IIb subunit and our model of the extended αIIb thigh-genu-calf-1 regions were used to analyze interdomain contacts between residues of the thigh and calf-1 domains in the bent conformation (i.e. distances between Cβ atoms within 10 Å) that would break upon extension. This analysis led to the identification of several candidate interactions, among them the thigh residue Arg597 and the calf-1 residue Tyr645, whose Cβ atoms are 5.7 Å apart in the bent conformation and 13.6 Å in the extended conformation. After these studies were completed, Zhu et al. (12) reported the crystal structure of a complete ectodomain of αIIbβ3. In that structure the Cβ atoms of αIIb Arg597 and Tyr645 are 7.1 Å apart. To clamp αIIb about the genu we mutated both of these residues to cysteines with the goal of creating a new disulfide bond (Fig. 1). We separately generated the β3 mutation N339S, which has been shown to constitutively activate αIIbβ3 and promote fibrinogen binding (23).
FIGURE 1.
Analysis of the region surrounding the αIIb genu in a model derived from the bent αVβ3 crystal structure identifies the Cβ carbons of Arg597 and Tyr645 as being ∼6 Å apart. See “Experimental Procedures” for details on the construction of the model.
pEF1/V5-His/αIIb and pCDNA3.1/Myc-His/β3 were generous gifts of Drs. Junichi Takagi and Timothy Springer (Harvard Medical School). The αIIb double mutant R597C/Y645C and the β3 N339S mutant were generated using a QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The mutant cDNA was transformed into XL-10 Gold Ultracompetent Escherichia coli bacteria. cDNA was purified (Maxi kit; Qiagen) and sequenced to confirm mutagenesis.
Stable Cell Line Generation
HEK293 cells were transfected with either normal or mutant cDNA using a cationic lipid transfection reagent according to the manufacturer's instructions (Cellipon 293; America Pharma Source, Gaithersburg, MD). The cells were selected in G418 for 2 weeks. To obtain populations of cells expressing high levels of αIIbβ3, the cells were sorted based on their binding of Alexa488-conjugated mAb 10E5 (FACSCalibur; BD Biosciences). The cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% nonessential amino acids. Expression of αIIbβ3 was assessed by the binding Alexa488–10E5 mAb on each day of experimentation.
Assessment of Cys597–Cyr645 Disulfide Bond Formation in Clamped αIIbβ3 by Mass Spectrometry
Clamped and normal recombinant αIIbβ3 were purified from HEK293 cells, and platelet αIIbβ3 was purified from washed platelets by solubilizing in 1% Triton X-100 and then immunoprecipitating the lysate with the anti-αIIbβ3 mAb 10E5 coupled to tosylated magnetic beads (Dynabeads M-280p; Invitrogen). The beads were washed, and the αIIbβ3 was eluted with diethylamine at pH 11.0 for 30 min at 22 °C, and then the pH of the eluate was immediately reduced to pH 8.0 with HCl. Purified αIIbβ3 was prepared for mass spectrometry by overnight digestion with trypsin (2 μg/ml; Promega sequencing grade modified trypsin), followed by two overnight digestions with endoproteinase Asp-N (2 μg/ml; Roche Applied Science). In some experiments, purified αIIbβ3 was treated with iodoacetic acid, DTT, or a combination of DTT and [13C]iodoacetic acid before mass spectrometry. The samples were analyzed by liquid chromatography-tandem mass spectrometry using a nano-reversed phase column coupled online with an LTQ-Orbitrap mass spectrometer (ThermoFisher, Waltham, MA).
Immunoprecipitation and Immunoblotting
Studies were performed to assess both the processing of the normal and mutant receptors during biogenesis and the biochemical composition of receptors on the surface of the cells. For the former, the cells were solubilized in 1% Triton X-100, and lysates were treated with SDS. Approximately 550 μg of protein was added to each well, and the samples were electrophoresed in 7.0% polyacrylamide gels (SDS-PAGE) under nonreducing or reducing (10% β-mercaptoethanol) conditions. After the proteins were transferred to polyvinylidene fluoride membranes, the membranes were incubated with the anti-αIIb mAb PMI-1. The membranes were washed and incubated with horseradish peroxidase-conjugated goat anti-mouse antibody (Jackson Immunoresearch, West Grove, PA) and visualized by chemiluminescence.
For analysis of surface proteins, the cells (1.5 × 106) were treated with biotin (1 mg/ml; EZ-Link Sulfo-NHS-LC-Biotin; Thermo Scientific, Rockford, IL) 30 min on ice, and then glycine (5 mm) was added for 10 min to quench the reaction. The cells were then washed and lysed with 1% Triton X-100; then αIIbβ3 was immunoprecipitated using mAb 10E5 and protein G-Sepharose beads (GE Healthcare). The samples were treated with SDS, and ∼550 μg of protein was added to each well. The samples were then electrophoresed in 7.0% polyacrylamide gels under nonreducing or reducing (10% β-mercaptoethanol) conditions. After the proteins were transferred to polyvinylidene fluoride membranes, the membranes were incubated with a streptavidin-horseradish peroxidase conjugate (Amersham Biosciences) and visualized by chemiluminescence.
Soluble Ligand Binding
HEK293 cells stably expressing either normal human β3 or β3 N339S in complex with either normal αIIb or clamped αIIb were washed and suspended in HEPES-modified Tyrode's buffer (HBMT; 138 mm NaCl, 2 mm NaHCO3, 10 mm HEPES, 2.7 mm KCl, 0.4 mm NaH2PO4, 0.1% glucose, 0.35% bovine serum albumin, pH 7.4) containing 2 mm CaCl2 and 1 mm MgCl2, and the cell count was adjusted to 5 × 106/ml. Activation of αIIbβ3 was achieved by treating cells with mAb PT25-2 (15 μg/ml) or Mn2+ (0.75 mm in HBMT without Ca/Mg). Preliminary experiments demonstrated that PT25-2 binds equally well to resting normal αIIbβ3 and clamped αIIbβ3 (supplemental Fig. S1). The αIIbβ3 antagonists 10E5 (40 μg/ml), 7E3 (40 μg/ml), or eptifibatide (100 μm) were used to assess the specificity of ligand binding. Alexa488-fibrinogen (200 μg/ml), FITC-PAC-1 (5 μg/ml), or Alexa488-kistrin (1 nm) was added to samples and incubated for 30 min. Unbound ligand was removed by washing and resuspending cells in HBMT; the samples were then diluted 1:10 with HBMT and analyzed by flow cytometry (FACSCalibur). Ligand binding was expressed as net normalized fluorescence intensity (NNFI) by subtracting the geometric mean fluorescence intensity (GMFI) observed in the presence of an αIIbβ3 antagonist from the GMFI in the absence of the αIIbβ3 antagonist and then normalizing for the αIIbβ3 receptor expression level determined by the binding of Alexa488-labeled 10E5.
