Abstract
Background
The cerebellum is a brain region recognized primarily in the coordination of movement and related accessory motor functions. In addition, emerging evidence implicates the cerebellum in cognitive processes and suggests that this brain region may be subject to experience-dependent plastic changes in structure. Therefore, the aim of this study was to evaluate the role of early environmental deprivation in the maturation of the cerebellum and aspects of cognitive development.
Methods
Structural MRI volumes of 12 cerebellar sub-regions from 31 previously-neglected and 30 typically developing children were compared to subjects’ corresponding neuropsychological test scores.
Results
Neglected children had smaller volume of the superior-posterior cerebellar lobes. Moreover, superior-posterior lobe volume was found to mediate neuropsychological test performance differences between groups, with larger volumes yielding better outcomes on tests of memory and planning.
Conclusions
These data support the importance of experience-dependent plastic changes in cerebellar structure and highlight the role of the cerebellum in higher cognitive functions.
Keywords: cerebellum, experience-dependent plasticity, deprivation, post-institutionalized children, cognitive development, superior-posterior lobes
Introduction
The cerebellum is a brain region that has been traditionally associated with motor control, physical coordination, and balance. Recent data suggest that distinct regions of the cerebellum have a significant role in cognition and learning (1-7). Still, relatively little is known about the development of the cerebellum with regard to higher-order cognition. Much research on the cerebellum has focused on individuals with congenital malformations, focal lesions, or localized degeneration of this brain region (8-10). Observational studies of these patients has resulted in the term “Cerbellar Cognitive Affective Syndrome,” which refers to a constellation of behavioral symptoms that include problems in executive functions (decision making, set-shifting, and working memory), visual-spatial deficits, emotional alterations (obsessive-compulsive behaviors, disinhibition, and flat affect), and linguistic abnormalities (11). Recent data suggests that the development of the cerebellum is more dependent upon environmental factors than most other brain regions (12). Therefore, the goal of the present study is to examine the potential role of experience-dependent plasticity in cerebellar development.
The role of the cerebellum in human cognition
Studies involving application of retrograde and anterograde neurotropic tracers have permitted researchers to observe reciprocal connectivity between the cerebellum and neocortex. Consequently, robust neural networks have been documented between specific cerebellar regions and neocortical areas involved in higher-order functions. Prior research suggested that the cerebellum provided output only to the primary motor cortex (M1) via a primary three-node pathway from cerebellum to thalamus to cerebrum (13-15). More recently, retrograde tracing procedures have revealed direct pathways between the dorsolateral prefrontal cortex (area 46) and cerebellum in nonhuman primates (16, 17). The application of anterograde tracer methods further suggests that the cerebellum exhibits a functional topographic alignment with the cerebral cortex. For example, dorsal portions of the cerebellar dentate nucleus project to primary motor and premotor regions of the cortex, whereas more ventral regions of this nucleus project to prefrontal and posterior parietal cortical areas, suggesting an anatomical segregation of motor and nonmotor processing domains in the cerebellum (18-21).
Functional neuroimaging studies also provide evidence that the cerebellum moderates some cognitive-behavioral processes. A variety of tasks in which participants are presented with emotional images (22), a pegboard puzzle (4), the Tower of London task (23), numbers to be remembered (24), and overt semantic discrimination between words (25) have all produced activation in the cerebellum that is not associated with motor responses. Transcranial magnetic stimulation (TMS) studies have also demonstrated an electrophysiological link between the cerebellum and higher-order behavior. Specifically, the administration of TMS pulses over the midline vermis of the cerebellum produced increased theta oscillations within areas of the prefrontal cortex, a correlate of complex cognitive processes (26).
Although much research demonstrates a role of the cerebellum in cognition, the precise neurobiological mechanisms by which the cerebellum regulates cognitive functioning is still unclear. Schmahmann, Weilburg, & Sherman (2007) (9) propose that the cerebellum acts as a specialized conflict monitor that serves to regulate behavior around a stable physiological level and to streamline it according to environmental context. According to the authors, if the cerebellum experiences damage or malformation within neural regions that subserve cognitive functioning, the behavioral symptoms of the Cerebellar Cognitive Affective Syndrome will result. Ito (2008) (27) further proposes that the cerebellum functions as a “unit learning machine” that encodes an internal model for a particular type of behavior. As mismatch occurs between behavioral output and the internal model, error signals are carried via climbing fibers to cerebellar microcomplexes until behavior is appropriately modified. Cognitive representations within the cerebellum appear to be facilitated by connections with temporo-parietal and prefrontal cortical areas (19, 28).
