Abstract
Phosphodiesterase type 5A (PDE5A) inhibitors acutely suppress beta-adrenergic receptor (β-AR) stimulation in left ventricular myocytes and hearts. This modulation requires cyclic GMP synthesis via nitric oxide synthase (NOS)-NO stimulation, but upstream and downstream mechanisms remain un-defined. To determine this, adult cardiac myocytes from genetically engineered mice and controls were studied by video microscopy to assess sarcomere shortening (SS) and fura2-AM fluorescence to measure calcium transients (CaT). Enhanced SS from isoproterenol (ISO, 10 nM) was suppressed ≥50% by the PDE5A inhibitor sildenafil (SIL, 1 µM), without altering CaT. This regulation was unaltered despite co-inhibition of either the cGMP-stimulated cAMP-esterase PDE2 (Bay 60-7550), or cGMP-inhibited cAMP-esterase PDE3 (cilostamide). Thus, the SIL response could not be ascribed to cGMP interaction with alternative PDEs. However, genetic deletion (or pharmacologic blockade) of β3-ARs, which couple to NOS signaling, fully prevented SIL modulation of ISO-stimulated SS. Importantly, both PDE5A protein expression and activity were similar in β3-AR knockout (β3-AR−/−) myocytes as in controls. Downstream, cGMP stimulates protein kinase G (PKG), and we found contractile modulation by SIL required PKG activation and enhanced TnI phosphorylation at S23, S24. Myocytes expressing the slow skeletal TnI isoform which lacks these sites displayed no modulation of ISO responses by SIL. Non-equilibrium isoelectric focusing gel electrophoresis showed SIL increased TnI phosphorylation above that from concomitant ISO in control but not β3-AR−/− myocytes. These data support a cascade involving β3-AR stimulation, and subsequent PKG-dependent TnI S23, S24 phosphorylation as primary factors underlying the capacity of acute PDE5A inhibition to blunt myocardial β-adrenergic stimulation.
Keywords: Phosphodiesterase, Sildenafil, Myocytes, Adrenergic, Contractility, Calcium, Troponin I, Beta-3 adrenergic receptor, Protein kinase G, Cyclic GMP
Introduction
Stimulation of cardiomyocyte beta-adrenergic receptors (β-AR) leads to the generation of cyclic adenosine monophosphate (cAMP) by adenylate cyclase and subsequent activation of protein kinase A (PKA). This is a primary mechanism for the acute enhancement of cardiac contractility. β-AR stimulation also engages another second messenger, cyclic guanosine monophosphate (cGMP), via a nitric oxide (NO) coupled pathway, which can modulate cAMP signaling in several ways [18, 30, 37]. First, cGMP activates protein kinase G (PKG) which phosphorylates troponin I to reduce myofilament responsiveness to Ca2+ [14] and the L-type Ca2+ channel that can reduce Ca2+ current [44]. Second, it can activate membrane bound phosphodiesterase (PDE) type 2 by binding to its N-terminus regulatory GAF domain thereby enhancing cAMP catabolism [17, 20, 46]. Third, it can compete for cAMP catabolism by PDE-3, in this case enhancing net cAMP levels [39]. The first mechanism has been proposed to underlie negative inotropic effects of higher levels of cGMP (or NO) stimulation [14, 32], the second for blunting of β-adrenergic (cAMP) stimulation [20, 46], and the third for positive inotropic effects of low levels of cGMP (or NO) [39].
Recent studies have identified an important role of the type-3 β-AR in coupling adrenergic with NOS stimulation [10, 21, 38]. Mice genetically lacking β3-AR exhibit little change in myocardial cGMP upon isoproterenol stimulation, nor an augmentation of the ISO-contractile response when NOS is inhibited [38]; both of these behaviors being observed in controls. NOS-stimulated cGMP coupled to β3-AR stimulation also activates PDE2-mediated cAMP hydrolysis, providing another mechanism countering adrenergic stimulation [20].
In addition to NOS stimulation, cGMP can be enhanced by stimulating natriuretic-peptide receptors (NPR) coupled to receptor-bound GC or by blunting catabolism by phosphodiesterases. However, we and others have shown in myocytes and intact hearts, that the cGMP pool coupled to NPR is highly compartmentalized [4, 25, 34] and does not modulate acute β-AR stimulation [34]. By contrast, inhibiting cGMP catabolism by phosphodiesterase type 5a (PDE5A) does suppress β-AR stimulated contraction in mammals [23, 31, 35], including humans [1]. This action is absent in hearts/cells lacking NOS3 or those with NOS inhibition [23, 35], though the latter can be offset by direct co-stimulation of sGC-derived cGMP [23], indicating the mechanism involves cGMP-dependent signaling rather than NO-protein modification. The potential role of various cGMP adrenergic modulation mechanisms with PDE5A inhibition remain unknown, but understanding them is important as PDE5A inhibitors are undergoing human trials to test their capacity to treat heart failure, and both β3-AR [10] and PDE5A expression [26] increase in this disorder.
The present investigation tested cellular mechanisms for modulation of β-AR by PDE5A inhibitors. Using a combination of pharmacologic and genetic approaches, we examined the role of PDE2 and PDE3 co-modulation, β3-AR signaling, and PKG distal targeting of the contractile apparatus. Our results reveal that anti-adrenergic effects of PDE5A inhibition are not modulated by PDE2 or PDE3, but rather require β3-AR stimulation, PKG activation, and consequent TnI phosphorylation.
Materials and methods
Myocyte isolation and physiologic analysis
Adult cardiac myocytes were freshly isolated from murine control species (C57BL/6, CD1, or FVB) and from corresponding genetically engineered mice (skeletal muscle TnI overexpressor (ssTnItg) and β3-AR−/−). The protocol followed U.S. National Institute of Health guidelines and was approved by the animal and care use committee of the Johns Hopkins Medical Institutions. Hearts were quickly removed from the chest after euthanasia and the aorta retroperfused at 100 cmH2O and 37°C for ~3 min with a Ca2+-free bicarbonate-based buffer containing (in mM) 120 NaCl, 5.4 KCl, 1.2 NaH2PO4, 20 NaHCO3, 1.6 MgCl2; glucose (1 mg/ml), 2, 3-butanedione monoxime (BDM, 1 mg/ml), and taurine (0.628 mg/ml), gassed with 95% O2–5% CO2. Enzymatic digestion was initiated by addition of 0.9 mg/ml collagenase type 2 (Worthington Biochemical Co., 299 U/mg) and 0.05 mg/ml protease type XIV (Sigma Chemical Co.) to the perfusion solution (6–7 min).
