Abstract
Plasmablastic lymphoma is a rare form of non-Hodgkin lymphoma. It is strongly associated with HIV infection, although it has been recognized in immunocompetent patients. Plasmablastic lymphoma has a predilection for the oral cavity. Its occurrence in the parotid gland has not been previously described. We report a case of an HIV positive man who developed a rapidly enlarging parotid mass. A core biopsy of the parotid mass was evaluated by routine microscopy, immunohistochemistry, and in situ hybridization. The tumor was comprised of sheets of large cells with abundant cytoplasm, eccentric nuclei and prominent nucleoli. The cells exhibited a plasmacytic immunophenotype including expression for CD38 and CD138. An in situ hybridization assay for Epstein-Barr virus was positive. These findings were diagnostic of plasmablastic lymphoma. Plasmablastic lymphoma is notoriously difficult to diagnose, particularly when it arises in unexpected sites outside of the oral cavity. As an aggressive lymphoma, plasmablastic lymphoma must be considered in the differential diagnosis of a high-grade malignant neoplasm not just in the oral cavity but at non-oral sites including the parotid gland, particularly in an HIV-positive individual.
Keywords: Plasmablastic lymphoma, Epstein-Barr virus, Parotid gland, Human immunodeficiency virus, Non-Hodgkin lymphoma
Introduction
Immunocompromised patients, including those infected with HIV, are at increased risk of developing malignancies. After Kaposi sarcoma, non-Hodgkin lymphomas are the most common malignant neoplasms affecting HIV-positive patients [1]. In addition to more routine lymphoid malignancies, HIV-positive patients are also predisposed to unique lymphoid malignancies (e.g. primary effusion lymphoma and large cell lymphoma arising in Kaposi virus-associated Castleman disease) that are rarely encountered in immunocompetent patients [2]. Plasmablastic lymphoma (PBL) is a rare and aggressive form of non-Hodgkin lymphoma originally described in 1997 [3]. Of the approximately 150 reported cases, PBL has most often been reported in the oral cavity of HIV-positive patients [1, 3–6]. Of the non-HIV-associated cases, one-third develop in patients with some other immunosuppressive condition such as steroid therapy or chemotherapy [6]. Like other HIV-associated lymphomas, the Epstein-Barr virus (EBV) is believed to play a role in the development of PBL [1, 3–6]. PBL is regarded as very aggressive neoplasm, and it usually presents at an advanced stage. Although the rarity of PBL has not permitted the development of a standardized treatment [4, 5], a combination of highly active anti-retroviral therapy and chemotherapy may lead to improved patient outcomes [5, 7].
Although most PBLs arise in the oral cavity, the anatomic distribution of PBL is not entirely restricted to this site. PBL has also been reported in the nasal cavity, skin, lymph nodes, lung, liver, stomach, anus, and heart [4, 6]. We report a PBL presenting as a parotid mass. This case expands the clinical profile of PBL and emphasizes that the diagnosis of PBL should not be dismissed solely on the basis of unusual clinical features such as origin in the parotid gland.
Case Report
A 44-year-old African American man presented with shortness of breath and right facial pain and swelling. His past medical history was remarkable for HIV infection with a history of non-compliance on highly active antiretroviral therapy. His CD4+ lymphocyte count was 160/μl. Although the facial mass had been present for ~2 months, the patient reported a dramatic increase in its size over the previous 3–4 days. Review of systems revealed that the patient had also lost about 30 lb over the past 3 months. On physical examination, a firm, rubbery, tender mass was palpated on the right cheek at the angle of the mandible. With the exception of poor dentition, the oral cavity and oropharynx were unremarkable. The initial clinical diagnosis was parotid sialadenitis, and the patient was prescribed antibiotics and corticosteroids.