To reverse the effect of the disulfide clamp, in some experiments the cells were incubated with the reducing agent DTT for 5 min at 37 °C before adding the activator and/or ligand. As previously shown by others, this concentration of DTT can partially activate αIIbβ3 (37).
Adhesion to Fibrinogen
Polystyrene 96-well microtiter plates (Nunc, Rochester, NY) were coated with fibrinogen (50 or 10 μg/ml; American Diagnostica, Stamford, CT) for 1 h at 22 °C and then washed and blocked with HBMT for 1 h. The cells expressing either normal or mutant αIIbβ3 were washed and resuspended in HBMT containing 2 mm CaCl2 and 1 mm MgCl2 at 1 × 106/ml. Some samples were treated with DTT (5 mm) for 5 min at 37 °C and then washed. Negative controls consisted of samples of cells treated with the αIIbβ3 antagonist mAb 7E3 (40 μg/ml). The cells (50 μl/well) were added to wells and allowed to adhere for 1 h at 37 °C. The wells were then washed with HBMT, and adhesion was quantified using an endogenous acid phosphatase activity assay (38). To assess any contribution to adhesion mediated by αVβ3, initial experiments were conducted in the presence of the αVβ3-blocking mAb LM609 (15 μg/ml). Because LM609 did not reduce the adhesion, we concluded that αVβ3 does not contribute significantly to adhesion under the conditions tested. Receptor expression was assessed prior to adhesion experiments on each of the 5 days of experimentation to ensure comparable surface levels of αIIbβ3 (normal αIIbβ3 GMFI = 138 ± 53; clamped αIIbβ3 = 148 ± 56).
mAb AP5 Binding
The cells were suspended in HBMT containing 2 mm CaCl2 and 1 mm MgCl2 at a cell count of 5 × 106/ml. Some cells were incubated with DTT (5 mm) at 37 °C for 5 min and were then washed and resuspended to the same count. The cells were incubated with Alexa488-AP5 (5 μg/ml) in the presence or absence of kistrin (200 nm) for 1 h at 37 °C. The samples were diluted 1:10 with HBMT and analyzed by flow cytometry. Nonspecific binding was determined in the presence of 25-fold excess unlabeled mAb AP5, and the data were normalized for receptor expression on each day of experimentation.
RESULTS
The Clamped Mutant αIIb Contains a Cys597–Cys645 Disulfide Bond and Migrates More Rapidly than Normal αIIb in SDS-PAGE under Nonreducing, but Not Reducing Conditions
Sequence analysis of the expected peptide cleavage products of the normal αIIbβ3 and clamped αIIbβ3 (assuming successful creation of the Cys597–Cys645 bond) led to the prediction of a unique disulfide-linked peptide (αIIb Asp589–Leu600 disulfide-linked to αIIb Asp636–Arg661) in the clamped αIIb but not in either the normal recombinant αIIb or platelet αIIb (supplemental Fig. S2). This disulfide-linked peptide was observed in the main spectrum of clamped αIIbβ3 at m/z = 1,031.21 corresponding to the MH44+. The identity of the peptide was confirmed by tandem mass spectrometry, in which fragments of both halves of the peptide were observed (data not shown). The peptide ion was not observed in mass spectra for either the recombinant normal αIIbβ3 or the αIIbβ3 purified from platelets.
To assess the extent of disulfide bond formation by Cys597 and Cys645 in clamped αIIb, the purified clamped αIIbβ3 was treated with iodoacetic acid to label free cysteines before and after reduction with DTT (45 mm). Although a Asp589–Leu600 peptide containing a free sulfhydryl at Cys597 could be identified in the nonreduced purified αIIb, the intensity of this peptide increased by at least 16-fold with reduction. A Asp636–Arg661 peptide containing a free sulfhydryl at Cys645 could not be detected in the nonreduced sample but was readily detectable after reduction. To further assess the extent of disulfide bond formation, a single sample of purified clamped αIIbβ3 was split in half and separately labeled with either iodoacetic acid or the combination of DTT and [13C]iodoacetic acid. The samples were then combined and analyzed by liquid chromatography-tandem mass spectrometry. The ratio of isotope labeling of the Asp589–Leu600 peptide (1:20) indicated that fewer than 5% of the Cys597 residues contained a free thiol, and the ratio of isotope labeling of the Asp636–Arg661 peptide (1:100) indicated that fewer than 1% of Cys645 residues contained a free thiol. To assess whether either the Cys597 or Cys645 created disulfide bonds with the nearest cysteine residues (Cys602 and Cys608), which are located adjacent to the genu, we analyzed the ratio of the disulfide-bonded peptide ions produced from this region by enzyme digestion and normalized the value by comparison with another internal standard peptide. The normalized intensities of the peptide ions were virtually identical in clamped αIIb, native recombinant αIIb, and platelet αIIb, indicating that neither Cys597 nor Cys645 cross-links in substantial amounts to either of these residues. We thus conclude that the Cys597–Cys645 bond is present in clamped αIIb and that ∼95% or more of the Cys597 and Cys645 residues are engaged in disulfide bonds.
