Abstract
Lager beers are traditionally made at lower temperatures (6–14 °C) than ales (15–25 °C). At low temperatures, lager strains (Saccharomyces pastorianus) ferment faster than ale strains (Saccharomyces cerevisiae). Two lager and two ale strains had similar maltose transport activities at 20 °C, but at 0 °C the lager strains had fivefold greater activity. AGT1, MTT1 and MALx1 are major maltose transporter genes. In nine tested lager strains, the AGT1 genes contained premature stop codons. None of five tested ale strains had this defect. All tested lager strains, but no ale strain, contained MTT1 genes. When functional AGT1 from an ale strain was expressed in a lager strain, the resultant maltose transport activity had the high temperature dependence characteristic of ale yeasts. Lager yeast MTT1 and MALx1 genes were expressed in a maltose-negative laboratory strain of S. cerevisiae. The resultant Mtt1 transport activity had low temperature dependence and the Malx1 activity had high temperature dependence. Faster fermentation at low temperature by lager strains than ale strains may result from their different maltose transporters. The loss of Agt1 transporters during the evolution of lager strains may have provided plasma membrane space for the Mtt1 transporters that perform better at a low temperature.
Keywords: brewer's yeast strains, evolution, α-glucoside transporters, temperature dependence of fermentation, temperature dependence of transport
Introduction
Among different beer types, ales are made by fermentation of brewer's wort at 15–25 °C, whereas lagers are traditionally made by fermentation at lower temperatures, 6–14 °C (Bamforth, 1998). Brewer's yeasts can be divided into ale strains (which cannot use melibiose) and lager strains (which can). Ale strains usually grow and ferment poorly at temperatures below about 12 °C, whereas lager strains perform well down to at least 7 °C (Walsh & Martin, 1977). Ale strains have been used for thousands of years, but it has been suggested that lager strains probably originated in low-temperature wort fermentations in Bavaria a few hundred years ago (see, e.g. Hornsey, 2003).
Most ale strains are thought to be varieties of Saccharomyces cerevisiae (Hammond, 1993; Tornai-Lehoczki & Dlauchy, 2000; Kobi et al., 2004;). However, it has been shown recently that they include strains (e.g. isolates from Trappist beers) that are hybrids between S. cerevisiae and Saccharomyces kudriavzevii (Gonzalez et al., 2008) and some other ale strains previously classified as S. cerevisiae may be hybrids (Querol & Bond, 2009). Lager strains have been variously described as Saccharomyces carlsbergensis, Saccharomyces uvarum and Saccharomyces pastorianus (Hammond, 1993). They have alloploid genomes and are hybrids of two species: S. cerevisiae and Saccharomyces bayanus (Naumova et al., 2005; Caesar et al., 2007; Dunn & Sherlock, 2008;). The hybrids are thought to have been selected during low-temperature wort fermentations. The cryophilic nature of lager yeasts probably derives from the S. bayanus partner (Sato et al., 2002). The complete sequencing of lager strain WS34/70 confirmed its hybrid nature: 36 chromosomes were found, 16 of S. cerevisiae type, 12 of S. bayanus type and 8 chimeric (Nakao et al., 2009). The presence of the chimeric chromosomes (part S. cerevisiae, part S. bayanus) suggested posthybridizational reorganization. Dunn & Sherlock (2008) also reported posthybridizational reorganization of lager strain chromosomes, and divided lager strains into two groups, originating from two separate hybridization events between S. cerevisiae and S. bayanus. They propose that distinct, but similar, S. cerevisiae strains were involved in the two events, and that hybridization was followed by the loss of a large portion of the S. cerevisiae genome from Group 1, whereas in Group 2, the loss of S. cerevisiae genes was much smaller.
The α-glucosides maltose and maltotriose together account for about 80% of the total fermentable sugars in brewer's wort. Efficient assimilation of these sugars is essential for fast and complete wort fermentations. All yeast α-glucoside transport systems characterized so far are H+-symporters that are driven by the electrochemical proton gradient across the plasma membrane. The active transport of maltose and maltotriose across the plasma membrane is a major rate-limiting step in the fermentation of wort (Kodama et al., 1995; Rautio & Londesborough, 2003; Stambuk et al., 2006;).
Several kinds of genes for α-glucoside transporters are found in Saccharomyces yeasts. MALx1 genes (x=1–4 and 6) occur in five unlinked MAL (maltose) loci (MAL1–MAL4 and MAL6). Most studies (e.g. Han et al., 1995; Salema-Oom et al., 2005; Alves et al., 2008;) indicate that the Malx1 transporters are narrowly specific for maltose (Km∼3 mM) and turanose, but activity towards maltotriose has been claimed (Day et al., 2002a). The identical MPH2 and MPH3 (maltose permease homologue) occur on different chromosomes and encode transporters able to carry maltose (Km∼4 mM), maltotriose (Km∼7 mM), α-methylglucoside and turanose (Day et al., 2002b). AGT1 (α-glucoside transporter) encodes the α-glucoside transporter with the widest substrate specificity reported so far (Han et al., 1995). The Agt1 transporter can carry trehalose and sucrose (Km∼8 mM) as well as maltose, maltotriose and α-methylglucoside (Km 20–35 mM) (Stambuk & de Araujo, 2001). A gene called MTT1 (mty-like transporter; Dietvorst et al., 2005) or MTY1 (maltotriose transport in yeast; Salema-Oom et al., 2005) encodes a transporter that has a higher affinity for maltotriose (Km∼20 mM) than for maltose (Km∼70 mM) and can also carry trehalose and possibly turanose (Salema-Oom et al., 2005). Nakao et al. (2009) have recently sequenced the genome of the lager strain, Weihenstephan 34/70. They found a gene, LBYG13187, that they believe to be the S. bayanus counterpart of the S. cerevisiae AGT1 because its closest homology was 79% identity to the AGT1 sequence in the Saccharomyces genome database (SGDB, where AGT1 is referred to as MAL11). Here, we call this gene Sb-AGT1, although nothing is known yet about the substrate specificity or other properties of the transporter it encodes.
