Abstract
Deciphering molecular events required for full transformation of normal cells into cancer cells remains a challenge. In T-cell acute lymphoblastic leukemia (T-ALL), the genes encoding the TAL1/SCL and LMO1/2 transcription factors are recurring targets of chromosomal translocations, whereas NOTCH1 is activated in >50% of samples. Here we show that the SCL and LMO1 oncogenes collaborate to expand primitive thymocyte progenitors and inhibit later stages of differentiation. Together with pre-T-cell antigen receptor (pre-TCR) signaling, these oncogenes provide a favorable context for the acquisition of activating Notch1 mutations and the emergence of self-renewing leukemia-initiating cells in T-ALL. All tumor cells harness identical and specific Notch1 mutations and Tcrβ clonal signature, indicative of clonal dominance and concurring with the observation that Notch1 gain of function confers a selective advantage to SCL-LMO1 transgenic thymocytes. Accordingly, a hyperactive Notch1 allele accelerates leukemia onset induced by SCL-LMO1 and bypasses the requirement for pre-TCR signaling. Finally, the time to leukemia induced by the three transgenes corresponds to the time required for clonal expansion from a single leukemic stem cell, suggesting that SCL, LMO1, and Notch1 gain of function, together with an active pre-TCR, might represent the minimum set of complementing events for the transformation of susceptible thymocytes.
Keywords: T-ALL, Notch1, SCL/TAL1, LMO1, leukemia-initiating cell, pre-TCR
Studies of human and murine cancers have identified multigenic hits that cooperate in cell transformation (Berns 1988; Hahn and Weinberg 2002). However, deciphering the multistep process that transforms normal cells into fully metastazing cancer cells remains a challenge. Strategies aimed at dissecting cancer genes indicate that they converge to enhance self-renewal, favor growth factor independence, and cause differentiation arrest (Jonkers and Berns 1996; Hanahan and Weinberg 2000). Interestingly, 70% of recurring chromosomal translocations in childhood T-cell acute lymphoblastic leukemia (T-ALL) involve genes encoding oncogenic transcription factors (Look 1997), which are “master” gene regulatory proteins (Rabbitts 1991) in hematopoiesis. These observations suggest that the process of cell transformation subverts cell fate determinants. Recent genome-wide approaches based on gene expression profiling, analysis of copy number alterations, array CGH, or second-generation deep-sequencing technology have identified a number of deletions, new mutations, or dysregulated gene expression in T-ALL (for review, see Mullighan and Downing 2009). These powerful approaches nonetheless likely overlook active, but unmutated, signaling pathways that are essential for the viability of transformed cells (Luo et al. 2009).
Elevated expression of the SCL/TAL1 transcription factor and/or its nuclear partner, LMO1 or LMO2, is found in 50%–60% of childhood T-ALL, associated with gene rearrangements (Begley et al. 1989; Boehm et al. 1991) or not (Ferrando et al. 2002; for review, see Aifantis et al. 2008). SCL is a member of the basic helix–loop–helix (bHLH) family of transcription factors that specifies the hematopoietic fate from mesodermal precursors, whereas LMO proteins are zinc-binding LIM domain proteins (for review, see Lecuyer and Hoang 2004). Inappropriate SCL expression in the thymus of SIL-SCL, CD2-SCL, or Sca1-SCL transgenic mice (SCLtg) impairs T-cell differentiation (Larson et al. 1996; Herblot et al. 2000) during the preleukemic stage without inducing leukemia (Aplan et al. 1997; Curtis et al. 1997; Goardon et al. 2002), whereas Lck-SCL transgenic mice develop T-cell lymphomas with low penetrance (Condorelli et al. 1996; Kelliher et al. 1996). LMO1tg or LMO2tg mice occasionally develop T-ALL after a long latency period of ∼18 mo (Larson et al. 1994). SCL collaborates with LMO1 (Aplan et al. 1997) or LMO2 (Larson et al. 1996) to accelerate disease onset (3–6 mo) and confer full penetrance. While SCL–LMO1/2 are sufficient to initiate leukemogenesis in transgenic mice, other events are required for emergence of full-blown leukemia, as evidenced by a highly variable latency period.
Gain-of-function mutations of the NOTCH1 gene are found in >50% of human T-ALL (Pear and Aster 2004; Weng et al. 2004) and in SCLtgLMO1tg mouse models of T-ALL (Lin et al. 2006; O'Neil et al. 2006; Gothert et al. 2007). Members of the NOTCH family are evolutionary conserved proteins that control cell fate. NOTCH1 signaling regulates the T-cell versus B-cell fate choice in prethymic progenitors (Sambandam et al. 2005; Aifantis et al. 2008), and also acts at later stages of T-cell development (Zweidler-McKay and Pear 2004). NOTCH1 cooperates with E2A (Ikawa et al. 2006) and the pre-T-cell antigen receptor (pre-TCR) (Ciofani et al. 2004; Aifantis et al. 2008) to specify T-lineage commitment. In the thymus, the pre-TCR (for review, see von Boehmer et al. 1999; Aifantis et al. 2008) controls the critical transition from the CD4−CD8− double-negative (DN) to the double-positive (DP; CD4+CD8+) stages, and coordinates cell proliferation, cell survival, and differentiation in the α/β lineage. In leukemogenesis, the contribution of the pre-TCR appears variable, as it is dispensable in some mouse models (Liao et al. 1998; Engel and Murre 2002) but important in others (Bellavia et al. 2002; dos Santos et al. 2007). In the latter, the precise contribution of the pre-TCR to the leukemogenic process remains unknown. Finally, the importance of the pre-TCR was reported as minor in SCL–LMO1-dependent leukemias (Fasseu et al. 2007), although SCL expression in childhood T-ALL is invariably associated with high TCR expression (Ferrando et al. 2002). Furthermore, SCL-dependent leukemias in mice harbor in-frame TCRβ rearrangements (Chervinsky et al. 2001). Hence, the pre-TCR or TCR pathway may be relevant to the pathophysiology of SCL-associated T-ALL.
Cell transformation results from the accumulation of multiple genetic anomalies (Hahn and Weinberg 2002), arguing for the likelihood that the initiating event occurs in a cell with extended proliferative potential, allowing for the acquisition of additional mutations. However, the cell of origin of leukemia need not be a normal stem cell (for review, see Jordan 2009). It was originally thought that ALLs could originate from a committed lymphoid progenitor arrested at the same stage as the bulk of tumor cells. However, oncogenes can modify cellular phenotypes, as observed in several transgenic mouse models in which the DN-to-DP progression can occur in the absence of pre-TCR signaling due to ectopic expression of BCL2 and NOTCH1/3 or inhibition of E-protein function (Linette et al. 1994; Engel and Murre 2002; Michie et al. 2007). Furthermore, evidence for the presence of a subpopulation of leukemia-initiating cells (LICs) with stem cell properties in T-ALL remains to be documented.
In the present study, we investigated the cellular and molecular events in SCL–LMO1-induced T-ALL using transgenic mice as a model.
Results
The SCL and LMO1 oncogenes expand early thymic precursor (ETP)/DN1 thymocytes
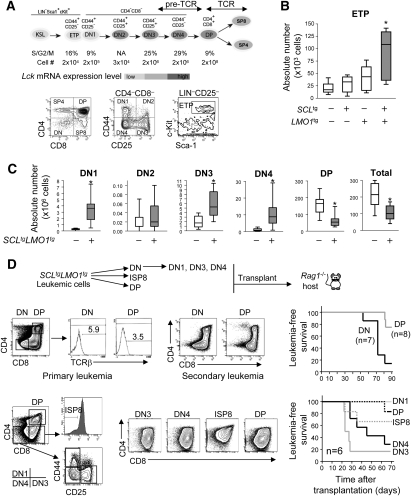
Circulating bone marrow-derived progenitors (Kit+Sca1+Lin− [KSL]) settle in the thymus, where they give rise to ETPs, a subset of DN1 cells with high T-lineage potential (Sambandam et al. 2005), endowed with the highest proliferative potential in the thymus. ETPs further differentiate into DN2, DN3, and DN4 cells (Fig. 1A). While transition from the ETP to DN2 and DN3 stages is associated with 10-fold expansion in cell numbers at each step, at the DN4-to-DP transition, thymocytes actively cycle under the influence of the pre-TCR, resulting in an ∼200-fold expansion of cell numbers (Fig. 1A; Supplemental Fig. S1A) when cells acquire the CD4 and CD8 markers (DP) as well as TCRα/β expression (Fig. 1A). To assess the earliest cell types that are affected by the oncogenes, we first analyzed progenitors in the bone marrow and thymus of SCLtgLMO1tg mice during the preleukemic stage; i.e., at 3–4 wk after birth. The SCL transgene is driven by the SIL promoter, which is active in all proliferating cells (Supplemental Fig. S1D), whereas LMO1 expression is driven by the Lck transgenic cassette. Expression levels of the endogenous Lck and the LMO1 transgene were assessed by RT–PCR. Both were absent in bone marrow KSL, but expression was initiated in thymic ETP/DN1 cells and was found at higher levels in all subsequent thymocyte subsets (Figs. 1A; Supplemental Fig. S1B,C), indicating that the Lck transgenic cassette is active in all thymic precursors, but inactive in bone marrow progenitors. Accordingly, bone marrow KSL numbers were not affected by the transgenes (Supplemental Fig. S2A). In the thymus, however, the SCL–LMO1 transgenes induced a 10-fold expansion of the ETP population compared with wild-type mice, whereas the variations induced by each transgene separately were not significant (Fig. 1B). This expansion was followed by an expansion of DN3/DN4 populations (Fig. 1C), whereas the DP population was decreased, consistent with previous reports indicating that SCL and LMO1 inhibit thymocyte differentiation (Larson et al. 1996; Aplan et al. 1997; Herblot et al. 2000). Consistent with decreased DP thymocytes, total cell numbers in the thymus of SCL–LMO1 transgenic mice were decreased compared with age-matched wild-type mice. Thus, the SCL–LMO1 transgenes induce an expansion of DN thymocytes, starting from the ETP, and inhibit the DN–DP transition.