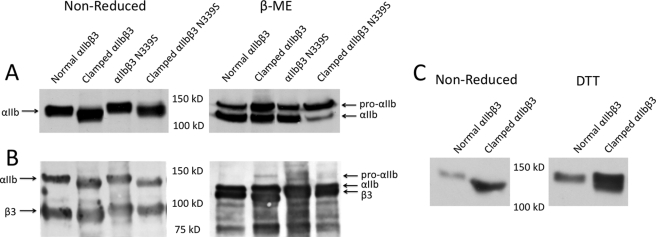
Further support for nearly complete disulfide bond formation by Cys597 and Cys645 in clamped αIIb came from SDS-PAGE studies (Fig. 2). In studies involving either both internal and surface receptors (Fig. 2A) or just surface receptors (Fig. 2B), normal αIIb migrated at a molecular mass of 140 kDa under nonreducing conditions, whereas the clamped αIIb molecules (in complex with either normal β3 or β3 N339S) migrated more rapidly (molecular mass, 130 kDa). After reducing the disulfide bonds, both the normal and mutant αIIb heavy chains migrated identically (molecular mass, 120 kDa). These data are consistent with the newly introduced disulfide bond producing a more compact structure, leading to more rapid migration of the clamped nonreduced αIIb. In the study involving both internal and external receptors (Fig. 2A), the ratio of uncleaved pro-αIIb (upper bands in right panel) to mature, cleaved αIIb (lower bands in right panel) was slightly greater for the clamped mutant, suggesting somewhat less efficient processing of the clamped receptor. Similar results were obtained when the clamped αIIb was expressed with β3 N339S, although the percentage of uncleaved αIIb was greater. In contrast, nearly all of the clamped αIIb subunits on the surface of cells expressing either clamped αIIb with β3 or clamped αIIb with β3 N339S were cleaved (Fig. 2B, right panel).
FIGURE 2.
Clamped αIIb migrates more rapidly under nonreducing conditions in SDS-PAGE than normal αIIb; reducing disulfide bonds with β-mercaptoethanol eliminates the difference in migration. A, total cell analysis. Lysates of cells expressing normal and clamped αIIb were heated to 100 °C in the presence or absence of 10% β-mercaptoethanol (β-ME) before loading on a 7% polyacrylamide gel. After protein transfer to polyvinylidene fluoride membranes, αIIb was detected by the anti-αIIb mAb PMI-1. B, surface receptor analysis. The cells were surface-labeled with biotin and then lysed and immunoprecipitated with a mAb to αIIbβ3. The proteins were separated by SDS-PAGE, and then biotin-labeled proteins were identified with avidin-horseradish peroxidase. C, nonreduced SDS-PAGE analysis of normal and clamped αIIb before and after treating cells with 5 mm DTT. Note that the increased migration of clamped αIIb is largely, but not completely, reversed by 5 mm DTT.
Clamped αIIbβ3 Has Reduced Ability to Bind Soluble Fibrinogen (Mr = 340,000) and the Soluble Ligand-mimetic mAb PAC-1 (Mr = 900,000) when Activated with mAb PT25-2, and DTT Reduction Partially Rescues the Defect
The αIIb-specific activating mAb PT25-2 bound equally well to normal and clamped αIIbβ3 (supplemental Fig. S1). Cells expressing normal αIIbβ3 bound only very small amounts of fibrinogen spontaneously but did bind fibrinogen in the presence of the PT25-2 (Figs. 3A and 4A). In sharp contrast, when incubated with PT25-2, cells expressing clamped αIIb in complex with normal β3 bound less than 9% of the amount of fibrinogen bound to the cells expressing normal αIIbβ3 (NNFI = 11 ± 5 for normal αIIbβ3 and 1 ± 1 for clamped αIIbβ3; n = 4, p < 0.001). Treating cells expressing normal αIIbβ3 with 5 mm DTT, even in the absence of PT25-2, partially enhanced fibrinogen binding. Similarly, treating cells expressing clamped αIIbβ3 with DTT led to an increase in fibrinogen binding in the absence of PT25-2. When PT25-2 was added to cells expressing normal αIIbβ3 after treatment with DTT, fibrinogen binding increased above the value in the absence of PT25-2, reaching a level similar to that with normal αIIbβ3 in the presence of PT25-2. Treating cells expressing clamped αIIbβ3 with the combination of DTT and PT25-2 further increased fibrinogen binding, but not to the level of normal αIIbβ3. SDS-PAGE analysis provided evidence that the DTT treatment substantially reduced the engineered disulfide bond with the majority of the clamped αIIb showing normalization of the migration pattern (Fig. 2C).
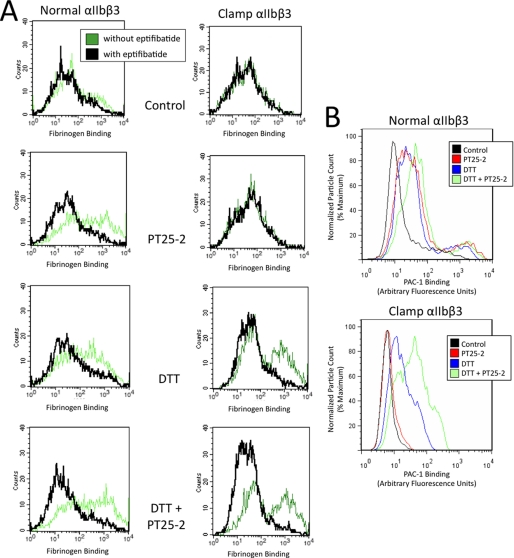
FIGURE 3.
Representative histogram plots of fibrinogen and PAC-1 binding to cells expressing either normal or clamped αIIbβ3. Normal αIIbβ3-expressing cells or cells expressing clamped αIIbβ3 were left untreated (control) or were treated with PT25-2, DTT, or DTT and PT25-2. Alexa488-fibrinogen (A) or FITC-PAC-1 (B) was then added, and binding was measured in the absence or presence of the αIIbβ3 antagonist eptifibatide. A, note that specific fibrinogen binding to cells expressing normal αIIbβ3 increased in the presence of PT25-2, but fibrinogen binding to clamped αIIbβ3 did not. DTT partially reversed the defect in fibrinogen binding to clamped αIIbβ3. αIIbβ3 and clamped αIIbβ3 surface expression levels were similar as judged by the binding of mAb 10E5 (GMFI = 107 for normal and 76 for clamped αIIbβ3). B, similar data to those in A, but with PAC-1 as the ligand. For simplicity, the values in the presence of eptifibatide are not displayed in B.