Several authors have compared maltose transport by individual ale and lager yeast strains. For example, Crumplen et al. (1996) found that glucose more strongly inhibited maltose transport by an ale strain than that by a lager strain. Rautio & Londesborough (2003) found that trehalose and sucrose (a substrate of only Agt1 among known maltose transporters) strongly inhibited maltose transport by an ale strain, but only weakly inhibited maltose transport by a lager strain. Vidgren et al. (2005) reported that α-methyl glucoside (a substrate of only Agt1 and Mphx transporters) inhibited maltose transport into two ale strains by 41–74%, but inhibited maltose transport into three lager strains by only 10–23%. Taken together, these results suggested that the dominant maltose transporters of the ale strains studied had a broader specificity than those of lager strains, and were probably Agt1 proteins.
However, hybridization studies showed that all the ale and lager strains tested contained AGT1 and several MALx1 genes (Jespersen et al., 1999; Vidgren et al., 2005;). This apparent discrepancy was partially resolved by the finding (Vidgren et al., 2005) that the AGT1 genes of two lager strains contained premature stop codons. The same defect has been found in other lager strains, but not in ale strains (Vidgren et al., 2007; Nakao et al., 2009;). MTT1 genes were found in all four lager strains examined by Dietvorst et al. (2005). MPHx sequences were found (usually on chromosome IV, corresponding to MPH2) in some, but not all lager strains and less frequently in ale strains (Jespersen et al., 1999; Vidgren et al., 2005;). The expression of MPHx genes seems to be strain specific. Expression was very low in several lager strains growing on maltose or glucose (Vidgren et al., 2005), but Gibson et al. (2008) and James et al. (2003) found a stronger expression of MPHx during wort fermentations with other lager strains.
The rates of most enzyme-catalysed reactions approximately double for each 10 °C increase in temperature. However, reactions catalysed by integral membrane proteins usually exhibit nonlinear Arrhenius plots with increased temperature dependence at lower temperatures, where the structure of the membrane lipids changes from a more fluid liquid-crystalline phase to a more rigid gel phase. This phase transition depends on the membrane lipid composition (e.g. the presence and type of sterols and fatty acids) and on external factors, such as the osmotic pressure (Guyot et al., 2006).
Rautio & Londesborough (2003) found strong temperature dependence for maltose transport (c. 70-fold between 0 and 20 °C) for an ale strain. However, Guimarães et al. (2006) reported a markedly smaller temperature dependence (c. 11-fold) for a lager strain. The apparent predominance of Agt1 transporters in ale strains, but not in lager strains, suggested that the different temperature dependencies of maltose transport (and, therefore, the fermentation rate) in ale and lager strains might reflect differences in the properties of their maltose transporters. This article presents evidence supporting this hypothesis. We report (1) the distribution of MTT1, defective S. cerevisiae-derived AGT1 and S. bayanus-derived AGT1 genes among ale and lager strains and (2) the temperature dependence of maltose transport into ale and lager strains and into genetically engineered yeasts expressing AGT1, MALx1 and MTT1 maltose transporter genes from particular brewer's yeast strains. A preliminary report of some of this work has been given (Vidgren et al., 2007).
Materials and methods
Materials
U-14C maltose was from Amersham Biosciences (Espoo, Finland). Maltose for uptake experiments (minimum purity, 99%) and trehalose were from Sigma-Aldrich (Helsinki, Finland), and maltotriose was from MP Biomedicals (Solon, OH). Maltose and glucose for growth media were from Fluka (Helsinki, Finland). G418 was from Invitrogen (Espoo, Finland).
Strains
The industrial strains used in this work are listed in Table 1. CMBS-33 was kindly provided by J.M. Thevelein (Katholieke Universiteit, Leuven, Belgium) and WS34/70 was from the Weihenstephan Brewery (Freising, Germany). The other strains were from the VTT Culture Collection. The frequently used strains A-63015, A-66024, A-75060 and A-10179 are hereafter referred to as A15, A24, A60 and A179, respectively. Two laboratory strains, CEN.PK2-1D (VW-1B; maltose-positive) and S150-2B (maltose-negative), were also used.
Table 1.
Distribution of AGT1, Sb-AGT1 and MTT1 genes in some industrial yeasts
| Strain | Origin | AGT1 | Sb-AGT1* | MTT1 |
|---|---|---|---|---|
| Lager strains | ||||
| A-60012 | Weihenstephan 1 | D | P | P |
| A-62013 | Weihenstephan 294 | D | P | P |
| A-63015 (A15) | Nordic brewery | D | P | P |
| A-66024 (A24) | Nordic brewery | D | P | P |
| A-82064 | Nordic brewery | D | P | P |
| A-85072 | Nordic brewery | D | P | P |
| A-95143 | Nordic brewery | D | P | P |
| WS34/70 | Weihenstephan | D | P | P |
| CMB33 | Belgium | D | P | P |
| Ale strains | ||||
| A-10179 (A179) | UK brewery | P | M | M |
| A-60055 | NCYC 1200 | P | M | |
| A-60056 | NCYC 240 | P | M | |
| A-75060 (A60) | Nordic brewery | P | M | M |
| A-93116 | NCYC 1087 | P | M | |
| Baker's yeasts | ||||
| B-62001 | Nordic baker's yeast | P | P | |
| B-62003 | Nordic baker's yeast | P | P | |
| Distiller's yeasts | ||||
| C-72051 | Nordic distillery | P | P | |
| C-77076 | Nordic distillery | M | M | |
| C-91180A | Nordic distillery | M | M | |
Sb-AGT1 indicates a gene with 79% identity to AGT1 recently discovered in WS34/70 by Nakao et al. (2009).
D, defective, frame shift mutation; P, present; M, missing.
PCR analyses
Primers are shown in Table 2. PCR reactions were performed using standard procedures. To test for the presence of MTT1 genes, total chromosomal DNA from each strain was used as a template with MTT1 Frw and MTT1 Rev primers to generate a 247-bp fragment. To test for the presence of S. cerevisiae-type AGT1 genes, the primers AGT1 Frw and AGT1 Rev were used to generate 986-bp fragments. These fragments (842–1828 of the AGT1 ORF) include the frame shift and premature stop codon (starting at nucleotide 1183) described previously (Vidgren et al., 2005) in lager strains A15 and A24. They were cloned to a pCR-TOPO vector (Invitrogen) and sequenced using the AGT1Sekv4 primer to test for the frame shift. The putative S. bayanus-type AGT1 gene (LBYG13187; Nakao et al., 2009) was also studied. Most of the sequence of this gene has been published (Nakao et al., 2009) and the 5′-terminal 400-bp sequence was kindly provided by Dr Y. Nakao. Total chromosomal DNA from several brewer's yeast strains and laboratory strain CEN.PK2-1D was used as a template with AGT1bay_Cl_Frw and AGT1bay_Cl_Rev primers to generate 1833-bp fragments corresponding to the complete ORF and stop codon. The fragments obtained were cloned into the pCR-TOPO vector and their sequences were determined using AGT1baySekv1–AGT1baySekv3 primers and universal M13 forward and reverse primers, which bind close to the cloning sites of pCR-TOPO.
