Abstract
Background
Meta-analyses of postpartum blood loss and the effect of uterotonics are biased by visually estimated blood loss.
Objectives
To conduct a systematic review of measured postpartum blood loss with and without prophylactic uterotonics for prevention of postpartum haemorrhage (PPH).
Search strategy
We searched Medline and PubMed terms (labour stage, third) AND (ergonovine, ergonovine tartrate, methylergonovine, oxytocin, oxytocics or misoprostol) AND (postpartum haemorrhage or haemorrhage) and Cochrane reviews without any language restriction.
Selection criteria
Refereed publications in the period 1988–2007 reporting mean postpartum blood loss, PPH (≥500 ml) or severe PPH (≥1000 ml) following vaginal births.
Data collection and analysis
Raw data were abstracted into Excel by one author and then reviewed by a co-author. Data were transferred to SPSS 17.0, and copied into RevMan 5.0 to perform random effects meta-analysis.
Main results
The distribution of average blood loss (29 studies) is similar with any prophylactic uterotonic, and is lower than without prophylaxis. Compared with no uterotonic, oxytocin and misoprostol have lower PPH (OR 0.43, 95% CI 0.23–0.81; OR 0.73, 95% CI 0.50–1.08, respectively) and severe PPH rates (OR 0.61, 95% CI 0.29–1.29; OR 0.74, 95% CI 0.52–1.04, respectively). Oxytocin has lower PPH (OR 0.65, 95% CI 0.60–0.70) and severe PPH (OR 0.71, 95% CI 0.56–0.91) rates than misoprostol, but not in developing countries.
Conclusion
Oxytocin is superior to misoprostol in hospitals. Misoprostol substantially lowers PPH and severe PPH. A sound assessment of the relative merits of the two drugs is needed in rural areas of developing countries, where most PPH deaths occur.
Keywords: Postpartum blood loss, postpartum haemorrhage, third stage of labour
Introduction
Haemorrhage is the single leading cause of maternal mortality.1 Postpartum haemorrhage (PPH) is most often attributed to uterine atony.2 Most births and maternal deaths occur in Africa and Asia, where home deliveries are common, infrastructure and transportation are limited, and where birth attendants are scarce or inadequately prepared to prevent and treat PPH.3 In such settings haemorrhage accounts for ≥30% of maternal deaths.1 The United Nation’s Millennium Development goal 5, to reduce 75% of maternal mortality by 2015, cannot be reached without the successful management of PPH.4,5
The conventional definition of PPH is a blood loss of ≥500 ml in the first 24 hours after delivery.6,7 By stimulating uterine muscle tone, prophylactic uterotonics reduce the incidence of PPH.2,8,9 Several factors influence PPH rates, including whether blood loss is measured, how the third stage of labour is managed (e.g. the provision of uterotonic, uterine massage and controlled cord traction), obstetric interventions carried out (e.g. episiotomy and mode of delivery), and study population (sample size, parity, urban/rural or facility/home delivery, and level of facility).10 Most clinicians (and studies) classify obstetric blood loss by visual estimation. Visually (clinically) assessed bleeding underestimates measured blood loss by an average of 100–150 ml, and substantially underestimates blood loss of ≥500 ml (by 30–50%).11–16
Underestimating blood loss ‘lowers’ PPH rates and the estimates of prevented PPH, as there is artificially less PPH to prevent. A recent systematic review found the prevalence of PPH was 10.55% in 19 studies that measured postpartum blood loss, compared with 7.23% in 22 studies where blood loss was estimated visually, suggesting a large underestimation of PPH.10 Thus, in meta-analyses such as the Cochrane reviews of the efficacy of prophylactic uterotonics to reduce postpartum blood loss and prevent its sequellae, the proportion of studies and subjects where blood loss was visually rather than objectively measured influences the PPH and severe PPH rates, and thus influences the estimates (relative risks or odds ratios) of the effectiveness of uterotonic agents in preventing or treating obstetric haemorrhage.2,8,17–19
Most women experiencing a loss of ≥500 ml of blood (PPH) do not receive clinical intervention or experience serious consequences.10,20,21 In fact, some suggest that the 500-ml definition of PPH should be considered an alert level, and that PPH may be better defined as the volume of blood loss requiring intervention to avert serious sequellae.22,23 Accordingly, a re-evaluation of PPH guidelines has been recommended.24–26 This article presents information about average blood loss, and the incidence of PPH (≥500 ml) and severe PPH (≥1000 ml) in studies where blood loss was measured, to clarify what we know about postpartum blood loss among women who received and did not receive uterotonic prophylaxis during the third stage of labour.
Methods
Searching
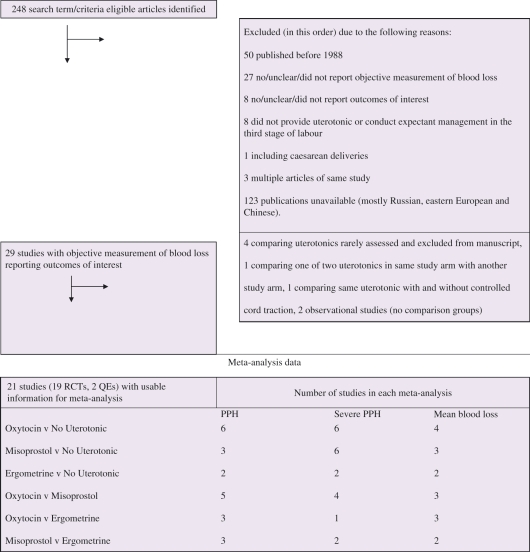
Nearly 250 observational and experimental studies published up to 31 December 2007 were identified by Medline and PubMed online search engines using the following search terms: (labour stage, third) AND (ergonovine, ergonovine tartrate, methylergonovine, oxytocin, oxytocics or misoprostol) AND (postpartum haemorrhage or haemorrhage), without language restriction (Figure 1). Articles were also identified and reviewed if cited by the Cochrane reviews on management of the third stage of labour.2,8,17,18
Figure 1.
Studies reviewed and included in the meta-analyses.
Inclusion and exclusion criteria
Studies were retained if there was objective measurement of blood loss after delivery, regardless of the duration of the blood measurement, augmentation or induction in the first or second stages of labour, or if other components of active management of the third stage of labour (AMTSL) were implemented (Figure 1). Articles published before 1 January 1988, with uncertain blood measurement, including one retrospective article,27 or articles published in journals that could not be accessed were excluded. Studies including caesarean deliveries were excluded to avoid biased comparisons should blood loss vary by delivery mode.10 However, studies with twin deliveries were included as twin and higher order births are relatively rare events. This review includes all eligible studies regardless of sample size. Twenty-three of the 59 study arms (39%) had sample arms of ≤200.
Assessment of methodological quality
Each study was classified as a randomised controlled trial (RCT), quasi-experiment (QE) or observational (Obs). Study group allocation concealment was classified as: adequate (i) if a method such as consecutively numbered sealed opaque envelopes was used; unclear (ii) if the concealment technique was not described; or inadequate (iii) if there was an open list of random numbers or no random assignment (e.g. QE) was used.
Data abstraction
All relevant raw data were abstracted from each eligible study by a single reviewer, and then reviewed by a co-author. Disagreements were resolved through verification against the publication and discussion. Data were then transferred to SPSS 17.0 and copied from the data extraction form into Review Manager (RevMan) 5.0 data analysis tables (The Cochrane Collaboration, 2008; The Nordic Cochrane Centre, Copenhagen, Denmark).