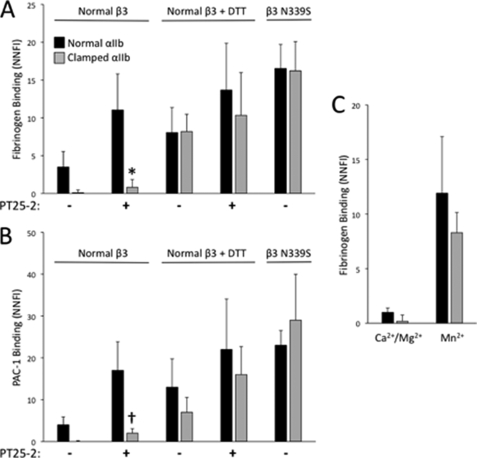
FIGURE 4.
Large ligands bind less well to clamped αIIbβ3 than normal αIIbβ3; Mn2+ and the β3 N339S mutation result in spontaneous ligand binding. A and B, cells expressing either clamped or normal αIIbβ3 were untreated or treated with DTT (5 mm) and incubated with either Alexa488-fibrinogen (200 μg/ml) or FITC-PAC-1 (5 μg/ml) in the presence or absence of activating antibody PT25-2. Nonspecific binding was determined in the presence of the αIIbβ3 inhibitor eptifibatide. Binding was assessed via flow cytometry and expressed as NNFI, in which the geometric mean fluorescence intensity after subtracting nonspecific binding is divided by the relative surface receptor expression as judged by the binding of mAb 10E5. *, p < 0.001 versus normal β3, n = 3; †, p < 0.001 versus normal β3, n = 4. C, cells expressing either clamped or normal αIIbβ3 were incubated with fluorescent fibrinogen in the presence of either Ca2+/Mg2+ or Mn2+. Nonspecific binding was determined in the presence of the αIIbβ3 inhibitor mAb 7E3. Binding was assessed via flow cytometry and expressed as NNFI (calculated as described above) (n = 4).
Data with the IgM ligand-mimetic mAb PAC-1 paralleled those with soluble fibrinogen (Figs. 3B and 4B). Thus, in the presence of mAb PT25-2, cells expressing normal αIIbβ3 had an NNFI of 17 ± 7, whereas cells expressing clamped αIIbβ3 had an NNFI of only 2 ± 1 (n = 3; p < 0.001). Binding of PAC-1 to clamped αIIbβ3 could be partially rescued by DTT treatment. Thus, after DTT treatment, PAC-1 binding to clamped αIIbβ3 receptors was ∼54% of the binding to normal αIIbβ3 in the presence of DTT. The further addition of PT25-2 increased PAC-1 binding to both normal and clamped αIIbβ3, with the latter reaching a level similar to that of normal αIIbβ3 in the presence of PT25-2 alone.
Fibrinogen Binding to Clamped αIIbβ3 Is Not Impaired when Clamped αIIb Is Expressed with a β3 Integrin Subunit Containing the Activating Mutation N339S or when Clamped αIIbβ3 Is Activated by Mn2+
Fibrinogen and PAC-1 bound spontaneously to cells expressing normal αIIb in complex with β3 N339S (Fig. 4, A and B). The specificity of the binding for αIIbβ3 was established by inhibition by eptifibatide and further confirmed in the case of fibrinogen by the lack of effect of mAb LM609 (anti-αVβ3) (data not shown). Expressing β3 N339S with clamped αIIb produced similar levels of spontaneous binding of fibrinogen and PAC-1. The addition of PT25-2 had little impact on ligand binding to cells expressing either normal αIIbβ3-N339S or clamped αIIbβ3-N339S (Fig. 4B and data not shown), suggesting that these receptors were already maximally activated. Normal and clamped αIIbβ3 bound fibrinogen to a similar extent in the presence of Mn2+, and the specificity of this binding was assessed by its inhibition by eptifibatide (Fig. 4C).
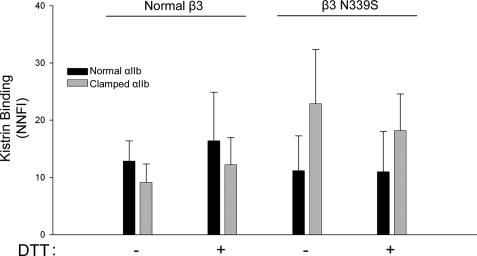
The RGD-containing Snake Venom Disintegrin Kistrin (Mr = 7,500) Binds Similarly to Normal and Clamped αIIbβ3
Fluorescently labeled kistrin bound to cells expressing clamped αIIb in complex with normal β3 somewhat less well than cells expressing normal αIIbβ3, but the difference was not statistically significant (Fig. 5). DTT treatment did not significantly affect kistrin binding. Expressing normal αIIb with β3 N339S did not affect kistrin binding, whereas there was an increase in kistrin binding when clamped αIIb was expressed with β3 N339S.
FIGURE 5.
Kistrin binds equally well to normal and clamped αIIbβ3 containing either normal β3 or β3 N339S. The cells were untreated or treated with DTT (5 mm) and incubated with Alexa488-kistrin (1 nm). Binding was assessed via flow cytometry and expressed as net normalized fluorescence intensity (n = 3).
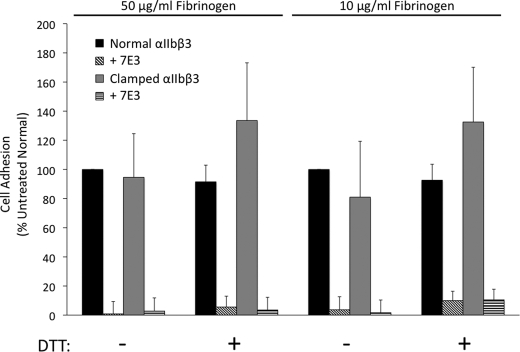
Clamped and Normal αIIbβ3 Adhere to Immobilized Fibrinogen
The number of cells expressing clamped αIIbβ3 receptors that adhered to fibrinogen immobilized at high density (coating concentration, 50 μg/ml) was 95 ± 30% of the value of cells expressing normal αIIbβ3 at similar levels of surface expression (mAb 10E5 GMFI 138 ± 53 and 148 ± 56 for normal and clamped cells, respectively; n = 5; Fig. 6). Adhesion of cells expressing clamped αIIbβ3 to fibrinogen immobilized at lower density (coating concentration, 10 μg/ml) was somewhat less than adhesion of cells expressing normal αIIbβ3, but the difference was not statistically significant (clamped cells 81 ± 38% relative to normal; p = 0.3). Treatment with DTT did not affect adhesion of normal cells to high or low density fibrinogen (92 ± 11 and 93 ± 11% relative to untreated normal cells, respectively). Treating cells expressing the clamped receptor with DTT did increase adhesion to both high and low density fibrinogen in five of five trials, by 41 and 64%, respectively, although there was variability in the degree of increase observed in each experiment (p = 0.06 for high density and p = 0.08 for low density). Adding mAb 7E3 blocked adhesion mediated by both normal and clamped αIIbβ3.