Table 2.
PCR primers
| Name | Primer sequence* | Sequence detected† |
|---|---|---|
| MTT1_Cl_Frw | 5′-CGAGATCTCGATGAAGGGATTATCCTCATT-3′ | 1–20 of MTT1 |
| MTT1_Cl_Rev | 5′-CGAGATCTCGTCATTTGTTCACAACAGATGG-3′ | 1828–1848 of MTT1 |
| MTT1Sekv1 | 5′-CTTTGAATAGCAATACAG-3′ | 404–421 of MTT1 |
| MTT1Sekv2 | 5′-AGAACTAGGATATAAGCT-3′ | 801–818 of MTT1 |
| MTT1Sekv3 | 5′-TATCCAATATTGTCTTGG′3′ | 1212–1229 of MTT1 |
| MTT1Sekv4 | 5′-GGTTATGTTTTGCCACTC-3′ | 1601–1618 of MTT1 |
| MTT1Frw | 5′-TTGGTAGGTTTGACCTTTAC-3′ | 1271–1290 of MTT1 |
| MTT1Rev | 5′-AGATGCCATATTATATGCGT-3′ | 1499–1518 of MTT1 |
| AGT1Frw | 5′-TTGCTTTACAATGGATTTGGC-3′ | 842–862 of AGT1 |
| AGT1Rev | 5′-CTCGCTGTTTTATGCTTGAGG-3′ | 1808–1828 of AGT1 |
| AGT1Sekv4 | 5′-AAAGCAGATTGAATTGAC-3′ | 1011–1028 of AGT1 |
| AGT1bay_Cl_Frw | 5′-CGAGATCTCGATGAAAAATATACTTTCGCTGG-3′ | 1–22 of Sb-AGT1 |
| AGT1bay_Cl_Rev | 5′-GCAGATCTCGTCATAACGCCTGTTGACTCG-3′ | 1814–1833 of Sb-AGT1 |
| AGT1baySekv1 | 5′-CCTACGATATCACTTCTC-3′ | 443–460 of Sb-AGT1 |
| AGT1baySekv2 | 5′-CGCCTTACAATGGATCTG-3′ | 843–860 of Sb-AGT1 |
| AGT1baySekv3 | 5′-ACGCTTGGTTCCTGGGTA-3′ | 1255–1272 of Sb-AGT1 |
BglII restriction sites are underlined.
The numbering is from the first nucleotide of the translational start. Sb-AGT1 refers to the putative Saccharomyces bayanus-derived counterpart of AGT1 (Nakao et al., 2009).
Laboratory strains bearing an ale or a lager strain AGT1 gene in a multicopy plasmid
The AGT1 genes from lager strain A15 and ale strains A60 and A179 were cloned by PCR using AGT1-F and AGT1-R primers. The sequences of these clones were verified as described earlier (Vidgren et al., 2009). AGT1-F and AGT1-R primers bear BglII restriction sites, which facilitated the next cloning step, i.e., the ligation of the PCR fragments to YEplac195 multicopy vectors (Gietz & Sugino, 1988) at the BglII site between the PGK1 promoter and terminator. In addition, the KanMX cassette (Wach et al., 1994) was introduced into the YEplac195 plasmid at the multiple cloning site to confer resistance to G418. The laboratory strain S150-2B was transformed with these YEplac195-PGK1-AGT1-KanMX constructs or with the empty YEplac195-KanMX plasmid as a control. The lithium acetate transformation procedure (Gietz et al., 1992) was used and transformants were selected using G418 selection.
Laboratory strains bearing a lager strain MTT1 or MALx1 gene in a multicopy plasmid
MTT1 and MALx1 genes were cloned from lager strain A15 by PCR with standard procedures using MTT1 Cl Frw and MTT1 Cl Rev primers, which contain BglII sites. Because the sequences of the MTT1 and MALx1 ORFs are identical to each other at both their starts and their ends, both genes were obtained with these primers. The PCR products were cloned into the pCR-TOPO vector and their sequences were determined using MTT1Sekv1–MTT1Sekv4 primers and universal M13 forward and reverse primers, which bind near the cloning site of pCR-TOPO. From the nine sequenced clones, four were >99% identical to the MTT1 sequence reported by Dietvorst et al. (2005) and five were >98% identical to the MAL31 type sequence in the SGDB. Clone 1 was 100% identical to the MTT1 sequence of Dietvorst et al. (2005) and was chosen to represent MTT1. Clone 2 was 99% identical to the MAL31 sequence in the SGDB and was chosen to represent MALx1. They were excised from the pCR-TOPO plasmid using the BglII enzyme and ligated between the PGK1 promoter and terminator at the BglII site in the YEplac195 multicopy vector. The laboratory strain S150-2B was transformed with either the YEplac195-PGK1-MTT1 or the YEplac195-PGK1-MALx1 construct using the lithium acetate transformation procedure (Gietz et al., 1992).
Construction of a lager yeast with an integrated, ale yeast-type AGT1 gene
Construction of Integrant 1 has been described previously (Vidgren et al., 2009). Briefly, the defective AGT1 gene in the lager strain A15 (with a premature stop codon at nucleotide 1183) was repaired using an integration cassette containing nucleotides 1–1478 of the AGT1 ORF from ale strain A60 functionally fused to a PGK1 promoter and flanked on the 5′-side by the AGT1 promoter sequence (−1 to −705). The ORF of the repaired gene has the ale yeast sequence from nucleotide 1 to somewhere between 1183 and 1478 (i.e. the frame shift and premature stop codon are removed), followed by the lager yeast sequence to the end of the AGT1 gene, and it is under the control of a PGK1 promoter.