Dispersed myocytes were filtered through a 150 µm mesh and gently centrifuged at 500 rpm for 30 s. The pellet was re-suspended in Tyrode’s solution with increasing Ca2+ (1 mM), and cells then incubated for 15 min with 3 µmol/l Fura2-AM (Invitrogen, Molecular Probes, Carlsbad CA) in Tyrodes (1 mM Ca2+). After rinsing, cells were placed in a perfusion chamber with a flow-through rate of 2 ml/min, and sarcomere length and whole cell Ca2+ transients recorded using an inverted fluorescence microscope (Nikon, TE2000), and IonOptix (Myocam®) software.
Western blot and PDE5 activity analysis
Protein lysates from isolated cardiac myocytes were extracted in lysis buffer (Cell Signaling) with protease inhibitor PMSF (1 mM). After 12,000g centrifugation for 10 min, protein concentrations were quantified by BCA assay (Pierce). The lysates were diluted in 4× NuPAGE lithium dodecyl sulfate sample buffer and electrophoresed on NuPAGE 4–12% gel (Invitrogen). The separated proteins were transferred onto membrane and incubated with specific PDE5 polyclonal antibody (Cell Signaling). PDE activity was measured at room temperature using 1 µM cGMP as substrate by fluorescence polarization (FP) method (Molecular Devices) under linear conditions with and without SIL (1 µM).
TnI phosphorylation analysis
Isolated cardiac myocytes were lysed in de-ionized (AG 501-X8 resin) sample buffer containing urea (8 M), thio urea (2 M), EDTA (10 mM pH 8.0), 3–10 ampholyte 1%, TBP (2 µM). Protein concentration was determined by the RC-DC assay (BioRad) to facilitate equal loading. Non-equilibrium pH gradient electrophoresis (NEGE) was performed in a Criterion gel system (Bio-Rad). The resolving gel was 5% acrylamide cross-linked with 3.3% bisacrylamide pH 8.8, urea (6 M), 2% X-100, 3–10 ampholyte 0.4% (150 µL), 7–9 ampholyte 1.6% (600 µL), glycerol 10% as previously described [12]. The resolving gel was de-gassed with vacuum for 30 min and then APS (30 µL), and Temed (30 µL) were added. The gel was run (with reversed power) at 100-V (20 min), 200-V (20 min), 400-V (20 min), 500-V (50 min) in upper chamber buffer H3PO4 (10 µM), and lower chamber buffer NaOH (100 µM). After electrophoresis, the gel was immediately placed in transfer buffer [10 mM 3-(cyclohexylamino)-1-propanesulfonic acid pH 11.0], and 10% methanol to equilibrate for 5–10 min. The separated proteins were transferred from the gel onto 0.2-µm polyvinylidene difluoride membrane at 20-V constant voltage for 2.5 h on ice. The membranes were blocked for 2 h at room temperature in 5% nonfat dry milk diluted in 50 mM Tris-base, 200 mM NaCl, 0.05% Tween 20 pH 7.5 (TBST). The primary monoclonal antibody specific for TnI Ser23/24 (from RDI, Flanders, NJ) was diluted 1:500, in 2.5% TBST, and blocked overnight at 4°C. The secondary antibody α-rabbit was diluted 1:20,000 in 2% BSA-TBST. After the membranes were washed, they were developed with an ECL plus kit (Amersham Biosciences, Piscataway, NJ), following the manufacturer’s recommendations.
Statistical analysis
Data are expressed as mean ± SE. Myocyte results were analyzed using a 1-way repeated measure ANOVA, with a Tukey test for multiple comparisons.
Results
