Abstract
Noonan-like/multiple giant cell lesion syndrome (NS/MGCLS) is a rare condition with phenotypic overlap with Noonan syndrome (NS). Once thought to be a specific and separate entity, it is now suggested to be a variant of the NS spectrum. We report a patient with classical cardinal features of NS, including short stature, mild ptosis, hypertelorism, down-slating palpebral fissures, low-set and posteriorly angulated ears, short neck, pectus excavatum, widely spaced nipples and cryptochidism, which were associated with bilateral central giant cell lesions in the mandible and germ-line mutation (C218T, Thr73Ile) in the exon 3 of the PTPN11 gene. The similar clinical and genetic aspects support the observation that NS/MGCLS is a variant of NS and giant cell lesions are an integrant part of this disorder.
Keywords: Noonan syndrome, Noonan-like/multiple giant cell lesion syndrome, Clinical features, Giant cell lesion, PTPN11 mutation
Introduction
Noonan syndrome (NS, MIM #163950) is an uncommon disorder characterized by short stature, craniofacial dysmorphism, short neck with webbing, deformity of the sternum, cardiac anomalies, and cryptorchidism [1]. The main craniofacial features include hypertelorism, downward eyeslant, low-set posteriorly rotated ears, and ptosis [2]. In 1991, Cohen and Gorlin [3] defined a syndrome characterized by NS features and multiple giant cell lesions, which was named Noonan-like/multiple giant cell lesion syndrome (NS/MGCLS, MIM #163955). However, recent studies demonstrated that NS/MGCLS are caused by mutations in PTPN11 or SOS1, the same genes related to NS, suggesting that giant cell lesions are a part of the clinical spectrum of NS and not a separate entity [4, 5].
Herein, we report the phenotype of one sporadic case of NS caused by heterozygous mutation in the PTPN11 gene, supporting that giant cell lesions should be considered as a clinical feature of the syndrome.
Case Report
A 8 year-old boy was referred for evaluation of a mandibular swelling causing facial asymmetry. According to the patient’s parents the lesion was first noted 10 months prior to the first consultation. Clinical examination revealed moderate hemi-facial asymmetry, secondary to an expansive mandibular growth, covered by intact mucosa (Fig. 1a). He also presented other facial features, including mild ptosis, hypertelorism, down-slating palpebral fissures and low-set and posteriorly angulated ears (Fig. 1b). Short stature, short neck, pectus excavatum, widely spaced nipples, and cryptochidism were noted (Fig. 1c). His medical history was unremarkable, and he has normal intelligence. Radiographic examination showed multilocular lesions bilaterally in the posterior region of the mandible (Fig. 2). The lesion on the right side was larger and caused expansion of the cortical and displacement of the canine, premolars and first and second molars. The mandibular canal was not evident on this side. On the left side, the lesion was small and appeared indolent. Under local anesthesia, incisional biopsies on both lesions were performed. Histological examination demonstrated a hypervascular fibroblastic proliferation with multiple unevenly distributed multinucleated giant cells. (Fig. 3). The proliferating fibroblasts showed bland nuclei, partly arranged in storiform pattern. The histopathological diagnosis of the biopsies taken from both radiolucencies was of central giant cell lesions.
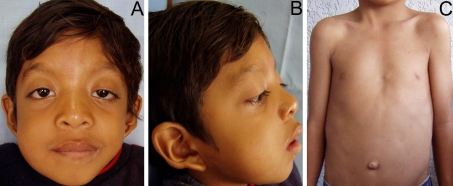
Fig. 1.
Clinical features of the patient at age of 8 year-old. a Note the short neck, ptosis, hypertelorism, and down-slating palpebral fissures. The patient also presented a swelling in the lower right cheek, which was his chief complain. b In the lateral view, the low-set and posteriorly angulated ear is evident. c Pectus excavatum and widely spaced nipples were observed
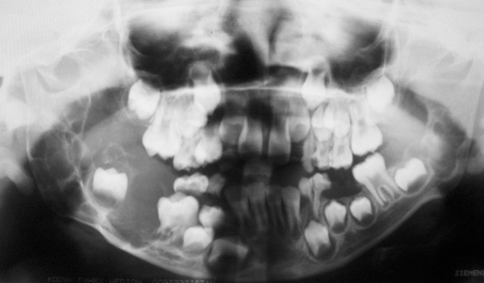
Fig. 2.