Study characteristics
Twenty-nine articles were eligible for the review.24–26,28–53Table 1 presents their characteristics (location, sample size, blood measurement technique and components of AMTSL implemented). Studies were conducted in low-, middle- and high-income settings in Africa (n = 6), the Middle East (n = 4), Asia (n = 4) and Europe (n = 5), with one multi-country study conducted in all of these regions and Latin America. All but five of the studies were conducted in tertiary hospitals. The remaining five studies included home births in rural Gambia,53 home or village subcentre births in India,24 home and district hospital births in Vietnam,32 rural primary health centre births in Guinea-Bissau,42 and rural health centre births in India.52 Most studies measured blood loss by placing a bedpan underneath the parturient woman immediately after delivery, usually after the cord was clamped and cut. The collected blood was generally poured into a jar for volume measurement, and all soaked gauze pads were counted and weighed. Relatively few (n = 6) studies used the fairly new blood collection sheet or delivery drape, sometimes tied around the woman’s waist, with a funnel portion hanging between her legs, including the BRASSS-V Drape™ (a calibrated plastic sheet, Excellent Fixable Drapes, Madurai, Tamil Nadu, India).54 Two studies used the bedpan and linens method for some women and the drape for others. Most studies measured blood loss until active bleeding stopped, regardless of a pre-specified duration for blood measurement.
Table 1.
Characteristics of studies reviewed (n = 29)
| Study | Allocation concealment | Blood measurement | Sample n | Prophylactic regimen | CCT UM Dele Col | Notes | In meta analysis |
|---|---|---|---|---|---|---|---|
| Angola: Strand, 200526 | QE, NotBlind, C | PanLinen, 2 hours | 782 | Expectant | Uncomp, AugL | X | |
| 814 | Oxytocin, 10 iu IM | CCT | |||||
| Egypt: Abdel-Aleem, 200648 | RCT, NotBlind, RanEnv, B | Drape, 1 hour | 102 | Oxytocin, 10 iu IM or IV | CCT | ||
| 98 | Oxytocin, 10 iu IM or IV | UM | |||||
| Egypt: Prata, 200634 | Obs, NotBlind, C | Drape, 4 hours | 1180 | Misoprostol, 600 μg, O | CCT, UM | ||
| France: Benchimol, 200147 | RCT, NotBlind, RanEnv, B | Drape, 1 hour | 220 | Expectant | AugL | X | |
| 196 | Oxytocin, 2.5 iu bolus IV | ||||||
| 186 | Misoprostol, 600 μg, O | ||||||
| Gambia, rural: Walraven, 200553 | RCT, Ran (block), Double, A | PanLinen, 1 hour | 630 | Misoprostol, 600 μg, O | CCT; UM for PPH tx | X | |
| 599 | Ergometrine, 4 mg, O | ||||||
| Guinea Bissau, rural: Høj, 200542 | RCT, Ran, Double, A | Both, 1 hour | 331 | Expectant, placebo | CCT | X | |
| 330 | Misoprostol, 600 μg, SL | CCT | |||||
| Hong Kong: Lam, 200439 | RCT (versus Other), Ran, NotBlind, C | PanLinen, 0th hour | 30 | Misoprostol, 600 μg, SL | CCT | LR | |
| Hong Kong: Yuen, 199530 | RCT, Ran (versus Other ), Double, A | PanLinen, 0th hour | 495 | Oxytocin, 10 iu IM | AugL | ||
| India, rural: Derman, 200624 | RCT, Ran (block), Double, A | Drape, 2 hours | 808 | Expectant, placebo | X | ||
| 812 | Misoprostol, 600 μg, O | ||||||
| India: Gupta, 200644 | RCT, Ran, Double, A | Drape, 1 hour | 100 | Oxytocin, 10 iu IM | CCT I, UM I | X | |
| 100 | Misoprostol, 600 μg, R | CCT I, UM I | |||||
| India: Verma, 200631 | RCT, Runsp, Double, B | Drape, 0th hour | 100 | Ergometrine, 200 μg IM | X | ||
| 100 | Misoprostol, 400 μg SL | ||||||
| India, rural: Vimala, 200452 | RCT, Ran, NotBlind, B | PanLinen, 0th hour | 60 | Ergometrine (nonHBP), 200 μg IV Oxytocin (HBP), 10 iu IV | CCT | LR | |
| 60 | Misoprostol, 400 μg SL | CCT | |||||
| India: Zachariah, 200629 | RCT, Ran, NotBlind, B | Both, 0th hour | 676 | Ergometrine, 2 mg IV | X | ||
| 617 | Oxytocin, 10 iu IM | ||||||
| 730 | Misoprostol, 400 μg, O | ||||||
| Ireland: Begley, 199049 | RCT, RanEnvBlock, NotBlind, C | PanLinen, 0th hour | 724 | Expectant | CCT | LR, AugL | X |
| 705 | Ergometrine, 0.5 mg IV | CCT | |||||
| Israel: Soriano, 199633 | Obs, NotBlind, C | PanLinen, 0th hour | 524 | Oxytocin, 10 iu IV | CCT | AugL | |
| Japan: Fujimoto, 200651 | QE, NotBlind, C | PanLinen, 2 hours | 82 | Oxytocin, 5 iu IV | CCT | LR | X |
| 95 | Oxytocin, 5 iu IV | CCT | |||||
| 70 | Ergometrine, 0.2 mg IV | CCT | |||||
| 79 | Ergometrine, 0.2 mg IV | CCT | |||||
| Mozambique: Bugalho, 200146 | RCT, RUnsp, Double, B | PanLinen, 0th hour | 329 | Oxytocin, 10 iu IM | X | ||
| 323 | Misoprostol, 400 μg, R | ||||||
| The Netherlands, multicentre: De Groot, 199650 | RCT, Ran Double (versus Ergometrine), NotBlind (versus Oxytocin), B | PanLinen, 1 hour | 143 | Expectant, placebo (O) | LR | X | |
| 146 | Ergometrine, 0.4 μg, O | ||||||
| 78 | Oxytocin, 0.5 μg, IM | ||||||
| The Netherlands: Poeschmann, 199135 | RCT, Runsp (block), Double, B | PanLinen, 1 hour | 24 | Expectant, placebo | Uncomp, LR | X | |
| 28 | Oxytocin, 5 iu IM | ||||||
| South Africa: Bamigboye, 199828 | RCT, Ran, Single, A | PanLinen, 1 hour | 272 | Expectant, placebo | LR | X | |
| 271 | Misoprostol, 400 μg R | ||||||
| South Africa: Hofmeyr, 199826 | RCT, Ran (block), Double, A | PanLinen, 1 hour | 250 | Expectant, placebo | CCT | X | |
| 250 | Misoprostol, 400 μg, O | CCT | |||||
| South Africa: Hofmeyr, 200143 | RCT, Ran (block), Double, A | PanLinen, 1 hour | 300 | Expectant, placebo | CCT | X | |
| 300 | Misoprostol, 600 μg, O | CCT | |||||
| Sweden: Nordstrom, 199725 | RCT, Ran, Double, A | PanLinen, 0th hour | 487 | Expectant, placebo | AugL | X | |
| 513 | Oxytocin, 10 iu IV | ||||||
| Turkey: Ozkaya, 200536 | RCT, Ran, Double (versus R) Single (versus V), A | PanLinen, 1 hour | 44 | Expectant, placebo, R | Xg | ||
| 45 | Misoprostol, 400 μg, V | ||||||
| 48 | Misoprostol, 400 μg, R | ||||||
| UAE: Khan, 199741 | RCT, Runsp, NotBlind, C | PanLinen, 0th hour | 821 | Oxytocin, DoseUnsp, IV | |||
| 827 | Oxytocin, 10 iu IM | CCT | |||||
| UK: Mitchell, 199338 | RCT (versus Other), Runsp, Double, B | PanLinen, 1 hour | 230 | Oxytocin, 5 iu IM | CCT | ||
| Vietnam, rural: Tsu, 200632 | QE, NotBlind, C | PanLinen, 0th hour | 2371 | Expectant | CCT, UM | AugL | X |
| 1236 | Oxytocin, 10 iu IM | CCT, UM | |||||
| Zimbabwe: Kundodyiwa, 200140 | RCT, Ran, Double, A | PanLinen, 0th hour | 256 | Oxytocin, 10 iu IM | X | ||
| 243 | Misoprostol, 400 μg, R | ||||||
| Multicentre Gulmezoglu, 200145 | RCT, Ran (centrally), Double, A | PanLinen, 1 hour | 9230 | Oxytocin, 10 iu IV or IM | CCT, UM | AugL | X |
| 9225 | Misoprostol, 600 μg, O | CCT, UM |
The format used for study identifiers was as follows: country, author, year.
