Abstract
NUT midline carcinomas (NMC) are a rare, recently described class of poorly-differentiated tumors that exhibit rapid onset and highly aggressive clinicopathologic behavior. These tumors are defined by rearrangement of the nuclear protein in testis (NUT) gene on chromosome 15q14, most commonly in a balanced translocation with the BRD4 gene on chromosome 19p13.1, resulting in the characteristic BRD4-NUT fusion gene and protein which blocks epithelial differentiation through chromatin binding. NMC frequently involve midline structures of adolescents and young adults and affect the head and neck region in 50% of cases. To our knowledge, only one case has been previously reported involving a salivary gland. Here, we present a case of a NMC of the salivary gland in an adolescent male presenting with an intermittently painful left submandibular mass of 3 months duration.
Introduction
NUT-associated midline carcinomas (NMC) are rare, poorly-differentiated, lethal tumors that uniformly involve midline structures, typically of the head and neck as well as mediastinum. Although the exact frequency is unknown, such tumors have been reported in all age groups with multiple studies identifying them predominantly in adolescents and young adults, with an average age of 25 years at presentation [6, 8]. Interestingly, however, a recent study by Stelow et al. showed NMC made up 18% of poorly differentiated carcinomas of the upper aerodigestive tract in all age groups with a median patient age of 47 years, suggesting a more uniform age distribution for NMC [16]. This group of tumors is defined by rearrangements of the nuclear protein in testis (NUT) gene on chromosome 15q14 [6–10, 13]. The majority of these cancers harbor the BRD4-NUT fusion oncogene resulting from a t(15;19) translocation, and the remaining cases harbor NUT-variant fusions. These translocations are diagnostic and, interestingly, usually the sole chromosomal abnormality. However, despite this pathognomonic translocation, many cases of NMC go unrecognized due to its lack of characteristic clinical or histologic features. While these tumors affect the head and neck region in 50% [4] of cases, only one case has been previously reported involving a salivary gland [2]. We report a case of a NUT-associated salivary gland carcinoma in an adolescent male presenting with an intermittently painful left submandibular mass.
Materials and Methods
Case Report
The patient is a 15 year-old male with no significant past medical history who presented with a left-sided submandibular mass of approximately 3 months duration. He reported waxing and waning pain as well as intermittent episodes of swelling and regression of the mass. The patient denied fever, otalgia, numbness, change in voice or recent dental procedures. The patient had received scheduled routine immunizations since birth and had no prior surgical history. An ultrasound showed no evidence of cystic degeneration, hemorrhage or invasion. He underwent uneventful removal of an encapsulated tumor, which was submitted for histopathological evaluation. A left neck dissection was subsequently performed. Postoperatively, the patient received radiation therapy to 66 Gy in 33 fractions, and remains free of disease 14 months after his initial diagnosis.
Methods
Histochemistry and Immunohistochemistry
The specimen was fixed in 10% formalin and representative sections of the gland, tumor and capsule were embedded in paraffin and stained with hematoxylin and eosin. Histochemical staining for mucicarmine and diastase periodic acid-Schiff (DPAS), and immunohistochemical staining with the following antibodies were performed per standard protocols: Ki-67, smooth muscle actin, calponin, cytokeratin AE1/AE3, CAM 5.2, p63, S100, p16, CD117, chromogranin, synaptophysin, CD34 and CD56. In situ hybridization for Epstein Barr encoded RNA (EBER) was performed using a probe complementary to a portion of EBER1 RNA sequence according to the method described by Chang et al. [1]. We used a negative control probe or random sequence 5′CAGCCATGATAGACGAGACACGCGTGGCGA/UU3′ similar in length and G/C content to the positive probe described by Chang et al. [1]. Briefly, 5 μm sections were deparaffinized with xylene, washed with 100% EtOH and treated with proteinase K (10–50 μg/mL in 10 mM Tris–HCl and 1 mM EDTA; pH 8.0). Oligonucleotide probes, labeled at their 3′ end with digoxigenin-11-dUTP purchased from Integrated DNA Technologies (Coralville, IA) were added to hybridization buffer [2× SSC; 50 mM NaPO4 (pH 7.4); 50% formamide; 100 mg/mL sheared salmon sperm DNA; 125 mg/mL yeast t-RNA] to a final concentration of 25 pmol/mL and incubated with the tissue overnight at 37C. The sections were washed with 2× SSC followed by 1× PBS (Dulbecco’s). The sections were covered with anti-digoxigenin-alkaline phosphatase conjugate (0.3U/mL; 1:2,300 dilution into 2.5 mL of a solution containing 1% sheep serum and 0.2% Tween 20 in 1× PBS) and incubated for 1 h at room temperature. The slides were washed twice in 1× PBS, once in 150 mM NaCl, 100 mM Tris–HCl (pH 7.5) and once in 150 mM NaCl, 100 mM Tris–HCl (pH 9.5) and 50 mM magnesium chloride and covered with color development solution (nitroblue tetrazolium salt solution and 5 bromo-4-chloro-3-indolyl phosphate) containing 50 mM levamisole, and incubated for 2 h at room temperature. Positive cells appear dark purple to black in color. All solutions are made in deionized water treated with diethylpyrocarbonate.