Maltose transport assays
For maltose transport studies, native ale and lager strains were grown in YP (10 g yeast extract and 20 g peptone L−1) containing 40 g maltose L−1. YP-40 g glucose L−1 was used for the growth of Integrant 1, so that its endogenous maltose transporters were repressed. YP-40 g glucose L−1 supplemented with G418 (200 mg L−1) was used for S150-2B derivatives transformed with a YEplac195-KanMX plasmid (with or without an AGT1 gene). S150-2B derivatives transformed with YEplac195 plasmids lacking KanMX (and with or without an MTT1 or MALx1) were grown in a synthetic complete medium (Sherman et al., 1983) lacking uracil and containing 20 g glucose L−1. Yeasts were grown in 100 mL of medium in 250-mL Erlenmeyer flasks at 150 r.p.m. and 24 °C. They were usually harvested at an OD600 nm between 4 and 7 (i.e. at 2±1 mg dry yeast mL−1) while sugar was still present, but were grown into the stationary phase when so stated. After centrifugation (10 min, 9000 g, 0 °C), the yeast pellets were washed with ice-cold water and then with ice-cold 0.1 M tartrate-Tris (pH 4.2) and finally suspended in the same buffer to 200 mg of fresh yeast mL−1. For standard assays, about 1-mL portions of yeast suspension were equilibrated for 10 min to assay temperature (0–20 °C) in a water bath. Zero-trans [14C]-maltose uptake rates were then determined at 5 mM maltose (unless stated otherwise) as described (Guimarães et al., 2006). Reactions were started by adding 40 μL of yeast suspension to 20 μL containing 15 mM [14C]-maltose (about 1000 c.p.m. nmol−1) and any inhibitors specified in the text. Reactions were stopped after 10–300 s by addition of 10 mL ice-cold water and immediate filtration through a (prewashed) HVLP membrane (Millipore). The membrane was rinsed with another 10 mL of ice-cold water and transferred to a scintillation cocktail and the radioactivity in the trapped yeast was counted. To ensure linearity with respect to time, two reaction times, t and 2t, were used, each in duplicate. Reaction times were chosen according to the yeast and temperature, so that the reaction rate calculated from the 2t assays was at least 90% of that calculated from the t assays.
Stimulation of maltose transport by glucose
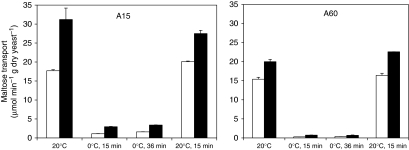
Where indicated, yeast suspensions were treated with glucose immediately before the maltose transport assays as described by Guimarães et al. (2008). The yeast suspension was mixed with 0.1 volume of 0.28 M glucose, incubated for 8 min at 20 °C and then maltose transport was assayed as described above. For the study of Fig. 1, the glucose-treated yeast was first assayed at 20 °C, then immediately transferred to an ice-water bath and assayed at 0 °C after 15 min and again after 36 min. The yeast suspension was then immediately returned to a 20 °C bath and assayed after a further 15 min.
Fig. 1.
Temperature dependence of maltose transport by lager (A15) and ale (A60) strains. Yeasts were harvested during growth on maltose at 24°C and their maltose transport activities were assayed at 20 and 0°C. For standard assays (white columns), the yeasts were equilibrated to 20°C for 8 min and then assayed at 20°C, transferred to 0°C and assayed at 0°C after 15 and 36 min and then returned to 20°C and reassayed at 20°C after 15 min. For glucose-activated assays (black columns), the same procedure was used, except that after 4 min at 20°C, glucose was added to 28 mM and incubation was continued for 8 min before the first 20°C assay. Results are averages±ranges of duplicate assays.
Results
Functionality and distribution of S. cerevisiae-type AGT1 genes among yeast strains
The AGT1 maltose transporter genes in two ale strains (A60 and A179) differ slightly from the sequence in the SGDB, but encode full-length proteins, whereas those in two lager strains (A15 and A24) encode truncated, 394 amino acid polypeptides because of a frame shift and a premature stop codon at nucleotide 1183 (Vidgren et al., 2005). To determine the functionality of these genes, the laboratory strain S150-2B (maltose-negative) was transformed with plasmids containing AGT1 genes from A15, A60 or A179 under PGK1 promoters or with the empty plasmid. Transformants were grown on glucose, harvested in the early stationary phase and assayed for maltose transport. Transformation with the empty plasmid or with AGT1 from lager strain A15 did not increase transport activity. Transformation with AGT1 from ale strains increased the maltose transport activity from <0.2 U g−1 dry yeast (control plasmid) to 13.1±0.4 U g−1 dry yeast (AGT1 from A60; mean±SD, n=3) or 10.0 U g−1 dry yeast (AGT1 from A179). Maltose transport by transformants carrying AGT1 genes from A60 or A179 was strongly inhibited by 50 mM maltotriose (85% and 79%, respectively) and 75 mM trehalose (94% and 85%, respectively), which is characteristic of maltose transport by the broad specificity Agt1 transporter. Between 0.5 and 55 mM maltose, the transporter encoded by AGT1 from A60 exhibited a single Km of 1.5 mM maltose, which is lower than that (5–10 mM) estimated by Han et al. (1995) and much lower than that reported (18 mM) by Stambuk & de Araujo (2001). These results show that the AGT1 genes from these two ale strains encode functional, broad-specificity α-glucoside transporters, whereas the defective AGT1 gene from lager strain A15 does not encode a functional maltose transporter.
The distribution of AGT1 genes in different kinds of industrial yeasts was studied by PCR (Table 1). All nine lager strains studied contained S. cerevisiae-type AGT1 genes with the same defect as strains A15 and A24. All five ale strains, both baker's strains and one of the three distiller's strains studied contained AGT1 genes without this defect. The other two distiller's strains lacked AGT1. It can be concluded that this particular AGT1 gene mutation, producing a premature stop codon, is characteristic of lager strains. These studied lager strains are not, to our knowledge, more closely related to each other than are lager strains in general.
Distribution of an S. bayanus-type AGT1 gene in brewer's yeast strains
The Sb-AGT1 gene (Nakao et al., 2009) is only 79% identical at the nucleotide level to AGT1 from S. cerevisiae, and so might not be revealed by earlier Southern hybridization and PCR studies using probes and primers designed for AGT1 from S. cerevisiae (Jespersen et al., 1999; Vidgren et al., 2005;). Using primers designed for Sb-AGT1, we found this gene in all the lager strains studied, but not in either studied ale strain (Table 1) or in the maltose-positive laboratory strain CEN.PK2-1D. In two tested lager strains, A15 and A24, the sequence of the Sb-AGT1 gene was 100% identical to that reported by Nakao et al. (2009) for Sb-AGT1 of WS34/70, which encodes a polypeptide of 610 amino acids.