Figure 1.
The SCL and LMO1 oncogenes expand the population of ETPs and induce CD3-dependent but antigen-independent T-ALL. (A) Schematic diagram of thymocyte populations and their cell surface markers. ETPs are Kit+Sca1+Lin−CD25−. Thymocyte populations (DN1–DN4, DP, SP4, and SP8) are Thy1.2+ and can be further differentiated on the basis of CD25, CD44, CD4, and CD8 expression. The pre-TCR is essential for DN-to-DP transition, whereas the TCR regulates negative and positive selection. Shown are the absolute numbers, the percentages of cells in S/G2/M phase, and Lck expression levels as assessed by real-time PCR for each subset of wild-type thymocyte. (B) The absolute number of ETPs was assessed by flow cytometry analysis of wild-type, SCLtg, LMO1tg, and SCLtgLMO1tg mice at the preleukemic stage (3 wk old). Shown are box plots delimited by the lower and upper 25 percentiles of the distribution with results from Student's t-test ([*] P < 0.001 compared with wild type). The line inside the box plots represents the median, and those outside represent the two extreme values. (C) The absolute number of DN1-to-DP populations was assessed by flow cytometry analysis in wild-type and preleukemic SCLtgLMO1tg mice as in B. (D) Diagram of the transplantation strategy. Indicated leukemic populations were purified from Rag1+/+SCLtgLMO1tg thymomas and transplanted into immunodeficient Rag1−/− mice at concentrations of 103 (DN and DP) or 104 (DN1, DN3, DN4, ISP8 and DP) cells. Examples of tumor phenotypes from primary and secondary leukemias, as well as Kaplan-Meier curves of the time to leukemia, are shown.
LICs are enriched in the DN population
At time of overt leukemia, the leukemic population is heterogeneous and comprises DN and DP cells, as reported (Larson et al. 1994; Kelliher et al. 1996; Chervinsky et al. 1999). Leukemia is sustained by self-renewing LICs that can be detected by transplantation into unirradiated immunodeficient mice (Hope et al. 2004). Leukemias arising in SCLtgLMO1tg mice were analyzed for their capacities to initiate secondary leukemias in Rag1−/− mice. We reasoned that the same clonal marker should appear in primary and secondary leukemias, as an indication of self-renewal. Since the rearrangement of the Tcrβ locus is a marker of clonality in T cells and of SCL–LMO1 leukemias (Chervinsky et al. 1999), these leukemias were analyzed for Vβ8 and Vβ5 rearrangement by PCR prior to and after transplantation (Supplemental Fig. S3). We found the same rearrangement in primary and secondary leukemias, indicating that the same clone has undergone self-renewal. We next assessed the presence of LIC in leukemic DN and DP populations purified from primary leukemias. Transplantation of 106 leukemic cells induced T-ALL in all secondary hosts (data not shown). There was, however, a significant difference among the two groups of mice receiving 103 leukemic cells. By 12 wk after transplantation, almost all mice transplanted with DN leukemic cells were moribund, whereas almost all mice grafted with DP leukemic cells remained healthy during this time frame (Fig. 1D). Furthermore, both leukemic DN and DP populations induced secondary leukemias that reproduced the phenotype of the primary tumor in transplanted hosts (Fig. 1D). Therefore, despite an apparent DN–DP hierarchy based on cell surface markers, LICs are found in both DN and DP populations. The leukemia-initiating potential of the DN population was nonetheless higher than that of the DP population, and was found mostly in the DN3 and DN4 populations (Fig. 1D). Even though the preleukemic DN1 population was expanded, the leukemic DN1 population lacks leukemia-initiating potential.
pre-TCR deficiency delays leukemia onset in SCLtgLMO1tg mice
The DN3-to-DP transition, which is affected by the transgenes during the preleukemic phase, is controlled by the pre-TCR. To address the importance of the pre-TCR in cell transformation, we exploited two genetic models in which thymocyte differentiation is blocked at the DN3 stage; i.e., Cd3ɛ−/− and Rag1−/− mice.
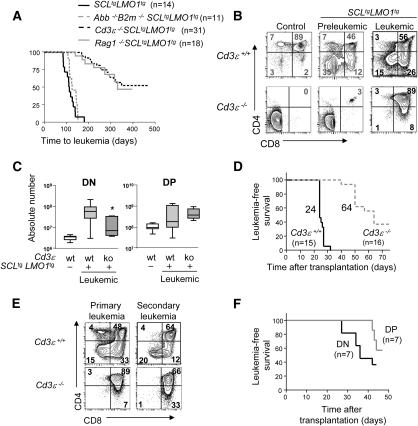
The pre-TCR and TCR require association with the CD3 complex for signaling (Malissen et al. 1995); therefore, Cd3-deficient cells have nonfunctional pre-TCR/TCRs. SCLtgLMO1tg mice develop T-ALL with 100% penetrance (Fig. 2A), as reported (Aplan et al. 1997). However, on a Cd3ɛ−/− background, the penetrance of the disease was decreased by 48% (Figs. 2A–4F) and the median survival increased by 234 d when compared with Cd3ɛ+/+ littermates. Our results indicate a strong genetic interaction between the SCL–LMO1 oncogenes and Cd3ɛ, but differ to some extent with the report by Fasseu et al. (2007) showing a complete abrogation of SCL–LMO1-induced leukemogenesis in Cd3ɛ−/− mice. This discrepancy is currently unresolved, but could be due to differences in genetic backgrounds. All of our mice have been backcrossed on a C57BL6 background for >10 generations, excluding the possibility of modifier loci introduced by genetic crossing between Cd3ɛ−/− and SCLtgLMO1tg mice. Together, these observations indicate that pre-TCR/TCR signaling collaborates with the SCL–LMO1 oncogenes to accelerate and increase the penetrance of leukemia.
Figure 2.
Leukemia initiation is reduced in the absence of CD3/pre-TCR signaling. (A) Kaplan-Meier curves of the time to leukemia for SCLtgLMO1tg, Cd3ɛ−/− SCLtgLMO1tg, Rag1−/−SCLtgLMO1tg, and Abb−/−B2m−/−SCLtgLMO1tg mice. All malignant thymic lymphomas were diagnosed at necropsy. The median survival times for each cohort of n mice are shown. (B) Preleukemic and leukemic phenotypes of SCLtgLMO1tg thymocytes. Representative flow cytometry analysis of thymocytes from wild-type and Cd3ɛ−/−SCLtgLMO1tg as well as nontransgenic control littermates was performed during the preleukemic stage (4 wk old) and at the time of overt leukemia. Numbers in gray are for preleukemic cells and numbers in black are for overt leukemia phenotype. (C) The absolute number of DN and DP populations was assessed by flow cytometry analysis for Cd3ɛ+/+SCLtgLMO1tg and Cd3ɛ−/−SCLtgLMO1tg tumors. Box plots shown as in Figure 1B ([*] P < 0.05 as compared with Cd3ɛ+/+SCLtgLMO1tg mice). (D) Time to leukemia after transplantation of 106 leukemic Cd3ɛ+/+ SCLtgLMO1tg or Cd3ɛ−/− SCLtgLMO1tg cells in Rag1−/− mice (n mice per group). Shown are the median survival times for each cohort. (E) Representative leukemic phenotypes of primary and secondary Cd3ɛ+/+ and Cd3ɛ−/− SCLtgLMO1tg tumors as in B. (F) Kaplan-Meier curves of the time to leukemia for purified DN or DP leukemic populations from Cd3ɛ−/−SCLtgLMO1tg thymomas transplanted into immunodeficient Rag1−/− mice at a concentration of 104. Data are shown as in Figure 1D.
Figure 4.