Fluorescent in situ hybridization (FISH). Dual-color, split-apart FISH assays were performed on formalin-fixed paraffin-embedded 4 μm tissue sections as described [9]. Probes used for the 15q14 NUT breakpoint, flanking a 181 kb region containing NUT, included 3′ telomeric BAC clones 1H8 and 64o3, and 5′ centromeric clones 412e10 and 3d4. Probes used for the 19p13.1 BRD4 breakpoint were 5′ centromeric BAC clone 187l3, and 3′ telomeric BAC clone 87m17. Probes used for the 9 BRD3 breakpoint were 5′ telomeric BAC clone 145e17, and 3′ centromeric BAC clone 2243H5. Sections in which >80% of cells contained hybridization signals in four areas (200 cells/area) were considered adequate for interpretation.
Results
Gross: The specimen was received unoriented and measured 4.5 × 2.7 × 2.4 cm. On cut section, a tan-white, rubbery, well-circumscribed lesion (2.5 × 2.3 × 1.8 cm) abutting the inked surgical margin was observed. On serial sectioning, no areas of necrosis, calcification, hemorrhage or cystic degeneration were identified.
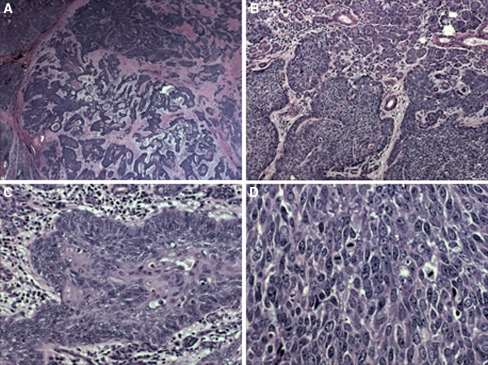
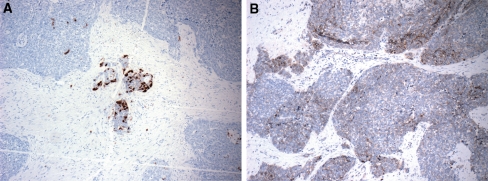
Microscopic: The tumor exhibited areas of solid, trabecular and cord-like growth patterns with infiltrative margins (Fig. 1a, b). Perineural and perivascular invasion were noted. Tumors cells were predominantly undifferentiated and characterized by enlarged nuclei with vesicular chromatin and prominent nucleoli with high mitotic activity, atypical mitoses and necrosis (Fig. 1c, d). Areas of cystic change, cholesterol granulomas and psammomatoid concretions were identified focally. Foci of abrupt keratinization and squamous differentiation including intercellular bridges, individual cell keratinization and squamous eddies were also noted. Left neck lymph nodes dissection revealed that one of 33 lymph nodes was positive for metastatic tumor. Histochemical stains for mucicarmine and DPAS, and immunohistochemical stains for calponin, smooth muscle actin, Chromogranin, synaptophysin, CD34 and CD56 were negative. EBER in situ hybridization was negative. The tumor was focally positive for S-100 and CD117 (Fig. 2a, b). Tumor cells showed variable reactivity with cytokeratin AE1/AE3, CAM 5.2, p63, and p16. Ki67 staining showed a proliferation rate of over 50%.
Fig. 1.
The tumor is relatively well-circumscribed (a, 10×), featuring an invasive margin which surrounds non-neoplastic salivary gland structures (b, 100×) with focal peripheral palisading of poorly-differentiated neoplastic cells (c, 200×) and high mitotic activity (d, 400×)
Fig. 2.