Concealment allocation code: randomised controlled trial (RCT); quasi-experiment (QE); double blinded (Double); single blinded (Single); not blinded (NotBlind); blinding method unspecified (BlindUnsp); compared with regimen (such as syntometrine) not included in manuscript analyses (versus Other); randomisation generated by computer or table (Ran); randomisation by drawn envelope containing treatments (RanEnv); randomisation method unspecified (Runsp); A, adequate; B, unclear; C, inadequate.
Dose in micrograms (μg); dose in international units (iu); placebo (P); unidentical placebo (PlaceboUn); dose unspecified (DoseUnsp); route of administration (B, buccal; IM, intramuscular; IV, intravenous; Oral, oral; Rec, rectal; SubL, sublingual; V, vaginal; tx, treatment).
Third-stage management technique (TSL technique): CCT, controlled cord traction; UM, uterine massage; I, indeterminate/’gave AMTSL’; Expectant, expectant management.
Blood loss measurement technique: PanLinen, bedpan/linens; Drape; Both, both bedpan/linens and drape; Pads, NumberHrs, number of hours of measured blood loss; 0th hour, other hours measured.
Notes: study included augmented or induced labour (AugL), low-risk sample (LR) and uncomplicated deliveries (Uncomp).
Misoprostol, 400 μg, V group not included in analysis to limit to double blind comparison.
Analysis
The range of average postpartum blood loss, rates of PPH and severe PPH, and ratio of severe PPH to PPH is presented for all eligible studies. In controlled studies comparing different prophylactic regimens, the effects of the regimen used to manage the third stage of labour on PPH, severe PPH and average postpartum blood loss were analysed by random-effects meta-analysis to avoid assumptions about similarity of study design or interventions. This systematic review presents Mantel–Haenszel odds ratios (ORs) for dichotomous (PPH and severe PPH) outcome, mean differences in blood loss and 95% confidence intervals (CIs). Heterogeneity across trials is evaluated using the chi-square test as calculated in MetaView. Subgroup analyses are presented for methodologically adequate studies, and figures with subgroup summary statistics are presented to demonstrate effects in individual studies and their settings. Observational studies or studies that compare different mechanisms of providing a single uterotonic are not included in the meta-analysis. In one study, only the comparison of the double-blind route was included when multiple routes of administration were studied to avoid over-counting the comparison group. Data on methergine were excluded as methergine was rarely assessed. Analyses were not stratified by dose or route (intravenous, intramuscular injection, oral, vaginal or rectal) to avoid reducing the analyses to single studies.
Role of the funding source
The funding source had no role in the study design, data collection, analysis, interpretation or report composition.
Results
Distribution of mean blood loss
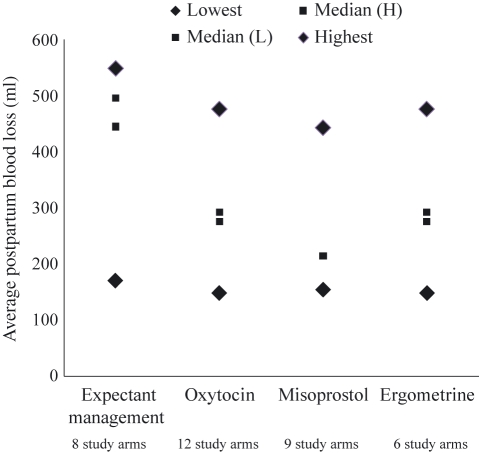
The average blood loss ranged from 149 to 548 ml (Table 2; 16 studies). The highest average blood loss (range 171–548 ml) was among women managed without uterotonic prophylaxis (eight studies). The range of average blood loss was similar in women receiving any prophylactic uterotonic: 151–499 ml for oxytocin (ten studies, 12 study arms), 155–443 ml for misoprostol (eight studies, nine study arms), and 149–476 ml in women receiving ergometrine (five studies, six study arms). The 95% CI of each study arm was equivalent to 4–28% of the average blood loss of the study arm. The median average blood loss in women managed without a uterotonic is about 150–200 ml higher than for those provided with uterotonics, whereas the median and range of those managed with uterotonics are fairly uniform (Figure 2).
Table 2.
Mean and 95% CI of measured postpartum blood loss by third-stage prophylactic regimen
| Regimen | Study (author, year) | Mean blood loss (ml) | 95% CI |
|---|---|---|---|
| No uterotonic | Angola: Strand, 200526 | 445 | 424–476 |
| Guinea Bissau, rural: Høj, 200542 | 496 | 475–517 | |
| India, rural: Derman, 200624 | 262 | 248–276 | |
| Ireland: Begley, 199049 | 235 | 218–251 | |
| The Netherlands, multicentre: De Groot, 199650 | 520 | 451–589 | |
| The Netherlands: Poeschmann, 199135 | 548 | 398–698 | |
| Sweden: Nordstrom, 199725 | 527 | 490–564 | |
| Turkey: Ozkaya, 200536 | 171 | 139–204 | |
| Oxytocin | Angola: Strand, 200526 | 224 | 211–238 |
| Egypt: Abdel-Aleem, 200648 | 282 | 248–315 | |
| Egypt: Abdel-Aleem, 200648 | 204 | 180–228 | |
| India: Gupta, 200644 | 151 | 137–165 | |
| India: Zachariah, 200629 | 183 | 173–193 | |
| Japan: Fujimoto, 200651 | 207 | 167–247 | |
| Japan: Fujimoto, 200651 | 288 | 244–332 | |
| Mozambique: Bugalho, 200146 | 157 | 142–172 | |
| The Netherlands, multicentre: De Groot, 199650 | 499 | 398–600 | |
| The Netherlands: Poeschmann, 199135 | 374 | 271–477 | |
| Sweden: Nordstrom, 199726 | 409 | 379–439 | |
| UK: Mitchell, 199341 | 252 | 229–275 | |
| Misoprostol | Gambia, rural: Walraven, 200553 | 281 | 267–295 |
| Guinea Bissau, rural: Høj, 200542 | 443 | 415–471 | |
| India, rural: Derman, 200624 | 214 | 204–224 | |
| India: Gupta, 200644 | 168 | 153–183 | |
| India, rural: Vimala, 200431 | 185 | 171–199 | |
| India: Zachariah, 200629 | 193 | 183–202 | |
| Mozambique: Bugalho, 200146 | 155 | 142–168 | |
| Turkey: Ozkaya, 200536 | 206 | 168–245 | |
| Turkey: Ozkaya, 200536 | 171 | 141–201 | |
| Ergometrine | Gambia, rural: Walraven, 200553 | 292 | 278–306 |
| India: Zachariah, 200629 | 188 | 178–198 | |
| Ireland: Begley, 199049 | 149 | 140–158 | |
| Japan: Fujimoto, 200651 | 338 | 289–387 | |
| Japan: Fujimoto, 200651 | 276 | 243–309 | |
| The Netherlands, multicentre: De Groot, 199650 | 476 | 421–531 |
Figure 2.