Anti-β-adrenergic effect from PDE5A inhibition is independent of PDE2 and PDE3
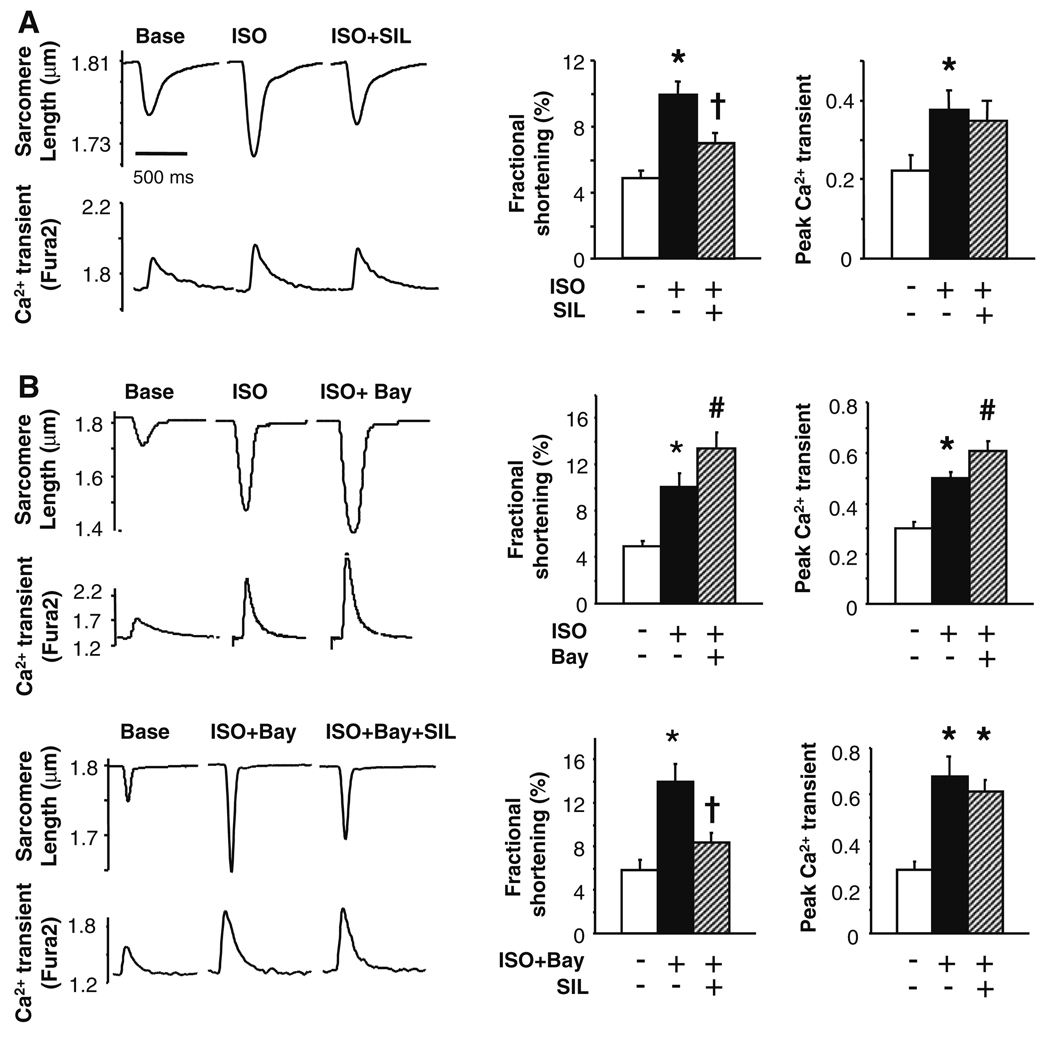
Figure 1a displays example sarcomere shortening and Ca2+ transient tracings (left) and summary data (right) for C57BL/6 myocytes exposed to isoproterenol (ISO, 10 nM) followed by ISO + sildenafil (SIL, 1 µM). SIL blunted the ISO response without significantly altering Ca2+ transients, consistent with prior studies [34, 35]. One potential mechanism for the SIL effect is that elevated cGMP concentrations activate PDE2 by binding to its regulatory domain, thereby stimulating cAMP hydrolysis. Enhanced sarcomere shortening and Ca2+ transients induced by ISO were further augmented by subsequent addition of the selective PDE2 inhibitor Bay 60-7550 (50 nM) (Fig. 1a). This is consistent with reduced cAMP hydrolysis due to PDE2 inhibition, as previously reported [20]. This result was similar if the order of exposure was switched. However, SIL-mediated suppression of ISO responses was unchanged despite PDE2 inhibition (Fig. 1b). This indicates that the cGMP-enhanced PDE2 hydrolysis of cAMP that accompanies β-AR stimulation is not further augmented by PDE5A inhibition. Bay 60-7550 alone had no effect on rest shortening or Ca2+ transients (supplemental Fig. A), and adding SIL to Bay 60–7550 (in the absence of ISO) also did not alter rest behavior (data not shown).
Fig. 1.
PDE2 modulation does not underlie suppression of isoproterenol stimulated contractility by the PDE5A inhibitor, sildenafil. a Example tracings (left) and summary results (right) for control C57BL/6 myocytes exposed to ISO (10 nM) followed by ISO + SIL (SIL, 1 µM). SIL suppressed the positive inotropic response to ISO without altering the Ca2+ transient. b Top cardiac myocytes were stimulated with ISO, then co-stimulated with ISO and PDE2 inhibitor (Bay 60-7550, 50 nM). The latter enhanced both contractile and Ca2+ responses to ISO, consistent with a rise in cAMP as PDE2 functions as a cGMP-stimulated cAMP-esterase [20]. Lower however, PDE2 inhibition did not block the suppressive effect of SIL on contraction stimulated by ISO. For all panels: *P < 0.01 versus control, #P < 0.02 versus control, † P < 0.01 versus middle condition (solid black bar). Data are mean ± SEM, n = 7–20 cells from at least four separate hearts in each group
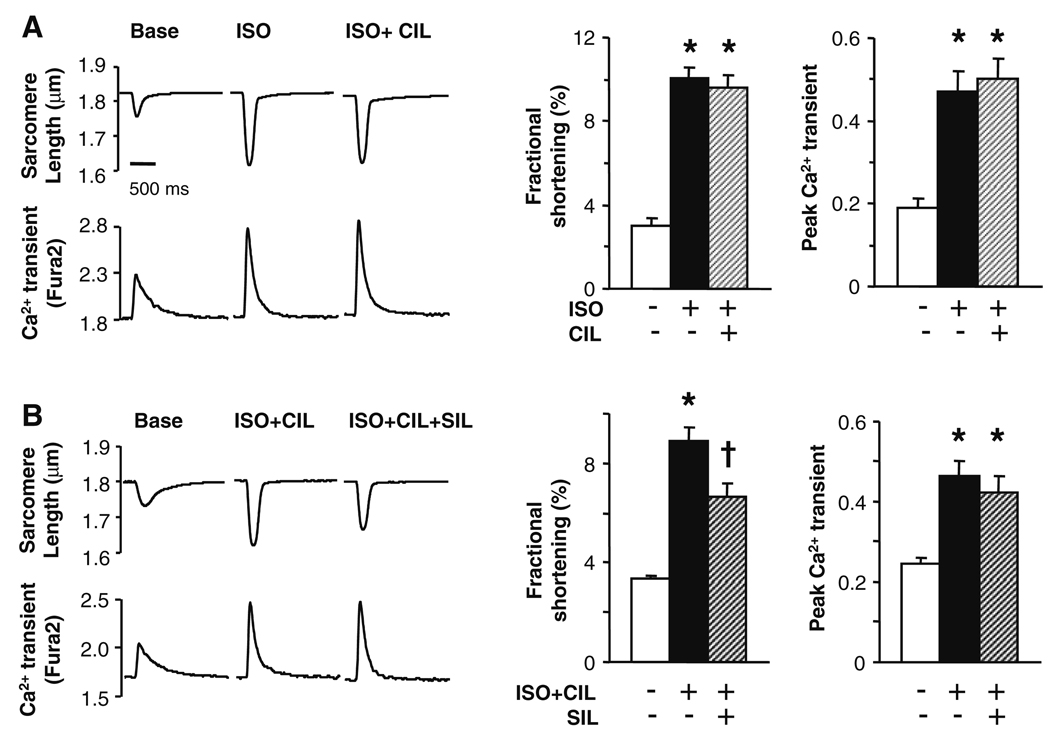
Another mechanism whereby SIL enhanced cGMP could potentially modify cAMP signaling is via competitive inhibition of PDE3 to elevate cAMP. This was suggested in a study of hypertrophied right ventricular myocardium [24], though this would be opposite to the net SIL effect observed in LV myocytes. To assess this possibility, cells were incubated with the selective PDE3 inhibitor cilostamide (CIL, 1 µM) [5]. The ISO/CIL order was randomized prior to SIL administration. CIL alone also did not impact rest shortening or Ca2+ transients (Supplemental Fig. B), nor did it significantly alter the contractile or calcium transient response to ISO (Fig. 2a), consistent with previous reports [15]. Importantly, CIL also had no impact on SIL-induced depression of ISO-contractile stimulation (Fig. 2b).