Panoramic radiograph revealed bilateral mandibular multilocular radiolucencies. On the right side occurred cortical expansion and displacement of the teeth, whereas on the left side the lesion was smaller
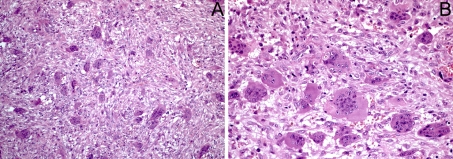
Fig. 3.
Histopathological features of the giant cell lesions. a Lesions were characterized by the presence of multiple multinucleated giant cells embedded in a fibrous stroma rich in blood vessels and proliferating spindle cells (×100). b High power view showing the giant cells with multiple nuclei and abundant cytoplasm (×200)
With the presumptive diagnosis of NS, further investigations such as echocardiogram, hematological and biochemical tests and DNA sequence analysis were performed. Echocardiogram revealed no cardiac alterations, and laboratory findings, including complete blood count, coagulation test and serum dosage of calcium, phosphate and parathormone, were all within normal limits. Mutation analysis of the PTPN11 gene and of the SH3BP2 exon 9 with genomic DNA extracted from oral mucosa cells were performed according to the published protocols [6, 7]. Genetic analysis revealed a heterozygous C to T transition at nucleotide position 218 of exon 3 of PTPN11 (C218T), which result in a threonine to isoleucine substitution at codon 3 (Thr73Ile) of the PTPN11 amino acid sequence (Fig. 4). No mutation within the exon 9 of SH3BP2 was identified (data not shown). Taking into account the genetic study, the diagnosis of NS with bilateral mandibular giant cell lesions was confirmed.
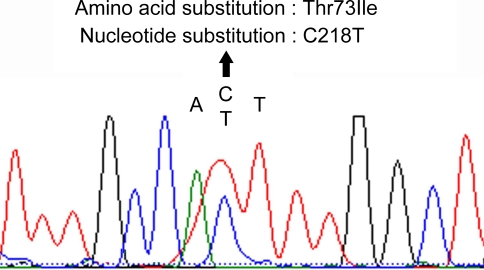
Fig. 4.
Detection of the PTPN11 mutation in the NS patient. Shown here is a portion of the DNA-sequence eletropherogram of the PTPN11 exon 3. Compared to the normal sequence, the affected patient has a heterozygous C to T transition at nucleotide position 218 of PTPN11 gene. This transition converts a threonine to isoleucine substitution at codon 3 (Thr73Ile) of the PTPN11 amino acid sequence
Surgical enucleation of both mandibular lesions was performed under general anesthesia, and no evidence of recurrence is seen after 2 years. In early adulthood, if necessary it is planned to carry out corrective orthodontic treatment. Patient also had undergone to orchidopexy, which was successfully performed. Echocardiograms are planned to be performed every 3 years as part of a continued follow up, since cardiology problems may arise at a later age. Although it represents a sporadic case, genetic counseling was given to the parents, and information regarding the pattern of gene transmission, possible ways of expression, and consequences of phenotypes were emphasized.
Discussion
Recent studies, based on clinical and genetic similarities, have suggested that NS/MGCLS is a variant of NS and giant cell lesions are clinical features of NS spectrum [4, 5, 8]. Thus, NS/MGCLS may not be a distinct entity. NS demonstrates an autosomal dominant mode of inheritance with complete penetrance and variable expressivity, although most cases are sporadic [9].
