Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a frequent and highly heritable disorder overrepresented in boys. In a recent study investigating boys only, we found that action monitoring deficits as reflected by certain behavioral and electrophysiological parameters were familially driven. As gender may also have an important impact, this was examined in the current study with nonaffected children aged 8 to 15 years having relatives suffering from ADHD (N=37, 21 ♀) and with age-matched controls without family history of ADHD (N=33, 11 ♀).
Extending our previous findings that action monitoring is a potential endophenotype for boys with ADHD, familially driven deficits were confirmed independently of gender. Thus, despite sharing the phenotype with controls, nonaffected siblings showed ADHD-like impairments albeit of smaller magnitude. However, girls performed generally more accurately, which in turn may have produced the differences between nonaffected siblings and controls in affective error processing that were not present in our boys-only assessment.
Keywords: error processing, N2, conflict monitoring, inhibition, endophenotype, familiality, ADHD
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a very common disorder, characterized by severe age-inappropriate levels of hyperactivity, impulsivity and inattention (American-Psychiatric-Association, 1994). Increased prevalence of ADHD among family members of ADHD patients and high heritability rates are frequently reported (Faraone et al., 2005), but developmental pathways to the phenotype, influenced by environmental and genetic factors are not well understood (Banaschewski et al., 2005). Numerous studies suggest that ADHD symptoms derive at least partly from dysfunctions in fronto-striatal dopaminergic networks which control attentional processes (Barkley, 1997; Sonuga-Barke, 2005). If the deficits found are due to genetic and environmental liability, nonaffected relatives of ADHD patients should also display some of these dysfunctions.
We recently proposed that an action monitoring deficit may be a suitable electrophysiological endophenotype of ADHD (Albrecht et al., 2008): boys with ADHD showed decreased performance and in the event-related potential (ERP) reduced N2-enhancement to stimulus conflict and decreased error monitoring in terms of error negativity (Ne) amplitudes as compared to controls without family history of ADHD. On each of these measures the responses of the nonaffected siblings were intermediate between those with ADHD and those without a family history of ADHD.
In contrast, the error-related positivity (Pe), that may reflect an affective component of error assessment (Van Veen & Carter, 2002) appeared to be intact in that study, possibly a consequence of the high error-rate elicited by this task. Similarly, in tasks that require intense conflict monitoring such as the Stop-Task, diminished N2 amplitudes or topographic alterations have consistently been reported for children with ADHD (Albrecht et al., 2005; Albrecht et al., 2008; Brandeis et al., 1998; Pliszka et al., 2000), while no impairments were detected with less demanding Continuous Performance Tests (CPT) or Go/Nogo tasks (Banaschewski et al., 2004; R. Wiersema et al., 2006). Decreased Ne in ADHD was also reported in other studies that used demanding tasks (Liotti et al., 2005; van Meel et al., 2007), but not with simpler tasks (Burgio-Murphy et al., 2007; J. R. Wiersema et al., 2005). Conversely, impaired Pe has been reported from tasks with lower overall error-rates (O’Connell et al., 2009; J. R. Wiersema et al., 2005), but not in tasks evoking less salient errors (Albrecht et al., 2008).
Virtually all these findings are based on studies with boys, as these are strongly overrepresented in ADHD (American-Psychiatric-Association, 1994; Tannock, 1998). Little is known about the effects of gender on ADHD, particularly for cognitive parameters. A number of studies on executive functions have indicated that girls with ADHD are impaired like their male counterparts (Gershon, 2002). In an ERP-study with the Stop-Task, no gender-differences were found for healthy controls and ADHD patients on performance data or with respect to the N2 amplitude (Liotti et al., 2007). However, it has also been reported that girls with ADHD act less impulsively on a CPT than boys (Newcorn et al., 2001).
The aim of this study was to extend our previous findings on a brain based endophenotype of ADHD displaying impaired action monitoring with an explicit assessment of gender effects on ADHD-familiality. Further, we hypothesized that female nonaffected siblings of patients suffering from ADHD would show impaired performance as well as diminished N2-enhancement with conflict and reduced Ne on the flanker task. We also explored whether girls showed a more accurate response-style, that in turn may lead to enhanced Pe.
Methods and Materials
Subjects
Recruitment of subjects was done as part of the International Multi-center ADHD Gene study (NIMH-grant R01MH062873 to S. Faraone). A total of 39 (14 ♀) control children and 42 (24 ♀) nonaffected siblings of children with ADHD, all aged 8 - 15 years and without a clinical diagnosis of ADHD were included in this analysis. The male subsample entered here is almost identical to that previously reported (Albrecht et al., 2008), but girls formed new groups. All subjects had normal or corrected to normal vision and displayed no susceptibility for ADHD as verified by a diagnostic procedure described previously. Ethical approval for this study was obtained from the local ethical review board.