Genetic interaction between SCL, LMO1, and a hyperactive Notch1 allele in leukemogenesis. (A) Survival analysis of SCLtgLMO1tg, Notch1tg, Notch1tgSCLtg, Notch1tgLMO1tg, and Notch1tg SCLtgLMO1tg mice. Results are presented as in Figure 2A ([*] P < 0.001 as compared with SCLtgLMO1tg mice). Cohorts of n mice were analyzed per genotype. (B) Preleukemic and leukemic phenotypes of Notch1tg and Notch1tgSCLtgLMO1tg mice. Representative flow cytometry analysis of thymocytes was performed at the indicated times as in Figure 2B. (C) Oligoclonal T-cell expansion detected by PCR analysis of Tcrβ gene rearrangements in Notch1tg and Notch1tgSCLtgLMO1tg tumors. Genomic DNA was amplified by PCR with primers for specific variability segments of Tcrβ. Shown is germline configuration, rearrangements in wild-type thymus, and seven independent Notch1tgSCLtgLMO1tg and five independent Notch1tg tumors. (D) Leukemic cells from SCLtgLMO1tg and Notch1tg SCLtgLMO1tg were transplanted through limiting dilution in Rag1−/− mice. Shown is the time to leukemia after transplantation of the indicated numbers of LSC equivalent. LIC frequency (Range LIC ± SE) for the indicated genotype was calculated by applying Poisson statistics using the Limit Dilution Analysis software (Stem Cell Technologies). (E) Purified preleukemic thymocyte populations from 7-d-old SCLtgLMO1tg and Notch1tgSCLtgLMO1tg mice were transplanted into sublethally irradiated coisogenic Pep3B mice at a concentration of 3 × 104. Shown are the numbers of mice presenting T-ALL 4 wk post-transplantation over the total numbers of transplanted mice. (F) Penetrance and median time to leukemia for SCLtgLMO1tg, Notch1tg SCLtgLMO1tg, Cd3ɛ−/− SCLtgLMO1tg, and Cd3ɛ−/−Notch1tgSCLtgLMO1tg.
We took an independent genetic approach with Rag1-deficient mice in which T-cell differentiation is arrested at the DN3 stage, because Rag1−/− cells are unable to rearrange their Tcr genes and therefore do not express the pre-TCR, the TCRα/β, or TCRγ/δ. Rag1-deficient mice expressing the SCL–LMO1 oncogenes developed leukemia with a similar kinetic as Cd3ɛ−/−SCLtgLMO1tg mice (Fig. 2A). Of note, the survival curves for Rag1−/−SCLtgLMO1tg and Cd3ɛ−/−SCLtgLMO1tg mice are almost superimposable. Together, our observations concur to indicate the importance of pre-TCR signaling as a collaborating event with the SCL and LMO1 oncogenes.
Major histocompatibility complex (MHC)-mediated antigen presentation and leukemia onset in SCLtgLMO1tg mice
Signaling by the pre-TCR and the TCR is important for thymocyte survival and proliferation at the DN and DP stages, respectively. The pre-TCR is antigen-independent (Irving et al. 1998; Gibbons et al. 2001), whereas the activation of TCR signaling is dependent on either MHC class I or MHC class II antigen presentation by antigen-presenting cells (APCs) (Matzinger and Bevan 1977; Marrack and Kappler 1988). Differentiation arrest at the DN3 stage in Rag1−/− and Cd3ɛ−/− mice did not allow for an assessment of the importance of the TCR in leukemogenesis, which is required later at the DP stage. To directly address the functional importance of the TCR, we examined SCL–LMO1-dependent T-ALL in mice deficient for both the H2-Ab1 gene (Abb) and the β2-microglobulin gene (β2m)—i.e., lacking MHC class I and class II (Grusby et al. 1993)—and therefore deficient for antigen presentation. Unlike Cd3ɛ−/− mice, the kinetics of SCL–LMO1-induced leukemia in Abb−/−β2m−/− mice was not affected when compared with wild-type littermates (Fig. 2A), despite impaired differentiation at the DP stage (Supplemental Fig. S4A), presumably due to defective antigen presentation and, hence, defective TCR function. Finally, the phenotype of these leukemia resemble those of Abb+/+β2m+/+SCLtgLMO1tg mice (Supplemental Fig. S4A). The collaboration between CD3 and the SCL–LMO1 oncogenes is therefore antigen-independent and, hence, due to pre-TCR signaling. Our observations indicate that antigen-dependent TCR signaling has no discernible contribution to leukemogenesis when the pre-TCR is functional, although we do not exclude the possibility that TCR signaling could be a collaborating event when the pre-TCR is dysfunctional; for example, in the absence of pTα (Fasseu et al. 2007).
Cosegregation between leukemia-initiating potential and Cd3 expression
To gain further insights into interactions between CD3 and SCL–LMO1, we analyzed thymocyte subsets in preleukemic and leukemic Cd3ɛ−/−SCLtgLMO1tg mice.
DP cells were absent in Cd3ɛ−/− (Fig. 2B) and Rag1−/− (Supplemental Fig. S4A) mice, as expected, due to the lack of pre-TCR signaling. Despite this, transgenic expression of SCL–LMO1 in Rag1−/− and Cd3ɛ−/− mice induced the appearance of a minor population of DP cells (2%–5%) in the thymus during the preleukemic stage, and a dramatic accumulation of DP cells in leukemic mice. Thus, the SCL and LMO1 transgenes allowed for progression to the DP stage independently of the pre-TCR, indicating that the phenotype of leukemic cells in this T-ALL does not reflect the developmental stage of the cell of origin of leukemia. Furthermore, the overwhelming progression to the DP stage at the time of overt leukemia suggests that a subpopulation of DN3 transgenic thymocytes has bypassed the DN–DP checkpoint. Surprisingly, leukemic DN populations were expanded in Cd3ɛ+/+ but not in Cd3ɛ−/− tumors, whereas DP populations were more significantly expanded in Cd3ɛ−/− tumors (Fig. 2B,C). In both genotypes, these DP cells are, however, nonfunctional due to the absence of CD3 or of low levels of TCRβ (data not shown).
To assess the presence of LICs in Cd3ɛ+/+ and Cd3ɛ−/− leukemias, we transplanted 106 leukemic cells in adult Rag1−/− mice (Fig. 2D,E). Mice grafted with Cd3ɛ+/+SCLtgLMO1tg leukemic cells reproducibly developed leukemia within a narrow window of 24–32 d. In sharp contrast, 35% of mice transplanted with Cd3ɛ−/−SCLtgLMO1tg leukemic cells remained healthy 75 d after transplantation. These observations indicate that Cd3ɛ+/+SCLtgLMO1tg leukemias have a higher potential to initiate leukemia in secondary hosts.
Despite the preponderance of the DP population, we noticed the presence of a minor population of DN cells in Cd3ɛ−/−SCLtgLMO1tg leukemias (Fig. 2B,C). We therefore purified DN and DP cells from these leukemias and found that DN leukemic cells have higher leukemia-initiating potential than DP leukemic cells (Fig. 2F). Furthermore, both DN and DP cells fully reconstitute the phenotypic composition of the primary tumor, giving rise to DP (70%), DN (4%), and immature single positive 8 (ISP8; 15%) cells in transplanted hosts (Supplemental Fig. S4C).
Cosegregation between Cd3 and Notch1 mutations in SCL-dependent leukemogenesis
This high capacity to initiate secondary T-ALL in Cd3ɛ+/+SCLtgLMO1tg leukemias suggests that additional genetic events might confer stem cell-like properties to populations that are expanded by the SCL–LMO1 oncogenes. Cytogenetic analysis of tumors from SCLtgLMO1tg mice by G-banding and SKY (Supplemental Figs. S5, S6) revealed that three out of four tumors exhibit a normal karyotype. One out of four tumors showed the presence of trisomy 7 and/or 16. Thus, the SCL–LMO1 oncogenes did not cause major chromosomal instability. Nonetheless, the clonal mark of rearranged Vβ5 and Vβ8 loci in primary leukemias that was also found in secondary leukemias indicated that the disease is monoclonal, regardless of Cd3 gene expression (Supplemental Fig. S3). These observations suggest that one or a few clones acquired a proliferative advantage, possibly through the occurrence of additional mutations that were not detected by cytogenetic analysis.
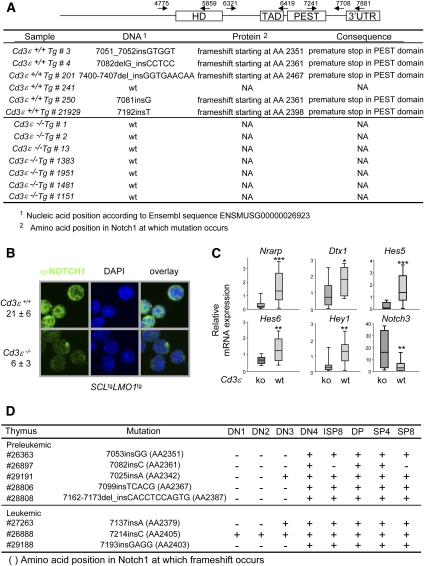
NOTCH1 gain-of-function mutations are frequently found in T-ALL, mostly in the PEST domain and the heterodimerization domain (Weng et al. 2004; Roy et al. 2007; Aifantis et al. 2008). Furthermore, NOTCH signals are required for the generation of ETPs (Sambandam et al. 2005), which we show here to be expanded by the SCL–LMO1 oncogenes, and to sustain the growth of LICs in human T-ALL (Armstrong et al. 2009). We therefore systematically sequenced the Notch1 proto-oncogene using cDNAs amplified from six SCLtgLMO1tg leukemias arising in Cd3ɛ+/+ mice and seven in Cd3ɛ−/− mice. We covered sequences upstream of the heterodimerization domain through to the 3′ untranslated region (UTR) in three amplicons (Fig. 3A). Frame-shift insertions and mutations that disrupt the PEST domain of Notch1 and that are associated with a premature stop were found in Cd3ɛ+/+ leukemias, except for sample #241, consistent with published reports (Fig. 3A; Lin et al. 2006; O'Neil et al. 2006). No other mutation was found outside the PEST domain, including the heterodimerization domain. In contrast, no mutation was detected in seven different Cd3ɛ−/− leukemias induced by SCL–LMO1. The cosegregation between Cd3 and Notch1 gain-of-function mutations suggests a functional link between Notch1 and Cd3 in T-ALL.