The patient reported in this study present many of the classical features of NS, including short stature, short neck, pectus excavatum, widely spaced nipples, cryptochidism, and typical craniofacial alterations, fulfilling the clinical diagnostic criteria proposed by Van der Burgt et al. [10]. In addition, the patient presented bilateral giant cell lesions in the mandible. Although mental retardation and delay on acquisition of developmental milestones are considered usual for patients with NS, our patient showed normal intellect [11]. Coagulation tests of the patient presented here were within normal limits, but bleeding diathesis is well documented in NS patients and involves either platelet dysfunction or factor XI or XII deficiencies [12]. This feature should be considered in the surgical procedures of the syndromic patients. Other cardinal feature of NS is congenital heart disease, which is seen in 50–75% of the NS patients. Pulmonary valve stenosis, hypertrophic cardiomyopathy and atrial septal alterations are the predominant associated-NS defects, but aortic coarctation is also reported in few cases [9]. Echocardiographic examination of our patient revealed no evidence of cardiac alterations, however, cardiac disorders may appear later in adulthood.
The craniofacial features of NS are variable and change throughout life [2]. In infants, hypertelorism, low nasal bridge, low-set and rotated ears, and broad and high forehead are the most expressive features. However, in the childhood the nasal bridge and forehead features are much less evident and ptosis becomes apparent, whereas in the adulthood craniofacial dimorphism is mild. We have seen the patient for the first time at age of 8 year-old, and his craniofacial features were characterized by ptosis, hypertelorism, down-slating palpebral fissures and low-set and posteriorly angulated ears. Malocclusions are frequent in NS patients, and most relate to high arched palate or micrognathia [8]. Malocclusion with tooth crowding was evident in our patient, but due probably to the giant cell lesion.
As multiple oral giant cell lesion can be acquired or genetic, detailed clinical examination and laboratory and genetic tests are important for the correct diagnosis. The presence of multiple and multifocal giant cell lesions is strongly suggestive of brown tumor due to hyperparathyroidism. Hyperparathyroidism is characterized by an increased production of parathyroid hormone, and it is classified in primary or secondary. Primary hyperparathyroidism is result of uncontrolled parathyroid hormone production usually associated with a parathyroid adenoma, parathyroid hyperplasia, and less frequently with a parathyroid carcinoma [13]. Secondary hyperparathyroidism is less common than primary hyperparathyroidism, and occurs when parathyroid hormone is continuously produced in response to low levels of serum calcium, as observed frequently in chronic renal failure [14]. Our patient showed normal serum levels of calcium, phosphate and parathormone, ruling out hyperparathyroidism causing brown tumors. Among the genetic disorders characterized by multiple oral giant cell lesions are NS, cherubism, type I neurofibromatosis and Schimmelpenning syndrome [15, 16]. Cherubism is the easiest confused with NS [17], mainly where NS characteristics are mild, since the other diseases have striking features that allow the differential diagnosis. Furthermore, giant cell lesions from both NS and cherubism are histologically and immunohistochemically indistinguishable. However, while cherubism is caused by mutations in SH3BP2 gene, NS is genetically heterogeneous and caused by mutations in PTPN11, KRAS, RAF1, MEK1 or SOS1 [12]. PTPN11 mutations are present in 29–60% of cases, and genetic testing should initially include screening of exons 3, 8 and 13, in which 75–80% of defects reside [9]. In our patient, gene mutation for cherubism was not identified, but the previous reported Thr73Ile missense mutation on exon 3 of PTPN11 was present. Thus, genetic testing may be important in situations of difficulty in distinguish those conditions.
There are several treatment choices for giant cell lesions, but no specific one is reported for those lesions in NS patients. Wolvius et al. [8] reported a recurrent and aggressive giant cell lesion in a patient with NS syndrome, which was treated by a combination of surgical curettage and nasal spray application of calcitonin. However, no information of the follow up was available. In the present case, we have opted to surgical enucleation, since the right side lesion was growing and might have caused cortical perforation.
In summary, we reported one case that showed typical features of NS and bilateral mandibular giant cell lesions, and that occurred as a de novo C218T mutation in PTPN11 gene. Proper evaluation with characterization of the clinical features in association with laboratory tests and genetic analysis were of utmost importance for the correct diagnosis and treatment of the affected patient. Although cryptochidism is a common feature of NS, orchidopexy traditionally results in normal fertility for most of the patients, marking early diagnosis/treatment and genetic counseling important.
