Abstract
Yes-associated protein (YAP) is a potent transcription coactivator acting via binding to the TEAD transcription factor, and plays a critical role in organ size regulation. YAP is phosphorylated and inhibited by the Lats kinase, a key component of the Hippo tumor suppressor pathway. Elevated YAP protein levels and gene amplification have been implicated in human cancer. In this study, we report that YAP is inactivated during embryonic stem (ES) cell differentiation, as indicated by decreased protein levels and increased phosphorylation. Consistently, YAP is elevated during induced pluripotent stem (iPS) cell reprogramming. YAP knockdown leads to a loss of ES cell pluripotency, while ectopic expression of YAP prevents ES cell differentiation in vitro and maintains stem cell phenotypes even under differentiation conditions. Moreover, YAP binds directly to promoters of a large number of genes known to be important for stem cells and stimulates their expression. Our observations establish a critical role of YAP in maintaining stem cell pluripotency.
Keywords: YAP, Hippo, stem cells, TEAD
The Yes-associated protein (YAP) was originally identified as a protein interacting with the c-Yes tyrosine kinase (Sudol 1994). Subsequent studies have demonstrated that YAP is a transcription coactivator with a potent transactivation domain in the C-terminal region (Yagi et al. 1999). In addition to that, there are one or two WW domains in the central region of YAP, depending on alternative splicing. The WW domains of YAP have been well characterized to bind the PPXY motif (Chen and Sudol 1995; Linn et al. 1997; Sudol et al. 1995), whereas the N-terminal region of YAP is responsible for interaction with TEAD transcription factors (Vassilev et al. 2001).
YAP is an oncogene located in the human amplicon 11q22 (Overholtzer et al. 2006; Zender et al. 2006). Ectopic expression of YAP promotes cell growth and induces oncogenic transformation in vitro (Overholtzer et al. 2006; Zhao et al. 2009). YAP also promotes epithelial–mesenchymal transition (EMT) (Overholtzer et al. 2006; J Zhang et al. 2008; Zhao et al. 2008), a property commonly associated with cancer metastasis. Notably, transgenic expression of YAP in the mouse liver results in a dramatic increase of liver mass in a reversible manner, and eventually leads to tumor formation (Camargo et al. 2007; Dong et al. 2007), confirming the important role of YAP in organ size regulation and tumorigenesis. Consistently, elevated YAP protein levels have been found in many human cancers, especially in the liver (Zender et al. 2006; Dong et al. 2007; Zhao et al. 2007; Steinhardt et al. 2008; Xu et al. 2009).
YAP itself has no DNA-binding activity; therefore, it must bind to DNA-binding transcription factors to stimulate gene expression. In fact, several transcription factors have been implicated as YAP targets, including the p53-related p73, RUNX2, the ErbB4 cytoplasmic domain, and TEAD (Yagi et al. 1999; Vassilev et al. 2001; Basu et al. 2003; Komuro et al. 2003). However, the significance of these transcription factors in mediating the physiological functions of YAP are not clear except for TEAD. Humans have four TEAD genes (TEAD1 to TEAD4) widely expressed in most tissues with different tissue distribution. However, most tissues express at least one TEAD gene. Recently, we demonstrated that the TEAD family members are key target transcription factors mediating YAP function in vivo (Zhao et al. 2008). Mutations of the TEAD-binding essential residues, such as S94, abolish the activity of YAP in gene induction (Zhao et al. 2008). Consistently, the TEAD-binding-defective mutant YAP fails to promote cell proliferation and EMT. Sveinsson's chorioretinal atrophy is a rare genetic disease caused by a mutation in the TEAD1 gene (Fossdal et al. 2004). Interestingly, the mutant TEAD1 completely lost its ability to interact with YAP (Kitagawa 2007; Zhao et al. 2008), further supporting the role of YAP–TEAD interaction in tissue growth. The three-dimensional structure of the YAP–TEAD1 complex has been solved recently (Chen et al. 2010; Li et al. 2010). Interestingly, the tyrosine residue mutated in TEAD1 of Sveinsson's chorioretinal atrophy directly forms a hydrogen bond with YAP Ser 94, which is also essential for YAP function. These data further support the functional importance of the YAP–TEAD partners.
Yki is the Drosophila homolog of YAP, and has also been shown to promote tissue growth (Huang et al. 2005). Yki stimulates cell proliferation and inhibits apoptosis at least in part by inducing the expression of cyclin E and Diap1. Consistent with the mammalian studies, Yki functions mainly through scalloped (Sd), which is the Drosophila TEAD ortholog, to promote tissue growth (Wu et al. 2008; L Zhang et al. 2008). Genetic studies have established that Yki acts at the end of, and is inhibited by, the Hippo tumor suppressor pathway, which is highly conserved in mammals (Huang et al. 2005). YAP is phosphorylated and inhibited by the Lats1/2 kinases, which are key components of the Hippo pathway (Dong et al. 2007; Hao et al. 2007; Zhao et al. 2007; Oka et al. 2008; J Zhang et al. 2008). The phosphorylated YAP is retained in the cytoplasm, and therefore is inactive to stimulate gene expression. The YAP nuclear–cytoplasmic shuttling is regulated dramatically by cell density, indicating that YAP inactivation may play a key role in contact inhibition in vitro (Zhao et al. 2007). Mutation of the Lats1/2 phosphorylation sites generates a constitutively active YAP that potently transforms NIH-3T3 cells in vitro (Zhao et al. 2009). Lats phosphorylation also inhibits YAP by promoting degradation (Zhao et al. 2010). Therefore, the Hippo pathway regulates YAP by both spatial (nuclear–cytoplasmic translocation) and temporal (degradation) mechanisms.
Stem cells are unique in that they have the ability of self-renewal as well as the potential to differentiate into cells of different lineage. The embryonic stem (ES) cells have the potential to generate all cells in an adult organism (Chambers and Smith 2004). Transcription regulation is key for ES self-renewal and differentiation (Nichols et al. 1998; Mitsui et al. 2003; Chambers and Smith 2004). This is supported by observations that expression of a defined set of transcription factors—such as Oct4, Sox2, Klf4 (OSK), and Myc—can reprogram differentiated adult cells into pluripotent stem cells (Takahashi and Yamanaka 2006). These induced pluripotent stem (iPS) cells resemble all properties of ES cells in self-renewal, differentiation, and capacity to generate adult mice. This remarkable discovery convincingly shows that transcription regulation is key for stem cell reprogramming and maintenance. Notably, genes important for stem cell induction have also been implicated in cancers (Zajac-Kaye 2001; Kinameri et al. 2008). Therefore, there are common features shared between iPS reprogramming and tumorigenesis.