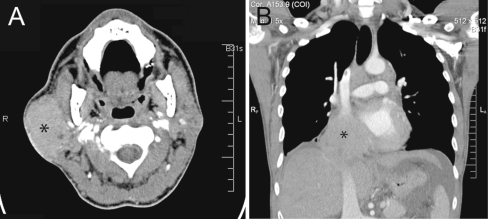
A computed tomography (CT) scan of the neck showed an enlarged right parotid gland measuring 5.9 × 5.2 × 3.2 cm with diffusely increased density (Fig. 1a). The differential diagnosis based on the imaging studies favored an abscess or some other inflammatory process. A CT of the chest revealed a soft tissue mass measuring 9.0 cm involving the inferior vena cava and right atrium (Fig. 1b). A core biopsy of the parotid mass was performed.
Fig. 1.
Computed tomography (CT) scans. a On the neck CT, there is an ~6 cm dense mass diffusely involving the right parotid gland (asterisk). b A 9 cm mass (asterisk) involving the inferior vena cava is seen on the concurrent chest CT
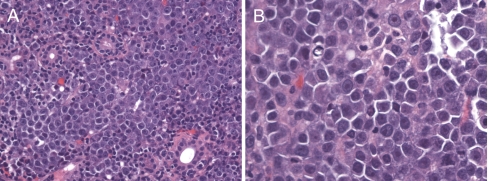
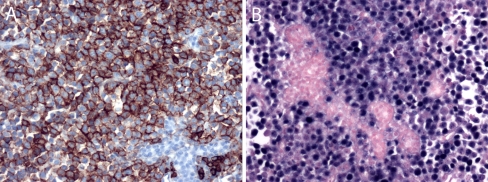
Microscopic examination of the parotid biopsy showed a diffuse infiltration of the parotid parenchyma by sheets of malignant cells (Fig. 2a). The cells were large with squared-off borders, eccentric nuclei, and a moderate amount of amphophilic cytoplasm. The nuclei had a single, large, eosinophilic nucleolus (Fig. 2b). The mitotic rate was high. Immunohistochemical staining showed that the malignant cells were immunoreactive for the plasmacytic markers CD38, CD138 (Fig. 3a), and MUM-1. The cells were only weakly and focally immunoreactive for CD45 (leukocyte common antigen), and they were entirely negative for the T lymphocyte markers CD3 and CD5 as well as the B lymphocyte markers CD20 and CD79a. Pancytokeratin and S-100 were also negative. The Ki-67 proliferation rate was approximately 90%. In situ hybridization for EBER was strongly positive in the tumor cells (Fig. 3b). The pathologic diagnosis was PBL.
Fig. 2.
Hematoxylin and eosin stained sections. a (original magnification ×200) There is a monotonous population of malignant cells diffusely infiltrating salivary gland parenchyma. b (original magnification ×400) The malignant cells have a plasmablastic appearance with clumped chromatin, often a single prominent “cherry red” nucleolus, smooth nuclear contours, and eccentric cytoplasm
Fig. 3.
a Immunohistochemistry for CD138 (original magnification ×200) shows strong membranous immunoreactivity of the neoplastic cells, but no reactivity in the remaining salivary gland acini. b In situ hybridization for Epstein-Barr virus-encoded RNA (EBER; original magnification ×200) shows strong nuclear signals in almost every tumor cell, but not in the background salivary gland acini
The patient’s condition rapidly progressed to multisystem organ failure despite systemic chemotherapy, the reintroduction of antiretroviral therapy, and aggressive supportive measures. He expired 18 days after initial presentation.