The data from 11 subjects were excluded as result of their showing too few correct responses or errors, or too many artifacts. However this did not differentially affect the factors “Group” (χ2(1)=0.21, p=.65) nor “Gender” (χ2(1)=0.30, p=.59). Groups and gender samples were age-matched and did not differ concerning prorated IQ, or on ADHD scales of the teacher-rated SDQ or Conners scales (all F(1, 66)<1.7, p>.20, see Table 1 for further details).
Table 1.
Sample description
| Measure | Controls (C) | Nonaffected Siblings (S) | ANOVA (F(1, 66)) | ||||
|---|---|---|---|---|---|---|---|
| male ♂ | female ♀ | male ♂ | female ♀ | ||||
| N = 22 | N = 11 | N = 16 | N = 21 | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Group | Gender | Group*Gender | |
| Age (in months) | 134.1 (20.6) | 142.0 (20.9) | 137.0 (24.5) | 142.1 (20.5) | 0.1 | 1.5 | <0.1 |
| Prorated-IQ | 110.3 (11.5) | 108.2 (12.7) | 107.9 (12.7) | 103.4 (8.6) | 1.7 | 1.4 | 0.2 |
| SDQ Teachera | |||||||
| Hyperactivity | 2.2 (2.8) | 2.4 (3.4) | 3.9 (3.1) | 2.2 (2.3) | 1.1 | 1.0 | 1.9 |
| Prosocial Behavior | 6.8 (1.8) | 8.7 (1.3) | 7.1 (1.5) | 7.7 (1.7) | 0.4 | 8.8** | 1.6 |
| Emotional Symptoms | 1.2 (1.8) | 0.6 (1.0) | 2.5 (2.8) | 2.1 (1.9) | 7.2** | 1.0 | <0.1 |
| Conduct Problems | 0.8 (2.1) | 0.3 (0.5) | 2.2 (2.0) | 0.8 (1.6) | 3.6+ | 4.3* | 1.0 |
| Peer Problems | 0.8 (1.5) | 1.1 (1.5) | 2.1 (2.0) | 1.7 (2.0) | 3.8+ | <0.1 | 0.4 |
| Conners Teacher | |||||||
| Global | 48.6 (7.9) | 52.6 (12.0) | 56.6 (9.5) | 52.4 (10.9) | 2.5 | <0.1 | 2.7 |
| DSM-IV ADHD | |||||||
| Inattention | 47.1 (6.6) | 54.7 (13.0) | 53.5 (9.1) | 54.5 (10.5) | 1.7 | 3.2+ | 1.9 |
| Hyperactive Impulsive | 48.0 (6.5) | 52.6 (10.9) | 52.4 (11.4) | 54.5 (14.1) | 1.3 | 1.5 | 0.2 |
| Total | 47.5 (6.0) | 54.4 (12.6) | 53.6 (9.9) | 55.1 (12.1) | 1.9 | 2.8+ | 1.2 |
α < .1
α < .05
α < .01
Strengths and difficulties Questionnaire, Raw-scores, not available for 6 subjects, df=1, 60
Stimuli and Task
Columns of three equilateral black arrowheads were presented in the centre of a CRT monitor against a light grey background subtending vertically 0.8° viewing angle per arrowhead and 3° per column. On each trial, the central fixation mark was replaced by flankers (two arrowheads pointing into the same direction above and below the fixation point) 100 ms before the target arrowhead appeared in between and remained there for another 150 ms. The children had to press response buttons with their index fingers corresponding to the target direction. On congruent trials, flankers and target pointed in the same, and on incongruent trials in opposite directions (Kopp et al., 1996). A trial was presented every 1650 ms, and the task with 400 trials took approximately 13 min. Standardized feedback depending on performance accuracy was given after every 40 trials on the screen for 8 sec in order to control for speed-accuracy trade-offs that can influence error processing (Falkenstein et al., 2000). Two 24 trial practice blocks were administered first. Congruent vs. incongruent stimuli and response directions were balanced and randomized.