In the mouse intestine, YAP expression is restricted in progenitor cells (Camargo et al. 2007). Transgenic overexpression of YAP in the intestine causes a significant expansion of undifferentiated progenitor cells, which undergo differentiation once YAP expression is reduced. YAP–TEAD has also been shown to induce expansion of neuroprogenitor cells (Cao et al. 2008). These data indicate a possible role of YAP in maintaining an undifferentiated progenitor cell population. Furthermore, modulation of TEAD4–YAP expression has been implicated in cell fate determination of trophectoderm (TE) from inner cell mass (ICM) during blastocyst development in early mouse embryos (Nishioka et al. 2009). Gene profiling has shown that YAP is highly expressed in ES cells (Ramalho-Santos et al. 2002). However, the precise functions of YAP in stem cells, especially in ES cells, have not been characterized.
In this study, we investigated the function of YAP in ES and iPS cells. YAP protein is induced during the induction of iPS cells, whereas YAP is inhibited during ES cell differentiation. YAP plays a critical role in ES cell self-renewal, as knockdown of either YAP or TEAD leads to loss of pluripotency of ES cells, while YAP overexpression suppresses ES cell differentiation. Chromatin immunoprecipitation (ChIP) and deep sequencing (seq) show that YAP binds to genes that are known to be important for ES cells. Our study reveals a novel function of YAP–TEAD in ES cells.
Results
YAP is inactivated during mouse ES cell differentiation
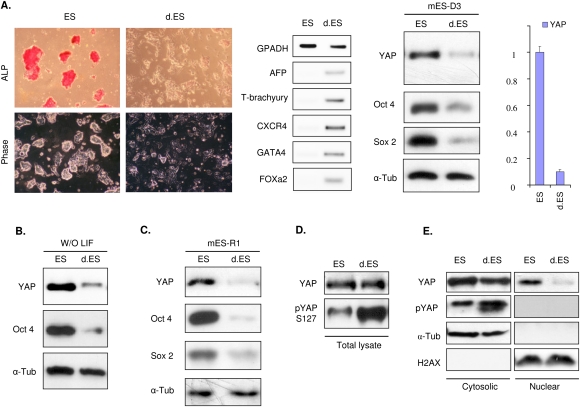
High YAP activity contributes to the expansion of intestinal epithelial progenitor cells (Camargo et al. 2007) and neuroprogenitor cells (Cao et al. 2008). To examine a possible role of YAP in ES cell maintenance and differentiation, we determined YAP protein levels in mouse ES cells before and after differentiation. As expected, under the condition that promotes differentiation (separated from undifferentiated population by differential adhesion, and switching from complete ES media to regular DMEM + 10% FBS), the mouse ES (mES-D3) cells lost their pluripotent stem cell markers, such as Oct4 and alkaline phosphatase (ALP), as well as morphological alterations (Fig. 1A, left panels). ES cell differentiation was also confirmed by the induction of various differentiation markers, such as mesoderm marker T-bruchyury, and endoderm markers AFP and GATA4. Concomitant with the decrease of stem cell markers and increase of differentiation markers, the YAP protein level was markedly reduced (Fig. 1A). Moreover, YAP mRNA was significantly lowered during differentiation (Fig. 1A, right panel). Under a slightly varied differentiation condition (feeder-free and leukemia inhibitory factor [LIF] withdrawal until cells differentiated), a similar trend of YAP reduction as well as loss of ES pluripotent markers were observed (Fig. 1B). We also tested another mES cell line, mES-R1, and found an identical pattern of YAP regulation during differentiation (Fig. 1C). These observations demonstrate that YAP expression is decreased during differentiation, and indicate a potential role of YAP in ES cells.
Figure 1.
YAP is inactivated during mES cell differentiation. (A) YAP expression is decreased during differentiation. mES-D3 cell line was cultured in normal ES medium or under differentiation conditions described in the Materials and Methods. The differentiation of ES cells was verified by the loss of ALP staining and colony morphology (left panels), as well as multiple lineage markers using RT–PCR (middle panel). YAP protein levels as well as ES cell pluripotent marker proteins Oct4 and Sox2 were examined under normal ES cell culture conditions and differentiation conditions. Western blot analysis was performed and α-tubulin blot was included for equal sample loading. (Right panel) YAP expression was also confirmed by quantitative RT–PCR. (B) Differentiation by LIF withdrawal decreases YAP protein levels. mES-D3 was grown in the ES media (ES) or absence of LIF (d.ES). The cell lysates were harvested and analyzed for YAP protein level and ES marker Oct4. (C) Reduction of YAP protein level during the mES cell line R1 differentiation. mES-R1 cell line was cultured in normal and differentiation conditions as described in A. Western blot analysis was performed to detect YAP and ES cell markers. (D) ES cell differentiation induces the inhibitory phosphorylation of YAP. Lysates from control and differentiated ES cells (more lysate from differentiated cells) were loaded to achieve equal amount of YAP protein. Western blotting with phosphoYAP S127-specific antibody and YAP antibody was performed. Relative YAP phosphorylation was increased in differentiated ES cells. (E) Differentiation deprives YAP from the nucleus. Both control and differentiated ES cells were fractionated into cytosolic and nuclear fractions as indicated. The samples were blotted for YAP protein (YAP) and S127 phosphorylation level (pYAP). α-Tubulin (α-Tub) and histone 2AX (H2AX) were used as positive controls for both loading and cytosolic and nuclear proteins, respectively.
Previously, we showed that phosphorylation of YAP, especially on S127, inhibits YAP activity (Zhao et al. 2007), which modulates YAP localization and degradation. We investigated whether ES cell differentiation also affected YAP phosphorylation. We observed that YAP S127 phosphorylation was increased in differentiated cells when an equal amount of YAP protein was compared (Fig. 1D). Phosphorylation of S127 results in YAP cytoplasmic retention. The control and differentiated ES cells were fractionated to cytoplasm and nuclear fractions, and then total YAP protein level and S127 phosphorylation were examined by specific antibodies. As expected, little YAP protein was found in the nuclear fraction of differentiated cells, while the undifferentiated ES cells had significant nuclear YAP protein (Fig. 1E). Moreover, the cytoplasmic YAP in differentiated cells was highly phosphorylated, and no phospho-YAP was present in the nuclear fractions of both undifferentiated and differentiated cells. Taken together, our data suggest that YAP is inactivated by both transcription repression and phosphorylation upon mES cell differentiation.