Figure 3.
Activating Notch1 mutations and high NOTCH1 protein levels in SCL–LMO1 T-ALL are dependent on Cd3 gene status. (A) The Notch1 gene from Cd3ɛ+/+SCLtgLMO1tg and Cd3ɛ−/− SCLtgLMO1tg tumors was sequenced in three overlapping amplicons. Shown are the positions of the primers used in the sequencing reactions. HD, TAD, and PEST represent the heterodimerization, transactivation, and protein degradation domains, respectively. (B) NOTCH1 intracellular staining in Cd3ɛ+/+SCLtgLMO1tg and Cd3ɛ−/−SCLtgLMO1tg primary tumor samples. The average fluorescence intensity per pixel from Z-stack confocal analysis from two Cd3ɛ+/+SCLtgLMO1tg and three Cd3ɛ−/−SCLtgLMO1tg different tumor samples is shown as mean ± SD. (C) Quantitative RT–PCR of the indicated NOTCH1 target genes were performed in Cd3ɛ+/+SCLtgLMO1tg and Cd3ɛ−/−SCLtgLMO1tg tumors. Relative mRNA expression levels in tumor cells are normalized by CT values obtained with Cd3ɛ+/+SCLtgLMO1tg cells and are presented as box plots with the median and extreme values of each distribution ([*] P < 0.05; [**] P < 0.01; [***] P < 0.001 as compared with Cd3ɛ−/−SCLtgLMO1tg tumors). (D) The Notch1 gene from purified late preleukemic and leukemic thymocyte subsets from Cd3ɛ+/+ SCLtgLMO1tg mice was sequenced as in A. (ISP8 corresponds to Thy1.2+CD8+CD4−Cd3ɛlo, SP4 corresponds to Thy1.2+CD4+CD8−, and SP8 corresponds to Thy1.2+CD4−CD8+.)
Cosegregation between Cd3 expression, NOTCH1 protein levels, and NOTCH1 activity in leukemic thymocytes
To verify the consequences of mutations in the PEST domain, we first determined NOTCH1 protein levels by immunofluorescence in Cd3ɛ+/+ and Cd3ɛ−/− SCL–LMO1 leukemias. We found fourfold higher NOTCH1 protein levels in Cd3ɛ+/+ tumors and strong nuclear staining (Fig. 3B; Supplemental Fig. S7A), indicating that mutations of the PEST domain result in increased NOTCH1 protein. Increased nuclear NOTCH1 in Cd3ɛ+/+ tumors was accompanied by higher expression of known NOTCH1 target genes in SCLtgLMO1tg leukemias (Fig. 3C; Supplemental Fig. S7B), Nrarp, Dtx1, Hes5, Hes6, and Hey1. Hes1, Il7r, Gata3, and Myc did not differ between Cd3ɛ+/+ and Cd3ɛ−/− leukemias. Finally, Notch3 and Ptcra showed higher expression levels in Cd3ɛ−/− leukemias. Since Hes1, Il7ra, Myc, and Ptcra are also controlled by NOTCH3 (Bellavia et al. 2002; Ong et al. 2006; Vacca et al. 2006), the elevated levels of Notch3 in Cd3ɛ−/− leukemias may compensate for lower NOTCH1 levels in these leukemias. Of note, Myc is expressed in both Cd3ɛ+/+ and Cd3ɛ−/− SCLtgLMO1tg tumors at levels comparable with Notch1tg tumors, consistent with an important role for c-Myc in leukemogenesis downstream from NOTCH1 (Sharma et al. 2006; Weng et al. 2006) and possibly NOTCH3. Furthermore, NOTCH1 target genes were low in leukemic samples from mouse #241, in which the Notch1 gene was wild type and for which the time of leukemia onset was the longest (183 d) within the SCL–LMO1 series. These results suggest that NOTCH1 activity is higher in Cd3ɛ+/+SCLtgLMO1tg tumors, concurring with higher NOTCH1 protein levels and PEST domain mutations in the Notch1 gene.
Strikingly, Notch1 mutations were found in DN4 cells during the late preleukemic stage, but not in more primitive thymocyte progenitors, except for sample #29191 (Fig. 3D). Furthermore, the same mutation was found in subsequent stages of thymocyte differentiation. However, mutations were found at different positions in five different preleukemic samples. Together, these observations further support the hypothesis that the acquisition of Notch1 gain of function confers clonal dominance. In sample #26897, we did not observe Notch1 mutations in the CD8 lineage, possibly because of the limit of detection of the PCR reaction, or, alternatively, these cells are not functional CD8+ cells, but represent more immature stages. These observations indicate that Notch1 mutations occur mostly at the DN4 stage during the preleukemic phase, and concur with the absence of Notch1 mutations in Cd3-deficient leukemias (Fig. 3A–D). At the time of overt leukemia, however, we found Notch1 mutations in DN3 cells in two out of three samples. Since DN3 cells are defined as CD25+CD44− and, furthermore, CD25 is a target of NOTCH (Deftos et al. 2000), it is possible that the acquisition of Notch1 gain-of-function mutations may cause abnormal CD25 expression. The presence of Notch1 mutations in DN1 and DN2 subpopulations in sample #26888 may be due to disrupted hierarchical organization and abnormal differentiation in leukemia (Jordan 2009), or, alternatively, that DN1 cells can occasionally acquire Notch1 mutations. Again, all subpopulations have the same mutation as observed during the preleukemic phase, consistent with clonal dominance. Together, our observations indicate that Notch1 gain of function as well as an active pre-TCR collaborate with the SCL and LMO1 oncogenes.
Genetic interactions between Notch1 and SCL–LMO1
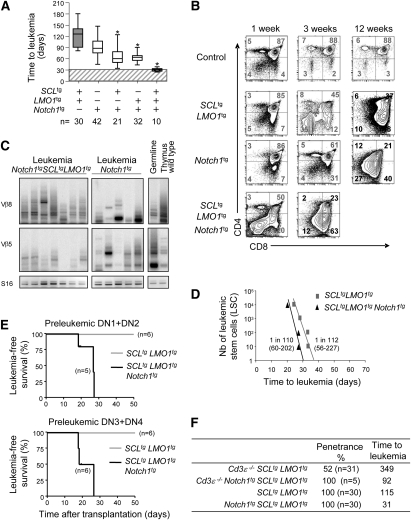
To determine whether Notch1 interacts genetically with the SCL and LMO1 oncogenes, we crossed SCLtgLMO1tg mice with mice overexpressing a constitutively active intracellular NOTCH1, lacking the PEST and part of the TAD domains (Notch1-ICΔP), under the control of the Lck promoter (Fowlkes and Robey 2002). Notch1tg or SCLtgLMO1tg mice developed acute leukemia with highly variable times of onset (Fig. 4A). The SCL or LMO1 transgene did not induce leukemia separately, but significantly accelerated leukemia onset in the presence of Notch1-ICΔP, suggesting that these genes operate in parallel pathways. In all of these series, the time of onset remains highly variable. Strikingly, triple Notch1tgSCLtgLMO1tg mice developed leukemia almost synchronously around day 31 after birth. This shortened time of onset (P < 0.01) indicates synergistic interactions. Furthermore, this narrow window of onset suggests that the introduction of Notch1-ICΔP is sufficient to bypass the time required for clonal evolution, which is highly variable.
We next assessed the cellular distribution within the DN population of leukemic cells. In contrast to the SCL and LMO1 transgenes, Notch1-ICΔP did not cause an expansion of the DN1/DN2 populations, but of the DN4 and ISP8 (Supplemental Fig. S6C), even though the Notch1-ICΔP transgene may be expressed at earlier stages. Since Notch1 mutations cosegregate genetically with Cd3 (Fig. 3A), and Notch1 mutations are found in DN4 cells and later stages, we surmise that the proliferative signal from the pre-TCR increases the likelihood of accumulating mutations that include the Notch1 locus. In leukemic thymi, coexpression of the SCL–LMO1 transgenes had expanded the numbers of DN1 and DN2 cells compared with leukemias arising in Notch1tg mice, whereas the other populations did not differ significantly between the two types of leukemias. Notably, the heterogenous distribution within each DN or DP subset was markedly reduced in triple-transgenic mice compared with Notch1tg or SCLtgLMO1tg mice (Supplemental Figs. S4B-S6C). In addition, Cd3ɛ−/− leukemias differ most from triple-transgenic mice; i.e., the numbers of DN1 and DN4 cells were lowest and the number of DP was highest, consistent with the absence of Notch1 mutations in these leukemias. However, the ISP8 population was in the same range, indicating that the SCL–LMO1 oncogenes can expand this population in the absence of Notch1 gain-of-function mutation. Overall, the total numbers of cells within the various DN subsets in leukemias arising in triple Notch1tgSCLtgLMO1tg mice was not higher than those arising in SCLtgLMO1tg or Notch1tg mice (Supplemental Fig. S6C). We next verified the clonality of leukemias arising in Notch1tgSCLtgLMO1tg mice and traced the presence of numerous clones (Fig. 4C), contrasting with the predominance of one or a few clones in SCLtgLMO1tg leukemias (Supplemental Figs. S3, S4B). The presence of multiple clones in leukemias from Notch1tgSCLtgLMO1tg mice suggests that T-ALL occurred in the absence of obvious clonal dominance.