Fig. 2.
PDE3 inhibition does not alter sildenafil-mediated suppression of acute β-adrenergic stimulation. a Cardiac myocytes stimulated with ISO followed by co-stimulation of ISO and the PDE3 inhibitor cilostamide (CIL, 1 µM). PDE3 inhibition did not alter the ISO response, supporting little basal esterase activity. b PDE3 inhibition had no effect on SIL suppression of ISO-stimulated contraction. Symbols and sample size for various studies are as in Fig. 1
β3-receptors are required for PDE5A negative modulation of β-stimulation
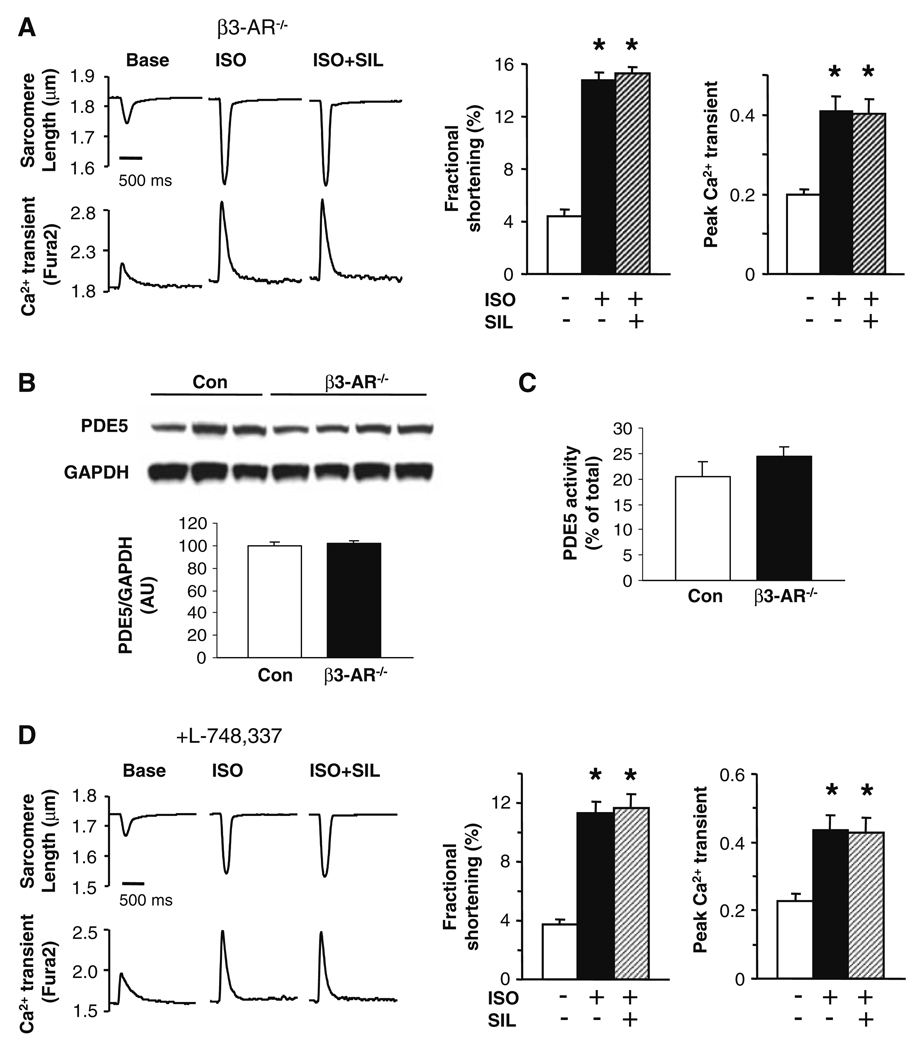
Prior studies have established that the efficacy of PDE5A inhibition to suppress ISO-stimulated contractility requires NOS-sGC generated cGMP [23, 35]. Coupling of adrenergic with NOS stimulation has been particularly linked to the activation of type 3 β-adrenergic receptors [9, 10]. We therefore tested if β3 receptors are also required for SIL to modulate ISO-stimulated contractility. First, myocytes from mice harboring a global gene deletion of the β3 receptor were compared with littermate (FVB) controls. Controls displayed SIL-induced depression of ISO-shortening similar to that present in C57BL/6 cells (data not shown); however, SIL did not suppress ISO-stimulated contraction in β3-AR−/− myocytes (Fig. 3a). The ISO response itself was about 30% greater in β3-AR−/− than control myocytes, consistent with the absence of the β3-coupled cascade. Both PDE5A protein expression (Fig. 3b) and activity (Fig. 3c) were similar between β3-AR−/− and control myocytes, and so unlikely to explain the lack of SIL effect. Since the β3-AR−/− model was global and embryonic, we also tested if control myocytes exposed to the selective β3-AR antagonist L-748,337 (1 µM × 20 min) might display a similar response. ISO-augmented contraction and Ca2+ transients in these cells (~threefold rise over baseline, similar to β3-AR−/− cells), and again SIL had no impact on this contraction response (Fig. 3d). Lastly, we tested if the lack of contractile suppression by SIL might result from enhanced competitive inhibition of PDE3 in β3-AR−/− cells. However, this was not the case, as addition of CIL did not alter the ISO or ISO + SIL response (Supplemental Fig. C).
Fig. 3.