Developmental factors in cerebellar organization
The development of the cerebellum may be highly influenced by environmental input. For example, heritable influences on cerebellar volume are less than for other brain regions (12). Evidence also suggests that the evolutionary development of associative neocortical areas occurred in parallel with the posterolateral cerebellar hemispheres (29). In addition, the cerebellum appears to have a very protracted period of development, suggesting that this region may be especially vulnerable to environmental factors. Neurogenesis continues in the cerebellum post-natally through the first two years of life, and limited post-mortem data indicate that it may continue to undergo important neurodevelopmental processes between late childhood and early adulthood (30).
Very few studies, however, have examined the relationship between post-natal experience and its influence on cerebellar development and cognitive outcomes. One study reported that children diagnosed with maltreatment-related post-traumatic stress disorder (PTSD) had smaller cerebellar volumes as compared with control subjects; cerebellum size was positively correlated with the age of onset of the trauma and negatively correlated with the duration of the trauma that led to PTSD. In addition, cerebellar volume was positively correlated with IQ measures (31). However, it is not known whether differences in brain volume and cognitive performance are related to maltreatment per se versus PTSD resulting from traumatic experience. The influence of physiological and social deprivation on the development of the pediatric cerebellum during a specific post-natal period is still relatively unknown. Here, we report on a sample of children who experienced severe deprivation for several months after birth followed by a drastic change in their environments. These children began their lives in institutionalized care and were then adopted into enriched family environments. The quality of care in institutionalized settings often involves many forms of social and physical deprivation that are insufficient to promote healthy child development (32). Not surprisingly, post-institutionalized children are known to exhibit a multitude of motor (33-35), cognitive (36, 37), and emotional difficulties (38, 39) even after years of nurturing experiences in their adoptive homes.
The present study sought to evaluate the role of early deprivation in the development of the cerebellum and aspects of cognitive development. Because distinct regions of the cerebellum appear to be related to higher-order behaviors, we used methods that allowed us to parse the cerebellum into anatomical subdivisions including three main cortical lobes, the vermis, and the bilateral corpora medullare. Based upon prior studies, we expected that the children who had experienced early deprivation would show delays in key neuropsychological tasks. Our main hypotheses evaluated whether these children also had smaller overall cerebellar volumes compared to control subjects and, importantly, whether specific volumetric deficits such as those in the posterior hemispheres mediated performance on cognitive tasks.
Method
Participants
Sixty-one children participated in this study. The post-institutionalized group consists of 15 males and 16 females (aged 9;0 to 14;11, M=10.9 years, SD=1.63). These children were compared with a group of 16 males and 14 females (aged 9;0 to 14;11, M=11.3 years, SD=1.68). Prior to adoption, post-institutionalized children were raised in orphanages in Romania (n=12), Russia (n=12), China (n=5), and other Eastern countries (n=2) for, on average, 31 months after birth (range 4 to 77 months, SD=16.97). The post-institutionalized children were drawn from the Wisconsin International Adoption Project Registry of families created through international adoption that expressed interest in being contacted about research participation, while control families were acquired through community advertisements in flyers. Post-institutionalized children were adopted at 10 months of age or older (range 10 to 92 months, M=38 months, SD=21.2) and remained with their adoptive families for at least 39 months prior to the study (range 39 to 126 months, M=93 months, SD=19.0). Children were not recruited for this study if they were diagnosed with a pervasive developmental disorder or fetal alcohol syndrome, or if the adolescent could not participate in the MRI procedures (e.g., ferromagnetic implants, braces, etc.). All subjects received fifty dollars for participating in the MRI session. Children's handedness was assessed with the Edinburgh Handedness Inventory. Ninety percent of children were right-handed, and handedness was not related to any dependent measure. Parents and children provided informed consent and assent, respectively, and the procedures were approved by the University of Wisconsin Health Science IRB.