Median and range of average measured blood loss by regimen used to manage the third stage of labour.
Distribution of PPH and severe PPH
The average PPH rate in the nine studies where women were managed expectantly (without uterotonic prophlyxis) ranged from 4 to 51% (Table 3). Where uterotonics were given, PPH ranged from 0 to 32% (17 studies, 19 study arms) for oxytocin, from 1 to 45% (12 studies) for misoprostol, and from 0 to 37% (seven studies, eight study arms) for ergometrine. Severe PPH ranged from 0.5 to 17% (12 studies) in women who were managed without prophylactic uterotonics, from 0.4 to 9% (12 studies, 13 study arms) for women managed with oxytocin, from 0 to 8% (11 studies) for women managed with misoprostol, and from 0 to 8% (five studies) for women managed with ergometrine.
Table 3.
PPH (≥500 ml) and severe PPH (≥1000 ml) by third-stage prophylactic regimen
| Prophylactic regimen | Study | % PPH | % Severe PPH | Ratio % Severe PPH to PPH |
|---|---|---|---|---|
| No uterotonic | Angola: Strand, 200526 | 40.41 | 7.42 | 18.36 |
| France: Benchimol, 200147 | 27.27 | 5.91 | 21.67 | |
| Guinea Bissau, rural: Høj, 200542 | 51.36 | 16.92 | 32.94 | |
| India, rural: Derman, 200624 | 12 | 1.24 | 10.33 | |
| Ireland: Begley, 199049 | 8.29 | 1.52 | 18.34 | |
| The Netherlands, multicenter: de Groot, 199650 | 38.46 | 11.19 | 29.10 | |
| The Netherlands: Poeschmann, 199135 | 41.67 | 12.5 | 30.00 | |
| South Africa: Bamigboye, 199828 | NR | 6.99 | ||
| South Africa: Hofmeyr, 199823 | NR | 9.2 | ||
| South Africa: Hofmeyr, 200143 | NR | 9.7 | ||
| Sweden: Nordstrom, 199725 | 35.93 | 8.83 | 24.58 | |
| Vietnam: Tsu, 200632 | 3.84 | 0.51 | 13.28 | |
| Oxytocin | Angola: Strand, 200526 | 8.23 | 0.98 | 11.91 |
| Egypt: Abdel-Aleem, 200648 | 4.08 | NR | ||
| Egypt: Abdel-Aleem, 200648 | 7.84 | NR | 41.38 | |
| France: Benchimol, 200147 | 14.79 | 6.12 | 41.38 | |
| Hong Kong: Yuen, 199530 | 12.12 | 2.02 | 16.67 | |
| India: Gupta, 200644 | 0 | NR | ||
| India: Zachariah, 200629 | 2.11 | 0.65 | 30.81 | |
| Israel: Soriano, 199633 | 9.73 | NR | ||
| Japan: Fujimoto, 200651 | 11.58 | NR | ||
| Japan: Fujimoto, 200651 | 7.32 | NR | ||
| Multicentre Gulmezoglu, 200140 | 13.53 | 2.85 | 21.06 | |
| The Netherlands, multicentre: de Groot, 199650 | 32.05 | 8.97 | 27.99 | |
| The Netherlands: Poeschmann, 199135 | 25.0 | 7.14 | 28.56 | |
| Sweden: Nordstrom, 199725 | 20.27 | 6.24 | 30.78 | |
| UAE: Khan, 199741 | 10.96 | 3.17 | 28.92 | |
| UAE: Khan, 199741 | 5.8 | 0.73 | 12.59 | |
| UK: Mitchell, 199341 | 7.39 | 0.43 | 5.82 | |
| Vietnam: Tsu, 200632 | 2.67 | 0.73 | 27.34 | |
| Zimbabwe: Kundodyiwa, 200140 | 13.28 | 1.95 | 14.68 | |
| Misoprostol | Egypt: Prata, 200634 | 1.61 | 0.08 | 4.97 |
| France: Benchimol, 200147 | 27.95 | 8.6 | 30.77 | |
| Gambia, rural: Walraven, 200553 | 10.97 | 0.32 | 2.92 | |
| Guinea Bissau, rural: Høj, 200542 | 45.45 | 11.21 | 24.66 | |
| Hong Kong: Lam, 200439 | 13.33 | NR | ||
| India, rural: Derman, 200624 | 6.4 | 0.25 | 3.91 | |
| India: Gupta, 200644 | 1 | |||
| India: Verma, 200631 | 1 | NR | ||
| India, rural: Vimala, 200452 | 3.33 | 0 | 0 | |
| India: Zachariah, 200629 | 2.6 | 0.14 | 5.38 | |
| South Africa: Bamigboye, 199828 | 4.81 | |||
| South Africa: Hofmeyr, 199823 | NR | 6.0 | ||
| South Africa: Hofmeyr, 200143 | NR | 9.0 | ||
| Zimbabwe: Kundodyiwa, 200140 | 15.23 | 3.7 | 24.29 | |
| Multicentre Gulmezoglu, 200140 | 19.46 | 3.97 | 20.40 | |
| Ergometrine | Gambia, rural: Walraven, 200553 | 12.02 | 0.67 | 5.57 |
| India: Verma, 200631 | 0 | NR | ||
| India, rural: Vimala, 200452 | 0 | 0 | 0 | |
| India: Zachariah, 200629 | 2.96 | 0.89 | 30.07 | |
| Ireland: Begley, 199049 | 1.99 | 0.14 | 7.04 | |
| Japan: Fujimoto, 200651 | 7.59 | NR | ||
| Japan: Fujimoto, 200651 | 18.57 | NR | ||
| The Netherlands, multicentre: de Groot, 199650 | 36.99 | 8.22 | 22.22 |
NR, not reported.
A subsample of studies reported both PPH and severe PPH (Table 3). The ratio of severe PPH to PPH should theoretically be similar regardless of how the third stage of labour was managed, unless a uterotonic has the characteristic of being more effective at preventing blood loss at lower or higher levels of blood loss. The ratio of severe PPH to PPH also varied from 10 to 33% for expectant management, from 6 to 41% for oxytocin, from 0 to 31% for misoprostol, and from 0 to 30% for ergometrine. In study sample arms with ≤200 women the range of severe PPH to PPH was 0–41%. In the larger study arms the range of severe PPH to PPH was slightly narrower: 4–33%.
Association of the management of the third stage of labour with blood loss measured
Oxytocin versus expectant management
In all controlled studies of measured blood loss (Figure S1; Table 4), oxytocin significantly reduced PPH (OR 0.43, 95% CI 0.23–0.81; six studies, n = 6892), and reduced mean blood loss by 140 ml (95% CI from −228 to −52 ml; four studies, n = 2833) and was associated with substantially but not significantly lower rates of severe PPH (OR 0.61, 95% CI 0.29–1.29; six studies, n = 6892), compared with expectant management (no uterotonic prophylaxis). Significant heterogeneity (differences between studies) was observed in these results. Limiting the analyses to studies qualified as methodologically adequate eliminates the heterogeneity, as it reduces the analyses to one study (n = 1000). In adequate studies, oxytocin significantly reduced PPH (OR 0.45, 95% CI 0.34–0.60), and reduced the mean blood loss by 118 ml (95% CI from −165 to −71 ml). The association with severe PPH is marginally significant (OR 0.76, 95% CI 0.52–1.09). Similarly, oxytocin substantially but not significantly lowered severe PPH in the two studies conducted in developing countries, both of which were quasi-experimental (and thus did not qualify as methodologically adequate) (OR 0.42, 95% CI 0.04–4.81; two studies, n = 5203).26,32
Table 4.