Distribution of MTT1 genes in ale and lager strains
MTT1 genes have earlier been demonstrated in lager strains PYCC4457 (the type strain of S. carlsbergensis) (Salema-Oom et al., 2005) and A15, CMBS33, OG2252 and WS34/70 (Dietvorst et al., 2005). We found MTT1 in all nine tested lager yeast strains (including three of the above-mentioned ones), but not in any of the five ale strains (Table 1). An MTT1 gene was also present in both tested baker's strains and in one of the three distiller's strain, the same that also contained an AGT1 gene.
The temperature dependence of maltose uptake by brewer's yeast strains
Brewer's yeasts were harvested during growth on maltose at 24 °C and their maltose transport activities were assayed at different temperatures. For lager strain A15, the activity measured in the standard way at 0 °C was 9.3±0.9% of that at 20 °C, whereas for ale strain A60, the activity at 0 °C was 2.1±0.1% of that at 20 °C (Fig. 1, open columns).
Maltose transport is active and depends on the transmembrane electrochemical potential. When yeast cells growing on fermentable sugar are harvested, washed and suspended in a medium lacking a carbon source, their intracellular adenylate energy charge (and therefore their membrane potential) can decrease. The adenylate energy charge and maltose transport rates of such cells can be increased by treatment with glucose for a few minutes immediately before the zero-trans maltose uptake assay (Guimarães et al., 2008). This activation with glucose increased the maltose transport activity of both A15 and A60, but did not eliminate the difference in temperature sensitivity between the two yeasts. For glucose-activated A15, the maltose transport rate at 0 °C was 10.9±1.4% of that at 20 °C, and for glucose-activated A60, it was 3.4±0.3% (Fig. 1, black columns). These results showed that there was a difference between the temperature sensitivities of maltose transport by the lager and ale strains that could not be explained by differences in adenylate energy charge. In further work, standard assays, without glucose activation, were used.
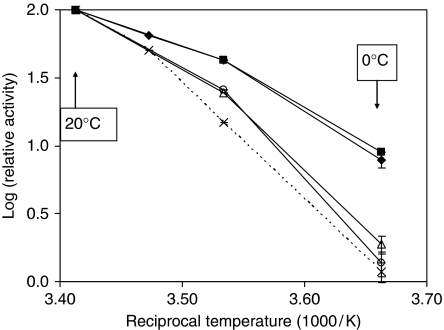
When harvested during growth on maltose, two lager strains and two ale strains had similar maltose transport activities at 20 °C, but the activities of both ale strains were markedly more temperature dependent than those of the lager strains (Fig. 2). At 20 °C, the difference between the lager and ale strains was not significant (in μmol min−1 g−1 dry yeast, 20.3±3.5 for the lager strains and 19.2±5.9 for the ale strains; means±SDs, n=5, P>0.72 (two-tail Student's t-test). However, at 0 °C, the lager strains had about fivefold greater activity than the ale strains and the difference was highly significant. Maltose transport activities at 0 °C in μmol min−1 g−1 dry yeast were 1.7±0.4 (8.4% of the 20 °C activity) for the lager strains and 0.31±0.05 (1.6% of the 20 °C activity) for the ale strains (means±SDs, n=5; P<0.002). The relatively smaller activities of the ale strains were also evident at 10 °C.
Fig. 2.
Arrhenius plots of maltose transport by the lager strains A15(♦) and A24(▪), ale strains A179(○) and A60(Δ) and the strain Integrant 1 (×). For each data set, rates are expressed as percentages of the rate at 20°C. Absolute rates (μmol min−1 g−1 dry yeast at 20°C) varied between 12 and 27 for A15, A24, A60 and A179 and between 2 and 4 for Integrant 1. Results at 0°C are means±SDs for A15 (n=4) and A60 (n=3) and means±ranges of independent duplicates for A179 and Integrant 1.
The temperature dependence of an Agt1-type transporter
Integrant 1 is a derivative of lager strain A15 containing a chimeric AGT1 in place of the defective native AGT1 of A15. The chimera consists of nucleotides 1 to x (where x is between 1183 and 1478) of an AGT1 gene from ale strain A60 and nucleotides x+1 to 1848 of the native AGT1 of strain A15, driven by a PGK1 promoter (Vidgren et al., 2009). It encodes a functional, 616 amino acid Agt1 transporter, with the same amino acid sequence as Agt1 of strain A60, because after nucleotide x, the ale and lager versions of AGT1 encode the same amino acid sequence (Vidgren et al., 2005). Compared with A15, Integrant 1 has considerably increased maltose and maltotriose transport activity during growth on glucose (when A15 has negligible activities) and slightly increased maltose transport activity, but considerably increased maltotriose transport activity during growth on maltose (Vidgren et al., 2009). Thus, Integrant 1 produces a functioning Agt1 transporter in a lager yeast background. When grown on glucose, this Agt1 is expected to be the only maltose transporter present (because glucose-grown A15 lacks maltose transport activity). The temperature dependence of maltose transport by glucose-grown Integrant 1 was much greater than that of the lager strains and at least as great as that of the ale strains (Fig. 2).
The temperature dependence of Malx1 and Mtt1 transporters
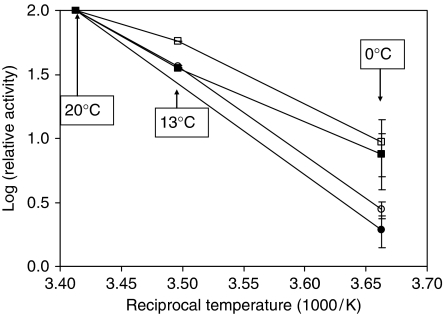
MALx1 (99% identical to MAL31 in the SGDB) and MTT1 (100% identical to the sequence reported by Dietvorst et al., 2005) were cloned from lager strain A15. The maltose-negative laboratory yeast, S150-2B, was transformed with plasmids containing these genes under the control of PGK1 promoters. Untransformed S150-2B had negligible maltose transport activity (<0.2 μmol min−1 g−1 dry yeast at 20 °C). MALx1 transformants had high activity (55 μmol min−1 g−1 dry yeast) during growth on glucose and lower activity (6 μmol min−1 g−1 dry yeast) in the stationary phase. Both growing- and stationary-phase transformants exhibited strong temperature dependence (Fig. 3). The rates at 0 °C compared with 20 °C were 2.8±0.4% (mean±SD, n=5) for growing cells and 1.9±0.6% (mean±range; n=2) for stationary-phase cells.