Procedures
Structural Imaging
High-resolution anatomical MRI images were obtained using a 3-Tesla GE SIGNA (40) scanner with a quadrature head RF coil. A three-dimensional, inversion recovery (IR) pulse sequence was used to generate T1-weighted images with the following parameters: TR/TE = 21/8 ms, flip angle = 308, 240 mm field of view, 256-192 in-plane acquisition matrix (interpolated on the scanner to 256-256), and 128 axial slices (1.2 mm thick) covering the whole brain. A three-dimensional Fast-Spin Echo pulse sequence was used to generate T2-weighted images. All acquired neuroimages were registered into standardized stereotaxic space using a linear transformation and corrected for nonuniformity artifacts (41). The registered and corrected volumes were then segmented into white matter, gray matter, CSF, and extraneous tissue via a specialized neural net classifier, a computer algorithm that can predict and define complex neuroanatomical boundaries (42). Before MRI scanning, participants were oriented to the MRI through the use of a mock-MRI simulator. During MRI acquisition, participants were instructed to stay as still as possible and were able to watch a movie of their choosing.
The total volume of the neocortex was measured by using an automated procedure developed at the Montreal Neurological Institute that combines voxel-intensity based tissue classification into gray matter, white matter, and CSF with a probabilistic atlas (43, 44). Because the cerebellum exhibits a complex gray-white matter border, it could not be classified using the above automated method. Instead, volumetric measurements of the cerebellum were conducted using BRAINS2 (45, 46). BRAINS2 implements a semi-automated neural net procedure to parcellate each lobe of the cerebellar cortex and corpus medullare. Specifically, 31 anatomical landmarks were manually selected before applying the neural net to generate surface masks of each cerebellar region. The parcellated masks were then reviewed and manually edited. Masks from five control and three post-institutionalized subjects were incomplete in the extreme regions of the inferior cerebellar lobes, since neural tissue was cutoff in these brain regions on subjects’ segmented neuroimages. These areas had to be manually traced after the neural net procedure by using each subject's intact T1 image as a guide. All left and right hemispheric divisions of the cerebellum had interrater intraclass correlations of 0.97 or greater. Volumes of the cerebellar areas were then measured and summed to obtain overall volume of the cerebellum.
Surface masks of the midline cerebellar vermis were manually traced. The sagittal plane was used to complete a guide trace that defined the superior, inferior, anterior, and posterior extent of the vermis, while the axial plane was implemented in order to place guide traces that defined the lateral vermal boundaries. The overall vermis was manually traced in each coronal slice by referencing these designated guide traces. Regional subdivision was completed in the sagittal plane by specifying limiting boundaries just at the points where the primary and prepyramidal cerebellar fissures became difficult to discern throughout the vermis.
In order to assess reliability of the manually defined vermis tracings, two authors (RKP and PMB) served as raters and compared each of their ROIs from five randomly selected subject brain images. The interrater intraclass correlations for the whole vermis and its individual anterior, superior, and inferior divisions were all greater than 0.72 and in agreement with accepted values in previous literature (47).
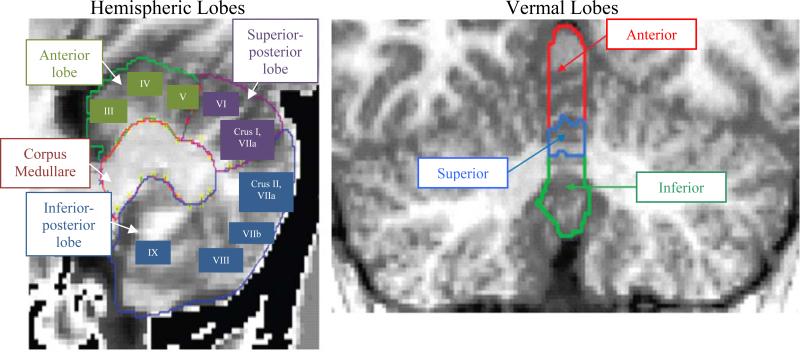
The left and right cerebellar hemispheres were each parcellated into anterior, superior-posterior, and inferior-posterior lobes in accordance with the anatomical nomenclature of Larsell and Jansen (48). The anterior lobe was defined as lobules I-V and demarcated by the primary fissure. The superior-posterior lobe included lobules VI and VIIA-folium in the midline vermis and lobule VI and crus I of VIIA in the hemispheres. The inferior-posterior lobe was composed of lobules VIIA-tuber through X. The superior- and inferior-posterior lobes were separated by the horizontal fissure. White matter extending into the folia was included in the three main cerebellar lobes, which are primarily composed of gray matter. In addition, the bilateral corpora medullare were defined to include the entirety of the central cerebellar white matter. The utilized neuroimaging acquisition procedure was not able to discern the deep nuclei embedded within the corpus medullare. The vermis was parcellated into anterior, superior, and inferior regions, respectively. The primary fissure defined the posterior extent of the anterior vermis, and the prepyramidal fissure specified the boundary between the superior and inferior vermis. All parcellated cerebellar regions are shown in Figure 1.