The tumor shows focal islands of cells staining positively for S-100 (a, 40×) and CD117 (b, 40×)
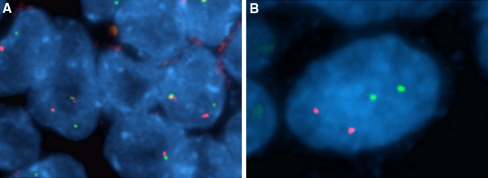
Fluorescent in situ hybridization: All tumor cells revealed rearrangement of NUT as evidenced by split apart of one allele (Fig. 3a). Neither rearrangement of BRD4 nor BRD3 was observed (Fig. 3b), consistent with this being a NUT-variant carcinoma.
Fig. 3.
Dual-color, split-apart fluorescent in situ hybridization (FISH) assay. Probes used for the 15q14 NUT breakpoint (a), flanking a 181 kb region containing NUT, included the 3′ telomeric (green) BAC clones 1H8 and 64o3, and the 5′ centromeric (red) clones 412e10 and 3d4. Probes used for the 19p13.1 BRD4 breakpoint (b) were the 5′ centromeric (red) BAC clone 187l3 and the 3′ telomeric (green) BAC clone 87m17
Discussion
Pediatric salivary gland tumors are exceedingly rare, comprising less than 0.5% of all malignancies [4]. Of these, benign and malignant tumors occur with approximately equal incidence, with pleomorphic adenoma occurring most commonly (60%). Mucoepidermoid carcinomas comprise approximately 25% of all pediatric salivary gland tumors and approximately 80% of salivary gland malignancies in children and adolescents [15]. Acinic cell carcinoma, adenocarcinoma, adenoid cystic carcinoma, metastases and poorly differentiated carcinoma NOS comprise the remaining minority of malignancies [4].
Poorly differentiated carcinomas of the salivary gland present diagnostic challenges and usually portend poor prognosis. Such tumors frequently exhibit high-grade anaplasia, increased mitoses, necrosis and cryptic differentiation that is difficult to resolve by immunohistochemistry. Features in this case included high-grade anaplasia with high mitotic rate, necrosis and focal squamoid differentiation, suggesting a poorly-differentiated squamous cell carcinoma. Although mucoepidermoid and lymphoepithelial carcinomas were initially considered in the differential diagnosis of this case, the absence of mucin and of a dense lymphocytic infiltrate with negative EBER in situ hybridization, excluded both possibilities. Subsequent molecular studies for NUT and BRD3/BRD4 gene rearrangements revealed a positive signal involving the NUT gene with negative BRD3/BRD4 signals, classifying this salivary gland tumor as a “NUT-variant” carcinoma.
The BRD4-NUT translocation defines a distinct tumor class with characteristic highly aggressive clinicopathologic behavior. Review of current known cases (Table 1) shows primarily adults affected (median age: 21) with ages ranging from 3 to 78 years. Males and females are approximately equally affected (M:F, 1:1.3). While the causes of the translocation remain unknown and the functions of both BRD4 and NUT proteins remain only partially understood, multiple studies by French et al. have shed some light on the molecular biology of the fusion protein and its role in tumorigenesis. Currently, BRD4-NUT rearrangement is thought to represent a tumor-initiating event since all tumor karyotypes to date have been simple. The BRD4 protein contains two bromodomains and has been found to bind chromatin during mitosis [3]. The normal role of BRD4 remains partially understood but it is thought to serve as a marker of active transcription, binding genes undergoing transcription immediately prior to mitosis to mark them for resumption following completion of cell division. The normal function of NUT protein is poorly understood. Studies by French et al. [7] have found that NUT remains bound to chromatin when fused to BRD4 or BRD3. As such, it is hypothesized that the BRD moiety tethers NUT to chromatin affecting transcription. siRNA knockout studies against the BRD4-NUT fusion protein by French et al. [7] have also shown induction of dramatic and irreversible squamous differentiation and arrested growth suggesting BRD4-NUT fusion protein blocks differentiation by binding to chromatin.
Table 1.