Fig. 3.
Arrhenius plots of maltose transport by Mtt1 and Malx1 transporters. Maltose transport was measured using S150-2B transformed with MTT1 (□, ▪) or MALx1 (○, •). The maltose concentration in the transport assay was 5 mM (□, ○, •) or 50 mM (▪) and transformants were harvested during growth on glucose (at OD600 nm of 3–5; □, ▪, ○) or in the stationary phase (OD600 nm of 10; •). Rates at 13 and 0°C are expressed as percentages of the rate at 20°C. Values at 0°C are (□, ○) means±SDs (n=4 or 5) or (▪, •) means±ranges of duplicate experiments.
Compared with MALx1 transformants, both growing and stationary-phase MTT1 transformants exhibited lower maltose transport activity at 20 °C, about 1.0 μmol min−1 g−1 dry yeast at 5 mM maltose and 6.5 μmol min−1 g−1 dry yeast at 50 mM maltose. Mtt1 is reported to have a high Km for maltose (60–90 mM, Salema-Oom et al., 2005; 40 mM, Multanen, 2008). At both maltose concentrations, the transport activity exhibited relatively small temperature dependence (Fig. 3). Activities at 0 °C compared with 20 °C were 9.4±4.4% (5 mM; mean±SD, n=4) and 7.5±2.5% (50 mM; mean±range, n=2). The absolute maltose transport activity at 50 mM maltose (6.5 μmol min−1 g−1 dry yeast) was similar to that at 5 mM maltose of stationary-phase cells transformed with MALx1 (6 μmol min−1 g−1 dry yeast). Thus, the marked differences in temperature dependence between cells transformed with MALx1 and cells transformed with MTT1 are not explained by differences in their absolute maltose transport activities.
Discussion
Because Agt1 is the only maltose transporter known to accept both trehalose and α-methyl glucoside as substrates (Day et al., 2002b; Salema-Oom et al., 2005;), the strong inhibition of maltose transport by both trehalose and α-methyl-glucoside in ale strains, but not lager strains (Rautio & Londesborough, 2003; Vidgren et al., 2005;), suggests that Agt1 transporters are the dominant maltose transporters in ale strains, but not lager strains. Vidgren et al. (2005) showed that two lager strains, A15 and A24, contain AGT1 genes with a premature stop codon starting at nucleotide 1183. Here, we show that the AGT1 from A15 does not encode a functional maltose transporter. Nakao et al. (2009) also found this premature stop codon in an AGT1 gene in a third lager strain, WS34/70. We extend these results to show that the premature stop codon at 1183 is present in the S. cerevisiae-type AGT1 genes of all nine tested lager strains, but is absent from all five tested ale strains. Because the premature stop codon is caused by an easily reversible point mutation, then if the Agt1 transporter were advantageous to lager strains in their normal habitat, one would expect to find lager strains in which this reversal has occurred. The observations that reversal has not occurred in any of the nine tested lager strains, whereas the mutation causing the premature stop codon was not present in any of the five ale strains, suggest that there has been selection pressure in favour of inactivation of AGT1 during the evolution of lager strains, but not during the evolution of ale strains. The main difference between the conditions under which ale and lager strains have evolved is the lower temperature of lager fermentations. MTT1 genes were present in all nine lager strains, but in none of the five ale strains. This suggests that the replacement of Agt1 transporters by Mtt1 transporters is an important difference between lager and ale strains, probably related to the lower temperature of lager fermentations.
We show that maltose transport is more strongly temperature dependent in two tested ale strains than in two tested lager strains (the yeasts were grown at 24 °C and then assayed at different temperatures). At 20 °C, all four strains had similar maltose transport activity, but at 0 °C, the ale strains showed about fivefold smaller activities. When single maltose transporters were studied, using genetically engineered strains, their temperature dependence decreased in the order Agt1≥Malx1>Mtt1. The temperature dependence of Mtt1 (in a laboratory strain) was similar to that of maltose transport by lager yeasts. An ale-type Agt1 transporter working in a lager yeast (Integrant 1) had the high temperature dependence of maltose transport observed for ale yeasts (Fig. 2). This suggests that the different temperature dependencies of maltose transport by ale and lager yeasts result from the different maltose transporters present in these yeasts rather than, for example, a hypothetical difference in the lipid composition of their plasma membranes. Thus, the Agt1 transporter in Integrant 1 had been inserted into a lager yeast membrane, but still exhibited high temperature dependence, which therefore was a property of the transporter protein itself rather than a property of the lipid membrane of ale strains.
We do not yet know what differences between Agt1 and Mtt1 transporters account for their different temperature dependencies. Membrane proteins are sensitive to membrane lipid composition and dynamics. They can have specific lipid requirements for their optimal activity (Opekarová & Tanner, 2003), correct orientation of transmembrane helices (Bogdanov et al., 2002), targeting to the yeast plasma membrane (Umebayashi & Nakano, 2003; Toulmay & Schneiter, 2007;) and stable localization in the plasma membrane (Mitsui et al., 2009). The high temperature dependence of reactions catalysed by enzymes embedded in lipid membranes may result from work carried out by the enzyme on surrounding lipid as a result of changes in protein shape during the catalytic cycle (Londesborough, 1980). Thus, one possibility is that Agt1 exhibits greater shape changes than Mtt1 during the catalytic cycle, and so performs more work on the surrounding lipid membrane. Nakao et al. (2009) found seven α-glucoside transporter genes in the genome of lager yeast WS34/70, two of which, S. bayanus-derived MALx1 and S. cerevisiae-derived AGT1, encoded truncated proteins. They also noted an increase in the copy number of MTT1, which was present on both the S. bayanus and the S. cerevisiae versions of chromosome VII. These results are consistent with the suggestion that in lager strains, Mtt1 transporters have become more important at the expense of Agt1 and Malx1 transporters. Nakao et al. (2009) located a MAL31 gene to S. cerevisiae chromosome II (Chr. Sc-II) and an MPH2 gene to Chr. Sc-IV. These loci were earlier observed in most, but not all, studied lager strains by hybridization of specific probes to chromosome blots (Jespersen et al., 1999; Vidgren et al., 2005;). These hybridization studies found binding of a MAL61-probe (expected to recognize all MALx1 genes) to Chr. VII from both lager and ale strains, whereas Nakao et al. (2009) found an MTT1 gene on both the S. cerevisiae and the S. bayanus versions of Chr. VII from lager strain WS34/70. MTT1 is 91% identical to MALx1, and so probably in the lager strains, the MAL61-probe bound to MTT1 genes, which were not known at the time of these hybridization studies. Nakao et al. (2009) found a truncated S. cerevisiae-derived AGT1 gene, but did not locate the gene. Presumably, this is the AGT1 detected on Chr. VII in both hybridization studies. Nakao et al. (2009) also reported an S. bayanus-derived AGT1 (Sb-AGT1) on Chr. Sb-XV-VIII and a truncated, S. bayanus-derived MAL31 on Chr. Sb-V. Neither of these genes was noted in the hybridization studies, which used probes based on S. cerevisiae sequences. Our present results show that Sb-AGT1 is present in all eight studied lager strains, but, as expected, in neither of the two ale strains studied. The sequence of the Sb-AGT1 in strains A15 and A24 was identical to that reported by Nakao et al. (2009). No information is available on the catalytic properties of the transporter encoded by this gene (it is only 79% identical to the AGT1 from S. cerevisiae).