Effect of prophylactic regimen of third stage of labour on PPH, severe PPH and mean blood loss (in ml)
| Outcome | Studies | n | Effect estimate OR/mean difference [95% CI] | P | Studies | n | Effect estimate OR/mean difference [95% CI] | P |
|---|---|---|---|---|---|---|---|---|
| All studies | Adequate quality RCT subgroup | |||||||
| Oxytocin versus no uterotonic | ||||||||
| PPH | 6 | 6892 | 0.43 [0.23, 0.81]* | <0.001 | 1 | 1000 | 0.45 [0.34, 0.60] | <0.001 |
| Severe PPH | 6 | 6892 | 0.61 [0.29, 1.29]* | 0.20 | 1 | 1000 | 0.76 [0.52, 1.09] | 0.12 |
| Mean blood loss | 4 | 2833 | −140.35 [−228.54, −52.16]* | 0.001 | 1 | 1000 | −118.00 [−165.23, −70.77] | <0.001 |
| Misoprostol versus no uterotonic | ||||||||
| PPH | 3 | 2687 | 0.73 [0.50, 1.08]* | 0.12 | 2 | 2281 | 0.63 [0.41, 0.99]** | 0.04 |
| Severe PPH | 6 | 4328 | 0.74 [0.52, 1.04] | 0.09 | 5 | 3922 | 0.67 [0.51, 0.89] | 0.005 |
| Mean blood loss | 3 | 2373 | −38.75 [−64.81, −12.70] | 0.004 | 3 | 2373 | −38.75 [−64.81, −12.70] | 0.004 |
| Ergometrine versus no uterotonic | ||||||||
| PPH | 2 | 1718 | 0.46 [0.11, 1.91]* | 0.29 | ||||
| Severe PPH | 2 | 1718 | 0.32 [0.04, 2.43]** | 0.27 | ||||
| Mean blood loss | 2 | 1718 | −84.07 [−102.47, −65.67] | <0.001 | ||||
| Oxytocin versus misoprostol | ||||||||
| PPH | 5 | 20868 | 0.65 [0.60, 0.70] | <0.001 | 3 | 19139 | 0.65 [0.60, 0.70] | <0.001 |
| Severe PPH | 4 | 19789 | 0.71 [0.56, 0.91] | 0.005 | 2 | 18941 | 0.70 [0.60, 0.83] | <0.001 |
| Mean blood loss | 3 | 2209 | −8.36 [−18.32, 1.61] | 0.10 | 1 | 200 | −16.70 [−36.96, 3.56] | 0.11 |
| Oxytocin versus ergometrine | ||||||||
| PPH | 3 | 1619 | 0.72 [0.34, 1.56] | 0.41 | ||||
| Severe PPH | 1 | 1293 | 0.73 [0.20, 2.59] | 0.63 | ||||
| Mean blood loss | 3 | 1619 | −36.97 [−106.47, 32.53]* | 0.30 | ||||
| Misoprostol versus ergometrine | ||||||||
| PPH | 3 | 2834 | 0.91 [0.67, 1.23] | 0.53 | 1 | 1228 | 0.90 [0.63, 1.28] | 0.56 |
| Severe PPH | 2 | 2634 | 0.30 [0.08, 1.15] | 0.08 | 1 | 1228 | 0.47 [0.09, 2.60] | 0.39 |
| Mean blood loss | 2 | 2634 | −1.64 [−16.50, 13.22] | 0.83 | 1 | 1228 | −11.00 [−30.75, 8.75] | 0.28 |
Significant heterogeneity.
Borderline significant heterogeneity (P = 0.06–0.10).
Misoprostol versus expectant management
Compared with no uterotonic prophylaxis, misoprostol was marginally associated with a substantial reduction in PPH (OR 0.73, 95% CI 0.50–1.08, three studies, n = 2687) and severe PPH (OR 0.74, 95% CI 0.52–1.04, six studies, n = 4328), and was significantly associated with a lower mean blood loss (−38.75 ml, 95% CI from −64.81 to −12.70 ml, three studies, n = 2833; Figure S2; Table 4). Significant heterogeneity was observed in the analysis of PPH. Limiting the analyses to studies qualified as methodologically adequate reduces the heterogeneity to marginally significant (P= 0.06), and confirms the effect of misoprostol on reducing PPH compared with expectant management (OR 0.63, 95% CI 0.41–0.99, two studies, n = 2281); both studies were conducted in rural areas of developing countries. In adequate studies (all in developing countries), misoprostol also significantly reduces severe PPH (OR 0.67, 95% CI 0.51–0.89, five studies, n = 3922) and mean blood loss (−39 ml, 95% CI from −65 to −13 ml, three studies, n = 2373).24,28,42,43,52
Ergometrine versus expectant management
Compared with no uterotonic, management with ergometrine was associated with a significant reduction in mean blood loss (−84 ml, 95% CI from −102 to −66 ml, two studies, n = 1718; Figure S3; Table 4). Whereas women receiving ergometrine had substantially lower PPH and severe PPH in all controlled studies (PPH, OR 0.46, 95% CI 0.11–1.91; severe PPH, OR 0.32, 95% CI 0.04–2.43, two studies, n = 1718), the differences were not statistically significant. None of the studies comparing ergometrine with expectant management was considered methodologically adequate, and none was conducted in developing countries.
Oxytocin versus misoprostol
Compared with misoprostol, oxytocin significantly reduced PPH (all controlled studies and adequate studies, OR 0.65, 95% CI 0.60–0.70, five studies, n = 20 868; Figure S4; Table 4) and severe PPH (all controlled studies, OR 0.71, 95% CI 0.56–0.91, four studies, n = 19 789; adequate studies, OR 0.70, 95% CI 0.60–0.83, two studies, n = 18 941). These odds ratios and 95% confidence limits of all studies and the adequate studies subgroup are identical, as the results are greatly influenced by the single WHO multicentre study.45 There was no considerable or significant difference between oxytocin and misoprostol in the two non-multicentre RCTs considered to be of adequate quality, which were conducted in much smaller tertiary care centres in developing countries (PPH, OR 0.83, 95% CI 0.51–1.37; severe PPH, OR 1.28, 95% CI 0.15–10.95, n = 1081) or in any of the studies solely conducted in developing countries (Figure S4). There was no difference in mean blood loss (all controlled studies, −8 ml, 95% CI from −18 to 2 ml, three studies, n = 2209; adequate studies, −17 ml, 95% CI from −37 to 4 ml, one study, n = 200). No statistical heterogeneity was observed in the comparisons. With the exception of one small study and the Ireland and Switzerland sites in the multicentre trial, these studies were conducted in developing country hospitals, none of which were in rural areas.
Oxytocin or misoprostol versus ergometrine
Oxytocin compared with ergometrine was associated with substantially lower PPH (oxytocin, OR 0.72, 95% CI 0.34–1.56, three studies, n = 1619) and severe PPH (oxytocin, OR 0.73, 95% CI 0.20–2.59, one study, n = 1293), although neither difference was statistically significant (Figure S5; Table 4). There was little difference in mean blood loss in women receiving oxytocin compared with ergometrine (−37 ml, 95% CI from −106 to 33 ml, three studies, n = 1619). In the single study in India (which was not of adequate quality), the results comparing oxytocin with ergometrine were almost identical to all studies comparing oxytocin with ergometrine (PPH, OR 0.71, 95% CI 0.35–1.43; severe PPH, OR 0.73, 95% CI 0.20–2.59; mean blood loss −5 ml, 95% CI from −20 to 10 ml, n = 1293).