Summary of clinicopathologic features in patients with reported NUT rearrangement positive carcinomas to date
| Patient | Diagnosis | Location | Histology | Molecular | Clinical course |
|---|---|---|---|---|---|
| 22 y.o. F [6, 12] | PD ca | Thymus | Poorly diff with very focal sq. diff | t(15;19) positive | Bone and lung mets; 14 wk survival |
| 13 y.o. F [6, 17] | UD ca | Epiglottis | High gr anaplasia w/o kerat | t(15;19) positive | LN and skin mets; 36 wk survival |
| 12 y.o. F [6, 17] | PD sq cell ca | Nasopharynx | Poorly diff with very focal sq. diff | t(15;19) positive | Bone mets; 13 wk survival |
| 15 y.o. M [6, 18] | PD sq cell ca | Thymus | Poorly diff with mod focal kerat | t(15;19) positive | Bone and lung mets; 24 wk survival |
| 3 y.o. M [6] | PD sq cell ca | Bladder | Poorly diff with very focal sq. diff | t(15;19) positive | Kidney and adrenal mets; 34 wk survival |
| 15 y.o. F [6] | PD sq cell ca | Orbit | Poorly diff with very focal sq. diff | t(15;19) positive | 28 wk survival |
| 26 y.o. M [6] | UD ca | Sinonasal | High gr anaplasia w/o kerat | t(15;19) positive | Bone mets; 67 wk survival |
| 35 y.o. F [6] | PD ca | Mediastinum | Poorly diff with very focal sq. diff | t(15;19) positive | Bone, soft tissue, pleura mets; 8 wk survival |
| 16 y.o. M [6] | Sq cell ca | Lung | Mod diff withextensive kerat | t(15) | Bone, LN, skin mets; 148 WK survival |
| 16 y.o. F [6] | PD ca | Trachea | Poorly diff with mod focal kerat | t(15) | LN, bone mets;100+ wks survival |
| 21 y.o. F [6] | Nasopharyngeal ca | Nasopharynx | Poorly diff with mod focal kerat | t(15) | Bone, LN, skin mets; 41 mets; 41 wk survival |
| 30 y.o. F [5] | PD ca | Mediastinum | Glycogenated poorly diff; poss thymic origin | t(15;19) positive | Cervical LN, vertebral column, epidural space; + response w/chemo |
| 10 y.o. M [14] | Small round cell tumor | Iliac bone | Small round cell, high MR, necrosis | t(15;19) positive | 4 cycles chemo (35 wks); remission (10 years) |
| 5 y.o. M [13] | PD sq cell ca | Mediastinum | Poorly diff w/mod focal kerat | t(15;19) positive | 14 wk survival |
| 11 y.o. F [11] | Undiff ca | Thorax | Undiff ca | t(15;19) positive | 18 wk survival |
| 31 y.o. M [16] | Sinonasal undiff ca | Nasal cavity | Undiff ca; high MR, necrosis | t(15;19) positive | |
| 34 y.o. F [6] | Unknown | Thorax | t(15;19) positive | ||
| 34 y.o. M [6] | PD ca | Mediastinum | Poorly diff ca | t(15;19) positive | 8 wk survival |
| 39 y.o. F [16] | PD sq cell ca | Nasal cavity/sinus | t(15;19) positive | ||
| 40 y.o. F [16] | PD sq cell ca | Nasal cavity/sinus | t(15;19) positive | ||
| 47 y.o. M [16] | Sinonasal undiff ca | Nasal cavity/sinus | t(15;19) positive | ||
| 78 y.o. F [16] | Undiff ca | Supraglottic larynx | t(15;19) positive | ||
| 15 y.o. M [2] | PD ca with mesenchymal differentiation | Parotid gland | Poorly diff ca w/atypical cartilaginous component | t(15;19) positive | Cervical LN mets; RT and chemo; alive 7 mo after surgery |
| 15 y.o. M (current) | PD ca | Salivary gland | Poorly diff with necrosis and very focal sq diff | t(15) | Ipsilateral neck LN mets; AWD, 15 wks |
AWD alive with disease, ca carcinoma, gr grade, kerat keratinization, LN lymph nodes, mets metastases, PD poorly differentiated, RT radiotherapy, UD undifferentiated, wk weeks, MR mitotic rate
Poorly differentiated tumors of the salivary gland, while diagnostically challenging, usually portend poor clinical outcome. BRD4-NUT positive carcinomas typically behave aggressively with median survival time of 28 wks [5]. By exception, Mertens et al. [14] report full remission in a case of a 10 year old boy with BRD4-NUT positive carcinoma of the iliac bone treated with VAI (vincristine, doxorubicin, and ifosfamide) alternating with one course of PAI (cisplatin, doxorubicin, and ifosfamide) at three-weekly intervals. NUT-variant carcinomas, as described by French et al. [6]. Demonstrate a longer median survival time of 96 weeks but still carry poor prognosis. Since poorly differentiated tumors, including BRD4-NUT positive and NUT-variant carcinomas, lack distinguishing histological or immunohistochemical features, molecular diagnosis of poorly differentiated midline or head and neck lesions in children and adolescents is essential for accurate categorization and treatment. As such, any poorly differentiated midline neoplasm or head and neck tumor that does not exhibit lineage-specific differentiation markers (except squamous) should be considered for NUT rearrangement testing.