FIGURE 6.
Cells expressing normal and clamped αIIbβ3 bind to immobilized fibrinogen; treatment with DTT increases adhesion of cells expressing clamped αIIbβ3. When added to wells coated with fibrinogen at either high density (50 μg/ml) or low density (10 μg/ml), cells expressing either normal or clamped αIIbβ3 adhered to the surface. Treatment with DTT (5 mm) did not affect adhesion of cells expressing normal αIIbβ3 but did increase adhesion of clamped αIIbβ3-expressing cells (n = 5).
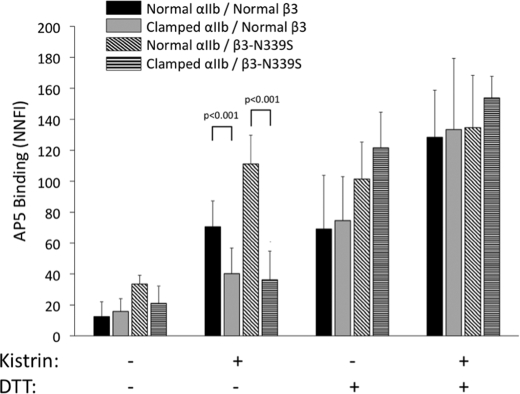
AP5 Binds Less Well to Clamped αIIbβ3 than Normal αIIbβ3 in the Presence of Kistrin, and Treatment with DTT Rescues the Clamped Mutant
AP5 binding to cells expressing normal αIIbβ3 and clamped αIIbβ3 was similarly low, reflecting ∼10% of maximal binding. Incubating cells expressing normal αIIbβ3 with kistrin increased AP5 binding ∼6-fold (Fig. 7) but produced only a 2–3-fold increase in binding to cells expressing the clamped αIIbβ3 (p < 0.001; n = 10). Treatment with DTT in the absence of kistrin increased the binding of AP5 to both normal and clamped αIIbβ3, with both reaching similar levels. Adding kistrin to DTT-treated cells of both types further increased AP5 binding. AP5 binding to αIIbβ3 N339S was higher than AP5 binding to normal αIIbβ3 (p < 0.001; n = 5) and clamped αIIbβ3 (p < 0.001; n = 5); it showed a trend to higher binding when compared with clamped αIIbβ3 N339S (p = 0.06; n = 5). Adding kistrin increased the binding of AP5 to αIIbβ3 N339S, reaching levels that were higher than those for normal αIIbβ3 (p < 0.001), clamped αIIbβ3 (p < 0.001), or clamped αIIbβ3 N339S (p < 0.001) in the presence of kistrin. After DTT treatment, the binding of AP5 to αIIbβ3 N339S was similar to its binding in the presence of kistrin, whereas DTT treatment of clamped αIIbβ3 significantly increased AP5 binding relative to its binding to the receptor in the presence of kistrin (p = 0.004). Adding kistrin in addition to DTT further increased binding to all of the receptors but to only a modest extent.
FIGURE 7.
Kistrin induces binding of AP5 to clamped αIIbβ3 to a lesser extent than to normal αIIbβ3; the defect is rescued by incubation with DTT. The fluorescently labeled ligand-induced binding site mAb AP5 was incubated with either untreated cells or cells pretreated with kistrin (200 nm) and/or DTT (5 mm) for 1 h at 37 °C. Antibody binding was assessed via flow cytometry and expressed as NNFI as indicated in the legend to Fig. 4, but using excess unlabeled AP5 to assess nonspecific binding. The values were further normalized for the ∼40% difference in the intensity of fluorescent labeling (F/P ratio) of the two AP5 preparations used in this study. The data for normal αIIbβ3 and clamped αIIbβ3 are from 10 experiments, and the data for αIIbβ3 N339S and clamped αIIbβ3 are from five experiments.
DISCUSSION
Our studies were designed to elucidate the role of αIIb extension about the genu in αIIbβ3 ligand binding. Previous studies clasping the αIIb β-propeller domain to the β3 IEGF-4 domain suggested a role for extension of αIIb, β3, or both in the binding of fibrinogen to αIIbβ3 when cells were activated by a combination of Mn2+ and an activating mAb (16). We have extended these studies by analyzing the effect of an αIIb clamp confined to the region immediately adjacent to the genu and assessing the binding of both high and low Mr soluble ligands, as well as the ability of the mutant receptor to support adhesion to immobilized fibrinogen and bind a ligand-induced binding site antibody whose epitope is on the β3 PSI domain.
The αIIb R597C-Y645C clamp was designed based on the predicted proximity of these residues in the αIIb molecular model we constructed using the crystal structure of the bent αVβ3 receptor ectodomains as a template. After these studies were completed, the close proximity of the residues was directly confirmed in the crystal structure of the ectodomain of αIIbβ3 (12). Support for the successful creation of the new disulfide bond came from analyzing both mass spectrometric data and the electrophorectic mobility of αIIb in SDS-PAGE under both nonreducing and reducing conditions.
We found that clamped αIIbβ3 has a dramatically reduced ability to bind the soluble ligands fibrinogen (Mr = 340,000) and IgM mAb PAC-1 (Mr = 900,000) induced by the activating mAb PT25-2, which binds to αIIb. The defect could be largely rescued by adding the reducing agent DTT. These studies have the inherit limitation that they depend on the ability of the mAb to bind equally to the normal and clamped receptor. We excluded the possibility that the reduction in ligand binding was due to the clamp altering the binding of PT25-2 by directly measuring PT25-2 binding to cells expressing normal or clamped αIIbβ3 and observing equivalent binding. DTT was able to partially activate the receptor itself and partially rescued the ability of the clamped receptor to increase ligand binding in response to PT25-2. SDS-PAGE analysis indicated that DTT treatment reduced the engineered disulfide bond in most, but not all, of the clamped αIIbβ3 molecules, providing an explanation for the partial rescue of ligand binding. We cannot, however, exclude the possibility that substituting cysteines for arginine at position 597 and/or tyrosine at position 645 affects ligand binding.