YAP and TEAD are required for mES cell pluripotency
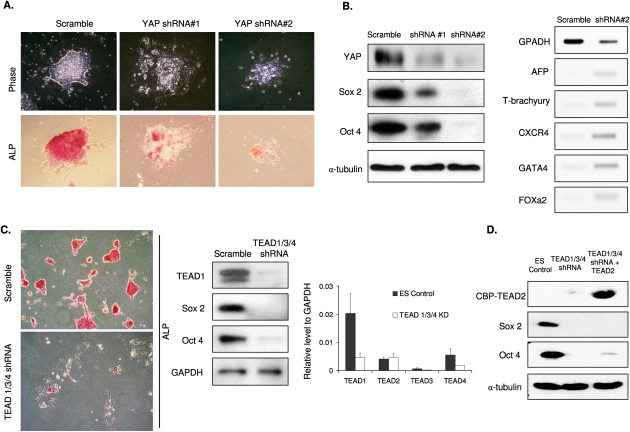
To investigate the potential roles of YAP in mES cells, we created YAP knockdown cell populations using two independent lentiviral shRNAs. The YAP knockdown ES cells displayed a flattened phenotype, indicating a loss of characteristic ES cell morphology. Similarly, ALP staining was also strongly reduced in the YAP knockdown cells (Fig. 2A). Knockdown of YAP protein was determined by Western blotting. The result indicated that YAP shRNA #2 resulted in more efficient YAP knockdown than shRNA #1 (Fig. 2B, left panel). Interestingly, both Sox2 and Oct4 were significantly decreased in these YAP knockdown cells, and the degrees of reduction of both ES markers were closely correlated with YAP knockdown efficiency. The loss of pluripotency in YAP knockdown cells was further confirmed by the marked increase of multiple differentiation markers (Fig. 2B, right panel). These data suggest that YAP protein is required to maintain ES cell stemness.
Figure 2.
YAP and TEAD are required for mES cell pluripotency. (A) YAP knockdown causes a loss of ES cell properties. ES cells were stably infected with lentiviral shRNA constructs targeting YAP as indicated. After selection, cells were grown in regular ES culture medium. Cell morphology (top panels) and ALP staining (bottom panels) are shown. (B) YAP knockdown results in ES cell differentiation. Cell lysates were prepared from control and YAP knockdown ES cells. (Left panel) Pluripotent ES markers Sox2 and Oct4 were examined by Western blotting. Protein level was normalized by α-tubulin. (Right panel) Several differentiation markers were determined by RT–PCR, including endoderm markers alphafetoprotein (AFP), CXC chemokine receptor 4 (CXCR4), Forkhead box a2 (Foxa2), and GATA4, and Mesoderm marker T-brachyury. (C) TEAD knockdown causes a loss of ES cell properties. Experiments were similar to those in A, except the shRNA targeting TEAD1/3/4 was used. (Left panel) Pluripotency was examined by ALP staining. (Middle panel) Expression of TEAD1, Oct4, and Sox2 was determined using specific antibodies along with the loading control GAPDH. (Right panel) Knockdown efficiencies of the shRNA were determined by quantitative RT–PCR. (D) TEAD2 overexpression does not rescue the loss of pluripotency in TEAD1/3/4 knockdown mES cells. Pluripotent ES markers Sox2 and Oct4 were examined by Western blotting along with loading control α-tubulin and the ectopically expressed TEAD2, which is tagged with calmodulin-binding protein (CBP).
Both genetic and biochemical studies have shown that TEAD family transcription factors are key to mediate the biological function of YAP in other cell types. We designed lentiviral an shRNA construct targeting a region conserved in mouse TEAD1, TEAD3, and TEAD4, and established a stable knockdown ES cell line. This construct, which had been shown previously to knock down TEADs and block the expression of YAP-dependent genes (Zhao et al. 2008), effectively reduced TEAD mRNA and protein in mES cells (Fig. 2C, right panels). As shown in Figure 2C, the TEAD knockdown cells exhibited a differentiated phenotype (left panels), indicated by the altered cell morphology and decreased ALP staining. Furthermore, the loss of ES cell characteristics was revealed by the dramatic reduction of Oct4 and Sox2 (Fig. 2C, middle panels). Because TEAD2 was not targeted by the shRNA, we examined whether overexpression of TEAD2 could rescue the phenotype of TEAD1/3/4 knockdown. TEAD2 was expressed in the TEAD1/3/4 knockdown ES cells. We found that TEAD2 overexpression could not rescue the effects caused by TEAD1/3/4 knockdown (Fig. 2D). Our data indicate that the functions of TEAD homologs are not completely redundant in ES cells, consistent with a previous report that each of the TEADs serves at least one nonredundant function in mammalian development (Yagi et al. 2007).
YAP promotes ES cell pluripotency
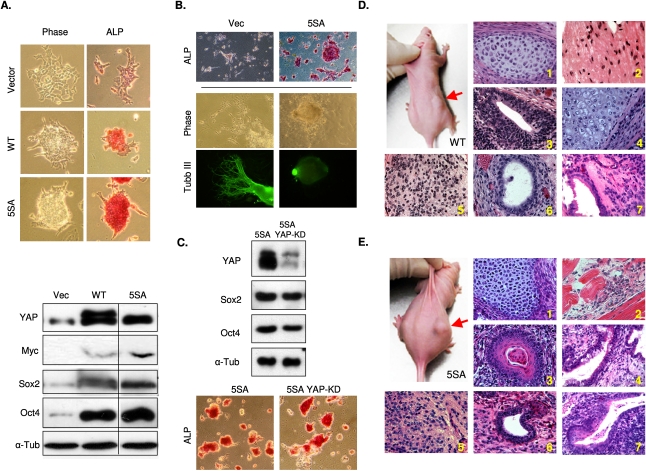
The observation that YAP is inactivated during mES differentiation prompted us to test whether overexpression of YAP is able to sustain pluripotency in ES cells, even under differentiation conditions. YAP contains five Lats inhibitory phosphorylation sites. Mutation of all of the Lats phosphorylation sites generates a constitutively active YAP-5SA, which is no longer inhibited by the Hippo pathway (Zhao et al. 2007). mES cells with stable expression of YAP wild type and the 5SA mutant were established and tested for differentiation. We found that expression of both YAP wild type and 5SA prevented ES cell differentiation in vitro, even under the differentiation conditions. The YAP-overexpressing cells, especially YAP-5SA, maintained ES cell morphology and ALP staining (Fig. 3A, top panels; Supplemental Fig. S1A). Moreover, Western blotting for ES cell markers also showed that Sox2 and Oct4 protein levels were still high in YAP-overexpressing cells (Fig. 3A, bottom panels); in contrast, ES cells expressing vector control exhibited a differentiated phenotype (Fig. 3A, top panels) and significantly reduced Sox2 and Oct4 levels (Fig. 3A, bottom panels). Taken together, our data showed that forced YAP expression inhibits ES cell differentiation and is sufficient to maintain stem cell characteristics.
Figure 3.