Electrophysiological recording and processing
The electroencephalogram from 23 sites according to an extended 10-20 system was recorded along with an electrooculogram (EOG, two electrodes placed above and below the right eye and at the outer canthi) with Ag/AgCl electrodes against FCz as recording reference using a BrainAmp amplifier. Sampling rate was 500 Hz with filters set to 0.016 – 100 Hz and a 50 Hz notch filter. Offline, the EEG was downsampled to 256 Hz, and electrodes were re-referenced to the average and filtered with 0.1 – 15 Hz (24 dB/oct Butterworth filters). Ocular artifacts were corrected using the method of Gratton and Coles without raw average subtraction (Gratton et al., 1983). If the amplitude at any EEG-electrode exceeded ±100 μV, a section -100 to +800 ms was excluded from further analyses. Response-locked (-500 ms to +1000 ms relative to the button press) and stimulus-locked (-200 to +1825 ms around the target-onset) segments were subsequently checked and averaged. The standard serial mouse used to record responses caused a response-trigger delay of 35 ms which was corrected for in the analyses. To avoid distortion of ERP topography, no baseline subtraction was applied. All averages contained at least 25 sweeps.
Statistical Analyses
Behavioral parameters were analysed with repeated measure ANOVAs with within-subject factor “Congruency” (congruent vs incongruent trials) and between subject factors “Group” (controls vs. nonaffected siblings) and “Gender” (boys vs. girls). Stimulus-locked N2 peaks scored 200-400ms after the stimulus-onset at FCz were tested in a repeated measure ANOVA with within factors “Congruency” (congruent correct vs. incongruent correct trials), “Site” (electrodes Fz, FCz, Cz) and between factors “Group” and “Gender”. Error negativity (Ne) and correct response negativity (Nc) were measured at FCz within 150ms following the response on incongruent trials with respect to the respective preceding positivity (Falkenstein et al., 2001; Nieuwenhuis et al., 2001). These peak-to-peak amplitudes were analysed in a repeated measure ANOVA with within factor “Correctness” (Ne vs. Nc) and between factors “Group” and “Gender”. The subsequent plateau-like Pe on incongruent error trials had a centro-parietal maximum. It was tested using the mean amplitude 200 to 500 ms after the response at electrodes Cz and Pz in an ANOVA with factors “Correctness” (error Pe vs. correct response Pc), “Site”, “Group”, and “Gender”.
In order to correct for violations from sphericity, Greenhouse-Geisser ε and adjusted p-values are reported with original degrees-of-freedom if appropriate. In case of significance, additional univariate ANOVAs and analyses of confidence intervals were conducted. Effect sizes in terms of partial eta square (part. η2) were computed and discussed following the notion that part. η2>.01 indicates small, part. η2>.06 medium, and part. η2>.14 large effects (Cohen, 1988).
Results
Error-rate
Error-rates were higher in incongruent than congruent trials (“Congruency” F(1,66)=399.0, p<.01, part. η2=.86; see Table 2). While the error-rates did not differ between the groups (“Group” F(1, 66)=0.3, p=.59, part. η2<.01), accuracy was higher in girls (“Gender” F(1, 66)=10.5, p<.01, part. η2=.14). The effect of congruency on error-rate (i.e. the differences in a two-factorial univariate ANOVA or the repeated measure interaction “Congruency*Gender”) reflected a larger error-rate increase under conflict in boys compared to girls (F(1, 66)=11.9, p<.01, part. η2=.15), and the effect of gender on error-rate was limited to incongruent trials (F(1, 66)=14.1, p<.01).
Table 2.