To determine whether the shortened time to leukemia in triple Notch1tgSCLtgLMO1tg compared with SCLtgLMO1tg mice might be due to increased LIC frequencies, we transplanted Rag1−/− mice with limiting dilutions of cells from these leukemias (Fig. 4D). We found that LIC frequency was not increased by the Notch1 transgene. Furthermore, this limiting dilution analysis indicates that the time to leukemia for one leukemic stem cell (LSC) equivalent is 31 d for Notch1tgSCLtgLMO1tg leukemias and 36 d for SCLtgLMO1tg leukemias. These similarities concur with the presence of spontaneously occurring Notch1 mutations in SCLtgLMO1tg leukemias and with the determining importance of these gain-of-function mutations on the biology of the leukemic clone. Strikingly, this time to leukemia was comparable with that observed in Notch1tgSCLtgLMO1tg mice (Fig. 4A). We therefore conclude that the 92-d difference between the median time to leukemia of double SCLtgLMO1tg and triple Notch1tgSCLtgLMO1tg mice represents the time required for acquisition of Notch1 mutations in a Cd3ɛ+/+ background.
To address the question of whether a constitutively active Notch1 gene confers leukemia-inducing potential to preleukemic SCL–LMO1-expressing DN or DP cells, we transplanted 3 × 104 purified DN1–DN2 and DN3–DN4 cells and 3 × 105 DP cells from double SCLtgLMO1tg and triple Notch1tgSCLtgLMO1tg mice into sublethally irradiated hosts (Fig. 4E; data not shown). The results show that purified thymocyte subsets from double SCLtgLMO1tg mice failed to induce leukemia after 70 d, indicating that these two oncogenes are not sufficient for cell transformation. This contrasts with the capacity of leukemic DN3/4 cells to initiate leukemia in transplanted hosts, possibly due to acquired Notch1 mutations. Indeed, purified DN1–DN2 and DN3–DN4 cells from triple Notch1tgSCLtgLMO1tg mice induced T-ALL in secondary recipients with high efficiency, indicating that Notch1 gain of function confers leukemogenicity to SCL–LMO1-expressing DN thymocytes. In contrast, the DP population was much less susceptible, and only one of six recipient mice developed leukemia. Moreover, the transformation of DN1–DN2 cells by the three oncogenes indicates that pre-TCR is not essential for leukemogenesis. Accordingly, we observed that the Notch1 transgene allows for a bypass of pre-TCR signaling deficiency to induce T-ALL with full penetrance and shortened latency on a Cd3-deficient background (Fig. 4F). Finally, all preleukemic triple-transgenic DN subsets induced T-ALL within 25 d, which corresponds to the time required for clonal expansion of fully transformed LSCs. Together, these experiments indicate that the three oncogenes—Notch1, SCL, and LMO1—are sufficient to convert susceptible thymocyte precursors into LICs without requiring pre-TCR signaling.
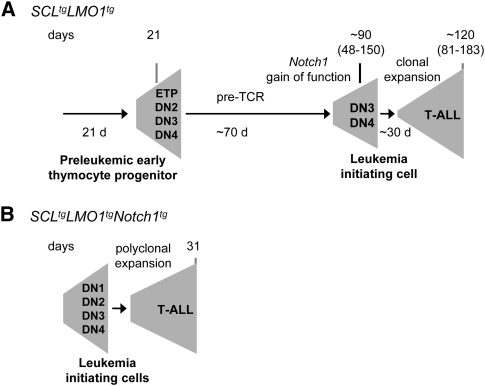
Our observations concur to support the conclusions that the variable times of leukemia onset in Cd3ɛ+/+SCLtgLMO1tg mice represents the time required for accumulation of Notch1 mutations (Fig. 5A). Accordingly, the presence of all three oncogenes at birth is sufficient for full transformation of primary thymocyte precursors. The time to leukemia therefore represents the time required for clonal expansion (Fig. 5B).
Figure 5.
Model of progression to leukemia. (A) At the time of weaning, thymic expression of the SCL–LMO1 oncogenes induces an expansion of the ETP/DN1 to DN4 population. A period of 92 d is necessary for acquisition of Notch1 gain-of-function mutations. The clonal expansion of the leukemic population takes another 31 d before overt leukemia. (B) The presence of all three oncogenes (Notch1, SCL, and LMO1) at birth is sufficient for full transformation of primary thymocyte precursors. The time to leukemia therefore represents the time required for clonal expansion.
Discussion
In the present study, we provide genetic evidence that Notch1 and the pre-TCR collaborate with the SCL–LMO1 oncogenes in T-ALL.
The pre-TCR and Notch1 as collaborating events in leukemogenesis
Primary oncogenic events such as those triggered by chromosomal translocations are generally insufficient by themselves to cause leukemias, and require secondary cooperative mutations to fully transform cells. These collaborating events may be different, depending on the initiating cytogenetic event and the target cell of transformation. Hence, T-ALL with MLL rearrangements exhibit high Flt3 levels (Armstrong et al. 2002; Brown et al. 2005), whereas NOTCH1-activating mutations were found in most molecular subgroups of T-ALL (Weng et al. 2004; Grabher et al. 2006).
Previous work indicates that pre-TCR or TCR signaling (Chervinsky et al. 2001; Fasseu et al. 2007) accelerates SCL-dependent leukemias in transgenic mouse models, and that SCL+ T-ALL constitutes a poor prognostic group in childhood leukemias (Ferrando et al. 2002), associated with elevated expression of genes of the TCR pathway. The relative importance of the pre-TCR or the TCR, however, remains debatable. Here, we show that abrogation of antigen presentation in the Abb−/−β2m−/− mice did not delay SCL–LMO1-induced leukemias, indicating that, when the pre-TCR is active, the contribution of antigen-dependent TCR signaling to leukemogenesis is minimal. In contrast, CD3 signaling is an important determinant, consistent with the report that all SCL–LMO1-induced T-ALL in SCID mice have an in-frame rearranged TCRβ and express the pTα (Chervinsky et al. 2001), suggesting a functional importance for the pre-TCR. Nonetheless, when the pre-TCR is inactive, as in pTα-deficient mice in which TCRα/β lymphocytes are still produced, albeit at reduced levels, it is possible that the TCRα/β or TCRγ/δ may provide an alternative collaborative signal for the SCL–LMO1 oncogenes, as reported (Fasseu et al. 2007).
Despite the recognition that pre-TCR signaling is important in T-ALL, the precise contribution of the pre-TCR to leukemogenesis remains to be documented. In the present study, we provide evidence that the aggressiveness of pre-TCR-proficient SCL–LMO1 T-ALL is associated with Notch1 mutations. Activating Notch1 mutations are a frequent occurrence in T-ALL and have been shown to coincide with the appearance of pre-LSCs in SCLtgLMO1tg mice (Lin et al. 2006). The absence of Notch1 gain-of-function mutations in Cd3ɛ−/− leukemias suggest that either Notch1 mutations do not provide a selective advantage to pre-TCR-deficient cells, or these cells are not permissive to Notch1 mutations. Here we provide several lines of evidence in support of the second hypothesis. First, we show that SCL–LMO1 expands ETP-DN1 cells and subsequent DN stages of thymocyte differentiation but inhibits the DN4–DP transition, thus favoring the accumulation of cells at the highly proliferative DN4 stage and, consequently, the appearance of additional mutations. Second, we found that activating Notch1 mutations cosegregate with Cd3ɛ in leukemogenesis. Third, in Cd3ɛ-proficient cells, Notch1 mutations are found in DN4 cells and later maturational stages, but not in DN1/2 and rarely in DN3 cells. Nonetheless, a hyperactive Notch1 allele bypasses the requirement for pre-TCR function in SCL–LMO1-induced leukemias. In summary, our observations indicate that the pre-TCR provides a permissive context for the appearance of mutations that include gain-of-function mutations of the Notch1 gene in primary thymocyte precursors expanded by the SCL–LMO1 oncogenes. Finally, our observation that, during the preleukemic phase, this hyperactive Notch1 allele confers leukemogenic potential to SCLtgLMO1tg DN1–DN2 subsets that are pre-TCR-independent indicates that Notch1 can provide a selective advantage in the absence of the pre-TCR (Campese et al. 2006).
A recent study indicates that LMO2 but not Notch3 favors the self-renewal of DN3 thymocytes (McCormack et al. 2010), leaving open the question of the role of Notch1 gain-of-function mutations in T-ALL. Our observations indicate that activating Notch1 mutations are essential for cell transformation induced by the SCL and LMO1 oncogenes. Previous work places the Myc oncogene downstream from NOTCH1 (Sharma et al. 2006; Weng et al. 2006; Li et al. 2008). As a corollary, Notch1 and Myc mutations are mutually exclusive in large-scale insertional mutagenesis screens (Kool and Berns 2009). Furthermore, SCL-induced leukemias are also accelerated by loss of Trp53 (Curtis et al. 1997), and Notch1 has been shown to suppress p53 in lymphomagenesis (Beverly et al. 2005), consistent with the observations that activating Notch1 mutations are not found in p53-deficient lymphomas (Uren et al. 2008). It will be interesting to assess whether p53 deficiency or c-Myc acts downstream from or in parallel to Notch1 in SCL–LMO1/2-induced leukemias. Although Notch1 mutations occur at a high frequency of ∼80% in Cd3ɛ+/+SCLtgLMO1tg mice (Lin et al. 2006; this study), cell transformation can also involve alternative pathways, such as the Wnt/β-catenin signaling pathway (Guo et al. 2007).