Beta-3 adrenergic stimulation is required for effectiveness of SIL to suppress acute β-adrenergic contractile stimulation. a Sarcomere shortening and corresponding Ca2+ transients in myocytes genetically lacking the β3-AR receptor. ISO stimulation increased both, but SIL had no suppressive effect on myocyte shortening. b PDE5A protein expression in isolated myocytes from control β3-AR−/− cells was similar. c PDE5A activity measured by fluorescence polarization (FP) as previously described [36]. Consistent with protein expression, PDE5A activity was similar in control and β3-AR−/− myocytes. Thus, the lack of SIL effects was not due to less PDE expression or activity. d Sarcomere shortening and corresponding Ca2+ transients with pre-incubation (20 min) of selective β3-AR antagonist (L-748,337, 1 µM) in control myocytes. SIL did not suppress ISO-stimulated shortening, similar to results in β3-AR−/− cells. Symbols and sample size for various studies are as in Fig. 1
Role of PKG activation and troponin I
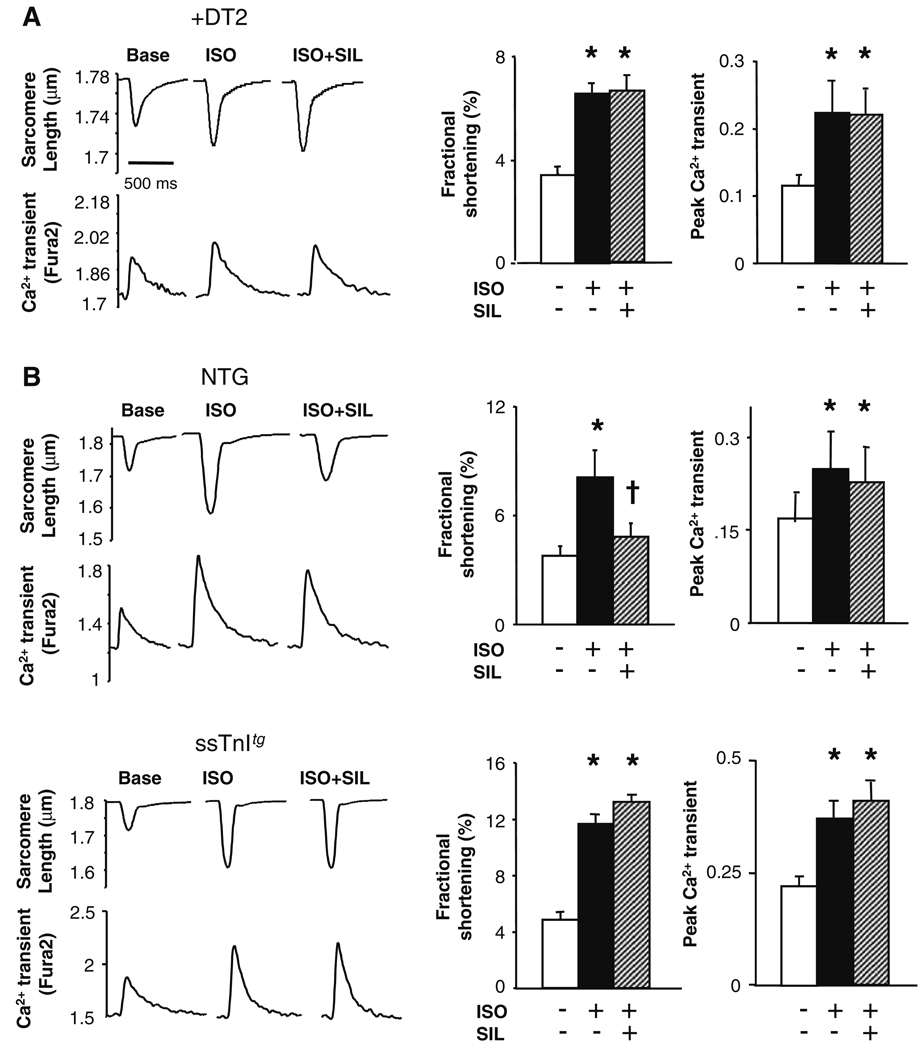
Since suppression of acute β-AR stimulation by inhibiting PDE5A could not be attributed to PDE2 or PDE3 regulation, we examined downstream signaling coupled to PKG. As shown in Fig. 4a, incubation with the PKG specific peptide blocker DT2 [6] (10 µM) fully blocked SIL modulation of ISO-stimulated contractility, consistent with prior results using the cGMP competitive analog 8Br-PET-cGMPs (another PKG antagonist) [34]. The ISO response was itself lower in these experiments as we used less ISO (1 nM), to match the dose used in our prior study. While PKG might suppress shortening by reducing L-type Ca2+ current, the lack of change in the whole cell Ca2+ transient suggested an alternative mechanism. One possibility is phosphorylation of troponin I (TnI) at S23, S24 to desensitize the myofilaments to Ca2+ [30, 41]. To test this, myocytes were isolated from mice for which cardiac TnI was largely replaced by slow skeletal TnI (ssTnItg). ssTnI lacks the N-terminus phosphorylation sites targeted by PKG and PKA [42]. Controls (CD1 strain) displayed identical negative modulation of ISO-stimulated contraction by SIL as in the other strains (Fig. 4b, upper panels). Though cells from ssTnItg hearts displayed ISO-augmented contraction and Ca2+ transients similar to controls, this could not be suppressed by SIL treatment (Fig. 4b, lower panels), identifying a critical role for TnI modulation.
Fig. 4.