Both hybridization studies detected MAL21 and MAL41 genes on Chr. III and Chr. XI, respectively. Such genes were not observed by Nakao et al. (2009) in Weihenstephan 34/70. The estimated sequence coverage was 95.8%, and so one or both genes might be in the unsequenced 4.2%. Alternatively, Weihenstephan 34/70 may lack these genes. Hybridization indicated that MAL21 was lacking from 13 of the 25 studied lager strains and MAL41 was lacking from one lager strain (Jespersen et al., 1999). Both our present results and those of Nakao et al. (2009) show that in lager strains, the number of different functional α-glucoside transporters is less than the number of different α-glucoside transporter (pseudo)genes. Inactivation of genes encoding less suitable transporters seems to be one way in which lager strains have evolved for low-temperature fermentations.
Acknowledgments
Silja Home, Jukka Kronlöf and Esko Pajunen are thanked for their interest and encouragement. Merja Helanterä, Outi Könönen, Aila Siltala and Pirjo Tähtinen are thanked for skilled technical assistance. Yoshihiro Nakao is thanked for sharing unpublished sequence data for LBYG1387. The financial support of the Finnish malting and brewing industry (PBL) is gratefully acknowledged.
Statement
Re-use of this article is permitted in accordance with the Terms and Conditions set out at http://www3.interscience.wiley.com/authorresources/onlineopen.html
References
- Alves SL, Jr, Herberts RA, Hollatz C, Trichez D, Miletti LC, de Araujo PS, Stambuk BU. Molecular analysis of maltotriose active transport and fermentation by Saccharomyces cerevisiae reveals a determinant role for the AGT1 permease. Appl Environ Microb. 2008;74:1494–1501. doi: 10.1128/AEM.02570-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth C. Tap into the Art and Science of Brewing. New York: Plenum Press; 1998. [Google Scholar]
- Bogdanov M, Heacock PN, Dowhan W. A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 2002;21:2107–2116. doi: 10.1093/emboj/21.9.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar R, Palmfeldt J, Gustafsson JS, Pettersson E, Hashemi SH, Blomberg A. Comparative proteomics of industrial lager yeast reveals differential expression of the cerevisiae and non-cerevisiae parts of their genomes. Proteomics. 2007;7:4135–4147. doi: 10.1002/pmic.200601020. [DOI] [PubMed] [Google Scholar]
- Crumplen RM, Slaughter JC, Stewart GG. Characteristics of maltose transporter activity in an ale and lager strain of the yeast Saccharomyces cerevisiae. Lett Appl Microbiol. 1996;23:448–452. doi: 10.1111/j.1472-765x.1996.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Day RE, Higgins VJ, Rogers PJ, Dawes IW. Characterization of the putative maltose transporters encoded by YDL247w and YJR160c. Yeast. 2002a;19:1015–1027. doi: 10.1002/yea.894. [DOI] [PubMed] [Google Scholar]
- Day RE, Rogers PJ, Dawes IW, Higgins VJ. Molecular analysis of maltotriose transport and utilization by Saccharomyces cerevisiae. Appl Environ Microb. 2002b;68:5326–5335. doi: 10.1128/AEM.68.11.5326-5335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietvorst J, Londesborough J, Steensma HY. Maltotriose utilization in lager strains: MTT1 encodes a maltotriose transporter. Yeast. 2005;22:775–788. doi: 10.1002/yea.1279. [DOI] [PubMed] [Google Scholar]
- Dunn B, Sherlock G. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 2008;18:1610–1623. doi: 10.1101/gr.076075.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BR, Boulton CA, Box WG, Graham NS, Lawrence SJ, Linforth RST, Smart KA. Carbohydrate utilization and the lager yeast transcriptome during brewery fermentation. Yeast. 2008;25:549–562. doi: 10.1002/yea.1609. [DOI] [PubMed] [Google Scholar]
- Gietz D, StJean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Barrio E, Querol A. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl Environ Microb. 2008;74:2314–2320. doi: 10.1128/AEM.01867-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães PMR, Virtanen H, Londesborough J. Direct evidence that maltose transport activity is affected by the lipid composition of brewer's yeast. J Inst Brew. 2006;112:203–209. [Google Scholar]
- Guimarães PMR, Multanen J-P, Domingues L, Teixeira JA, Londesborough J. Stimulation of zero-trans rates of lactose and maltose uptake into yeasts by pre-incubation with hexose to increase the adenylate energy charge. Appl Environ Microb. 2008;74:3076–3084. doi: 10.1128/AEM.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot S, Ferret E, Gervais P. Yeast survival during thermal and osmotic shocks is related to membrane phase change. J Agric Food Chem. 2006;54:8450–8455. doi: 10.1021/jf0620158. [DOI] [PubMed] [Google Scholar]
- Hammond JRM. Brewer's yeasts. In: Rose AH, Harrison JS, editors. The Yeasts. Vol. 5. London: Academic Press; 1993. pp. 7–67. [Google Scholar]
- Han EK, Cotty F, Sottas C, Jiang CH, Michels CA. Characterization of AGT1 encoding a general α-glucoside transporter from Saccharomyces. Mol Microbiol. 1995;17:1093–1107. doi: 10.1111/j.1365-2958.1995.mmi_17061093.x. [DOI] [PubMed] [Google Scholar]
- Hornsey I. A History of Brewing. Cambridge: RSC Paperbacks; 2003. [Google Scholar]
- James TC, Campbell S, Donnelly D, Bond U. Transcription profile of brewery yeast under fermentation conditions. J Appl Microbiol. 2003;94:432–448. doi: 10.1046/j.1365-2672.2003.01849.x. [DOI] [PubMed] [Google Scholar]