YAP overexpression promotes ES cell self-renewal and suppresses differentiation. (A) Ectopic YAP expression maintains ES cell properties even under differentiation conditions. mES cells were infected with vector control (VEC), YAP wild-type (WT), or the constitutive active YAP-5SA (5SA) viruses. Stable pools were selected and maintained in normal ES cell medium. (Top panels) Cells were induced to the differentiation and their morphology and ALP activity (Supplemental Fig. S1A) were examined. (Bottom panel) In addition, cell lysates were analyzed for YAP, Oct4, and Sox2 protein levels by Western blotting. (B) YAP overexpression retards neuronal differentiation of ES cells. Vector or YAP-5SA-overexpressing ES cells were induced to neuronal-specific differentiation for 5 d after EB formation. Pluripotency was determined by ALP staining (ALP panel). Immunostaining with β-tubulin III (Tubb III panel), a neuronal marker protein, was used to determine the progress of neuronal differentiation. (C) Forced YAP expression rescued YAP knockdown-induced differentiation. ES cells were infected with a retrovirus harboring a gene encoding constitutively active YAP-5SA, which is resistant to the YAP knockdown shRNA construct targeting the YAP 3′UTR region. The YAP-5SA-expressing ES cells were infected with scramble or the YAP knockdown shRNA construct (YAPKD). Knockdown of endogenous YAP protein was confirmed by Western blot (note that the epitope-tagged YAP-5SA migrated more slowly than the endogenous YAP). Pluripotency of the ES cells was determined by Oct4 and Sox2 protein levels (top panel) as well as positive ALP activity (bottom panel). (D) Teratoma formation from YAP-WT-overexpressed ES cells. ES cells were grown under differentiation conditions and then injected subcutaneously into the nude mice. Teratomas were observed in mice injected with YAP-WT ES cells (right side, indicated by a red arrow) but not in the control (vector-only ES cells, left side). Shown is a representative of five injected mice. Teratoma were harvested and processed by H&E staining. Cells resembling characteristics of three germ layers were observed including, cartilage (mesoderm) (panel 1), muscle (mesoderm) (panel 2), retinal epithilium (ectoderm) (panel 3), squamous (ectoderm) (panel 4), neuroepithelium (ectoderm) (panel 5), epithelium (ectoderm) (panel 6), and cilia (endoderm) (panel 7). (E) Teratoma formation from YAP-5SA-overexpressed ES cells. Experiments were similar to D except YAP-5SA-expressing ES cells were used. (Panel 1) Cartilage (mesoderm). (Panel 2) Muscle (mesoderm). (Panel 3) Epidermal (ectoderm). (Panel 4) Goblet (endoderm). (Panel 5) Neuroepithelium (ectoderm). (Panel 6) Epithelial (ectoderm). (Panel 7) Epithelium squamous cilia (ecdoderm).
To further confirm the importance of YAP in maintaining the pluripotency of mES cells, we examined neural-specific differentiation in YAP-overexpressing ES cells following an established protocol (Haegele et al. 2003). The ES cells were first set to form embryoid bodies (EBs), followed by a 7-d incubation with retinoic acid (RA) to induce neural differentiation. The control ES cells were differentiated as indicated by the loss of ALP and morphological changes. In contrast, the YAP-expressing ES cells still showed a high level of ALP staining and a maintained high nucleus to cytoplasm ratio, consistent with the ES phenotype (Fig. 3B, top panels). Moreover, the vector control ES cells clearly exhibited a neuroepithelial-like phenotype with long pseudopods (Fig. 3B, bottom panels). Neural marker β-tubulin III staining convincingly demonstrated a successful differentiation of vector control cells into the neural progenitor lineage, while 5SA cells were resistant to the differentiation under the same conditions. The observations that YAP knockdown leads to a loss of ES stemness while YAP overexpression prevents ES differentiation strongly support the idea that YAP plays a critical role in maintenance of ES cell pluripotency.
In order to investigate whether forced expression of YAP can rescue the knockdown phenotype shown in Figure 2A, we infected the YAP-5SA-expressing ES cells with YAP shRNA. The YAP shRNA construct used in this experiment targeted the 3′ noncoding region and therefore would not affect the ectopically expressed YAP-5SA, which did not have the endogenous 3′ noncoding region. Western blot indicated an effective knockdown of endogenous YAP (Fig. 3C, top panel). Notably, YAP knockdown in the YAP-5SA-expressing cells did not significantly reduce the expression of Sox2 and Oct4, or reduce the ALP staining (Fig. 3C). Our data show that expression of YAP-5SA was sufficient to rescue the phenotype caused by YAP knockdown, and thus excluded possible off-target effects of the YAP shRNA.
To examine whether YAP-overexpressing ES cells cultured under differentiating conditions (without pluripotent extrinsic factors LIF and other supplements) were still pluripotent, we injected YAP-WT and 5SA ES cells subcutaneously into nude mice (right dorsolateral sites) and injected the vector-infected cells (left dorsolateral sites) as controls to observe teratoma formations. Consistent with our in vitro findings, both YAP-WT-expressing (Fig. 3D) and YAP-5SA-expressing (Fig. 3E) ES cells gave rise to teratomas in vivo within 5 wk, while the vector control cells, which had been cultured in differentiation medium, did not (Fig. 3D E; Supplemental Fig. S1D). These results show that YAP activation maintains ES pluripotency even under in vitro differentiation conditions.
YAP enhances reprogramming efficiencies of mouse iPS cells and is elevated in human iPS cells
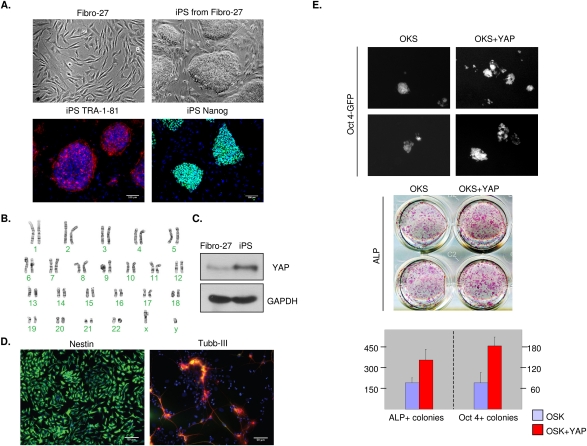
Generation of iPS cells by reprogramming of differentiated adult cells with a defined set of transcription factors has been established, and represents an important breakthrough in stem cell research (Takahashi and Yamanaka 2006; Maherali et al. 2007; Okita et al. 2007; Wernig et al. 2007). To compare the level of YAP protein during human iPS reprogramming, we studied iPS cells made from normal human fibroblasts with four transcription factors (=OSK and Myc; OSK + cMyc). The human iPS cells displayed ES cell-like morphology (Fig. 4A, top panels) as well as normal karyotype (Fig. 4B), and expressed pluripotent-associated markers such as TRA-1-81 and Nanog (Fig. 4A, bottom panels), while the parental fibroblasts did not (data not shown). These human iPS cells were further derived into neuro stem cells (Fig. 4D, left panel) and neurons (Fig. 4D, right panel), indicating their differentiation potentials. Comparison of YAP protein levels between the iPS cells and fibroblasts indicated that iPS cells expressed significantly higher YAP protein than the parental fibroblasts (Fig. 4C). These data show that YAP is activated during human iPS reprogramming, and further support a role of YAP in pluripotent stem cells.
Figure 4.