Women who received misoprostol had similar PPH rates and mean blood loss to those receiving ergometrine (PPH, OR 0.91, 95% CI 0.67–1.23, three studies, n = 2834; mean blood loss −2 ml 95% CI from −17 to 13 ml, two studies, n = 2634; Figure S6; Table 4). However, women receiving misoprostol had substantially and marginally significantly lower rates of severe PPH than those receiving ergometrine (OR 0.30, 95% CI 0.08–1.15, P= 0.08, two studies, n = 2634). Only one study on rural Gambian home deliveries, comparing misoprostol with ergometrine, was considered to be adequate: there was no substantial difference in PPH or mean blood loss, but misoprostol was associated with a large yet not statistically significantly lower rate of severe PPH (OR 0.47, 95% CI 0.09–2.60, n = 1228).53 All studies comparing misoprostol with ergometrine were conducted in developing countries.
Discussion
The WHO recommends oxytocin as the uterotonic of choice for PPH prevention, and that oxytocin or misoprostol be offered by a health worker trained in its use in the absence of oxytocin and other components of AMTSL, e.g. provision of a uterotonic, uterine massage and controlled cord traction.8 These recommendations are currently based upon a body of studies that do not distinguish between visual and measured blood loss, and are influenced by the sample for which blood loss was visually assessed. Similarly, analyses upon which policy recommendations are based do not separate studies for other factors that influence bleeding. The American College of Obstetricians and Gynecologists has suggested functional definitions of severe blood loss, including a 10% decline from ante- to post-partum haematocrit, or the need for red blood cell transfusion;55 however, too few studies measuring blood loss exist to support such functional definitions.
By reviewing only articles of measured postpartum blood loss, this article provides comparisons unbiased by the proportion of studies using visual compared with measured blood loss. Most of the presented analyses show similar effects to those published in meta-analyses that pool visually estimated and measured blood loss; however, our analyses clarify some important discrepancies.17 Comparing oxytocin with no uterotonic, our analyses of all studies show a slightly stronger and still significant effect for PPH and mean blood loss (PPH, OR 0.43, 95% CI 0.23–0.81 versus Cochrane OR 0.50, 95% CI 0.43–0.59; mean blood loss of −140 ml, 95% CI from −229 to −52 ml versus Cochrane blood loss of −102 ml, 95% CI from −135 to −69 ml), with the same effect on severe PPH (OR 0.61, 95% CI 0.29−1.29 versus Cochrane 95% CI 0.44–0.87). As a smaller subgroup, our analyses of severe PPH do not reach statistical significance.17 Our analyses of studies of adequate quality compared with the Cochrane subgroup of RCTs demonstrate a significant and much stronger reduction of PPH with oxytocin compared with no uterotonic (OR 0.45, 95% CI 0.34–0.60 versus Cochrane OR 0.61, 95% CI 0.51–0.72), and a reduction in mean blood loss (−118 ml, 95% CI from −165 to −71 ml versus Cochrane mean blood loss of −109 ml, 95% CI from −152 to −66 ml), whereas the effect on severe PPH was similar, and was still marginally significant (OR 0.76, 95% CI 0.52−1.09 versus Cochrane OR 0.72, 95% CI 0.49–1.05).
The Cochrane comparisons of misoprostol with no or other uterotonics are less methodologically similar to our analyses.8 The Cochrane review of prostaglandins for PPH prevention does not provide estimates summarising the overall effect comparing misoprostol with no uterotonic; however, the estimate we calculate from the data they present for this comparison, excluding the Gambian study53 (as the comparison group received oral ergometrine) and the Turkish study36 (which compared a combination of oxytocin and misoprostol with no uterotonic), was Cochrane OR 0.75 (95% CI 0.49–1.14) for severe PPH, very similar to our results from all studies (OR 0.74, 95% CI 0.52–1.04), although our adequate-quality studies showed a stronger and highly significant effect (OR 0.67, 95% CI 0.51–0.89). The Cochrane review found that compared with sublingual misoprostol, any injectable uterotonic had a similar yet marginally significant effect on PPH (Cochrane OR 0.93, 95% CI 0.79–1.11), and was inferior to sublingual misoprostol for severe PPH (Cochrane OR 1.85, 95% CI 0.79–4.35). In contrast, the Cochrane review found any injectable uterotonic to be superior to oral misoprostol for severe PPH (Cochrane OR 0.76, 95% CI 0.66–0.86).8
The effects of uterotonics on severe PPH are particularly important, as maternal death as a result of PPH usually occurs when blood loss is >1000 ml.19 Distinct from existing reviews, we found that prophylactic oxytocin significantly reduces PPH, but is only marginally associated with lower severe PPH compared with expectant management. This might be attributable to insufficient statistical power, as severe PPH is a relatively rare condition. In addition, a small portion of PPH and severe PPH would not be responsive to uterotonics (for example, if caused by trauma), thereby minimising the incidence of potentially responsive severe bleeding. However, compared with no uterotonic, misoprostol significantly lowered severe PPH in adequate-quality studies with a much smaller total sample size than that of all studies evaluating the effects of oxytocin on severe PPH. The data from adequate-quality studies or developing country data are too scant to draw conclusions about the effects of oxytocin compared with no uterotonic, or misoprostol, in these contexts.
Prophylactic misoprostol significantly reduces PPH and severe PPH, compared with expectant management, only when analyses are limited to adequate-quality studies, or in studies solely conducted in developing countries. In the WHO multicentre study comparing oxytocin with misoprostol in hospital settings, oxytocin reduces PPH and severe PPH significantly more than misoprostol, but does not differentially affect maternal death.45 Four studies of misoprostol have been conducted in rural, developing country settings: two compared with ergometrine and two with no uterotonic. There is only one quasi-experimental study of oxytocin in a rural developing country setting. No studies compare oxytocin with misoprostol in home birth or primary care centre settings, or in rural areas of developing countries, where misoprostol being simpler, and therefore more feasible to administer and study, may be relatively more effective because of greater coverage.
Distinct from other reviews, this review of measured blood loss, complementing meta-analyses with broader epidemiologic data, and providing sufficient stratification of information, demonstrates that women experience a large range of postpartum blood loss, even when bleeding was carefully measured. The median of reported average blood loss in women receiving any prophylactic uterotonics was similar, and was approximately 40% lower than that of women not receiving prophylactic uterotonics. However, the range of average blood loss, PPH and severe PPH was large, and fairly consistent, across women receiving and not receiving prophylactic uterotonics. The difference between the lowest and highest mean blood loss, incidence of PPH and severe PPH, and the ratio of severe PPH to PPH within each regimen for managing the third stage of labour is greater than the discrepancy in these ranges across the regimens. Variation in blood loss was only slightly larger in study arms with ≤200 women compared with larger study arms.
Women’s characteristics, obstetric practices and other factors associated with setting could account for some of the blood loss variation, and for the differences in the relative effectiveness of uterotonics on blood loss and haemorrhage.10 Eligible studies generally excluded high-risk or complicated pregnancies. Labour augmentation and/or induction were permitted in about half of the reviewed studies with 2–47% (median 27%) of women having augmentation or induction. In women otherwise managed without uterotonic prophylaxis, study arms that permitted augmentation or induction had lower levels of blood loss than those without. Measuring blood loss is more difficult than visual estimation, and thus has been implemented less frequently. Although blood loss measurement could in theory influence observed blood loss, few studies used the drape, and both the bedpan/linens and drape methods are direct measurements that are found to be quite accurate and similar.56,57 Exclusion of studies where the incidence of PPH was extremely high, or limiting analyses to studies measuring blood loss for 1 hour, only slightly modified the incidence of severe PPH for all regimens.