- Jespersen L, Cesar LB, Meaden PG, Jakobsen M. Multiple α-glucoside transporter genes in brewer's yeast. Appl Environ Microb. 1999;65:450–456. doi: 10.1128/aem.65.2.450-456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobi D, Zugmeyer S, Potier S, Jaquet-Gutfreund L. Two-dimensional protein map of an ‘ale’-brewing yeast strain: proteome dynamics during fermentation. FEMS Yeast Res. 2004;5:213–230. doi: 10.1016/j.femsyr.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Fukui N, Ashikari T, Shibano Y, Morioka-Fujimoto K, Hiraki Y, Nakatani K. Improvement of maltose fermentation efficiency: constitutive expression of MAL genes in brewing yeasts. J Am Soc Brew Chem. 1995;53:24–29. [Google Scholar]
- Londesborough J. The causes of sharply bent or discontinuous Arrhenius plots for enzyme-catalysed reactions. Eur J Biochem. 1980;105:211–215. doi: 10.1111/j.1432-1033.1980.tb04491.x. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Hatakeyama K, Matsushita M, Kanazava H. Saccharomyces cerevisiae Na+/H+antiporter Nha1p associates with lipid rafts and requires sphingolipid for stable localization to the plasma membrane. J Biochem. 2009;145:709–720. doi: 10.1093/jb/mvp032. [DOI] [PubMed] [Google Scholar]
- Multanen J-P. 2008. Genetic and environmental factors affecting α-glucoside uptake by lager yeasts. Master's Thesis, Helsinki University of Technology, Helsinki.
- Nakao Y, Kanamori T, Itoh T, Kodama Y, Rainieri S, Nakamura N, Shimonaga T, Hattori M, Ashikari T. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 2009;16:115–129. doi: 10.1093/dnares/dsp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova ES, Naumov GI, Masneuf-Pomarède I, Aigle M, Dubourdieu D. Molecular genetic study of introgression between Saccharomyces bayanus and S. cerevisiae. Yeast. 2005;22:1099–1115. doi: 10.1002/yea.1298. [DOI] [PubMed] [Google Scholar]
- Opekarová M, Tanner W. Specific lipid requirements of membrane proteins-a putative bottleneck in heterologous expression. Biochim Biophys Acta. 2003;1610:11–22. doi: 10.1016/s0005-2736(02)00708-3. [DOI] [PubMed] [Google Scholar]
- Querol A, Bond U. The complex and dynamic genomes of industrial yeasts. FEMS Microbiol Lett. 2009;293:1–10. doi: 10.1111/j.1574-6968.2008.01480.x. [DOI] [PubMed] [Google Scholar]
- Rautio J, Londesborough J. Maltose transport by brewer's yeasts in brewer's wort. J Inst Brew. 2003;109:251–261. [Google Scholar]
- Salema-Oom M, Pinto VV, Gonçalves P, Spencer-Martins I. Maltotriose utilization by industrial Saccharomyces strains: characterization of a new member of the α-glucoside transporter family. Appl Environ Microb. 2005;71:5044–5049. doi: 10.1128/AEM.71.9.5044-5049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kishimoto M, Watari J, Takashio M. Breeding of brewer's yeast by hybridization between a top-fermenting yeast Saccharomyces cerevisiae and a cryophilic yeast Saccharomyces bayanus. J Biosci Bioeng. 2002;5:509–511. doi: 10.1016/s1389-1723(02)80101-3. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink G, Hicks JB. Methods in Yeast Genetics. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1983. [Google Scholar]
- Stambuk BU, de Araujo PS. Kinetics of active α-glucoside transport in Saccharomyces cerevisiae. FEMS Yeast Res. 2001;1:73–78. doi: 10.1111/j.1567-1364.2001.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Stambuk BU, Alves SL, Jr, Hollatz C, Zastrow CR. Improvement of maltotriose fermentation by Saccharomyces cerevisiae. Lett Appl Microbiol. 2006;43:370–376. doi: 10.1111/j.1472-765X.2006.01982.x. [DOI] [PubMed] [Google Scholar]
- Tornai-Lehoczki J, Dlauchy D. Delimination of brewing yeast strains using different molecular techniques. Int J Food Microbiol. 2000;62:37–45. doi: 10.1016/s0168-1605(00)00356-1. [DOI] [PubMed] [Google Scholar]
- Toulmay A, Schneiter R. Lipid-dependent surface transport of the proton pumping ATPase: a model to study plasma membrane biogenesis in yeast. Biochimie. 2007;89:249–254. doi: 10.1016/j.biochi.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Umebayashi K, Nakano A. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J Cell Biol. 2003;161:1117–1131. doi: 10.1083/jcb.200303088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidgren V, Ruohonen L, Londesborough J. Characterization and functional analysis of the MAL and MPH loci for maltose utilization in some ale and lager yeast strains. Appl Environ Microb. 2005;71:7846–7857. doi: 10.1128/AEM.71.12.7846-7857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidgren V, Ruohonen L, Londesborough J. Proceedings of the 31st European Brewing Convention Congress, Venice, 6–10 May 2007. Vol. 46. Nurnberg: Fachverlag Hans Carl; 2007. Lager yeasts lack AGT1 transporters, but transport maltose at low temperatures faster than ale yeasts; pp. 438–444. [Google Scholar]
- Vidgren V, Huuskonen A, Virtanen H, Ruohonen L, Londesborough J. Improved fermentation performance of a lager yeast after repair of its AGT1 maltose and maltotriose transporter genes. Appl Environ Microb. 2009;75:2333–2345. doi: 10.1128/AEM.01558-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pöhlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;13:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Walsh RM, Martin PA. Growth of Saccharomyces cerevisiae and Saccharomyces uvarum in a temperature gradient. J Inst Brew. 1977;83:169–172. [Google Scholar]