Figure 1.
Regional Cerebellar Parcellations for Measurement
Behavior
Each adolescent was assessed with seven standardized tests from the Cambridge Neuropsychological Test Automated Battery (49). This subset of CANTAB tests probes domains of cognition that have been documented as difficulties for post-institutionalized children (50).
Prior to administration of the cognitive tests, a CANTAB screening of general motor ability was presented to subjects. The Motor Screening test (MOT) exploits motor problems by requiring subjects to touch a flashing cross at different locations on the screen. Difficulties in motor movement are measured by each subject's time response latency.
Memory functions were measured with the Delayed Matching to Sample (DMS), Paired Associates Learning (PAL), and Pattern Recognition Memory (PRM) tasks. DMS presents subjects with a complex abstract pattern and after a period of brief delay, requires subjects to select the correct pattern previously seen from four similar patterns. Test performance is assessed by the correct number of responses the subject makes. The PAL task begins with a series of boxes presented on the computer screen. The contents of the boxes are then shown in randomized order, with one or more of them containing an abstract pattern. The patterns are then presented in the middle of the screen one at a time, and subjects are then required to select the box containing the respective pattern's correct location. If a subject makes an error, the patterns are shown again to remind him or her of their locations. The difficulty of the trials progresses throughout the test, and subject performance is measured via the total number of errors made. PRM presents subjects with twelve visual images one at a time, which cannot easily be given verbal labels. Subjects are subsequently asked to discriminate between a previously viewed pattern and a new image. The order of pattern presentation is reversed from the original order. Subject performance is assessed through the number of correct trials completed.
Executive functions were assessed with the Intra-Extra Dimensional Set Shift (IED), Stockings of Cambridge (SOC), and Spatial Working Memory (SWM) tests. These tests specifically examine working memory and planning abilities. During the IED test, participants are shown two different dimensions, including solid color-filled shapes and white lines. Simple stimuli consist of only one of these dimensions, while more complex stimuli include both, specifically the overlay of white lines upon solid shapes. Participants are initially shown two different simple stimuli and are asked to select the shape which matches the rule, which is then altered. These rule shifts are initially intra-dimensional (colored shapes are the only relevant dimension) and then become extra-dimensional (white lines become the only relevant dimension). The total number of errors during this task is used as a measurement of performance. SOC is a version of the Tower of London spatial planning test. Participants are shown two displays, each of which contains three colored balls. The displays are presented in such a way that they can be perceived as stacks of colored balls held in stockings, or socks, suspended from a beam. The subject is required to use the balls in the lower display to match the pattern in the upper display. The balls can be moved one at a time by touching a ball on the screen and then moving it to the correct position. Test performance is measured by the correct number of matches each subject completes in the minimum number of moves. SWM tests an individual's ability to retain spatial information and to manipulate items in working memory. By touching boxes and applying a process of elimination, participants will find one blue ‘token’ in each of a quantity of boxes and use them to fill an empty column on the right side of the screen. A participant's score is based upon total errors (touching boxes that have already been found to be empty and revisiting boxes which have already been revealed to contain a token).
Attentional abilities were measured with the Match to Sample test (MTS). This test assesses a child's reaction time to identifying a stimulus. Children are shown a complex visual pattern in the middle of a computer screen, and then, after a brief delay, a varying number of similar patterns are shown in a circle of boxes around the edge of the screen. Only one of these boxes matches the pattern in the center of the screen, and the subject must indicate which it is by touching it. The correct number of trials is used as an assessment of subject performance.