In summary, this case of an adolescent with NMC of the salivary gland identifies NUT midline carcinomas as a diagnostic consideration in poorly differentiated salivary gland carcinomas in children and adolescents. Ancillary molecular studies are essential for accurate diagnosis, prognosis and treatment regimens and are recommended for any pediatric salivary gland malignancy with poor differentiation or unclassifiable features.
References
- 1.Chang KL, Chen YY, Shibata D, Weiss LM. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol. 1992;1(4):246–255. [PubMed] [Google Scholar]
- 2.Bakker MA, Beverloo BH, Heuvel-Eibrink MM, et al. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. 2009;33(8):1253–1258. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- 3.Dey A, Ellenberg J, Farina A, et al. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G2-to-M transition. Mol Cell Bio. 2000;20(17):6537–6549. doi: 10.1128/MCB.20.17.6537-6549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias da Cruz Perez DE, Romoa PF, Abreu AF, et al. Salivary gland tumors in children and adolescents: a clinicopathologic and Immunohistochemical study of fifty-three cases. Int J of Ped Otorhinolaryngology. 2004;68(1):895–902. doi: 10.1016/j.ijporl.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Engleson J, Soller M, Panagopoulos I, Dahlén A, Dictor M, Jerkeman M. Midline carcinoma with t(15;19) and BRD4-NUT fusion oncogene in a 30-year-old female with response to docetaxel and radiotherapy. BMC Cancer. 2006;6:69. doi: 10.1186/1471-2407-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22(20):4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 7.French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27(15):2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 8.French CA. Molecular pathology of NUT midline carcinomas. J Clin Pathol. 2008 Jun 13. [DOI] [PubMed]
- 9.French CA, Miyoshi I, Kubonishi I, et al. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63(2):304–307. [PubMed] [Google Scholar]
- 10.French CA, Miyoshi I, Aster JC, et al. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19) Am J Pathol. 2001;159(6):1987–1992. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year old girl showing a translocation t(15;19) Am J Pediatr Hematol Oncol. 1991;13(4):459–464. doi: 10.1097/00043426-199124000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19)(q15; p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 1991;51(12):3327–3328. [PubMed] [Google Scholar]
- 13.Lee AC, Kwong YI, Fu KH, et al. Disseminated mediastinal carcinoma with chromosomal translocation (15;19). A distinctive clinicopathologic syndrome. Cancer. 1993;72(7):2273–2276. doi: 10.1002/1097-0142(19931001)72:7<2273::AID-CNCR2820720735>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Mertens F, Wiebe T, Adlercreutz C, et al. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer. 2007;49(7):1015–1017. doi: 10.1002/pbc.20755. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro NL, Bhattacharyya N. Clinical characteristics and survival for major salivary gland malignancies in children. Otolaryngology-Head and Neck Surgery. 2006;134(4):631–634. doi: 10.1016/j.otohns.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32(6):828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 17.Toretsky JA, Jenson J, Sun CC, et al. Translocation (11;15;19): a highly specific chromosome rearrangement associated with poorly differentiated thymic carcinoma in young patients. Am J Clin Oncol. 2003;26(3):300–306. doi: 10.1097/00000421-200306000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Vargas SO, French CA, Faul PN, et al. Upper respiratory tract carcinoma with chromosomal translocation 15;19: evidence for a distinct disease entity of young patients with rapidly fatal course. Cancer. 2001;92(5):1195–1203. doi: 10.1002/1097-0142(20010901)92:5<1195::AID-CNCR1438>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]