PKG-dependent signaling targeting troponin I phosphorylation is central to effect of PDE5A inhibition. a Inhibition of PKG by peptide antagonist DT2 blocks modulation of ISO (1 nM)- stimulated sarcomere shortening by SIL, while augmentation in the Ca2+ transient is unaltered. b Effect of SIL on ISO stimulation in control CD-1 myocytes (upper panels) and myocytes from transgenic hearts expressing the skeletal TnI isoform in myocytes (ssTnItg) (lower panels). Controls behaved as in other mouse strains, with SIL markedly suppressing shortening without altering the calcium transient. However, SIL had no impact on the ISO-stimulated changes in ssTnItg myocytes. Symbols and sample size similar to that for Fig. 1
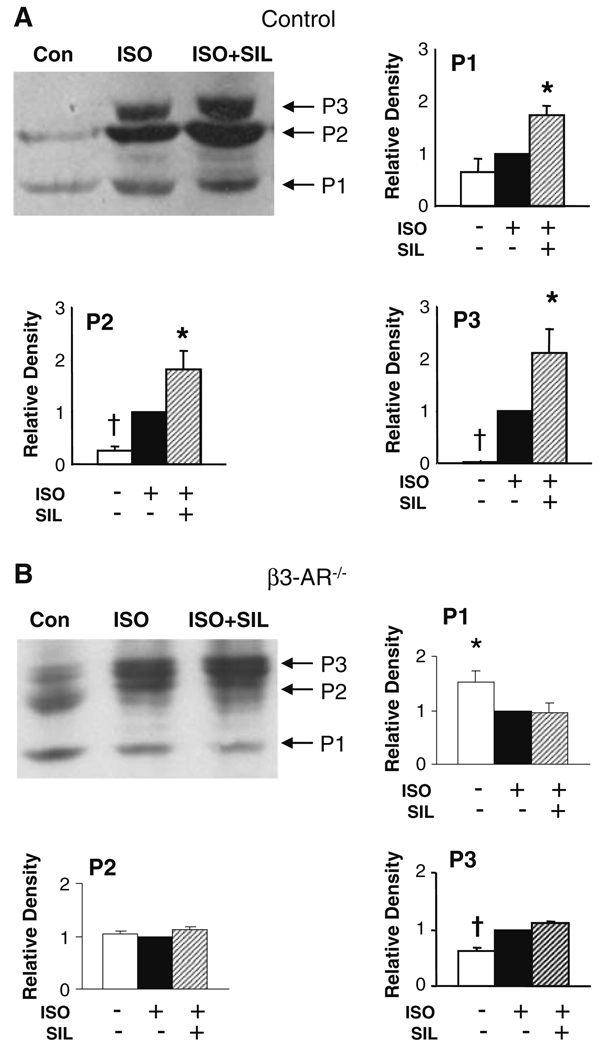
To directly confirm altered TnI phosphorylation with SIL, we performed non-equilibrium gel electrophoresis using isolated myocytes exposed to ISO or ISO + SIL. TnI phosphorylation was detected with a phospho-S23/S24 antibody. Three dominant bands representing increasing phosphorylation states were identified (Fig. 5a shows example gel and provides summary data, n = 7). In unstimulated controls, modest basal phosphorylation was detectable in two states (P1, P2). ISO increased TnI phosphorylation revealed in P2 and a further phosphorylated P3 state. Each of these states was further enhanced in cells co-incubated with SIL, indicating similar targeting by PKG and PKA on TnI. Thus, PKG-dependent SIL modulation was coupled to greater phosphorylation of TnI, beyond that targeted by PKA, consistent with further reduction of myofilament Ca2+ sensitivity.
Fig. 5.
TnI phosphorylation is augmented by SIL above that obtained with ISO in isolated control (C57BL/6), but not in β3-AR−/− cardiomyocytes. Cells isolated from C57BL/6 hearts were stimulated with ISO or ISO + SIL, and TnI phosphorylation was examined using a TnI phospho-S23/S24 antibody. Three primary phosphorylation states were identified, and summary data are shown normalized to the ISO response. a In control myocytes, resting cells displayed weak P1 and P2 states, and ISO stimulation mostly enhanced P2 and revealed an additional P3 state. SIL increased phosphorylation levels in all three states. *P < 0.03 versus ISO, P < 0.005 versus control, †P < 0.05 versus ISO. b In β3-AR−/− myocytes, there was greater resting phosphorylation, shown by a balance favoring P2 and appearance of P3. ISO stimulation resulted in largely a rise in the highest phosphorylation state—P3, and importantly, SIL did not impact this any further. *P < 0.0001 versus other groups, †P < 0.002 versus other two groups. ANOVAs performed using data adjusted for mean value per group
If augmented TnI phosphorylation was indeed a primary mechanism for SIL modulation of acute β-AR stimulation, then the lack such modulation in β3-AR−/− myocytes predicted that such cells would also show little change in ISO-stimulated TnI phosphorylation by SIL. To test this, the same gel experiments were performed in β3-AR−/− myocytes (Fig. 5b, n = 6). Basal TnI phosphorylation was somewhat greater in these cells than in WT, as noted by a stronger P2 band and presence of a P3 band. ISO induced a shift mostly to the high phosphorylation (P3) state. However, the addition of SIL to ISO induced no further significant changes as compared to ISO alone. This results supports our proposed link between SIL modulation of acute β-AR stimulation and β3-coupled, PKG dependent, TnI phosphorylation.
Discussion
Despite its low level of myocardial expression, accumulating evidence supports a role for PDE5A in the regulation of cardiac stress signaling, including acute β-adrenergic reserve, ischemia–reperfusion [2], doxorubicin toxicity [8], myocardial infarction [29], and pressure-overload [36]. Among these, the acute suppression of β-adrenergic contractile stimulation by PDE5A inhibitors is the only one confirmed thus far in multiple species including human [1, 23, 31, 35]. Given the increasing use of SIL in the treatment of clinical heart disease and ongoing trials with this drug for the therapy of heart failure with a preserved ejection fraction (RELAX TRIAL; Clinicaltrials.gov: NCT00763867), elucidating the mechanisms is important.