In sharp contrast to the findings with fibrinogen and PAC-1, the binding of the lower Mr snake venom kistrin, which contains an Arg-Gly-Asp (RGD) sequence and does not require exogenous receptor activation, was unaffected by the clamp, indicating receptor extension about the genu is not required for its binding. The smaller Mr of kistrin compared with fibrinogen and PAC-1 may account for the difference in its ability to bind to αIIbβ3 because it is established that short peptides containing the RGD sequence can bind to unactivated platelets (39).
Because activating αIIbβ3 with a mAb may not simulate activation by physiologic agonists (10), especially because it introduces a large molecule that may cause steric hindrance, we also activated the receptor by coexpression with the activating β3 subunit mutant N339S and by incubation with Mn2+. Cells expressing normal αIIb with the β3 mutant demonstrated high level spontaneous binding of both fibrinogen and PAC-1, and adding an activating mAb had little or no effect. Fibrinogen and PAC-1 both also showed high level binding to cells expressing the clamped αIIb in combination with the β3 activating mutant, and adding an activating mAb also did not affect the results. Additional structural studies are required to assess the mechanisms by which N339S activates the receptor, but it is strategically located in the region of β3 encompassing the β6-α7 loop and α7 helix; the latter have been implicated in controlling ligand affinity because they occupy different locations in the unliganded and liganded β3 crystal structures (11–14). Because size-dependent access appears to play a role in αIIbβ3 ligand binding (40), it is possible that the β3 N339S mutation alters the conformation of the receptor so as to allow greater access to the ligand-binding pocket; alternatively or additionally, it is possible that β3 N339S directly affects the affinity for the ligands either by reorienting Asp251 (12) or by facilitating the movement of the α1-β1 loop toward the MIDAS so as to bring two of the loop's backbone N atoms close enough to interact with the ligand carboxyl oxygen that does not coordinate the MIDAS (12). Our studies of AP5 binding to the normal and mutant αIIbβ3 receptors provided some data that bear on these issues. Thus, in the absence of ligand, AP5 bound equally well to normal αIIbβ3 and clamped αIIbβ3; αIIbβ3 N339S, however, bound more AP5 than the other receptors, suggesting that the β3 N339S mutation induces conformational changes in the β3 βA (I-like) domain that are propagated to the PSI domain.
Mn2+ activation was also able to overcome the defect in binding soluble fibrinogen induced by clamping αIIb. The mechanism by which Mn2+ activates integrin receptors is unknown, but studies swapping different regions of αIIb and αV implicated the calf-2 region as controlling the process (41). Ye et al. (10) reported that purified αIIbβ3 reconstituted into liposomes did not undergo a major change in height after activation by Mn2+ despite increased adhesion of the liposomes to immobilized fibrinogen. They interpreted their data as inconsistent with the need for integrin extension for ligand binding, but their study had a number of limitations, including lack of quantitation of the percentage of receptors that mediated ligand binding, the identification of a small subpopulation of receptors that did assume a more upright conformation in the presence of Mn2+, and the absence of cytoskeletal and contractile elements in their system that may play an important role in generating the conformational changes in αIIbβ3 (12). Regardless of the precise mechanisms, the data from the β3 N339S mutant and Mn2+ activation indicate that it is possible under certain circumstances for high Mr ligands to bind to αIIbβ3 receptors that have limited mobility about the αIIb genu.
If, as suggested by our data, ligand size is an important determinant of its ability to bind to integrin receptors that have not undergone extension about the α-subunit genu, this may provide an explanation for the finding by Adair et al. (22) that an ∼50-kDa fragment of fibronectin, which is between kistrin and fibrinogen in Mr, can bind to αVβ3 without evidence of headpiece extension. However, because Mn2+ was used to induce the binding of the fibronectin fragment, and we found that Mn2+ could rescue the ability of the clamped αIIbβ3 receptor to bind soluble fibrinogen, it is not possible to separate the potential contributions of ligand Mr and Mn2+ activation.
In contrast to our finding that Mn2+ activation could rescue soluble ligand binding to clamped αIIbβ3, Takagi et al. (16) found reduced soluble fibrinogen binding to cross-clasped αIIbβ3 even in the presence of both Mn2+ and PT25-2. Thus, the alteration induced by cross-clasping differs from that induced by our clamping procedure. Collectively our data and those of Takagi et al. could be explained by postulating that PT25-2 (and perhaps AP5) induces activation via an initial effect on αIIb extension, with subsequent effects on the β3 subunit. The effect of the β3 N339S mutant and Mn2+ may thus be downstream of the effect of PT25-2, but the Mn2+ effect may require mobility between regions of αIIb and β3 that are restricted by the cross-clasp introduced by Takagi et al.
Fibrinogen is unusual among αIIbβ3 ligands in that the binding of soluble fibrinogen requires receptor activation, whereas adhesion to immobilized fibrinogen does not (42–46). One possible explanation for this behavior is that activation-induced αIIbβ3 extension is required for the binding of soluble fibrinogen, but not for mediating adhesion to immobilized fibrinogen. To test this hypothesis, we also studied the effect of clamping αIIb about the genu on αIIbβ3-mediated adhesion to fibrinogen. Clamping αIIbβ3 produced little or no reduction in adhesion to fibrinogen immobilized at high density and only a slight reduction in adhesion to fibrinogen immobilized at low density. Thus, αIIb extension about the genu had much less of an effect on αIIbβ3 interactions with immobilized fibrinogen than on binding of soluble fibrinogen. A number of mechanisms have been proposed to account for the difference in requirements for αIIbβ3 binding of soluble fibrinogen versus mediating adhesion to immobilized fibrinogen, including conformational changes in fibrinogen induced by immobilization (47) and the potential for multivalent interactions between receptors and ligand molecules stemming from the higher density of fibrinogen achieved with immobilization (43). We noted a greater impact of clamping αIIb at the lower fibrinogen coating density, suggesting a correlation with ligand density, but these data need to be interpreted in light of the known impact of fibrinogen coating density on the conformational changes induced by immobilizing the ligand (43, 47). The recent study by Zhu et al. (12) provides an additional hypothesis, namely adhesion-mediated engagement of the cytoplasmic region of β3 with the cytoskeleton, leading to activation of the receptor via contractile traction forces initiating receptor extension and β3 swing-out. Thus, even an initial low affinity interaction with immobilized fibrinogen could induce higher affinity by this traction activation mechanism. If this is a contributing factor, our data indicate that higher affinity can be achieved even without extension at the genu. Most recently, Ye et al. found that binding of the talin head domain to the cytoplasmic domain of β3 was sufficient to induce both αIIbβ3 extension and PAC-1 binding (10), but this does not exclude an additional contribution to enhanced αIIbβ3 affinity via a traction mechanism (12).