Target cell of transformation
The nature of the target cells of transformation by oncoproteins remains an open question. The DP population is transient in the thymus, and most DP thymocytes are eliminated by apoptosis. Thus, even if oncogene activation can occur in these cells, the probability of survival and subsequent clonal evolution is limited. Our genetic approach, combined with transplantation of purified thymocyte progenitors during the preleukemic phase (i.e., 1 wk after birth), indicates that the collaboration between the three oncogenes (SCL, LMO1, and Notch1) is sufficient to transform susceptible target cells; i.e., DN1–DN4 thymocyte progenitors. In principle, all hematopoietic progenitors that can give rise to thymocytes could be targeted by the Notch1 oncogene. Indeed, retroviral delivery of a constutively active NOTCH1 gene in hematopoietic stem cells (HSCs) has been shown to exclusively induce T-ALL (Pear et al. 1996). In contrast, our observations indicate that DP cells are less susceptible to cell transformation by these oncogenes. Since the DP stage represents a critical checkpoint in the thymus, it is possible that only DP cells that have successfully undergone selection are prone to oncogene transformation, whereas cells that are fated to be eliminated may require additional collaborating events.
Since SCL–LMO1/2 leukemias are mostly DP, these observations are consistent with the view that the surface phenotype of the bulk of leukemic cells may not be indicative of the cell of origin of leukemia. This is further supported by our genetic approach that ectopic expression of the SCL–LMO1 oncogenes in the thymus of Rag1- or Cd3ɛ-deficient mice induces tumors that “illegitimately” express the CD4 and/or CD8 markers (DP or ISP8), even though these thymocytes should be arrested at the DN3 stage due to pre-TCR deficiency.
Similar to Notch1, ectopic LMO2 expression in bone marrow HSCs by retroviral gene transfer (our unpublished results) or by LTR-mediated activation of the endogenous gene (for review, see Hoang 2010) also gives rise to T-ALL. Thus, bone marrow-derived HSCs and all DN thymic populations are susceptible to transformation by NOTCH1, SCL, and LMO1. Nonetheless, in the double SCL–LMO1 transgenic mouse model, Notch1 mutations accumulate at the DN4 stage, possibly due to intensive cell proliferation. Since Notch1 gain of function confers leukemia-initiating potential to SCL–LMO1 transgenic thymocytes, it is quite possible that SCL–LMO1 favors the accumulation of DN4 cells by expanding the DN1 population, but also by preventing the DN4–DP transition, thereby facilitating the acquisition of complementing Notch1 mutations.
LSCs have been studied extensively in acute myeloid leukemia (AML), where they are found in primitive populations (Lapidot et al. 1994). In contrast, the identification of LSCs in ALL is still controversial (Jordan 2009). A recent study in human B-ALL showed that most populations of leukemic blasts were able to reconstitute the tumor phenotype (le Viseur et al. 2008). Transformed leukemic cells could follow a disorganized differentiation pattern associated with an illegitimate expression of surface markers resulting from oncogenic protein misexpression, such as NOTCH1/3 (Michie et al. 2007). Despite the fact that preleukemic DP cells rarely initiate leukemias in transplanted hosts, we show here that both leukemic DN and DP populations in SCL–LMO1 leukemias have leukemia-initiating potential, even though leukemic DN3–DN4 cells have the highest potential in our model. During the preleukemic phase, Notch1 mutations occur in DN4 thymocytes. At time of overt leukemia, however, Notch1 mutations were identified in leukemic cells with a more primitive phenotype, which could be explained by either a developmental flexibility (Jordan 2009) or oncogene-mediated surface marker misexpression. Indeed, in Notch1tg thymocytes, we observed an increase in Cd25 expression (Deftos et al. 2000; Fowlkes and Robey 2002; data not shown) that could confer a DN3 surface phenotype to cells that are actually at the DN4 stage. Together, our results show that different subpopulations of leukemic cells have leukemia-initiating potential, suggesting that leukemic cells in T-ALL do not follow an orderly developmental structure.
Significance of the time to leukemia
In our transgenic model, SCL and LMO1 gains of functions precede the acquisition of Notch1 mutations (Fig. 5; Lin et al. 2006), although the sequence of events may also be inverted, as reported in some cases of childhood T-ALL (Eguchi-Ishimae et al. 2008; Hong et al. 2008). It has been proposed that acquiring the appropriate numbers of complementary genetic hits may be more important than the sequence of events in cell transformation, even though these genetic alterations may occur according to a preferred sequence (Fearon and Vogelstein 1990). In the present study, we provide several lines of evidence supporting the hypothesis that Notch1 gain-of-function mutations confer clonal dominance to SCL–LMO1 transformed cells. First, the clonality of leukemic cells as assessed by the Tcrβ rearrangement indicates the emergence of a single clone. Second, seven of eight Cd3ɛ-proficient leukemias exhibit Notch1-activating mutations. Third, in the absence of Cd3ɛ and activating Notch1 mutations, tumor cells have decreased leukemia-initiating potential.
At the time of overt leukemia, tumor cells, in principle, have acquired the minimum numbers of transforming events. Therefore, the time taken by a single LSC to induce leukemia in secondary congenic hosts represents the time required for clonal expansion. We found that the time to leukemia for a single LSC is 36 d, consistent with results published for another genetic model of T-ALL in mice caused by PTEN deficiency (Guo et al. 2008). Interestingly, leukemia develops with a latency of 31 d in Notch1tgSCLtgLMO1tg mice. Furthermore, transplantation of 7-d-old preleukemic triple-transgenic thymocytes gives rise to leukemia in recipient mice after 3 wk, thus recapitulating the above time of tumor onset. This latency corresponds to the production of ∼1010 cells from a single LSC, assuming a 24-h division time, comparable with the tumor mass collected from the thymi and lymph nodes of these mice. We therefore surmise that pre-LSCs might appear during the late prenatal period, as reported for ALL in monochorionic twins (Eguchi-Ishimae et al. 2008; Hong et al. 2008). Furthermore, our observations suggest that SCL, LMO1, and Notch1 gains of functions, together with an active pre-TCR, might be sufficient to transform thymocytes. As a corollary, the 92-d difference in median survival times between the double SCLtgLMO1tg model and the triple Notch1tgSCLtgLMO1tg model likely represents the time required for the occurrence of Notch1 mutations in appropriate target cells. Our observations are consistent with the view that SCL–LMO1 oncogenes expand the DN populations, increasing the likelihood of acquisition of Notch1 mutations in DN4 thymocytes, thereby conferring clonal dominance to transformed cells.
Materials and methods
Mouse models
We used the previously described pSil-TSCL (SCLtg) (Aplan et al. 1997) and Lck-LMO1 (LMO1tg) (McGuire et al. 1992) transgenic mouse lines (Herblot et al. 2000). Both mouse lines were backcrossed onto a C57BL6/J background for >12 generations. All mouse strains were on a C57BL6/J background: Rag1−/− (Jackson Laboratory), Abb−/−B2m−/− (Taconic), Lck-NotchIC9 (Notch1tg) (Fowlkes and Robey 2002) (NIAID/Taconic Repository, Bethesda), and Cd3ɛ−/− kindly given by P. Hugo (Malissen et al. 1995). Mice cohorts were generated by cross-breeding, and their genotypes were verified by PCR. Transplantation assays were performed by injection of leukemic thymoma cells into unirradiated Rag1−/− mice or purified subpopulations of preleukemic cells into isogenic Pep3B mice. All animals were maintained in pathogen-free conditions according to institutional animal care and use guidelines. Kaplan-Meier survival and statistical analysis was performed using GraphPad Prism 4.0 software (GraphPad Software, Inc.).
Notch1 sequencing
Amplification of Notch1 exons 26, 27, and 34 from cDNA of tumor cells was performed by PCR. Amplification products were sequenced in both directions. Primer sequences are shown in Supplemental Table S1. Residues are numbered according to their location in Notch1 sequence (Ensembl sequence ENSMUSG00000026923).
Clonality analysis
Genomic DNA was obtained from digestion of leukemic cell extracts with proteinase K, followed by phenol/chloroform extraction. Tcrb rearrangements were determined by PCR amplification (Aplan et al. 1997).
RNA analysis
Total RNA was prepared from 10,000 sorted cells as described previously (Herblot et al. 2000) using RNeasy extraction kit (Qiagen). Primer sequences used are shown in Supplemental Table S1. Real-time quantitative PCR was done with SYBR Green detection kits (Qiagen) on a Stratagene Mx3000 apparatus (Stratagene).