Discussion
PBL is an uncommon form of non-Hodgkin lymphoma that classically arises in the oral cavity of HIV-positive patients. PBL is a rapidly progressive lymphoma, and delay in treatment can adversely impact patient survival [7]. Although prompt and accurate recognition of PBL is imperative, the diagnosis of PBL is not easy due to a variety of contributing factors. First, PBL is rare, and its description as a distinct tumor entity is relatively recent. Prior to its original description in 1997 [3], most PBLs were likely diagnosed as CD20-negative large B cell lymphomas, intermediate forms of Burkitt lymphoma, or some poorly differentiated non-lymphoid malignancy [1, 3]. Indeed, even after its description as a distinct entity, Folk et al. [1] found that up to 40% of cases submitted for pathologic consultation were initially regarded as poorly differentiated carcinomas. Second, PBLs have an unusual immunophenotype that renders them susceptible to pathologic misdiagnosis. PBL is classified as a B cell lymphoma and its cellular morphology resembles immunoblastic diffuse large B-cell lymphoma, yet these tumors fail to consistently and strongly express leukocyte common antigen and the B cell markers CD20 and CD79a [1, 4, 8]. Diagnosis of PBL requires the use of additional markers to confirm plasmacytic differentiation. PBLs are immunoreactive for the plasmacytic markers CD138 and CD38. Unlike plasmacytomas, most PBLs are EBV related such that in situ hybridization for Epstein-Barr encoded RNA (EBER) is useful in distinguishing between these two plasma cell neoplasms [4, 6].
Finally, PBL may be entirely unanticipated when it occurs outside of the oral cavity. Although PBL has a strong predilection for the oral cavity, a growing experience has confirmed that its distribution is not limited to this single site. PBL has also been reported in the nasal cavity, skin, lymph nodes, lung, liver, stomach, anus, heart, and orbit [4, 6, 9]. Accordingly, PBL should be included in the differential diagnosis of any high grade malignant neoplasm with plasmacytoid features, irrespective of anatomic site. This concept is underscored by our report of a PBL involving the parotid gland—a previously unreported site for PBL. At this site, PBL could be mistaken for poorly differentiated salivary gland carcinoma, metastatic malignant melanoma, or a plasmacytoid variant of a myoepithelial neoplasm. Accurate diagnosis requires some familiarity with this recently described entity, judicious use of immunohistochemical studies including markers for plasmacytic differentiation, and a readiness to include PBL in the differential diagnosis of parotid masses.
In conclusion, our report of PBL of the parotid gland further expands the list of anatomic sites involved by PBL, and it highlights the principle that this aggressive lymphoma must not be eliminated from the differential diagnosis based on extra-oral location, particularly in the setting of an HIV-positive patient. Awareness of this entity and its unusual immunophenotype can prevent misdiagnosis.
Contributor Information
Justin A. Bishop, Phone: +1-410-9553980, FAX: +1-410-6149011, Email: jbisho16@jhmi.edu
William H. Westra, Email: wwestra@jhmi.edu
References
- 1.Folk GS, Abbondanzo SL, Childers EL, et al. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8–12. doi: 10.1016/j.anndiagpath.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Carbone A, Cesarman E, Spina M, et al. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113:1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 3.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413–1420. [PubMed] [Google Scholar]
- 4.Stein H, Harris NL, Campo E, et al. Plasmablastic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Washington, DC: IARC Press; 2008. p. 256. [Google Scholar]
- 5.Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804–809. doi: 10.1002/ajh.21250. [DOI] [PubMed] [Google Scholar]
- 6.Rafaniello Raviele P, Pruneri G, Maiorano E. Plasmablastic lymphoma: a review. Oral Dis. 2009;15:38–45. doi: 10.1111/j.1601-0825.2008.01493.x. [DOI] [PubMed] [Google Scholar]
- 7.Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, et al. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008;8:261–267. doi: 10.1016/S1473-3099(08)70067-7. [DOI] [PubMed] [Google Scholar]
- 8.Kane S, Khurana A, Parulkar G, et al. Minimum diagnostic criteria for plasmablastic lymphoma of oral/sinonasal region encountered in a tertiary cancer hospital of a developing country. J Oral Pathol Med. 2009;38:138–144. doi: 10.1111/j.1600-0714.2008.00673.x. [DOI] [PubMed] [Google Scholar]
- 9.Morley AM, Verity DH, Meligonis G, et al. Orbital plasmablastic lymphoma—a comparison of a newly reported entity with diffuse large B cell lymphoma of the orbit. Orbit. 2009;28:429. doi: 10.3109/01676830903177427. [DOI] [PubMed] [Google Scholar]