YAP is induced during iPS reprogramming and enhances iPS induction. (A) YAP expression is elevated during iPS reprogramming. Human fibroblasts were infected with OSK and c-Myc to produce iPS as documented. The iPS properties were characterized by cell morphology and staining for pluripotent stem cell markers TRA-1-81 (red) and Nanog (green). (B) Karyotype of iPS cells generated from human fibroblast (Fibro-27). (C) YAP expression in the parental fibroblasts and iPS cells were determined by Western blotting. (D) iPS cells generated from human fibroblasts (Fibro-27) can be differentiated successfully to neural stem cells (NSCs) stained by Nestin (green, left panel) and neurons stained by βIII-tubulin (red, right panel), MAP-2 (green), and DAPI (blue). (E) YAP increases iPS induction. Mouse embryonic fibroblasts (MEFs) were infected by three factors (OSK) in the presence or absence of YAP coinfection. (Top panel) Nineteen days after viral infection, cells were stained for ALP. (Bottom panel) The Oct4-GFP reporter expression was examined to verify iPS induction (note the high magnification to show GFP-positive colonies). Expression of ALP and GFP were quantified by ALP-positive (ALP+) and GFP-positive (Oct4-GFP+) colony numbers.
To investigate the effect of YAP in the reprogramming process, we coinfected YAP with retroviral vectors containing OSK by following a standard protocol described in the Materials and Methods. Infection with the three factors induced ALP-positive and Oct4-GFP-positive iPS cells, indicating the establishment of iPS cell colonies (Fig. 4E). We found that addition of YAP increased the reprogramming efficiency twofold compared with the three-factor control (Fig. 4E; Supplemental Fig. S1C). These data support that YAP positively contributes to the pluripotency of iPS during the reprogramming process.
YAP binds to promoters of genes important for ES cells
In order to understand the mechanism of YAP in promoting ES cell self-renewal, we performed genome-wide analysis of YAP-binding targets in pluripotent and differentiated mES cells, aiming to identify YAP target genes and the relationship between YAP and other factors that are known to be important in ES cells. ChIP-seq of endogenous YAP was performed. Notably, the ChIP-seq output showed significantly more YAP-binding targets (both in the intensity and number of hits) (Supplemental Fig. S2) in undifferentiated ES cells (14,125 hits, 7098 genes) (Supplemental Table S1) compared with the differentiated cells (5038 hits, 3052 genes) (Supplemental Table S2), consistent with a higher YAP protein level in the undifferentiated ES cells. It should be noted that the in vitro differentiation was not 100% complete, and some undifferentiated ES cells would still remain. To obtain a functional taxonomy of the YAP-bound genes, we performed molecular concept map (MCM) analysis for enrichment of these genes in ∼20,000 molecular concepts/gene sets either collected from the literature or predefined in the Oncomine database (http://www.oncomine.org; Scott et al. 2007). Out of all available molecular concepts, a total of 1368 (<7%) concepts showed significant enrichment (P < 0.001) with the YAP-bound genes (Supplemental Table S3). Interestingly, many of the most significantly enriched concepts (P < 1.0 × 10−28) relate to ES cells; these include target genes of Polycomb group (PcG) proteins, Nanog, Oct4, and Sox2 (Fig. 5A; Supplemental Table S3). The PcG protein EZH2 is a histone methyltransferase specific to H3K27 trimethylation, and PcGs are known to play key roles in stem cell pluripotency (Bernstein et al. 2006; Boyer et al. 2006). Similarly, Nanog, Oct4, and Sox2 are essential transcription factors for ES cells. Our data strongly indicate that YAP binds to many targets commonly shared with ES critical transcription factors.
Figure 5.
YAP binds to and induces genes important for ES cell functions. (A) An enrichment network linking YAP1-bound genes to gene sets regulated in ES cells. YAP1-bound genes (hollow node with black ring) were derived from ChIP-seq analysis of YAP in undifferentiated ES cells, and were compared against all other concepts available in the MCM in Oncomine for significant overlap. Each node represents one molecular concept or gene set with node size proportional to the number of genes. Each edge represents a statistically significant overlap (P < 1 × 10−10) of genes in the two linked nodes. Significance of overlap between two concepts was determined by Fisher's exact test comparing the chance and expected overlap (Rhodes et al. 2007). The odds ratio and P-value of such tests are shown in the table. The P-values for pairs between YAP and all concepts in this network are <3.8 × 10−34 (Supplemental Table S3). (B) YAP binds preferentially to gene promoters containing TEAD sites. The intensity of YAP binding as determined by the number of sequence hits is grouped and plotted on the X-axis. The Y-axis indicates the percentage of genes containing TEAD sites in a given intensity group. The data indicate a positive correlation between the YAP-binding intensity and the presence of TEAD sites (R = 0.92 for control mES cells [light-gray bars] and R = 0.66 for differentiated mES cells [dark-gray bars]). (C) YAP regulates a large set of ES cell important genes. mRNAs were isolated from undifferentiated, differentiated, YAP-5SA-expressing, and YAP knockdown ES cells. Expression of YAP ChIP-positive genes were determined by quantitative RT–PCR. The top panel shows gene expression between control and differentiated ES cells. The middle panel shows expression of corresponding genes in YAP knockdown cells. The bottom panel shows gene expression in YAP-5SA ES cells under differentiation conditions. Quantitative PCR data were normalized to GAPDH control then scaled proportionally in the Y-axis. Samples to the left of the dashed line follow the scale on the left, while samples to the right of the dashed lines follow the scale on the right Y-axis, respectively.
We next focused on the genes with YAP binding within their 5′ untranslated region (UTR) or close to their transcriptional start sites (TSS). Among these hits, we noticed that the strength of YAP binding (represented by the height of the ChIP-seq peaks) is tightly correlated with the presence of TEAD recognition motif XDGHATXT, where X = A, T, C, or G; D = A or T; and H = A, T, or C (Fig. 5B; Anbanandam et al. 2006). Notably, a great majority of the top hits (high ChIP-seq peak value) from the undifferentiated mES cells contain TEAD-binding sites. Within 640 unique genes with YAP binding in 5′UTR/TSS in undifferentiated mES cells, a subset of these genes are known to be important for ES cell pluripotency. A selected set of these genes, including all targets in LIF and bone morphorgenic protein (BMP) signaling pathways, were validated by ChIP followed by PCR (ChIP-PCR) (Supplemental Fig. S2). Our data show that YAP indeed binds to these targets, as predicted by the ChIP-seq experiments. Moreover, the bindings of YAP to these genes were abolished or reduced in differentiated cells, further supporting a specific and direct role of YAP in the expression of these genes.
We also searched for putative TEAD-binding sites in genes that were not identified by the YAP ChIP-seq but were important for ES cells. We found that Sox2, ID1, Esrrb, and Gab1 contain one or more putative TEAD sites in their promoters (Supplemental Fig. S3C;Supplemental Table S4). ID1 is a well-characterized transcription target of BMP signaling (Hollnagel et al. 1999), Esrrb binds to the Nanog promoter in an Oct4-dependent manner (Van Den Berg et al. 2008), and Gab1 regulates LIF-induced embryonic gene expression through interaction with SHP2 (Nakaoka et al. 2003). ChIP-PCR showed that the TEAD-binding sites in Sox2, Esrrb, and Gab1 could be precipitated specifically with YAP antibody, while the control actin promoter was not precipitated by YAP (Supplemental Fig. S3C, right panel). In line with this ChIP-PCR data, the Sox2 protein level was changed upon YAP overexpression and knockdown conditions. However, the results for ID1 were inconclusive because the control Flag antibody produced a high background. Our data indicate that Sox2, Esrrb, and Gab1 could be YAP target genes in mES cells.