Conclusions
A better understanding of postpartum blood loss could improve our strategies to prevent and manage PPH, particularly in the rural developing country settings where most maternal deaths occur, yet where few adequate-quality studies have taken place. Our results of measured blood loss indicate that although oxytocin is superior to misoprostol in hospitals, misoprostol substantially lowers PPH and severe PPH in developing countries. The relative merits of oxytocin and misoprostol continue to require sound assessment in rural areas of developing countries, where most PPH deaths occur.
Acknowledgments
This body of work was financially supported by the Bill & Melinda Gates Foundation.
Disclosure of interests
We declare that we have primary responsibility for the composition of the report and the data analyses. The sponsor of the study had no role in the study design, data collection, analysis or interpretation, or in the composition of the manuscript.
Contribution to authorship
NLS assumes primary responsibility for data analysis and first authorship. BW originally conceptualised the article, and as the senior contributor elected the final author placement. NLS, JD and TA were responsible for extracting the reviewed data. In reflection of time and effort spent on the manuscript, JD is the second author, TA is the third author and JB is the fourth author. All authors have participated in composing the report, interpreting and presentating the analyses, and have seen and approved the final version of the manuscript.
Details of ethics approval
No original research was conducted. Summary data from published peer-review articles are presented.
Funding
This body of work was financially supported by the Bill & Melinda Gates Foundation. The Foundation had no role in the study design, data collection, analysis, interpretation or report composition.
Supporting information
The following supplementary materials are available for this article:
Figure S1. Oxytocin versus expectant management.
Figure S2. Misoprostol versus expectant management.
Figure S3. Ergometrine versus expectant management.
Figure S4. Oxytocin versus misoprostol.
Figure S5. Oxytocin versus ergometrine.
Figure S6. Misoprostol versus ergometrine.
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Cotter AM, Ness A, Tolosa JE. Prophylactic oxytocin for the third stage of labour. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001808.pub2. DOI: 10.1002/14651858.CD001808. [DOI] [PubMed] [Google Scholar]
- 3.WHO, UNICEF, UNFPA. The World Bank World Health Organization . WHO Press, World Health Organization. Geneva: World Health Organization; 2005. Maternal mortality in 2005: estimates developed by WHO, UNICEF, UNFPA, and the World Bank; pp. 16–17.pp. 23–27.pp. 29–38. editor. [Google Scholar]
- 4.UN . The Millennium Development Goals Report 2008. New York: United Nations; 2008. [Google Scholar]
- 5.FIGO-ICM 2006. Prevention and Treatment of Post-partum Haemorrhage: New Advances for Low Resource Settings Joint Statement: International Confederation of Midwives (ICM), International Federation of Gynaecology and Obstetrics (FIGO)
- 6.World Health Organization . Managing Complications in Pregnancy and Childbirth: A guide for midwives and doctors. Department of Reproductive Health and Research. Geneva: World Health Organization; 2003. [Google Scholar]
- 7.Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD, editors. Williams Obstetrics. 21st edn. New York: McGraw-Hill Professional; 2001. Obstetrical hemorrhage; pp. 619–70. [Google Scholar]
- 8.Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2007:CD000494. doi: 10.1002/14651858.CD000494.pub3. DOI: 10.1002/14651858.CD000494.pub3. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO Recommendations for the Prevention of Postpartum Haemorrhage. Geneva: World Health Organization/MPS; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum hemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999–1012. doi: 10.1016/j.bpobgyn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Prasertcharoensuk W, Swadpanich U, Lumbiganon P. Accuracy of the blood loss estimation in the third stage of labor. Int J Gynecol Obstet. 2000;71:69–70. doi: 10.1016/s0020-7292(00)00294-0. [DOI] [PubMed] [Google Scholar]
- 12.Glover P. Blood loss at delivery: how accurate is your estimation? Aust J Midwifery. 2003;16:21–4. doi: 10.1016/s1031-170x(03)80005-3. [DOI] [PubMed] [Google Scholar]
- 13.Duthie SJ, Ven D, Yung GL, Guang DZ, Chan SY, Ma HK. Discrepancy between laboratory determination and visual estimation of blood loss during normal delivery. Eur J Obstet Gynecol Reprod Biol. 1991;38:119–24. doi: 10.1016/0028-2243(91)90188-q. [DOI] [PubMed] [Google Scholar]
- 14.Razvi K, Chua S, Arulkumaran S, Ratnam SS. A comparison between visual estimation and laboratory determination of blood loss during the third stage of labour. Aust NZ J Obstet Gynaecol. 1996;36:152–4. doi: 10.1111/j.1479-828x.1996.tb03273.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel A, Goudar SS, Geller SE, Kodkany BS, Edlavitch SA, Wagh K, et al. Drape estimation vs. visual assessment for estimating postpartum hemorrhage. Int J Gynaecol Obstet. 2006;93:220–4. doi: 10.1016/j.ijgo.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Pritchard J. Changes in the blood volume during pregnancy and delivery. Anesthesiology. 1965;26:393–292. doi: 10.1097/00000542-196507000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Elbourne DR, Prendiville WJ, Carroli G, Wood J, McDonald S. Prophylactic use of oxytocin in the third stage of labour. Cochrane Database Syst Rev. 2001:CD001808. doi: 10.1002/14651858.CD001808. DOI: 10.1002/14651858.CD001808. [DOI] [PubMed] [Google Scholar]
- 18.McDonald S, Abbott JM, Higgins SP. Prophylactic ergometrine-oxytocin versus oxytocin for the third stage of labour. Cochrane Database Syst Rev. 2004:CD000201. doi: 10.1002/14651858.CD000201.pub2. DOI: 10.1002/14651858.CD000201.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmeyr GJ, Gulmezoglu AM, Novikova N, Linder V, Ferreira S, Piaggio G. Misoprostol to prevent and treat postpartum haemorrhage: a systematic review and meta-analysis of maternal deaths and dose-related effects. Bull World Health Organ. 2009;87:666–77. doi: 10.2471/BLT.08.055715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selo-Ojeme DO. Primary postpartum hemorrhage. J Obstet Gynaecol. 2002;22:463–9. doi: 10.1080/0144361021000003555. [DOI] [PubMed] [Google Scholar]
- 21.Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. Br Med J. 1988;297:1295–300. doi: 10.1136/bmj.297.6659.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coker A, Oliver R. Definitions and Classifications. In: B-Lynch C, Keith L, Lalonde A, Karoshi M, editors. A Textbook of Postpartum Hemorrhage. United Kingdom: Sapiens Publishing; 2006. pp. 11–6. [Google Scholar]
- 23.Hofmeyr JG, Nikodem V, de Jager M, Gelbart BR. A randomized placebo controlled study of oral misoprostol in the third stage of labour. Br J Obstet Gynaecol. 1998;105:971–5. doi: 10.1111/j.1471-0528.1998.tb10259.x. [DOI] [PubMed] [Google Scholar]
- 24.Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet. 2006;368:1248–53. doi: 10.1016/S0140-6736(06)69522-6. [DOI] [PubMed] [Google Scholar]
- 25.Nordström L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labor: a placebo-controlled study randomised trial. Br J Obstet Gynaecol. 1997;104:781–6. doi: 10.1111/j.1471-0528.1997.tb12020.x. [DOI] [PubMed] [Google Scholar]