Behavioral data
| Measure | Controls (C) | Nonaffected Siblings (S) | ANOVA (F(1, 66)) Gender | |||||
|---|---|---|---|---|---|---|---|---|
| male ♂ | female ♀ | male ♂ | female ♀ | |||||
| N = 22 | N = 11 | N = 16 | N = 21 | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Group | Gender | Group*Gender | Repeated Measure | |
| Error-rate (%) | Congruency: 399.0** Group: 0.3 Gender: 10.5** Group*Gender: 0.9 |
|||||||
| congruent trials | 4.5 (5.7) | 3.4 (2.7) | 5.7 (4.8) | 5.7 (3.7) | 2.4 | 0.3 | 0.3 | |
| incongruent trials | 31.5 (11.6) | 21.5 (5.6) | 29.3 (9.8) | 23.2 (3.9) | <0.1 | 14.1** | 0.9 | |
| difference | 27.0 (11.8) | 18.1 (6.1) | 23.5 (9.9) | 17.5 (3.4) | 0.9 | 11.9** | 0.4 | |
| Reaction-times of correct responses (ms) | Congruency: 663.0** Group: 3.5+ Gender: 3.0+ Group*Gender: <0.1 |
|||||||
| congruent trials | 335 (56.7) | 366 (67.0) | 363 (72.7) | 387 (50.7) | 2.7 | 3.3+ | <0.1 | |
| incongruent trials | 433 (71.7) | 457 (56.8) | 463 (83.2) | 493 (55.4) | 3.9* | 2.5 | <0.1 | |
| difference | 98 (33.2) | 91 (21.7) | 100 (36.2) | 105 (27.5) | 1.2 | <0.1 | 0.6 | |
| Reaction-time variability of correct responses (ms) | Congruency: 1.0 Group: 11.3** Gender: 0.2 Group*Gender: <0.1 |
|||||||
| congruente trials | 91 (39.6) | 95 (37.4) | 135 (78.4) | 129 (32.3) | 10.4** | <0.1 | 0.2 | |
| incongruent trials | 94 (42.1) | 85 (26.4) | 137 (84.6) | 124 (25.2) | 11.1** | 0.7 | <0.1 | |
| difference | 3 (19.1) | -10 (16.7) | 1.6 (26.8) | -5 (21.2) | 0.1 | 3.5+ | 0.3 | |
α < .1
α < .05
α < .01
Reaction-times of correct responses
Incongruent items elicited slower reaction-times (“Congruency” F(1,66)=663.0, p<.01, part. η2=.91). In general, reaction-times tended to be slower in nonaffected siblings (“Group” F(1, 66)=3.5, p=.07, part. η2=.05), and in girls compared to boys (“Gender” F(1, 66)=3.0, p=.09, part. η2=.04). As the girls also committed fewer errors, possible differences in speed-accuracy trade-offs were considered by adding age and error-rate on incongruent trials as covariates into the general linear model. After that, the trend towards gender-differences on RT disappeared, but the other effects remained significant.
Reaction-time variability of correct responses
Reaction-time variability was higher in nonaffected siblings compared to normal controls (“Group” F(1,66)=11.3, p<.01, part. η2=.15) with a trend towards diverging effects of conflict across gender (“Congruency*Gender” F(1, 66)=3.5, p=.07, part. η2=.05).
Stimulus-locked ERP
Latencies of N2 did not differ between groups, gender or between congruent and incongruent trials nor were there any interactions (all F<1.4, p>.24; see Table 3). N2 amplitude was enhanced in incongruent compared to congruent trials (“Congruency” F(1, 66)=27.8, p<.01, part. η2=.30), particularly at Fz and FCz compared to Cz (“Congruency*Site” F(2, 132)=29.2, ε=.64**, p<.01, part. η2=.31). However, there were also interactions “Conflict*Site*Gender” (F(2, 132)=5.8, ε=.64**, p=.01, part. η2=.08) and “Congruency*Site*Group*Gender” (F(2, 132)=3.5, ε=.64**, p=.05, part. η2=.05). Analyses of the congruency effect (the N2 amplitude difference between congruent and incongruent trials) for each electrode separately, revealed more N2-enhancement in controls compared to nonaffected siblings at electrodes Fz and FCz (F(1, 66)=5.5, p=.02, part. η2=.08 and F(1, 66)=4.1, p=.05, part. η2=.06, respectively), but not at Cz (F(1, 66)=.01, p=.90, part. η2<.01). Conversely, boys’ N2-enhancement was higher at electrodes FCz and Cz (F(1, 66)=3.5, p=.06, part. η2=.05 and F(1, 66)=9.5, p<.01, part. η2=.13, respectively), but not at Fz (F(1, 66)>0.1, p>93, part. η2<.01). There were no interactions “Group*Gender” at any electrode for N2-enhancement (all F(1, 66)<2.4, p>.13, see Fig. 1).
Table 3.