To investigate whether knockdown of TEAD transcription factors has an effect on the YAP binding to the pluripotent targets, we performed ChIP-PCR on the TEAD1/3/4 knockdown mES cells and revealed that YAP association with several genes—including TEAD4, LIFr, Smad2, BMPr1b, and Mgat1—was reduced or eliminated, while the bindings to NCOA1 and TBPL1 were not affected (Supplemental Fig. S3D).
After identifying a panel of YAP targeting transcripts in undifferentiated ES cells by ChIP-seq and ChIP-PCR analyses, we examined whether their transcription could be regulated by YAP. We selected a set of genes that have been implicated to have a role in ES cells. Quantitative RT–PCR was conducted using RNA isolated from control or differentiated mES cells, and YAP-overexpressing or knockdown ES cells. Consistent with their predicted roles in ES cells, expression of most of the selected genes were decreased in differentiated ES cells (Fig. 5C, top panel). Under differentiation conditions, in cells overexpressing YAP-5SA, a majority of the genes were up-regulated over the control, with a few genes that were not significantly affected (Fig. 5C, bottom panel). Notably LIFr1, Gab1, Jak1, Sox2, BMPr1b, and Smad2 in the LIF and BMP signaling, as well as TEAD4 and transcription repressor Dnmt3l, were among the most significantly up-regulated transcripts by YAP-5SA. An opposite trend was observed in the YAP knockdown cells that showed a reduced expression for most of the genes (Fig. 5, middle panel). Therefore, YAP activity was important to sustain the expression of genes important for ES maintenance, while YAP knockdown decreased the expression of those genes. Together, our data support the notion that YAP serves as a positive regulator of self-renewal by directly binding to and stimulating pluripotent genes in mES cells.
Discussion
Molecular basis of pluripotency in ES cells is an ongoing subject of intense interest in stem cell research. A delicate network of regulation—including transcription factors, repressors, signal transduction pathways, and epigenetic regulators such as miRNA and chromatin modifiers—has been shown to play an important role in maintaining the pluripotency of ES cells (Jaenisch and Young 2008). Although remarkable progress has been made in recent years toward understanding the overall picture in the pluripotency regulatory network, novel factors are still being revealed in the context of underlying molecular mechanisms. In this study, we demonstrate that YAP promotes mES cell stemness by participating in the pluripotent transcription network.
This study establishes an essential role of YAP in mES cell self-renewal and pluripotency based on the following evidence. First, YAP is inactivated during mES cell differentiation. YAP mRNA and protein levels are decreased dramatically in differentiated cells compared with the control ES cells. Further contributing to YAP inactivation, phosphorylation of S127 is also increased, and, consequently, little YAP protein is present in the nuclei of differentiated cells, indicating the low level of YAP in the differentiated cells is inactive. Second, experimental manipulation of YAP expression dramatically affects ES cell properties. We found that YAP overexpression inhibits ES cell differentiation and maintains ES cell markers even under differentiation conditions in vitro. Conversely, YAP knockdown results in a rapid loss of ES cell properties, including the morphological changes and disappearance of molecular markers of ES cells. However, the YAP-overexpressing ES cells efficiently generate teratomas in injected mice, indicating that high YAP level is not incompatible with ES cell differentiation in vivo. Third, YAP protein is elevated during iPS reprogramming. YAP coexpression potentiates the efficiency of iPS induction by OSK. These data indicate that YAP activation is associated with iPS induction. Fourth, genome-wide analysis shows that YAP binds to a large number of genes important for ES cell pluripotency and self-renewal. For example, the YAP-enriched genes overlap significantly with targets of prominent factors important for ES cells. Notably, targets of PCG proteins (which function to repress gene expression to maintain stem cell pluripotency), Nanog, Sox2, and Oct4 show the highest overlap with YAP targets. Finally, YAP overexpression or knockdown affect the expression of a large set of genes important for stem cell functions, including those involved in LIF and BMP signaling. Many of those YAP-inducible genes are likely to be direct YAP target genes because they contain TEAD-binding sites that are bound by YAP, as verified by ChIP-PCR. We propose that YAP has a critical role in ES cell biology.
The relationship between YAP and Oct4 or Sox2 is rather interesting and complex. YAP activation positively regulates the expression of Oct4 and Sox2. Reciprocally, reprogramming of iPS cells by Oct4 and Sox2 also increases YAP protein levels. Therefore, YAP and Oct4 or Sox2 exist in a positive regulatory loop. Given the fact that the targets of Nanog, Sox2, and Oct4 display a high degree of overlaps with YAP targets, it is likely that these transcription factors corporately regulate the expression of their common target genes. However, whether YAP modulates the binding of these ES cells, important transcription factor binding to target genes requires further investigation.
Consistent with a role in ES cells, previous gene profiling has shown that YAP is highly expressed in human ES cells (Ramalho-Santos et al. 2002). However, it should be noted that YAP is also widely expressed in many tissues. Therefore, YAP function is not restricted to ES cells, as opposed to Oct4. In fact, YAP has been well established in organ size control and tumorigenesis (Camargo et al. 2007; Dong et al. 2007). It has been observed that many genes expressed in ES cells are also commonly expressed in cancer cells (Dreesen and Brivanlou 2007), indicating some common features between ES cells and tumorigenesis. Tumor cells are often dedifferentiated and have the capability to divide indefinitely, two properties similar to ES cells. Therefore, it is not surprising that part of the ES cell transcriptome is shared with cancer cells. Moreover, some stem cell-promoting transcription factors, such as Myc, are also bona fide oncogenes. On the other hand, expression of Oct4 or Nanog generally does not cause cancer. A link between cancer and stem cells is also consistent with the observation that p53 inactivation enhances iPS reprogramming (Hong et al. 2009; Kawamura et al. 2009). YAP is one such gene, acting in both stem cells and cancer cells. We speculate that YAP also has a role in tissue-specific stem cells. Indeed, YAP is expressed preferentially in the progenitor compartment of the intestine (Camargo et al. 2007). Forced expression of YAP results in expansion of progenitor cells and dysplasia (Camargo et al. 2007; Cao et al. 2008). Therefore, YAP may promote organ size by increasing tissue-specific progenitor cells.