Demographic characteristics
Several individual background variables describing the post-institutionalized children were collected via parent report. Information about the duration of institutionalization, height or weight at time of adoption, country of origin, and condition of the orphanage setting for each child was assessed and correlated with cerebellar lobe volumes.
Results
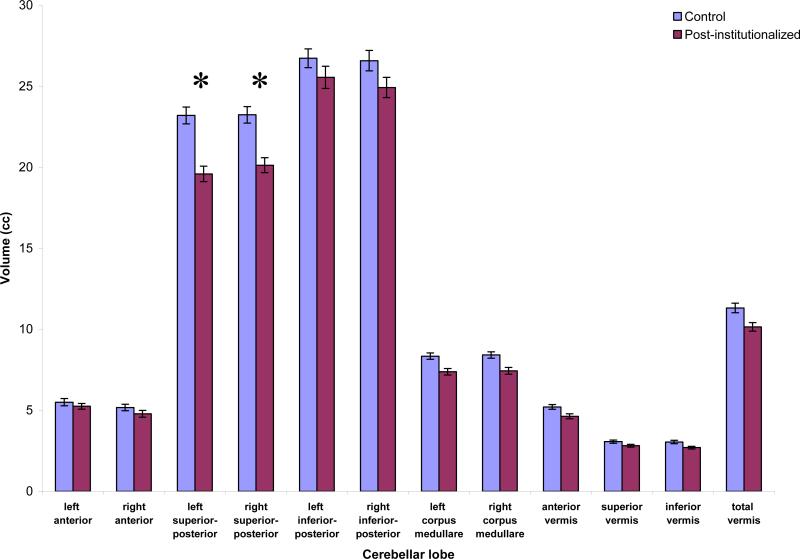
Determination of cerebellar volumetric differences between groups was conducted using a multivariate analysis of variance (MANOVA), with group (Post-institutionalized, Control) as a between-subject factor and the five cerebellar regions as within-subject factors. Cerebellar volumetric analyses were corrected for whole brain volume. Post-institutionalized children exhibited smaller volume of the left, F (1,58) = 10.9, p = .002, and right, F (1, 58) = 7.4, p = .009, superior-posterior lobes compared to control subjects. These effects remained after applying a Greenhouse-Geisser correction for repeated measures, F (3.1, 181.5) = 4.3, p = .005. The two groups of children had similar volumes for other cerebellar regions (Figure 2). To evaluate the relationships between left and right superior-posterior lobe volumes within each group, we used paired-samples t-tests. While controls showed similar volumes in the left and right superior-posterior lobes, the post-institutionalized children had smaller volumes in the left, as compared to the right, t(30) = -2.53, p = .017. Superior-posterior lobe volumes among the post-institutionalized children were not related to any demographic variables.
Figure 2.
Group Comparison of Cerebellar Regional Volumes
Next, we examined the relation between cerebellar volumes and the adolescent's cognitive performance using a multivariate analysis of variance (MANOVA). As shown in Table 1, and consistent with previous reports, the post-institutionalized children displayed impaired performance relative to age-matched controls on a number of tasks, Wilk's Lambda F (7, 53) = 5.4, p < .001. In order to formally test whether left and right superior-posterior cerebellar lobes mediated cognitive performance, we used the joint significance test (51). Linear regression analyses revealed that group status accounted for 16% of the variance in left superior-posterior lobe volume, b = -.40, p = .001, and for 9% of right superior-posterior lobe volume, b = -.30, p = .020. We next regressed each neuro-cognitive task on group status and left and right superior-posterior lobe volumes. Across subjects, left superior-posterior lobe volume mediated subjects’ performance on the Delayed Match to Sample task, b = .25, p = .032. Together, group status and left superior-posterior lobe volume accounted for 35% of the variance in children's DMS performance, F (1, 59) = 25.3, p < .001. Across subjects, right superior-posterior lobe volume predicted children's performance on the Stockings of Cambridge task, b = .28, p = .023. Together, group status and right superior-posterior lobe volume accounted for 23% of the variance in SOC performance, F (1, 59) = 11.0, p = .002. In a group comparison of performance on the Motor Screening test, the post-institutionalized children (M=821, SD=349) did worse than controls (M=656, SD=133), F (1, 59) = 5.9, p = .018. However, both left and right superior-posterior lobe volumes did not mediate performance on this task across subjects.