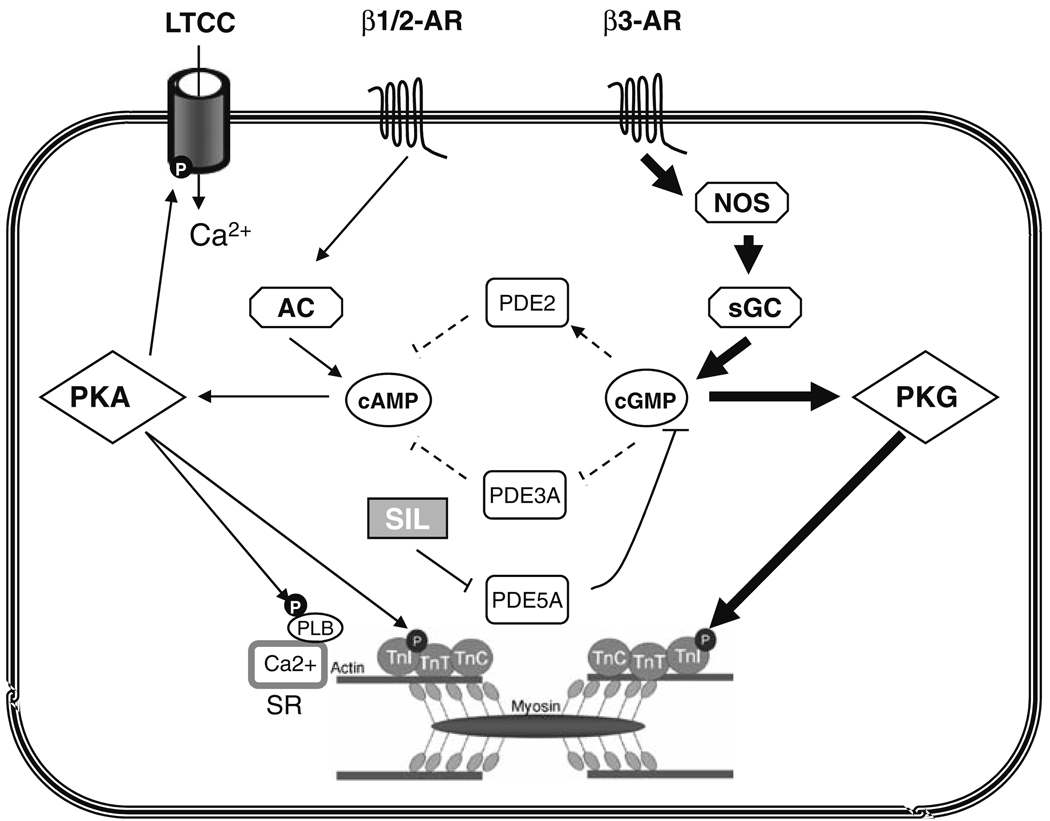
Here we reveal an essential role for proximal activation of β3-ARs that stimulate the NOS-sGC pathway, and identify PKG phosphorylation of TnI as central to the PDE5A inhibitory effect. In contrast, cGMP-mediated changes in either PDE2 or PDE3 activity, that could also alter cAMP signaling, do not appear involved. This indicates that the compartmentalized cGMP pool and/or magnitude of this pool enhanced by sildenafil is largely independent of regulation by these alternative PDEs. Figure 6 summarizes this proposed signaling, showing the potential pathways and identifying those felt responsible on the basis of the current data by bold arrows/lines, and those found not significantly involved by dashed arrows/lines.
Fig. 6.
Schematic of proposed signaling underlying PDE5A inhibitory effects of acute β-adrenergic stimulation. Potential contributors are shown, with those identified by solid lines/arrows supported by prior and/or present results, while the dashed lines/arrows indicate pathways found unimportant to the SIL modulation in the current analysis. On the left side, activation of β1/β2 predominantly stimulates adenylate cyclase coupled to cAMP generation, which in turn activates PKA phosphorylating the L-type Ca2+ channel (LTCC), phospholamban (PLB), and troponin I (TnI) and enhancing contractility and Ca2+ transients. Co-stimulation of the β3-AR predominantly stimulates nitric oxide synthase (NOS) to activate soluble guanylate cyclase (sGC) generating cGMP. This in turn activates PKG with the dominant effect (related to suppression of contraction) being TnI phosphorylation. Potential regulation of cAMP levels by PDE2 or PDE3—activated or inhibited by cGMP respectively—are shown in the center, but do not appear to regulate SIL effects on β-stimulated contraction
PDE2: unidirectional regulation of β-AR stimulated cAMP?
Myocyte cGMP is generated by two different cyclases, and growing evidence supports a high degree of localized compartmentation for their respective signaling [7, 46]. In this regard, the role of PDE2 is intriguing, as it is a dualsubstrate esterase with a lower Km for cGMP catalysis, but a much lower Km for cGMP binding to a regulatory GAF domain which results in preferential hydrolysis of cAMP [43]. In resting rat myocytes, Castro et al. [4] showed PDE2 hydrolyzed a cGMP pool principally generated by natriuretic-peptide-coupled signaling with little effect on cGMP generated by NO-sGC. However, in the presence of β-adrenergic stimulation, there is sufficient co-generation of cGMP to favor PDE2 hydrolysis of cAMP, and this pool of cGMP has been linked to β3-AR stimulation [20]. The present study shows this same cGMP pool is essential for PDE5A anti-adrenergic regulation as well. Given this, one might have anticipated that by further enhancing this pool via PDE5A inhibition, PDE2 would be even more activated to suppress cAMP stimulation. Yet, SIL was equally effective whether PDE2 was blocked or not. This could mean that to interact with PDE5A, β3-AR/NOS-derived cGMP diffuses to subcellular region away from the membrane where it no longer interacts with PDE2 to modify cAMP hydrolysis (i.e., unidirectional modulation). Alternatively, PDE2 selectivity for cAMP (i.e., triggered by cGMP to switch substrate preference) may be established at lower levels of cGMP, so that further enhancement by higher concentrations (e.g., following SIL) is negligible. This would be consistent with reported non-linear responses of PDE2 to cGMP [33, 43]. Teasing out of these possibilities will require localized imaging of cGMP pools in adult myocytes, something that remains elusive.
Linking β3-AR, NOS, and PDE5A
The expression of β3-ARs in healthy myocytes is low, making it hard to detect by ligand binding assays. Nonetheless, data from mice lacking the β3-AR and studies with fairly selective β3-AR small molecule agonists and inhibitors support the link between myocyte activation of the receptor and NOS-cGMP stimulation in ventricular myocytes [3, 9, 38]. In our prior studies, we established a mandatory role of NOS-sGC-derived cGMP for PDE5A modulation of acute adrenergic stimulation, as mice lacking NOS3 or those with NOS acutely inhibited (by L-NAME) show no change in the ISO response with subsequent PDE5 inhibition [23, 34, 35]. The current findings link these two behaviors, showing a central role of β3-signaling to PDE5A-inhibitory effects of broad β-AR stimulation.
While this physiology was studied in normal myocytes, it is worth considering how this coupling could impact pathological conditions. Unlike β1 or β2-AR, β3 receptors appear upregulated in experimental and human heart failure [10, 28]. This may depress contractile function and lower adrenergic responsiveness, but may also provide a protective mechanism, as mice lacking β3-AR exhibit marked maladaptive responses to pressure-overload [19]. PDE5A is also upregulated in heart failure [26, 36] which may be in response to enhanced cGMP synthesis. In this setting, PDE5A inhibition might have an even greater impact due both to enhanced levels of resting esterase expression/activity, and greater stimulation via β3-ARs to provide the hydrolysis substrate.