Microscopy
Thymoma cells were rinsed twice in PBS (pH 7.4), fixed in 2% paraformaldehyde for 10 min at room temperature, washed, blocked, and permeabilized with 10% FBS and 0.1% NP40 in PBS (pH 7.4). Cells were then incubated with rabbit anti-NOTCH1 antibody (C20R, sc-6014R, Santa Cruz Biotechnologies) in blocking buffer for 2 h. After incubation with primary antibodies, the cells were washed three times in 1× PBS (pH 7.4) and incubated with FITC-conjugated donkey anti-rabbit antibody (Jackson ImmunoResearch Laboratories) for 45 min at room temperature. The coverslips were then washed three times, mounted in Vectashield with DAPI (Vector Laboratories, Inc.), and sealed. Fluorescence was analyzed on a confocal microscope LSM 510 META (Zeiss) with a 100× objective, further magnified by a zoom of 4.
FACS analysis
Single-cell suspensions were prepared from thymi or thymoma of mice at the indicated ages. Flow cytometry analysis and cell sorting were done as described previously (Herblot et al. 2000) using Pharmingen antibodies (BD Biosciences) against CD44 (IM7), CD25 (PC61.5), CD4 (RM4-4), CD8 (53-6.7), Thy1.2 (30-H12), TCRβ (H57-597), and CD3ɛ (145-2C11). Bone marrow KSL population was stained as described previously (Lacombe et al. 2009). ETPs (Lin−Sca1+CD25−) were analyzed by staining thymocytes with c-KIT (eBioscience, 2B8), SCA1 (BD, E13-161.7), and CD25 antibodies and excluding Lin+ cells stained with biotinylated antibodies against B220 (RA3-6B2), TER119 (eBioscience, TER119), CD11b (M1/70), GR1 (RB6-8C5), CD8a (53-6.7), CD3ɛ (145-2C11), NK1.1 (PK136), TCRβ (H57-597), TCRγδ (GL3), and CD11c (HL3). Dead cells were excluded by propidium iodide staining. FACS analysis was performed on an LSRII cytometer, and cell sorting was performed on FACSAria (BD Biosciences).
Acknowledgments
We thank Danièle Gagné (IRIC) for her assistance with cell sorting, and Veronique Litalien for mice handling. This work was funded by grants from the Canadian Cancer Society Research Institute (T.H.) and the Canada Research Chair program (T.H.). The Quebec Leukemia Cell Bank is supported in part by funds from the Fonds de Recherche en Santé du Québec (FRSQ) and by the Cole Foundation (J.H.). The flow cytometry service was supported by a Multiuser grant from the Canadian Institute for Health Research (CIHR), and the infrastructure was supported in part by an FRSQ group grant (IRIC). M.T. received doctoral awards from CIHR and the Cole Foundation, S.H. received a post-doctoral fellowship from the Leukemia Research Fund of Canada, and C.S.T. received a post-doctoral Research Fellowship of the Terry Fox Foundation Award (#700153).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1897910.
Supplemental material is available at http://www.genesdev.org.
References
- Aifantis I, Raetz E, Buonamici S 2008. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev Immunol 8: 380–390 [DOI] [PubMed] [Google Scholar]
- Aplan PD, Jones CA, Chervinsky DS, Zhao X, Ellsworth M, Wu C, McGuire EA, Gross KW 1997. An scl gene product lacking the transactivation domain induces bony abnormalities and cooperates with LMO1 to generate T-cell malignancies in transgenic mice. EMBO J 16: 2408–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong SA, Staunton JE, Silverman LB, Pieters R, denBoer ML, Minden MD, Sallan SE, Lander ES, Golub TR, Korsmeyer SJ 2002. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet 30: 41–47 [DOI] [PubMed] [Google Scholar]
- Armstrong F, Brunet de la Grange P, Gerby B, Rouyez MC, Calvo J, Fontenay M, Boissel N, Dombret H, Baruchel A, Landman-Parker J, et al. 2009. NOTCH is a key regulator of human T-cell acute leukemia initiating cell activity. Blood 113: 1730–1740 [DOI] [PubMed] [Google Scholar]
- Begley CG, Aplan PD, Davey MP, Nakahara K, Tchorz K, Kurtzberg J, Hershfield MS, Haynes BF, Cohen DI, Waldmann TA, et al. 1989. Chromosomal translocation in a human leukemic stem-cell line disrupts the T-cell antigen receptor δ-chain diversity region and results in a previously unreported fusion transcript. Proc Natl Acad Sci 86: 2031–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia D, Campese AF, Checquolo S, Balestri A, Biondi A, Cazzaniga G, Lendahl U, Fehling HJ, Hayday AC, Frati L, et al. 2002. Combined expression of pTα and Notch3 in T cell leukemia identifies the requirement of preTCR for leukemogenesis. Proc Natl Acad Sci 99: 3788–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns A 1988. Provirus tagging as an instrument to identify oncogenes and to establish synergism between oncogenes. Arch Virol 102: 1–18 [DOI] [PubMed] [Google Scholar]
- Beverly LJ, Felsher DW, Capobianco AJ 2005. Suppression of p53 by Notch in lymphomagenesis: Implications for initiation and regression. Cancer Res 65: 7159–7168 [DOI] [PubMed] [Google Scholar]
- Boehm T, Foroni L, Kaneko Y, Perutz MF, Rabbitts TH 1991. The rhombotin family of cysteine-rich LIM-domain oncogenes: Distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci 88: 4367–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Levis M, Shurtleff S, Campana D, Downing J, Small D 2005. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood 105: 812–820 [DOI] [PubMed] [Google Scholar]
- Campese AF, Garbe AI, Zhang F, Grassi F, Screpanti I, von Boehmer H 2006. Notch1-dependent lymphomagenesis is assisted by but does not essentially require pre-TCR signaling. Blood 108: 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervinsky DS, Zhao XF, Lam DH, Ellsworth M, Gross KW, Aplan PD 1999. Disordered T-cell development and T-cell malignancies in SCL LMO1 double-transgenic mice: Parallels with E2A-deficient mice. Mol Cell Biol 19: 5025–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervinsky DS, Lam DH, Melman MP, Gross KW, Aplan PD 2001. scid Thymocytes with TCRβ gene rearrangements are targets for the oncogenic effect of SCL and LMO1 transgenes. Cancer Res 61: 6382–6387 [PubMed] [Google Scholar]
- Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, Maryanski JL, Zuniga-Pflucker JC 2004. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J Immunol 172: 5230–5239 [DOI] [PubMed] [Google Scholar]
- Condorelli GL, Facchiano F, Valtieri M, Proietti E, Vitelli L, Lulli V, Huebner K, Peschle C, Croce CM 1996. T-cell-directed TAL-1 expression induces T-cell malignancies in transgenic mice. Cancer Res 56: 5113–5119 [PubMed] [Google Scholar]
- Curtis DJ, Robb L, Strasser A, Begley CG 1997. The CD2-scl transgene alters the phenotype and frequency of T-lymphomas in N-ras transgenic or p53 deficient mice. Oncogene 15: 2975–2983 [DOI] [PubMed] [Google Scholar]
- Deftos ML, Huang E, Ojala EW, Forbush KA, Bevan MJ 2000. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity 13: 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos NR, Rickman DS, de Reynies A, Cormier F, Williame M, Blanchard C, Stern MH, Ghysdael J 2007. Pre-TCR expression cooperates with TEL-JAK2 to transform immature thymocytes and induce T-cell leukemia. Blood 109: 3972–3981 [DOI] [PubMed] [Google Scholar]
- Eguchi-Ishimae M, Eguchi M, Kempski H, Greaves M 2008. NOTCH1 mutation can be an early, prenatal genetic event in T-ALL. Blood 111: 376–378 [DOI] [PubMed] [Google Scholar]
- Engel I, Murre C 2002. Disruption of pre-TCR expression accelerates lymphomagenesis in E2A-deficient mice. Proc Natl Acad Sci 99: 11322–11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasseu M, Aplan PD, Chopin M, Boissel N, Bories JC, Soulier J, von Boehmer H, Sigaux F, Regnault A 2007. p16INK4A tumor suppressor gene expression and CD3ɛ deficiency but not pre-TCR deficiency inhibit TAL1-linked T-lineage leukemogenesis. Blood 110: 2610–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B 1990. A genetic model for colorectal tumorigenesis. Cell 61: 759–767 [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, Behm FG, Pui CH, Downing JR, Gilliland DG, et al. 2002. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 1: 75–87 [DOI] [PubMed] [Google Scholar]
- Fowlkes BJ, Robey EA 2002. A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. J Immunol 169: 1817–1821 [DOI] [PubMed] [Google Scholar]
- Gibbons D, Douglas NC, Barber DF, Liu Q, Sullo R, Geng L, Fehling HJ, von Boehmer H, Hayday AC 2001. The biological activity of natural and mutant pTα alleles. J Exp Med 194: 695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goardon N, Schuh A, Hajar I, Ma X, Jouault H, Dzierzak E, Romeo PH, Maouche-Chretien L 2002. Ectopic expression of TAL-1 protein in Ly-6E.1-htal-1 transgenic mice induces defects in B- and T-lymphoid differentiation. Blood 100: 491–500 [DOI] [PubMed] [Google Scholar]
- Gothert JR, Brake RL, Smeets M, Duhrsen U, Begley CG, Izon DJ 2007. NOTCH1 pathway activation is an early hallmark of SCL T leukemogenesis. Blood 110: 3753–3762 [DOI] [PubMed] [Google Scholar]
- Grabher C, von Boehmer H, Look AT 2006. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat Rev Cancer 6: 347–359 [DOI] [PubMed] [Google Scholar]