Table 1.
Descriptive Data for Individual CANTAB Measures
| Groups Post-institutionalized | Control | Group Comparisons | |
|---|---|---|---|
| Memory Tasks | |||
| DMS (number correct trials) | 78.4 (11.3) |
89.9 (5.4) |
p < 0.001 PI < Control |
| PAL (adjusted total errors, z-score) | 0.1 (0.4) |
0.3 (0.2) |
p = 0.005 PI < Control |
| PRM (total correct trials) | 0.2 (0.6) |
0.7 (0.5) |
p = 0.002 PI < Control |
| Executive Function Tasks | |||
| IED (adjusted total errors, z-score) | 0.3 (0.6) |
0.7 (0.6) |
p = 0.005 PI < Control |
| SOC (correct trials in minimum moves, z-score) | - 0.4 (1.0) |
0.4 (0.8) |
p = 0.002 PI < Control |
| SWM (total errors, z-score) | 0.3 (0.8) |
1.1 (0.6) |
p < 0.001 PI < Control |
| Attention Tasks | |||
| MTS (number correct trials) | 95.3 (5.1) |
96.0 (3.6) |
p = 0.554 PI = Control (NS) |
Discussion
This study explored the effects of early deprivation on the development of the human cerebellum as well as the association between cerebellar development and components of cognitive functioning. These data indicate that children who experienced early deprivation had smaller left and right superior-posterior cerebellar lobe volumes than controls, and that development of these brain regions was related to two aspects of cognition. Specifically, the left superior-posterior lobe of the cerebellum was associated with visual-spatial memory and the right superior-posterior lobe of the cerebellum mediated a planning component of executive functioning. These data suggest specificity in these effects in that other regions of the cerebellum were similar in the neglected and control groups, and cerebellar volume did not account for all of the cognitive performance differences that emerged between groups. Such a result suggests that the cerebellum may have a distinct functional topography, with the superior-posterior regions mediating some specific cognitive processes.
Both the DMS and SOC tasks tap processes involving frontal and medial temporal lobe function. There is some evidence of reciprocal connectivity between the cerebellum and prefrontal cortex in nonhuman primates. Purkinje cells within the superior-posterior lobe, specifically within crus I of VIIA in the posterior hemispheres, project to prefrontal areas via the ventral dentate and thalamus (17, 27). In a recent meta-analysis of neuroimaging studies exploiting functional topography within the cerebellum, both lobule VI and crus I of VIIA in the superior-posterior lobes were found to be consistently activated during executive function tasks. Lobule VI was specifically activated during spatial tasks (52). In addition, the cerebellum and medial temporal lobes have been implicated in both memory processing and skill learning (53-55). Projections from the cerebellum to the temporo-parietal cortex within nonhuman primates further suggest a cerebellar contribution to memory (28).
As with most studies of clinical populations, the present findings are tempered by a number of factors. The difficulty in adequately assessing post-institutionalized children's pre-adoption living conditions remains a limitation of the work with this population of children. While more “objective” measures such as number of pre-adoption placements, length in the institution, or height/weight may be useful and specific, it is unlikely that these variables alone are broad enough to capture a complete understanding of the early living conditions that post-institutionalized children experienced. Development of more extensive interviews or questionnaires for parents may be useful in addressing this limitation, although this issue will likely always be problematic in research with post-institutionalized children given the very nature of their early rearing experiences.
There are also some challenges specific to MRI acquisition in pediatric populations. Because of the short distance between children's necks, shoulders, and the hindbrain areas, it is often difficult to contain a child's head completely in the MRI head coil. Consequently, some subjects had images with poor resolution in more inferior cerebellar regions. In these cases tracings had to be performed manually (but with subject's group status masked) by following borders between the cerebellum and extraneous tissue.