Stimulus-locked effects of congruency on electrophysiological parameters
| Measure | Controls (C) | Nonaffected Siblings (S) | ANOVA (F(1, 66)) Gender | |||||
|---|---|---|---|---|---|---|---|---|
| male ♂ | female ♀ | male ♂ | female ♀ | |||||
| N = 22 | N = 11 | N = 16 | N = 21 | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Group | Gender | Group*Gender | Repeated Measure | |
| Stimulus-locked N200 Amplitude (μV) | Congruency: F(1, 66)=27.8** Congruency*Group: F(1, 66)=2.9+ Congruency*Gender: F(1, 66)=4.8* Site: F(2, 132)=127.1** Site*Gender: F(2, 132)=4.7* Congruency*Site: F(2, 132)=29.2** Congruency*Site*Group: F(2, 132)=2.5 Congruency*Site*Gender: F(2, 132)=5.8* Congr.*Site*Group*Gender: F(2, 132)=3.5* Group: F(1, 66)=1.7 |
|||||||
| congruent correct | ||||||||
| Fz | -4.5 (3.5) | -5.3 (2.7) | -4.5 (3.8) | -5.9 (3.2) | 0.2 | 1.7 | 0.1 | |
| FCz | -3.8 (2.5) | -4.3 (3.2) | -3.4 (3.4) | -3.9 (3.3) | 0.3 | 0.4 | <0.1 | |
| Cz | -.3 (3.3) | 0.3 (3.5) | 0.7 (3.0) | 0.9 (2.9) | 1.0 | 0.3 | 0.1 | |
| incongruent correct | ||||||||
| Fz | -7.0 (3.7) | -8.6 (2.2) | -6.5 (3.3) | -7.0 (3.8) | 1.4 | 1.5 | 0.5 | |
| FCz | -7.5 (4.2) | -6.6 (2.2) | -5.6 (3.6) | -4.8 (3.7) | 4.3* | 0.9 | <0.1 | |
| Cz | -2.0 (3.7) | 1.9 (4.0) | 0.0 (4.2) | 1.6 (3.6) | 0.8 | 8.5** | 1.5 | |
| mean difference | ||||||||
| Fz | -2.5 (3.0) | -3.4 (2.0) | -2.0 (2.4) | -1.1 (1.7) | 5.5* | 0.1 | 2.4 | |
| FCz | -3.7 (3.7) | -2.3 (3.4) | -2.2 (2.5) | -0.9 (1.9) | 4.1* | 3.5+ | <0.1 | |
| Cz | -1.7 (2.9) | 1.6 (4.1) | -0.7 (3.3) | 0.8 (2.5) | 0.1 | 9.5** | 1.4 | |
α < .1
α < .05
α < .01
Figure 1. Stimulus-locked N2 to congruent and incongruent correct answered trials.
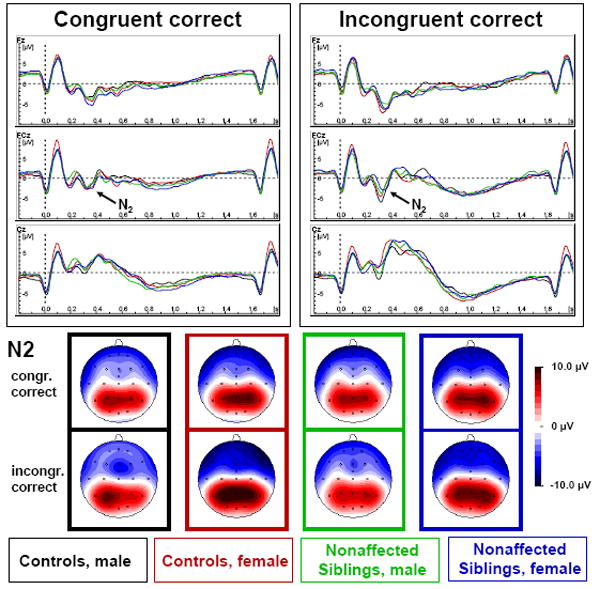
Stimulus-locked grand average waves of male (black) and female (red) controls and male (green) and female (blue) nonaffected siblings with spline-interpolated maps of N2 evoked by correct congruent (above) and incongruent (below) trials at the respective grand average latency.
Response-locked ERP
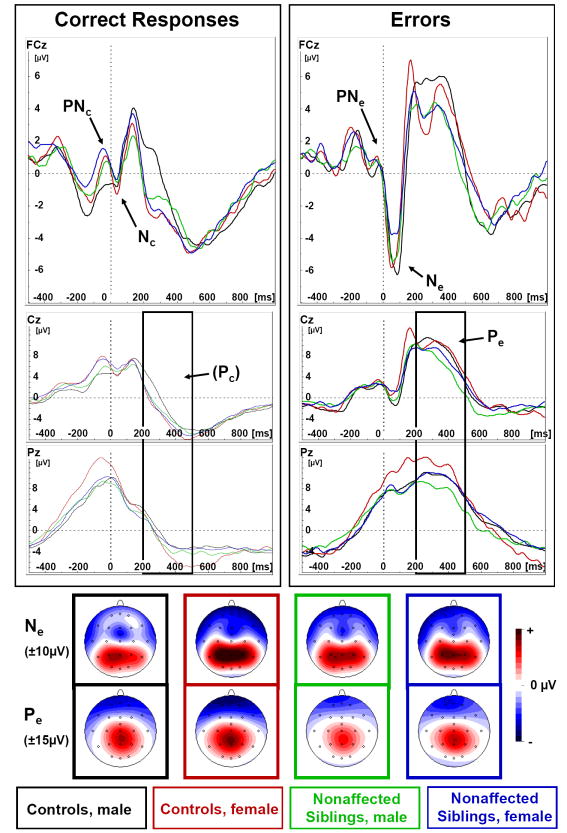
The latency of the Ne was shorter than that of Nc in control boys and nonaffected girls (“Correctness*Group*Gender”, F(1, 66)=4.7, p=.03, part. η2=.07).