- Grusby MJ, Auchincloss H Jr, Lee R, Johnson RS, Spencer JP, Zijlstra M, Jaenisch R, Papaioannou VE, Glimcher LH 1993. Mice lacking major histocompatibility complex class I and class II molecules. Proc Natl Acad Sci 90: 3913–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Dose M, Kovalovsky D, Chang R, O'Neil J, Look AT, von Boehmer H, Khazaie K, Gounari F 2007. β-Catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood 109: 5463–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Lasky JL, Chang CJ, Mosessian S, Lewis X, Xiao Y, Yeh JE, Chen JY, Iruela-Arispe ML, Varella-Garcia M, et al. 2008. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature 453: 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA 2002. Rules for making human tumor cells. N Engl J Med 347: 1593–1603 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA 2000. The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Herblot S, Steff AM, Hugo P, Aplan PD, Hoang T 2000. SCL and LMO1 alter thymocyte differentiation: Inhibition of E2A-HEB function and pre-T α chain expression. Nat Immunol 1: 138–144 [DOI] [PubMed] [Google Scholar]
- Hoang T 2010. Of mice and men: How an oncogene transgresses the limits and predisposes to acute leukemia. Science Translational Medicine 2: 21ps10 doi: 10.1126/scitranslmed.3000885 [DOI] [PubMed] [Google Scholar]
- Hong D, Gupta R, Ancliff P, Atzberger A, Brown J, Soneji S, Green J, Colman S, Piacibello W, Buckle V, et al. 2008. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science 319: 336–339 [DOI] [PubMed] [Google Scholar]
- Hope KJ, Jin L, Dick JE 2004. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol 5: 738–743 [DOI] [PubMed] [Google Scholar]
- Ikawa T, Kawamoto H, Goldrath AW, Murre C 2006. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J Exp Med 203: 1329–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving BA, Alt FW, Killeen N 1998. Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science 280: 905–908 [DOI] [PubMed] [Google Scholar]
- Jonkers J, Berns A 1996. Retroviral insertional mutagenesis as a strategy to identify cancer genes. Biochim Biophys Acta 1287: 29–57 [DOI] [PubMed] [Google Scholar]
- Jordan CT 2009. Cancer stem cells: Controversial or just misunderstood? Cell Stem Cell 4: 203–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MA, Seldin DC, Leder P 1996. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIα. EMBO J 15: 5160–5166 [PMC free article] [PubMed] [Google Scholar]
- Kool J, Berns A 2009. High-throughput insertional mutagenesis screens in mice to identify oncogenic networks. Nat Rev Cancer 9: 389–399 [DOI] [PubMed] [Google Scholar]
- Lacombe J, Herblot S, Rojas-Sutterlin S, Haman A, Barakat S, Iscove NN, Sauvageau G, Hoang T 2009. Scl regulates the quiescence and the long-term competence of hematopoietic stem cells. Blood 115: 792–803 [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE 1994. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648 [DOI] [PubMed] [Google Scholar]
- Larson RC, Fisch P, Larson TA, Lavenir I, Langford T, King G, Rabbitts TH 1994. T cell tumours of disparate phenotype in mice transgenic for Rbtn-2. Oncogene 9: 3675–3681 [PubMed] [Google Scholar]
- Larson RC, Lavenir I, Larson TA, Baer R, Warren AJ, Wadman I, Nottage K, Rabbitts TH 1996. Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice. EMBO J 15: 1021–1027 [PMC free article] [PubMed] [Google Scholar]
- Lecuyer E, Hoang T 2004. SCL: From the origin of hematopoiesis to stem cells and leukemia. Exp Hematol 32: 11–24 [DOI] [PubMed] [Google Scholar]
- le Viseur C, Hotfilder M, Bomken S, Wilson K, Rottgers S, Schrauder A, Rosemann A, Irving J, Stam RW, Shultz LD, et al. 2008. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell 14: 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gounari F, Protopopov A, Khazaie K, von Boehmer H 2008. Oncogenesis of T-ALL and nonmalignant consequences of overexpressing intracellular NOTCH1. J Exp Med 205: 2851–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao MJ, Zhang XX, Hill R, Gao J, Qumsiyeh MB, Nichols W, Van Dyke T 1998. No requirement for V(D)J recombination in p53-deficient thymic lymphoma. Mol Cell Biol 18: 3495–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Nichols RA, Letterio JJ, Aplan PD 2006. Notch1 mutations are important for leukemic transformation in murine models of precursor-T leukemia/lymphoma. Blood 107: 2540–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linette G, Grusby M, Hedrick S, Hansen T, Glimcher L, Korsmeyer S 1994. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity 1: 197–205 [DOI] [PubMed] [Google Scholar]
- Look AT 1997. Oncogenic transcription factors in the human acute leukemias. Science 278: 1059–1064 [DOI] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ 2009. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell 136: 823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B 1995. Altered T cell development in mice with a targeted mutation of the CD3-ɛ gene. EMBO J 14: 4641–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J 1988. The T-cell repertoire for antigen and MHC. Immunol Today 9: 308–315 [DOI] [PubMed] [Google Scholar]
- Matzinger P, Bevan MJ 1977. Hypothesis: Why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol 29: 1–5 [DOI] [PubMed] [Google Scholar]
- McCormack MP, Young LF, Vasudevan S, de Graaf CA, Codrington R, Rabbitts TH, Jane SM, Curtis DJ 2010. The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science 327: 879–883 [DOI] [PubMed] [Google Scholar]
- McGuire EA, Rintoul CE, Sclar GM, Korsmeyer SJ 1992. Thymic overexpression of Ttg-1 in transgenic mice results in T-cell acute lymphoblastic leukemia/lymphoma. Mol Cell Biol 12: 4186–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie A, Chan A, Ciofani M, Carleton M, Lefebvre J, He Y, Allman D, Wiest D, Zuniga-Pflucker J, Izon D 2007. Constitutive Notch signalling promotes CD4 CD8 thymocyte differentiation in the absence of the pre-TCR complex, by mimicking pre-TCR signals. Int Immunol 19: 1421–1430 [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Downing JR 2009. Genome-wide profiling of genetic alterations in acute lymphoblastic leukemia: Recent insights and future directions. Leukemia 23: 1209–1218 [DOI] [PubMed] [Google Scholar]
- O'Neil J, Calvo J, McKenna K, Krishnamoorthy V, Aster JC, Bassing CH, Alt FW, Kelliher M, Look AT 2006. Activating Notch1 mutations in mouse models of T-ALL. Blood 107: 781–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CT, Cheng HT, Chang LW, Ohtsuka T, Kageyama R, Stormo GD, Kopan R 2006. Target selectivity of vertebrate notch proteins. Collaboration between discrete domains and CSL-binding site architecture determines activation probability. J Biol Chem 281: 5106–5119 [DOI] [PubMed] [Google Scholar]
- Pear WS, Aster JC 2004. T cell acute lymphoblastic leukemia/lymphoma: A human cancer commonly associated with aberrant NOTCH1 signaling. Curr Opin Hematol 11: 426–433 [DOI] [PubMed] [Google Scholar]
- Pear WS, Aster JC, Scott ML, Hasserjian RP, Soffer B, Sklar J, Baltimore D 1996. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med 183: 2283–22918642337 [Google Scholar]
- Rabbitts TH 1991. Translocations, master genes, and differences between the origins of acute and chronic leukemias. Cell 67: 641–644 [DOI] [PubMed] [Google Scholar]
- Roy M, Pear WS, Aster JC 2007. The multifaceted role of Notch in cancer. Curr Opin Genet Dev 17: 52–59 [DOI] [PubMed] [Google Scholar]
- Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A 2005. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol 6: 663–670 [DOI] [PubMed] [Google Scholar]
- Sharma VM, Calvo JA, Draheim KM, Cunningham LA, Hermance N, Beverly L, Krishnamoorthy V, Bhasin M, Capobianco AJ, Kelliher MA 2006. Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc. Mol Cell Biol 26: 8022–8031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren AG, Kool J, Matentzoglu K, de Ridder J, Mattison J, van Uitert M, Lagcher W, Sie D, Tanger E, Cox T, et al. 2008. Large-scale mutagenesis in p19(ARF)- and p53-deficient mice identifies cancer genes and their collaborative networks. Cell 133: 727–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca A, Felli MP, Palermo R, Di Mario G, Calce A, Di Giovine M, Frati L, Gulino A, Screpanti I 2006. Notch3 and pre-TCR interaction unveils distinct NF-κB pathways in T-cell development and leukemia. EMBO J 25: 1000–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H, Aifantis I, Azogui O, Saint-Ruf C, Grassi F 1999. The impact of pre-T-cell receptor signals on gene expression in developing T cells. Cold Spring Harb Symp Quant Biol 64: 283–289 [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JP IV, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC 2004. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306: 269–271 [DOI] [PubMed] [Google Scholar]
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H, Tobias J, et al. 2006. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev 20: 2096–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweidler-McKay PA, Pear WS 2004. Notch and T cell malignancy. Semin Cancer Biol 14: 329–340 [DOI] [PubMed] [Google Scholar]