The findings reported here suggest an important role of experience-dependent plasticity in the brain-behavior relationships supported by the cerebellum. The fact that post-institutionalized children demonstrate smaller cerebellar volumes and worse cognitive performance than typically developing subjects suggest that physiological and social deprivation have a profound influence on cerebellar neurodevelopment. Deprivation serves as an environmental stressor that may disrupt early cerebellar organization and maturation and consequently lead to underdeveloped neural pathways between cerebellum and cortex. This faulty connectivity is then manifested during adolescence as deficits in cognitive-behavioral functioning. Establishing a firm relationship between anatomy and function will provide further insight into the functional role of the cerebellum and may improve our understanding of the biological mechanisms through which early adverse experiences constitute risks for children's cognitive development.
Acknowledgements
This project was supported by grants from the National Institute of Mental Health to SDP (R01 MH068858) and to RJD (P50-MH084051). Infrastructure support was provided by the Waisman Center, University of Wisconsin through the National Institute of Child Health and Human Development (P30-HD03352, M. Seltzer, Director). Mary Schlaak, Barb Roeber, Elizabeth Shirtcliff, Geoffrey Borman, and Daniel Bolt provided invaluable assistance for this project. We also greatly appreciate the children and their families whose participation made this research possible.
Footnotes
Financial Disclosures:
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 2.Ito M. Movement and thought: identical control mechanisms by the cerebellum. Trends Neurosci. 1993;16:448–50. doi: 10.1016/0166-2236(93)90073-u. discussion 453-4. [DOI] [PubMed] [Google Scholar]
- 3.Ito M, editor. How does the cerebellum facilitate thought? Oxford University Press; Oxford: 1993. [Google Scholar]
- 4.Kim SG, Ugurbil K, Strick PL. Activation of a cerebellar output nucleus during cognitive processing. Science. 1994;265:949–951. doi: 10.1126/science.8052851. [DOI] [PubMed] [Google Scholar]
- 5.Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44:113–128. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- 6.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 7.Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- 8.Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- 9.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 10.Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- 11.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 12.Giedd JN, Schmitt JE, Neale MC. Structural brain magnetic resonance imaging of pediatric twins. Hum Brain Mapp. 2007;28:474–481. doi: 10.1002/hbm.20403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks VB. Comment: on functions of the “cerebellar circuit” in movement control. Can J Physiol Pharmacol. 1981;59:776–778. doi: 10.1139/y81-113. [DOI] [PubMed] [Google Scholar]
- 14.Eccles JC. Physiology of motor control in man. Appl Neurophysiol. 1981;44:5–15. doi: 10.1159/000102178. [DOI] [PubMed] [Google Scholar]
- 15.Evarts EV, Thach WT. Motor mechanisms of the CNS: cerebrocerebellar interrelations. Annu Rev Physiol. 1969;31:451–498. doi: 10.1146/annurev.ph.31.030169.002315. [DOI] [PubMed] [Google Scholar]
- 16.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taber KH, Strick PL, Hurley RA. Rabies and the cerebellum: new methods for tracing circuits in the brain. J Neuropsychiatry Clin Neurosci. 2005;17:133–139. doi: 10.1176/jnp.17.2.133. [DOI] [PubMed] [Google Scholar]
- 18.Dum RP, Li C, Strick PL. Motor and nonmotor domains in the monkey dentate. Ann N Y Acad Sci. 2002;978:289–301. doi: 10.1111/j.1749-6632.2002.tb07575.x. [DOI] [PubMed] [Google Scholar]
- 19.Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- 20.Middleton FA, Strick PL. Dentate output channels: motor and cognitive components. Prog Brain Res. 1997;114:553–566. doi: 10.1016/s0079-6123(08)63386-5. [DOI] [PubMed] [Google Scholar]
- 21.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner BM, Paradiso S, Marvel CL, Pierson R, Boles Ponto LL, Hichwa RD, et al. The cerebellum and emotional experience. Neuropsychologia. 2007;45:1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schall U, Johnston P, Lagopoulos J, Juptner M, Jentzen W, Thienel R, et al. Functional brain maps of Tower of London performance: a positron emission tomography and functional magnetic resonance imaging study. Neuroimage. 2003;20:1154–1161. doi: 10.1016/S1053-8119(03)00338-0. [DOI] [PubMed] [Google Scholar]