Binding of ligands to αIIbβ3 is known to induce conformational changes in the receptor (4–7), resulting in outside-in signaling that leads to profound cellular changes, most dramatically cytoskeletal reorganization and cell spreading (2). Although receptor extension has been postulated to affect ligand binding, little is known about its potential contribution to post-ligand binding events. Therefore, we assessed the effect of clamping αIIb on the ability of the snake venom kistrin (which we demonstrated binds similarly to both normal and clamped αIIbβ3) to expose the AP5 epitope, which is located in the PSI domain of β3 (amino acids 1–6) and becomes more available with ligand binding (5). As expected, AP5 binding to normal αIIbβ3 was low in the absence of kistrin and increased ∼6-fold in the presence of kistrin. AP5 binding to clamped αIIbβ3 was similarly low in the absence of kistrin, but the addition of kistrin produced less enhancement than was observed with normal αIIbβ3. This was not due to an irreversible alteration in the clamped molecule or reduced expression of surface receptors because treatment with DTT completely rescued the response. Clamping the αIIbβ3 N339S mutant also decreased kistrin-induced AP5 binding. In the crystal structure of the complete ectodomain of αIIbβ3, the AP5 epitope is masked by IEGF-2 in the bent conformation, but it is predicted to be accessible with headpiece extension (12). Thus, our data provide evidence that AP5 epitope exposure secondary to ligand binding requires extension at the genu and raises the possibility that the latter also contributes to outside-in signaling. Moreover, because Zhu et al. reported that the hybrid and PSI domains bury a total 822 Å2 of solvent-accessible surface area in the bent receptor compared with a model of the extended receptor, it is also possible that headpiece extension initiated by inside-out signaling contributes to enhanced ligand affinity by reducing the energy barrier for the swing-out motion of the hybrid and PSI domains.
It is of interest to compare our data on the clamped receptor with observations on the platelets of a patient with Glanzmann thrombasthenia who had intact αIIbβ3 ectodomains but whose receptors were insensitive to inside-out signaling as a result of a mutation in the β3 cytoplasmic domain (26, 36). The patient's receptors were able to support platelet adhesion to immobilized fibrinogen but not soluble fibrinogen binding in response to agonist stimulation. The similarities between the patient data and the data using clamped αIIbβ3 are consistent with a model in which inside-out signaling acts, at least in part, by inducing extension at the αIIb genu.
In conclusion, our data support a role for αIIb extension about the genu in the binding of high Mr, but not lower Mr ligands under some, but not all conditions of activation. Additional data are required to define which pathways of activation are initiated by physiologic and pathologic stimuli. Although there is support for αIIb extension controlling binding of high Mr ligands via an effect on access to the ligand-binding site (40), it remains possible that αIIb extension affects the activation responsiveness and/or affinity of the ligand-binding pocket in addition to, or instead of, affecting size-dependent access. One possibility is that the loss of the headpiece-tailpiece interactions that stabilize the β3 hybrid and PSI domains in the bent structure frees the β3 subunit to undergo the swing-out motion and the movement of the α1-β1 loop toward the MIDAS. These data also provide a potential explanation for the difference in activation dependence of soluble fibrinogen binding versus adhesion to immobilized fibrinogen. Finally, our data raise the novel possibility that αIIb extension also contributes to post-ligand binding outside-in signaling by facilitating conformational change in β3.
Supplementary Material
Acknowledgments
We thank Joseph Fernandez and Haiteng Deng of the Rockefeller University Proteomics Core Facility for performing the mass spectrometry studies.
This work was supported, in whole or in part, by National Institutes of Health Grant 19278. This work was also supported by Clinical and Translational Science Award UL1-RR024143 from the National Center for Research Resources at the National Institutes of Health and by funds from Stony Brook University.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- PSI
- plexin-semaphorin-integrin
- mAb
- monoclonal antibody
- IEGF
- integrin epidermal growth factor
- DTT
- dithiothreitol
- FITC
- fluorescein isothiocyanate
- HBMT
- HEPES-modified Tyrode's buffer
- NNFI
- net normalized fluorescence intensity
- GMFI
- geometric mean fluorescence intensity.