Ne was more negative in peak-to-peak amplitude than Nc (“Correctness” F(1, 66)=95.2, p<.01, part. η2=.59, see Table 4). This difference between correct- and error-related negativities was larger in controls compared to nonaffected siblings (“Correctness*Group” F(1, 66)=3.9, p=.05, part. η2=.06); and in boys compared to girls (“Correctness*Gender” F(1, 66)=4.0, p=.05, part. η2<=.06) without showing a higher-order interaction (“Correctness*Group*Gender” F(1, 66)=0.4, p=.56, part. η2<.01). Moreover, a “Group” main effect was found, indicating that both response-locked negativities were enhanced in controls compared to nonaffected siblings (F(1, 66)=6.5, p=.01, part. η2=.09.
Table 4.
Response-locked electrophysiological data of error processing
| Measure | Controls (C) | Nonaffected Siblings (S) | ANOVA (F(1, 66)) Gender | |||||
|---|---|---|---|---|---|---|---|---|
| male ♂ | female ♀ | male ♂ | female ♀ | |||||
| N = 22 | N = 11 | N = 16 | N = 21 | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Group | Gender | Group*Gender | Repeated Measure | |
| Early Response-locked Negativity (peak-to-peak, μV) | Correctness: F(1, 66)=95.2** Corr.*Group: F(1, 66)=3.9* Corr.*Gender: F(1, 66)=4.0* Group: F(1, 66)=6.5* Group*Gender: F(1, 66)=2.3 |
|||||||
| Ne | -9.9 (4.2) | -10.0 (6.0) | -8.4 (4.0) | -5.8 (3.7) | 6.9** | 1.4 | 1.6 | |
| Nc | -2.6 (2.2) | -4.3 (3.3) | -2.7 (1.7) | -2.9 (2.7) | 1.0 | 2.4 | 1.3 | |
| Late Response-locked Positivity (+200 to +500ms, μV) | Correctness: F(1, 66)=549.8** Corr.*Group: F(1, 66)=3.9* Corr.*Gender: F(1, 66)=7.1* Site: F(1, 66)=24.2** Site*Gender: F(1, 66)=4.5* Corr.*Site: F(1, 66)=1.8 Group: F(1, 66)=6.8** Gender: F(1, 66)=3.2+ Group*Gender: 0.2 |
|||||||
| Pe Mean Amplitude | ||||||||
| at Cz | 9.1 (4.1) | 9.1 (3.9) | 6.5 (3.1) | 7.7 (4.1) | 4.4* | 0.4 | 0.4 | |
| at Pz | 9.8 (4.0) | 12.0 (2.9) | 7.7 (2.5) | 9.9 (3.1) | 6.5* | 7.2** | <0.1 | |
| Pc Mean Amplitude | ||||||||
| at Cz | -1.5 (3.0) | -4.2 (2.2) | -3.0 (2.3) | -3.6 (3.1) | 0.4 | 5.7* | 2.2 | |
| at Pz | -1.1 (2.9) | -3.2 (2.1) | -2.2 (2.7) | -1.6 (2.5) | 0.1 | 1.5 | 4.5* | |
α < .1
α < .05
α < .01
The mean amplitude of the Pe component was larger than for the Pc component (F(1, 66)=549.8, p<.01, part. η2=.89). Moreover, this effect of “Correctness” was enhanced in controls compared to nonaffected siblings (“Correctness*Group” F(1, 66)=3.9, p=.05, part. η2=.06) and in girls compared to boys (“Correctness*Gender” F(1, 66)=7.1, p=.01, part. η2=.10) without showing a higher order interaction (F(1, 66)=0.8, p=.38, part. η2=.01). Component amplitudes were generally higher at Pz than Cz (“Site” F(1, 66)=24.2, p<.01, part. η2=.27), particularly in girls (“Site*Gender” F(1, 66)=4.5, p=.04, part. η2=.06, see Fig. 2).
Figure 2. Response-locked error-related components.