LIF and BMP signaling are critical in mES cell maintenance, although LIF may not be required for human ES cells (Dahéron et al. 2004). YAP induces expression of several genes in these two signaling pathways, including Gab1, LIFR, Smad2, Jak1, BMPR1, and Sox2. Therefore, one mechanism by which YAP promotes ES cell pluripotency is to enhance LIF and BMP signaling. On the other hand, YAP itself is regulated by LIF signaling. When LIF is removed in the culture medium, YAP protein level drops significantly. These results indicate an intricate network between YAP and LIF signaling. TAZ is a transcription coactivator sharing significant homology with YAP, although it may have different physiological functions. Notably, TAZ has been reported to bind directly to Smad2/3, and may play a role in human ES cells (Varelas et al. 2008). Reduced TAZ activity leads to neuronal differentiation of human ES cells. Therefore, both YAP and TAZ are new factors that contribute to ES cell biology.
An important open question is how YAP regulation contributes to ES cell biology. Transcription regulation is clearly one of the mechanisms involved in YAP regulation in ES cells, as YAP mRNA dramatically decreased during differentiation. In other cells, such as fibroblasts, YAP activity is also inhibited by Lats-dependent phosphorylation. Apparently, YAP S127 phosphorylation is also increased during ES cell differentiation. It would be interesting to learn if the Hippo pathway is suppressed in ES cells, and how this pathway contributes to ES cell pluripotency.
Material and methods
Cell culture and differentiation
The mES-D3 line (American Type Culture Collection) was maintained and expanded on a confluent feeder layer of irradiated SVJ-129 murine fibroblasts in knockout DMEM (KO-DMEM) supplemented with 20% (v/v) knockout serum replacement, 1% nonessential amino acid, and 5 mM glutamax (Invitrogen), with 0.1 mM 2-mercaptoethanol (Sigma) and 10 ng/mL recombinant mouse LIF (Sigma). ES-D3 cells were harvested by gentle trypsinization and were plated onto either feeder layers or 0.1% gelatin for short-term experiments. Feeder and differentiated cells were separated from undifferentiated ES cell culture by differential adhesion (plating ES cell suspension on a nongelatinized plate for 30 min at 37°C, then aspirating relatively nondifferentiated ES cells in supernatant). ES cells were induced to differentiate by culturing on feeder-free, 0.1% gelatin-coated plates without LIF or without both LIF and other supplements (10% FBS + high glucose DMEM only). Cells were triptinized and replated every 48–72 h until they exhibited a flattened and differentiated phenotype. Pluripotency was assayed by ALP kit (Millipore) according to the manufacturer's protocol, as well as by Oct4 and Sox2 antibodies (Santa Cruz Biotechnologies, sc5279 and sc-17320, respectively) in Western blot. Primers for germline-specific differentiation markers are listed in Supplemental Table S7. mES-R1 cell line was maintained under identical condition to the ES-D3 cells. To initiate EB formation and differentiation, ES-D3 cells were cultured in the absence of LIF on 35-mm bacteriological-grade plastic petri dishes (Fisher Scientific). RA-induced (1 μM) differentiation was carried out for 7 d after EB formation according to the manufacturer's instructions by supplementing Neurobasal Medium (Gibco 21103) supplemented with B27 (Gibco 17504) and G5 (Gibco 17503).
Lentiviral and retroviral infection
To generate YAP and TEAD1/3/4 knockdown cells, mES cells were infected with lentivirus containing shRNA targeting YAP and TEAD, respectively (Zhao et al. 2008). TEAD1/3/4 shRNAs were designed in a region conserved in TEAD1, TEAD3, and TEAD4. Plasmids were propagated in and purified from Stbl2-competent cells (Invitrogen). Lentiviral packaging plasmids psPAX2 and pMD2.G were cotransfected into HEK293-T cells for virus production. Cells were selected in culture medium containing 5 μg/mL puromycin (Sigma). To generate wild-type, mutant YAP-, and TEAD2-expressing mES cells, retrovirus infection was carried out by transfecting 293 Phoenix retrovirus packaging cells with empty vector or pQCXIX constructs harboring the indicated genes (Zhao et al. 2008). Forty-eight hours after transfection, retroviral supernatant was supplemented with 5 μg/mL polybrene, filtered through a 0.45-μm filter, and used to infect ES-D3 mES cells. Twenty-four hours after infection, cells were selected with 200 μg/mL hygromycin (Invitrogen).
Teratoma formation assay
Mouse procedures were performed according to the guidelines of approved animal protocol and based on the methods described previously (Prokhorova et al. 2009). Control and YAP-overexpressed mES cells were grown at differentiating conditions described above, harvested by trypsinization, washed in phosphate-buffered saline (PBS), and resuspended in PBS supplemented with 30% Matrigel (BD Bioscienses). Cells (1.5 × 106) in 200 μL per injection site were used. Nude mice (Nu-foxn1nu; Charles Rivers Laboratories) were injected on both sides (control on the left, YAP-WT/5SA on the right) of the dorsolateral sites subcutaneously. Teratomas were harvested, fixed, and stained in hematoxylin and eosin (H&E) 5 wk post-injection.
Retroviral transduction and generation of iPS cells
The pMX-based retroviral vectors Oct3/4, Klf4, Sox2, and c-Myc were obtained from Addgene. Viral production and transduction process was performed as described (Takahashi et al. 2007). Mouse embryonic fibroblasts (MEFs) were derived from transgenic mice [B6;CBA-Tg(Pou5f1-EGFP)2Mnn; Jackson Laboratory] that express enhanced GFP under the control of the Oct4 promoter and distal enhancer. To generate iPS cells, MEFs previously seeded on gelatinized 12-well plates were infected overnight with combinations of the pMX-based retroviral vectors OSK and c-Myc, with or without the pQCXIX-based retroviral vector expressing YAP. Three days after infection, the MEF media was switched to ES cell culture media (KO-DMEM, 10% FBS, 10% knockout serum replacement, 1× nonessential amino acids, 2 mM L-glutamine, 0.1 mM β-mercaptoethanol, 103 U/mL LIF [Chemicon]). All reagents were from Invitrogen, unless otherwise mentioned. iPS cells and YAP-overexpressed cells were karyotyped via service of Cell Line Genetics, LLC.
ChIP, ChIP-seq, RT–PCR, and quantitative PCR
ChIP was carried out as described previously (Yu et al. 2007) using an anti-YAP antibody (gift from Dr. Marius Sudol). ChIP-enriched DNA was prepared into libraries and sequenced using the Genome Analyzer (Illumina) following the manufacturer's protocols. The raw sequencing image data were analyzed by the Illumina analysis pipeline, aligned to the unmasked reference genome (NCBI version 37, mm9) using ELAND (Illumina) to generate genomic coordinates of sequence reads, which were further analyzed by HPeak, a Hidden Markov Model (HMM)-based peak-identifying algorithm that we developed (http://www.sph.umich.edu/csg/qin/HPeak), to identify ChIP-enriched binding peaks. ChIP-identified targets were confirmed by RT–PCR and assayed for expression by semiquantitative PCR using the primers listed in Supplemental Tables S5 and S6, respectively.