References
- 1.Mendez HM, Opitz JM. Noonan syndrome: a review. Am J Med Genet. 1985;21:493–506. doi: 10.1002/ajmg.1320210312. [DOI] [PubMed] [Google Scholar]
- 2.Noordam K. Expanding the genetic spectrum of Noonan syndrome. Horm Res. 2007;68(Suppl 5):24–27. doi: 10.1159/000110468. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MM, Jr, Gorlin RJ. Noonan-like/multiple giant cell lesion syndrome. Am J Med Genet. 1991;40:159–166. doi: 10.1002/ajmg.1320400208. [DOI] [PubMed] [Google Scholar]
- 4.Hanna N, Parfait B, Talaat IM, Vidaud M, Elsedfy HH. SOS1: a new player in the Noonan-like/multiple giant cell lesion syndrome. Clin Genet. 2009;75:568–571. doi: 10.1111/j.1399-0004.2009.01149.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Tartaglia M, Gelb BD, et al. Phenotypic and genotypic characterisation of Noonan-like/multiple giant cell lesion syndrome. J Med Genet. 2005;42:e11. doi: 10.1136/jmg.2004.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai Y, Kanno K, Moriya T, et al. A missense mutation in the SH3BP2 gene on chromosome 4p16.3 found in a case of nonfamilial cherubism. Cleft Palate Craniofac J. 2003;40:632–638. doi: 10.1597/1545-1569(2003)040<0632:AMMITS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Kosaki K, Suzuki T, Muroya K, et al. PTPN11 (protein-tyrosine phosphatase, nonreceptor-type 11) mutations in seven Japanese patients with Noonan syndrome. J Clin Endocrinol Metab. 2002;87:3529–3533. doi: 10.1210/jc.87.8.3529. [DOI] [PubMed] [Google Scholar]
- 8.Wolvius EB, Lange J, Smeets EE, Wal KG, Akker HP. Noonan-like/multiple giant cell lesion syndrome: report of a case and review of the literature. J Oral Maxillofac Surg. 2006;64:1289–1292. doi: 10.1016/j.joms.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Tartaglia M, Gelb BD. Noonan syndrome and related disorders: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2005;6:45–68. doi: 10.1146/annurev.genom.6.080604.162305. [DOI] [PubMed] [Google Scholar]
- 10.Burgt I, Berends E, Lommen E, Beersum S, Hamel B, Mariman E. Clinical and molecular studies in a large Dutch family with Noonan syndrome. Am J Med Genet. 1994;53:187–191. doi: 10.1002/ajmg.1320530213. [DOI] [PubMed] [Google Scholar]
- 11.Jongmans M, Sistermans EA, Rikken A, et al. Genotypic and phenotypic characterization of Noonan syndrome: new data and review of the literature. Am J Med Genet A. 2005;134A:165–170. doi: 10.1002/ajmg.a.30598. [DOI] [PubMed] [Google Scholar]
- 12.Jorge AA, Malaquias AC, Arnhold IJ, Mendonca BB. Noonan syndrome and related disorders: a review of clinical features and mutations in genes of the RAS/MAPK pathway. Horm Res. 2009;71:185–193. doi: 10.1159/000201106. [DOI] [PubMed] [Google Scholar]
- 13.DeLellis RA, Mazzaglia P, Mangray S. Primary hyperparathyroidism: a current perspective. Arch Pathol Lab Med. 2008;132:1251–1262. doi: 10.5858/2008-132-1251-PHACP. [DOI] [PubMed] [Google Scholar]
- 14.Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Edwards PC, Fantasia JE, Saini T, Rosenberg TJ, Sachs SA, Ruggiero S. Clinically aggressive central giant cell granulomas in two patients with neurofibromatosis 1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:765–772. doi: 10.1016/j.tripleo.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Ernst LM, Quinn PD, Alawi F. Novel oral findings in Schimmelpenning syndrome. Am J Med Genet A. 2007;143A:881–883. doi: 10.1002/ajmg.a.31663. [DOI] [PubMed] [Google Scholar]
- 17.Jafarov T, Ferimazova N, Reichenberger E. Noonan-like syndrome mutations in PTPN11 in patients diagnosed with cherubism. Clin Genet. 2005;68:190–191. doi: 10.1111/j.1399-0004.2005.00475.x. [DOI] [PubMed] [Google Scholar]