- 26.Strand RT, Da Silva F, Jangsten E, Bergstrom S. Postpartum hemorrhage: a prospective, comparative study in Angola using a new disposable injection device for oxytocin administration. Acta Obstet Gynecol Scand. 2005;84:260–5. doi: 10.1111/j.0001-6349.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 27.Phillip H, Fletcher H, Reid M. The impact of induced labour on postpartum blood loss. J Obstet Gynaecol. 2004;24:12–5. doi: 10.1080/01443610310001620215. [DOI] [PubMed] [Google Scholar]
- 28.Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo-controlled trial. Am J Obstet Gynecol. 1998;179:1043–6. doi: 10.1016/s0002-9378(98)70212-1. [DOI] [PubMed] [Google Scholar]
- 29.Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labor. Int J Gynecol Obstet. 2006;92:23–6. doi: 10.1016/j.ijgo.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Yuen PM, Chan NS, Yim SF, Chang AM. A randomised double blind comparison of Syntometrine and Syntocinon in the management of the third stage of labour. Br J Obstet Gynaecol. 1995;102:377–80. doi: 10.1111/j.1471-0528.1995.tb11288.x. [DOI] [PubMed] [Google Scholar]
- 31.Verma P, Aggarwal N, Jain V, Suri V. A double-blind randomized controlled trial to compare sublingual misoprostol with methylergometrine for prevention of postpartum hemorrhage. Intl J Gynecol Obstet. 2006;94(Suppl. 2):137–8. doi: 10.1016/S0020-7292(06)60013-1. [DOI] [PubMed] [Google Scholar]
- 32.Tsu VD, Mai TT, Nguyen YH, Luu HT. Reducing postpartum hemorrhage in Vietnam: assessing the effectiveness of active management of third-stage labor. J Obstet Gynaecol Res. 2006;32:489–96. doi: 10.1111/j.1447-0756.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 33.Soriano D, Dulitzki M, Schiff E, Barkai G, Mashiach S, Seidman DS. A prospective cohort study of oxytocin plus ergometrine compared with oxytocin alone for prevention of postpartum hemorrhage. Br J Obstet Gynaecol. 1996;103:1068–73. doi: 10.1111/j.1471-0528.1996.tb09584.x. [DOI] [PubMed] [Google Scholar]
- 34.Prata N, Hamza S, Gypson R, Nada K, Vahidnia F, Potts M. Misoprostol and active management of the third stage of labor. Int J Gynecol Obstet. 2006;94:149–55. doi: 10.1016/j.ijgo.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Poeschmann RP, Doesburg WH, Eskes TK. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. Br J Obstet Gynaecol. 1991;98:528–30. doi: 10.1111/j.1471-0528.1991.tb10364.x. [DOI] [PubMed] [Google Scholar]
- 36.Ozkaya O, Sezik M, Kaya H, Desdicioglu R, Dittrich R. Placebo-controlled randomized comparison of vaginal with rectal misoprostol in the prevention of postpartum hemorrhage. J Obstet Gynaecol Res. 2005;31:389–93. doi: 10.1111/j.1447-0756.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 37.Nellore V, Mittal S, Dadhwal V. Rectal misoprostol vs. 15-methyl prostaglandin F2alpha for the prevention of postpartum hemorrhage. Int J Gynecol Obstet. 2006;94:45–6. doi: 10.1016/j.ijgo.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell GG, Elbourne DR. Oxytocin plus ergometrine versus oxytocin alone in the active management of the third stage of labor. Online J Curr Clin Trials. 1993;2 Doc No. 83. [PubMed] [Google Scholar]
- 39.Lam H, Tang O, Lee CP, Ho PC. A pilot-randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstet Gynecol Scand. 2004;83:647–50. doi: 10.1111/j.0001-6349.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 40.Kundodyiwa TW, Mojoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. Int J Gynecol Obstet. 2001;75:235–41. doi: 10.1016/s0020-7292(01)00498-2. [DOI] [PubMed] [Google Scholar]
- 41.Khan GQ, John IS, Wani S, Doherty T, Sibai BM. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. Am J Obstet Gynecol. 1997;177:770–4. doi: 10.1016/s0002-9378(97)70266-7. [DOI] [PubMed] [Google Scholar]
- 42.Høj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, Aaby P. Effect of sublingual misoprostol on severe postpartum hemorrhage in a primary health centre in Guinea-Bissau: randomized double blind clinical trial. Br Med J. 2005;331:723–7. doi: 10.1136/bmj.331.7519.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofmeyr GJ, Nikodem VC, de Jager M, Drakely A. Side-effects of oral misoprostol in the third stage of labour--a randomised placebo-controlled trial. S Afr Med J. 2001;91:432–5. [PubMed] [Google Scholar]
- 44.Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum hemorrhage—A pilot study. Int J Gynecol Obstet. 2006;94(Suppl. 2):139–40. doi: 10.1016/S0020-7292(06)60014-3. [DOI] [PubMed] [Google Scholar]
- 45.Gülmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised study of misoprostol in the management of the third stage of labour. Lancet. 2001;358:689–95. doi: 10.1016/s0140-6736(01)05835-4. [DOI] [PubMed] [Google Scholar]
- 46.Bugalho A, Daniel A, Faúndes A, Cunha M. Misoprostol for prevention of postpartum hemorrhage. Int J Gynecol Obstet. 2001;73:1–6. doi: 10.1016/s0020-7292(01)00346-0. [DOI] [PubMed] [Google Scholar]
- 47.Benchimol M, Gondry J, Mention J-E, Gagneur O, Boulanger JC. Place du misoprostol dans la direction de la deliverance. J Gynecol Obstet Biol Reprod. 2001;30:576–83. [PubMed] [Google Scholar]
- 48.Abdel-Aleem H, Hofmeyr G, Shokry M, El-Sonoosy E. Uterine massage and postpartum blood loss. Intl J Gynecology Obstet. 2006;93:238–9. doi: 10.1016/j.ijgo.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Begley C. A comparison of ‘active’ and ‘physiological’ management of the third stage of labour. Midwifery. 1990;6:3–17. doi: 10.1016/s0266-6138(05)80091-9. [DOI] [PubMed] [Google Scholar]
- 50.De Groot ANJ, van Roosmalen J, van Dongen PW, Borm GF. A placebo-controlled study of oral ergometrine to reduce postpartum hemorrhage. Acta Obstet Gynecol Scand. 1996;75:464–8. doi: 10.3109/00016349609033355. [DOI] [PubMed] [Google Scholar]
- 51.Fujimoto M, Takeuchi K, Sugimoto M, Maruo T. Prevention of postpartum hemorrhage by uterotonic agents: comparison of oxytocin and methylergometrine in the management of the third stage of labor. Acta Obstet Gynecol Scand. 2006;85:1310–4. doi: 10.1080/00016340600756912. [DOI] [PubMed] [Google Scholar]
- 52.Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Int J Gynaecol Obstet. 2004;87:1–5. doi: 10.1016/j.ijgo.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 53.Walraven G, Blum J, Dampha Y, Sowe M, Morison L, Winikoff B, et al. Misoprostol in the management of the third stage of labour in the home delivery setting in rural Gambia: a randomised controlled trial. Br J Obstet Gynaecol. 2005;12:1277–83. doi: 10.1111/j.1471-0528.2005.00711.x. [DOI] [PubMed] [Google Scholar]
- 54.Kodkany BS, Derman RJ, Goudar SS, Geller SE, Edlavitch SA, Naik VA, et al. Initiating a novel therapy in preventing postpartum hemorrhage in rural India: a joint collaboration between the United States and India. Int J Fertil Women’s Med. 2004;49:91–6. [PubMed] [Google Scholar]
- 55.American College Obstetricians and Gynecologists Task Force on Quality Assurance . Quality Assurance in Obstetrics and Gynecology. Washington DC: ACOG; 1989. [Google Scholar]
- 56.Schorn MN. Measurement of blood loss: review of the literature. J Midwifery Womens Health. 2010;55:20–7. doi: 10.1016/j.jmwh.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Ambardekar S, Coyaji K, Otiv S, Bracken H, Winikoff B. A comparison of drape estimation and a standardized weight method for the measurement of postpartum blood loss. Int J Gynaecol Obstet. 2009;107(Suppl. 2):S10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.