Acknowledgments
We thank Marius Sudol for YAP antibody, and UCSD Histology Core (Nissi Varki) for the teratoma analysis. This work is supported by grants from NIH (to K.-L.G.).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1903310.
Supplemental material is available at http://www.genesdev.org.
References
- Anbanandam A, Albarado DC, Nguyen CT, Halder G, Gao X, Veeraraghavan S 2006. Insights into transcription enhancer factor 1 (TEF-1) activity from the solution structure of the TEA domain. Proc Natl Acad Sci 103: 17225–17230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J 2003. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14–3–3 and attenuation of p73-mediated apoptosis. Mol Cell 11: 11–23 [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K 2006. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125: 315–326 [DOI] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. 2006. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441: 349–353 [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR 2007. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060 [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage F 2008. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev 22: 3320–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Smith A 2004. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 23: 7150–7160 [DOI] [PubMed] [Google Scholar]
- Chen HI, Sudol M 1995. The WW domain of yes-associated protein binds a proline-rich ligand that differs from the consensus established for src homology 3-binding modules. Proc Natl Acad Sci 92: 7819–7823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chan SW, Zhang XQ, Walsh M, Lim CJ, Hong W, Song H 2010. Structural basis of YAP recognition by TEAD4 in the Hippo pathway. Genes Dev 24: 290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahéron L, Opitz SL, Zaehres H, Lensch WM, Andrews PW, Itskovitz-Eldor J, Daley GQ 2004. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem Cells. Stem Cells 22: 770–778 [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesen O, Brivanlou AH 2007. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev 3: 7–17 [DOI] [PubMed] [Google Scholar]
- Fossdal R, Jonasson F, Kristjansdottir GT, Kong A, Stefansson H, Gosh S, Gulcher JR 2004. A novel TEAD1 mutation is the causative allele in Sveinsson's chorioretinal atrophy helicoid peripapillary chorioretinal degeneration. Hum Mol Genet 1: 975–981 [DOI] [PubMed] [Google Scholar]
- Haegele L, Ingold B, Naumann H, Tabatabai G, Ledermann B, Brandner S 2003. Wnt signalling inhibits neural differentiation of embryonic stem cells by controlling bone morphogenetic protein expression. Mol Cell Neurosci 24: 696–708 [DOI] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X 2007. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem 283: 5496–5509 [DOI] [PubMed] [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A 1999. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274: 19838–19845 [DOI] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S 2009. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature 460: 1132–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434 [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Young R 2008. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132: 567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Belmonte JC 2009. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460: 1140–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinameri E, Inoue T, Aruga J, Imayoshi I, Kageyama R, Shimogori T, Moore AW 2008. Prdm proto-oncogene transcription factor family expression and interaction with the Notch–Hes pathway in mouse neurogenesis. PLoS One 3: e3859 doi: 10.1371/journal.pone.0003859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M 2007. A Sveinsson's chorioretinal atrophy-associated missense mutation in mouse Teadi affects its interaction with the co-factors YAP and TAZ. Biochem Biophys Res Commun 361: 1022–1026 [DOI] [PubMed] [Google Scholar]
- Komuro A, Nagai M, Navin NE, Sudol M 2003. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem 278: 33334–33341 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhao B, Want P, Chen F, Dong Z, Yang H, Guan KL, Xu Y 2010. Structural insights into the YAP and TEAD complex. Genes Dev 24: 235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn H, Ermekova KS, Rentschler S, Sparks AB, Kay BK, Sudol M 1997. Using molecular repertoires to identify high-affinity peptide ligands of the WW domain of human and mouse YAP. Biol Chem 378: 531–537 [DOI] [PubMed] [Google Scholar]
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, et al. 2007. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1: 367–368 [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S 2003. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113: 631–642 [DOI] [PubMed] [Google Scholar]
- Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, Sugiyama S, Matsuda S, Koyasu S, Yamauchi-Takihara K, et al. 2003. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res 93: 221–229 [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A 1998. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95: 379–391 [DOI] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson R, Ogonuk N 2009. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 16: 398–410 [DOI] [PubMed] [Google Scholar]
- Oka T, Mazack V, Sudol M 2008. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J Biol Chem 283: 27534–27546 [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S 2007. Generation of germline-competent induced pluripotent stem cells. Nature 448: 313–317 [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA 2006. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci 103: 12405–12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhorova TA, Harkness LM, Frandsen U, Ditzel N, Schroder HD, Burns JS, Kassem M 2009. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev 18: 47–54 [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA 2002. ‘Stemness’: Transcriptional profiling of embryonic and adult stem cells. Science 298: 597–600 [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Kalyana-Sundaram S, Tomlins SA, Mahavisno V, Kasper N, Varambally R, Barrette TR, Ghosh D, Varambally S, Chinnaiyan AM 2007. Molecular concepts analysis links tumors, pathways, mechanisms, and drugs. Neoplasia 9: 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AT, Mehra R, Rhodes DR,Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, et al. 2007. Integrative molecular concept modeling of prostate cancer progression. Nat Genet 39: 41–51 [DOI] [PubMed] [Google Scholar]
- Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA 2008. Expression of Yes-associated protein in common solid tumors. Hum Pathol 39: 1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M 1994. Yes-associated protein YAP65 is a proline rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene 9: 2145–2152 [PubMed] [Google Scholar]
- Sudol M, Bork P, Einbond A, Kastruy K, Druck T, Negrini M, Huebner K, Lehman D 1995. Characterization of the mammalian YAP Yes-associated protein gene and its role in defining a novel protein module, the WW domain. J Biol Chem 270: 14733–14741 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast culture by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe1 K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Van Den Berg D, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot R 2008. Estrogen-related receptor β interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol 28: 5986–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL 2008. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10: 837–848 [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML 2001. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev 15: 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R 2007. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448: 318–324 [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D 2008. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 14: 388–398 [DOI] [PubMed] [Google Scholar]
- Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM 2009. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 115: 4576–4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y 1999. A WW domain-containing yes-associated protein YAP is a novel transcriptional co-activator. EMBO J 18: 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A 2007. Transcription factor TEAD4 speicifies the trophecoderm lineage at the beginning of mammalian development. Development 134: 3827–3836 [DOI] [PubMed] [Google Scholar]
- Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, Wang X, Ghosh D, Shah RB, Varambally S, et al. 2007. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res 6722: 10657–10663 [DOI] [PubMed] [Google Scholar]
- Zajac-Kaye M 2001. Myc oncogene: A key component in cell cycle regulation and its implication for lung cancer. Lung Cancer 34: S43–S46 doi: 10.1016/S0169-5002(01)00343-9 [DOI] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. 2006. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125: 1253–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Smolen GA, Haber DA 2008. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res 68: 2789–2794 [DOI] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J 2008. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell 14: 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al. 2008. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Kim J, Ye X, Lai ZC, Guan KL 2009. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of Yes-associated protein. Cancer Res 69: 1089–1098 [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL 2010. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ–TRCP. Genes Dev 24: 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]