Response-locked grand average waves of correct responses and errors to incongruent trials from male (black) and female (red) controls and male (green) and female (blue) nonaffected siblings with spline-interpolated maps of Ne at the respective grand average peak latency (above) and Pe mean activity 200-500ms post error response (below). The response-locked Ne has its maximum at FCz (even more prominent when measured peak-to-peak), while Pe was maximal at centro-parietal electrodes.
Discussion
The main findings of our previous study with boys only were confirmed with the present comparison between controls and nonaffected siblings, taking gender effects into account. The reaction-time variability was markedly larger in the nonaffected siblings than in the controls, and thus resembled levels of variability reported frequently for patients with ADHD (Tannock, 1998). We also found that the error-rates differed between the genders. Thus, while participants of both groups and genders showed very low error-rates and accordingly no statistical effects on congruent trials (floor effect), girls made fewer errors on incongruent trials. This finding may indicate that girls displayed a response-style shifted towards accuracy, which was less well controlled by feedback than in the subsample of boys analysed previously. Gender-differences in response speed disappeared after controlling for accuracy. Thus, in this statistical sense (assuming a linear relation between response speed and accuracy) there are no differences in performance capabilities between genders. Indeed, a similar, slightly slower but more accurate response-style has been described previously (Newcorn et al., 2001) and may prevent children (and especially girls) susceptible for ADHD from showing clinically relevant symptoms.
Action monitoring triggered by stimulus conflict yielded enhanced N2 amplitudes with similar latency after incongruent and congruent stimuli. Controls showed more N2-enhancement than nonaffected siblings at fronto-central sites, whereas boys showed more N2-enhancement than girls particularly at central sites. The different topography of the effects of group and gender on this N2-enhancement following stimulus conflict suggests that at least partly different neural networks are involved, and thus that the basic influence of familial ADHD differs from the effect of gender. Since no differences between groups and gender were found for congruent trials, these effects rather reflect the impact of higher stimulus conflict in incongruent trials than some differences in processing the low conflict congruent trials.
The negative deflection immediately following responses to incongruent stimuli was generally larger for controls than nonaffected siblings. This may well reflect that targets that elicit correct responses can also be in conflict with the flankers. However, error-specific subprocesses or modulations were also active as the negativity was enhanced following errors(Yordanova et al., 2004). Accordingly, nonaffected siblings compared to controls and girls compared to boys showed medium effects of reduced error-specific negativity amplitude. Importantly, no interactions between factors Group and Gender were found. Thus, nonaffected siblings of both genders show consistent medium effect distortions in a parameter associated with early error detection and action monitoring.
The later response-locked positivity was similarly enhanced following errors, which was both with medium effect size more pronounced in controls and in girls. This in line with findings by Wiersema et al., who reported a diminished error-specific Pe and higher error-rates on a Go-nogo task in ADHD subjects compared to controls: unfortunately they did not examine the effects of gender (J. R. Wiersema et al., 2005). The difference between genders on Pe may be related to differences in the speed-accuracy trade-off and presumably indexes gender-differences in error evaluation. The impaired Pe in nonaffected siblings may be a consequence of either girls’ generally higher accuracy or a general gender effect, but this needs to be disentangled in studies using tasks that explicitly manipulate accuracy.
Taken together, the impacts of ADHD-familiality and Gender on performance measures as well as on electrophysiological parameters of action monitoring were generally additive, even though the latter was accompanied by topographical differences concerning location of effects. As a further new finding, we detected diminished Pe amplitude in nonaffected siblings. Since this effect was also additive, we have a concise, although given the relatively small sample size still tentative result concerning our main hypotheses: nonaffected siblings of both genders differ from controls in parameters reflecting diminished action monitoring, and girls tend to perform more accurately and do show enhanced activity in brain processes related to affective error processing.
The current study supplements our previous investigation of action monitoring in boys with ADHD by explicitly assessing ADHD-familiality and gender effects. Although the current findings are suggestive, evidence from a sample of girls with ADHD is needed before parameters of action monitoring can be regarded as gender-independent endophenotypes of ADHD.
Acknowledgments
The authors thank all the children and their families for participation. Christa Dahlmann and Renate Kolle conducted ERP-recordings, Anke Fillmer-Otte, Anne Reiners and Nicola Wöstmann performed IQ- and further neuropsychological testings. Robert D. Oades proofread the manuscript.