REFERENCES
- 1.Phillips D. R., Charo I. F., Scarborough R. M. (1991) Cell 65, 359–362 [DOI] [PubMed] [Google Scholar]
- 2.Shattil S. J., Newman P. J. (2004) Blood 104, 1606–1615 [DOI] [PubMed] [Google Scholar]
- 3.Parise L. V., Helgerson S. L., Steiner B., Nannizzi L., Phillips D. R. (1987) J. Biol. Chem. 262, 12597–12602 [PubMed] [Google Scholar]
- 4.Kouns W. C., Wall C. D., White M. M., Fox C. F., Jennings L. K. (1990) J. Biol. Chem. 265, 20594–20601 [PubMed] [Google Scholar]
- 5.Honda S., Tomiyama Y., Pelletier A. J., Annis D., Honda Y., Orchekowski R., Ruggeri Z., Kunicki T. J. (1995) J. Biol. Chem. 270, 11947–11954 [DOI] [PubMed] [Google Scholar]
- 6.Frelinger A. L., 3rd, Cohen I., Plow E. F., Smith M. A., Roberts J., Lam S. C., Ginsberg M. H. (1990) J. Biol. Chem. 265, 6346–6352 [PubMed] [Google Scholar]
- 7.Shattil S. J., Hoxie J. A., Cunningham M., Brass L. F. (1985) J. Biol. Chem. 260, 11107–11114 [PubMed] [Google Scholar]
- 8.Du X., Gu M., Weisel J. W., Nagaswami C., Bennett J. S., Bowditch R., Ginsberg M. H. (1993) J. Biol. Chem. 268, 23087–23092 [PubMed] [Google Scholar]
- 9.Litvinov R. I., Nagaswami C., Vilaire G., Shuman H., Bennett J. S., Weisel J. W. (2004) Blood 104, 3979–3985 [DOI] [PubMed] [Google Scholar]
- 10.Ye F., Hu G., Taylor D., Ratnikov B., Bobkov A. A., McLean M. A., Sligar S. G., Taylor K. A., Ginsberg M. H. (2010) J. Cell. Biol. 188, 157–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao T., Takagi J., Coller B. S., Wang J. H., Springer T. A. (2004) Nature 432, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J., Luo B. H., Xiao T., Zhang C., Nishida N., Springer T. A. (2008) Mol. Cell 32, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong J. P., Stehle T., Diefenbach B., Zhang R., Dunker R., Scott D. L., Joachimiak A., Goodman S. L., Arnaout M. A. (2001) Science 294, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong J. P., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman S. L., Arnaout M. A. (2002) Science 296, 151–155 [DOI] [PubMed] [Google Scholar]
- 15.Beglova N., Blacklow S. C., Takagi J., Springer T. A. (2002) Nat. Struct. Biol. 9, 282–287 [DOI] [PubMed] [Google Scholar]
- 16.Takagi J., Petre B. M., Walz T., Springer T. A. (2002) Cell 110, 599–611 [DOI] [PubMed] [Google Scholar]
- 17.Luo B. H., Takagi J., Springer T. A. (2004) J. Biol. Chem. 279, 10215–10221 [DOI] [PubMed] [Google Scholar]
- 18.Salas A., Shimaoka M., Phan U., Kim M., Springer T. A. (2006) J. Biol. Chem. 281, 10876–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J., Boylan B., Luo B. H., Newman P. J., Springer T. A. (2007) J. Biol. Chem. 282, 11914–11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocco M., Rosano C., Weisel J. W., Horita D. A., Hantgan R. R. (2008) Structure. 16, 954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnaout M. A., Goodman S. L., Xiong J. P. (2007) Curr. Opin. Cell Biol. 19, 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adair B. D., Xiong J. P., Maddock C., Goodman S. L., Arnaout M. A., Yeager M. (2005) J. Cell Biol. 168, 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng M., Foo S. Y., Shi M. L., Tang R. H., Kong L. S., Law S. K., Tan S. M. (2007) J. Biol. Chem. 282, 18225–18232 [DOI] [PubMed] [Google Scholar]
- 24.Coller B. S. (1985) J. Clin. Invest. 76, 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. (1983) J. Clin. Invest. 72, 325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y. P., O'Toole T. E., Ylänne J., Rosa J. P., Ginsberg M. H. (1994) Blood 84, 1857–1865 [PubMed] [Google Scholar]
- 27.Tokuhira M., Handa M., Kamata T., Oda A., Katayama M., Tomiyama Y., Murata M., Kawai Y., Watanabe K., Ikeda Y. (1996) Thromb. Haemost. 76, 1038–1046 [PubMed] [Google Scholar]
- 28.Cheresh D. A. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 6471–6475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shadle P. J., Ginsberg M. H., Plow E. F., Barondes S. H. (1984) J. Cell Biol. 99, 2056–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shattil S. J., Cunningham M., Hoxie J. A. (1987) Blood 70, 307–315 [PubMed] [Google Scholar]
- 31.Dennis M. S., Henzel W. J., Pitti R. M., Lipari M. T., Napier M. A., Deisher T. A., Bunting S., Lazarus R. A. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 2471–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell W. B., Li J., Murcia M., Valentin N., Newman P. J., Coller B. S. (2007) Blood 109, 3725–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiser A., Sali A. (2003) Methods Enzymol. 374, 461–491 [DOI] [PubMed] [Google Scholar]
- 34.Xiong J. P., Stehle T., Goodman S. L., Arnaout M. A. (2004) J. Biol. Chem. 279, 40252–40254 [DOI] [PubMed] [Google Scholar]
- 35.Shi M., Sundramurthy K., Liu B., Tan S. M., Law S. K., Lescar J. (2005) J. Biol. Chem. 280, 30586–30593 [DOI] [PubMed] [Google Scholar]
- 36.Chen Y. P., Djaffar I., Pidard D., Steiner B., Cieutat A. M., Caen J. P., Rosa J. P. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 10169–10173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zucker M. B., Masiello N. C. (1984) Thromb. Haemost. 51, 119–124 [PubMed] [Google Scholar]
- 38.Law D. A., Nannizzi-Alaimo L., Ministri K., Hughes P. E., Forsyth J., Turner M., Shattil S. J., Ginsberg M. H., Tybulewicz V. L., Phillips D. R. (1999) Blood 93, 2645–2652 [PubMed] [Google Scholar]
- 39.Beer J. H., Springer K. T., Coller B. S. (1992) Blood 79, 117–128 [PubMed] [Google Scholar]
- 40.Coller B. S. (1986) J. Cell Biol. 103, 451–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamata T., Handa M., Sato Y., Ikeda Y., Aiso S. (2005) J. Biol. Chem. 280, 24775–24783 [DOI] [PubMed] [Google Scholar]
- 42.Coller B. S. (1980) Blood 55, 169–178 [PubMed] [Google Scholar]
- 43.Lindon J. N., McManama G., Kushner L., Merrill E. W., Salzman E. W. (1986) Blood 68, 355–362 [PubMed] [Google Scholar]
- 44.Savage B., Ruggeri Z. M. (1991) J. Biol. Chem. 266, 11227–11233 [PubMed] [Google Scholar]
- 45.Zucker M. B., Vroman L. (1969) Proc. Soc. Exp. Biol. Med. 131, 318–320 [DOI] [PubMed] [Google Scholar]
- 46.Stanford M. F., Munoz P. C., Vroman L. (1983) Ann. N.Y. Acad. Sci. 416, 504–512 [DOI] [PubMed] [Google Scholar]
- 47.Moskowitz K. A., Kudryk B., Coller B. S. (1998) Thromb. Haemost. 79, 824–831 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.