- 24.Hayter AL, Langdon DW, Ramnani N. Cerebellar contributions to working memory. Neuroimage. 2007;36:943–954. doi: 10.1016/j.neuroimage.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Xiang H, Lin C, Ma X, Zhang Z, Bower JM, Weng X, et al. Involvement of the cerebellum in semantic discrimination: an fMRI study. Hum Brain Mapp. 2003;18:208–214. doi: 10.1002/hbm.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schutter DJ, van Honk J. An electrophysiological link between the cerebellum, cognition and emotion: frontal theta EEG activity to single-pulse cerebellar TMS. Neuroimage. 2006;33:1227–1231. doi: 10.1016/j.neuroimage.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 27.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 28.Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP’. Cereb Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- 29.Whiting BA, Barton RA. The evolution of the cortico-cerebellar complex in primates: anatomical connections predict patterns of correlated evolution. J Hum Evol. 2003;44:3–10. doi: 10.1016/s0047-2484(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 30.Verbitskaya LB. Neurobiology of cerebellar evolution and development. In: Llinas R, editor. Neurobiology of Cerebellar Evolution and Development: Proceedings of the First International Symposium of the Institute for Biomedical Research. American Medical Association/Education & Research Foundation; Chicago: 1969. pp. 859–874. [Google Scholar]
- 31.De Bellis MD, Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2006;60:697–703. doi: 10.1016/j.biopsych.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 32.Mason P, Narad C. International adoption: a health and developmental prospective. Semin Speech Lang. 2005;26:1–9. doi: 10.1055/s-2005-864211. [DOI] [PubMed] [Google Scholar]
- 33.Albers LH, Johnson DE, Hostetter MK, Iverson S, Miller LC. Health of children adopted from the former Soviet Union and Eastern Europe. Comparison with preadoptive medical records. JAMA. 1997;278:922–924. [PubMed] [Google Scholar]
- 34.Cermak SA, Daunhauer LA. Sensory processing in the postinstitutionalized child. Am J Occup Ther. 1997;51:500–507. doi: 10.5014/ajot.51.7.500. [DOI] [PubMed] [Google Scholar]
- 35.Diamond GW, Senecky Y, Schurr D, Zuckerman J, Inbar D, Eidelman A, et al. Pre-placement screening in international adoption. Isr Med Assoc J. 2003;5:763–766. [PubMed] [Google Scholar]
- 36.Beckett C, Maughan B, Rutter M, Castle J, Colvert E, Groothues C, et al. Scholastic attainment following severe early institutional deprivation: a study of children adopted from Romania. J Abnorm Child Psychol. 2007;35:1063–1073. doi: 10.1007/s10802-007-9155-y. [DOI] [PubMed] [Google Scholar]
- 37.Nelson CA, 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 38.Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, et al. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Dev Psychopathol. 2008;20:547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- 39.Fries AB, Pollak SD. Emotion understanding in postinstitutionalized Eastern European children. Dev Psychopathol. 2004;16:355–369. doi: 10.1017/S0954579404044554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.General Electric Medical Systems; Milwaukee, WI: [Google Scholar]
- 41.Wellcome Department of Cognitive Neurology Statistical Parametric Mapping; London, England: [Google Scholar]
- 42.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 43.Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Human Brain Mapping. 1995;3:190–208. [Google Scholar]
- 44.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 45.Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 46.Pierson R, Corson PW, Sears LL, Alicata D, Magnotta V, Oleary D, et al. Manual and semiautomated measurement of cerebellar subregions on MR images. Neuroimage. 2002;17:61–76. doi: 10.1006/nimg.2002.1207. [DOI] [PubMed] [Google Scholar]
- 47.Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- 48.Larsell O. The human cerebellum, cerebellar connections, and cerebellar cortex. In: Jansen J, editor. The Comparative Anatomy and Histology of the Cerebellum. University of Minnesota Press; Minneapolis, MN: 1972. pp. 203–208. [Google Scholar]
- 49.Cambridge Cognition; Cambridge, England: [Google Scholar]
- 50.Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, et al. Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Dev. 2007 doi: 10.1111/j.1467-8624.2009.01391.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 53.Poldrack RA, Gabrieli JD. Functional anatomy of long-term memory. J Clin Neurophysiol. 1997;14:294–310. doi: 10.1097/00004691-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Thompson RF, Kim JJ. Memory systems in the brain and localization of a memory. Proc Natl Acad Sci U S A. 1996;93:13438–13444. doi: 10.1073/pnas.93.24.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson RF. In search of memory traces. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]