Abbreviations
- ACC
anterior cingulate cortex
- ADHD
attention-deficit/hyperactivity disorder
- ERP
event-related potential
- Ne
error negativity or error related negativity
- Nc
negativity following correct responses
- Pe
error positivity
- Pc
positivity to correct responses
Footnotes
Financial Disclosures Recruitment of nonaffected siblings of patients suffering from ADHD was supported by NIMH-grant R01MH062873 to S. Faraone. Daniel Brandeis received support from the Swiss National Science Foundation grant 32-109591. Dipl. Psych. Albrecht, Dr. Banaschewski, Dr. Brandeis, Dr. Heinrich, Dr. Heise, Dr. Uebel and Dr. Rothenberger reported no direct or indirect financial or personal relationships, interests, and affiliations relevant to the subject matter of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the productionprocess errors may be discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
References
- Albrecht B, Banaschewski T, Brandeis D, Heinrich H, Rothenberger A. Response inhibition deficits in externalizing child psychiatric disorders: An erp-study with the stop-task. Behav Brain Funct. 2005;1:22. doi: 10.1186/1744-9081-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B, Brandeis D, Uebel H, Heinrich H, Mueller UC, Hasselhorn M, et al. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: Evidence for an endophenotype. Biol Psychiatry. 2008;64:615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American-Psychiatric-Association. Diagnostic and statistical manual of mental disorders. 4. Washington: American Psychiatric Association; 1994. [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Questioning inhibitory control as the specific deficit of adhd--evidence from brain electrical activity. J Neural Transm. 2004;111(7):841–864. doi: 10.1007/s00702-003-0040-8. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Hollis C, Oosterlaan J, Roeyers H, Rubia K, Willcutt E, et al. Towards an understanding of unique and shared pathways in the psychopathophysiology of adhd. Dev Sci. 2005;8(2):132–140. doi: 10.1111/j.1467-7687.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of adhd. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Brandeis D, van Leeuwen TH, Rubia K, Vitacco D, Steger J, Pascual-Marqui RD, et al. Neuroelectric mapping reveals precursor of stop failures in children with attention deficits. Behav Brain Res. 1998;94(1):111–125. doi: 10.1016/s0166-4328(97)00174-5. [DOI] [PubMed] [Google Scholar]
- Burgio-Murphy A, Klorman R, Shaywitz SE, Fletcher JM, Marchione KE, Holahan J, et al. Error-related event-related potentials in children with attention-deficit hyperactivity disorder, oppositional defiant disorder, reading disorder, and math disorder. Biol Psychol. 2007;75(1):75–86. doi: 10.1016/j.biopsycho.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD. Statistical power analysis for the behavioral sciences. second edition. Hillsdale, New York: Erlbaum; 1988. [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. Erp components on reaction errors and their functional significance: A tutorial. Biol Psychol. 2000;51(2-3):87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related erps with age. Exp Brain Res. 2001;138(2):258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in adhd. J Atten Disord. 2002;5(3):143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33(3):282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, 3rd, Luus B, Glahn D, Semrud-Clikeman M. Electrophysiological correlates of response inhibition in children and adolescents with adhd: Influence of gender, age, and previous treatment history. Psychophysiology. 2007;44(6):936–948. doi: 10.1111/j.1469-8986.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with adhd. Cortex. 2005;41(3):377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Halperin JM, Jensen PS, Abikoff HB, Arnold LE, Cantwell DP, et al. Symptom profiles in children with adhd: Effects of comorbidity and gender. J Am Acad Child Adolesc Psychiatry. 2001;40(2):137–146. doi: 10.1097/00004583-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. [PubMed] [Google Scholar]
- O’Connell RG, Bellgrove MA, Dockree PM, Lau A, Hester R, Garavan H, et al. The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (adhd) Neuropsychologia. 2009;47(4):1149–1159. doi: 10.1016/j.neuropsychologia.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: Event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biol Psychiatry. 2000;48(3):238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Tannock R. Attention deficit hyperactivity disorder: Advances in cognitive, neurobiological, and genetic research. J Child Psychol Psychiatry. 1998;39(1):65–99. [PubMed] [Google Scholar]
- van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (adhd): The role of error processing. Psychiatry Res. 2007 Jun 30;151(3):211–20. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. Erp correlates of impaired error monitoring in children with adhd. J Neural Transm. 2005;112(10):1417–1430. doi: 10.1007/s00702-005-0276-6. [DOI] [PubMed] [Google Scholar]
- Wiersema R, van der Meere J, Antrop I, Roeyers H. State regulation in adult adhd: An event-related potential study. J Clin Exp Neuropsychol. 2006;28(7):1113–1126. doi: 10.1080/13803390500212896. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004;22(2):590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]