There are additional mechanisms by which this regulation maybe altered in diseased hearts. In our first report conducted in a canine heart failure model (pacing-tachycardia), we reported that PDE5 inhibition failed to alter dobutamine-stimulated contraction unlike the response in normal controls [31]. One contributor may have been reduced NOS function which is reported in this model [27], but another is dislocalization of PDE5 away from the z-disk region [31]. This change to a more diffuse myocyte PDE5 distribution was recapitulated when NOS was inhibited or eNOS genetically deleted [23, 35], and reversed by restoring sGC-derived cGMP synthesis, indicating the availability of NOS-derived cGMP is important to localizing PE5 in the sarcomere.
PDE5A inhibition targets troponin I
The results from the ssTnItg mice and phosphorylation analysis in both normal and β3-AR−/− myocytes establish TnI phosphorylation by PKG as central to the ability of PDE5A to antagonize acute β-stimulation. Had there been a primary influence of PKG on the L-type calcium current, as suggested [40], this should have persisted in ssTnItg myocytes, yet the removal of PKG (and PKA) TnI phosphorylation sites was sufficient to prevent SIL from reducing ISO-stimulated contraction. To our knowledge, the data shown in Fig. 5 are the first to directly document that TnI is further phosphorylated by a cGMP-PKG pathway when superimposed on pre-existing changes due to PKA. Basal effects of SIL are negligible as the resting cyclase activity is low, so there is minimal cGMP to modulate with PDE5A inhibition. The presence of multiple phosphorylation states from PKA stimulation is consistent with prior studies [12], but importantly, the pattern observed with SIL + ISO in control cells was identical to that with ISO itself, suggesting additive but similar targeting by both PKG and PKA.
Skeletal TnI differs from cardiac TnI in more than just the lack of the unique N-terminal segment containing PKA (PKG) phosphorylation sites, including a proline instead of a threonine at position 144 thought important for PKC modulation [13], and a histidine instead of an alanine at position 164 linked to preserved contractile force despite acidosis [11]. Thus, it is possible that the lack of SIL efficacy in the ssTnItg myocytes reflected more than just the absence of S23, S24 sites. However, as PDE5A is not known to interact acutely with PKC or alter pH, the mechanism most likely relates to the S23, S24 sites.
The fact that both PKG and PKA modify TnI and thus reduce myofilament sensitivity raises potentially important issues with respect to heart failure. In this syndrome, PKA phosphorylation of TnI has been found by some to be reduced, whereas PKC phosphorylation is increased [16]. The state of TnI PKG phosphorylation in diseased hearts is unknown, but given the importance of this modification to myocyte relaxation [45] and apparent specificity to NOS-sGC-PDE5A regulation, such analysis seems warranted.
Limitations
Our study has several limitations. One is that the analysis was performed at ambient rather than physiologic temperature. However, we have repeatedly shown that myocyte modulation of β-AR by PDE5A-inhibition (or lack of change by ANP) determined under such conditions is fully concordant with responses of fully intact in vivo hearts [22], so the results are unlikely to be substantively different if measured at 37°. Second, we used pharmacologic inhibition of PDEs, although this is generally done due to availability of highly selective inhibitors and a lack of genetic deletion models for the three PDEs in question. We did not directly assess the L-type Ca2+ channel, though SIL has been shown to slightly reduce channel current [35, 40]. Importantly, net whole cell transients were very little altered even in this study. Third, we generally applied SIL last after ISO stimulation (or ISO+ another modulator) in this as in prior published studies. Giving SIL first would likely have little impact until cGMP generation was augmented, as resting sGC activity is very low [35]. All the doses of pharmaceuticals used are based on documented dose–response dependencies. Gene silencing by viral transfection in adult myocytes poses its own limitations since such primary culture studies is difficult, and receptor-coupled signaling may itself be altered.
Conclusion
We have demonstrated that acute PDE5A inhibition suppresses concomitant β-AR stimulation by a β3-AR and PKG-dependent pathway that requires TnI phosphorylation. Alternative mechanisms, including activation of PDE2A to blunt cAMP signaling or inhibition of PDE3A to enhance cAMP signaling, do not appear to play a major role. As PDE5A inhibitors are further explored as potential therapies in cardiac failure and hypertrophy, examining how modifications of these signaling pathways may influence their net efficacy will be important. Additional functional studies on the different localization and expressional levels of PDE5 and myocardial cGMP compartmentalization may provide a more valuable approach to determine the optimal conditions for pharmacological therapies in patients with heart failure.
Supplementary Material
Acknowledgments
We thank Chad M. Warren for his assistance in performing some of the isoelectric gel electrophoresis studies. This work was supported by Public Health Service NHLBI grants: HL-089297, HL-095408 (DAK), and T32 HL-07227 (DAK, SV), and RO1 HL-022231, and PO1-HL-062426 (RJS).
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s00395-010-0084-5) contains supplementary material, which is available to authorized users.
Conflict of interest statement None.
Contributor Information
Dong I. Lee, Ross 858, Division of Cardiology, Department of Medicine, Johns Hopkins University Medical Institutions, 720 Rutland Ave, Baltimore, MD 21205, USA
Susan Vahebi, Ross 858, Division of Cardiology, Department of Medicine, Johns Hopkins University Medical Institutions, 720 Rutland Ave, Baltimore, MD 21205, USA.
Carlo Gabriele Tocchetti, Ross 858, Division of Cardiology, Department of Medicine, Johns Hopkins University Medical Institutions, 720 Rutland Ave, Baltimore, MD 21205, USA.
Lili A. Barouch, Ross 858, Division of Cardiology, Department of Medicine, Johns Hopkins University Medical Institutions, 720 Rutland Ave, Baltimore, MD 21205, USA
R. John Solaro, Department of Physiology and Biophysics, University of Illinois at Chicago, Chicago, IL, USA.
Eiki Takimoto, Ross 858, Division of Cardiology, Department of Medicine, Johns Hopkins University Medical Institutions, 720 Rutland Ave, Baltimore, MD 21205, USA.
David A. Kass, Ross 858, Division of Cardiology, Department of Medicine, Johns Hopkins University Medical Institutions, 720 Rutland Ave, Baltimore, MD 21205, USA, dkass@jhmi.edu
References
- 1.Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation. 2005;112:2642–2649. doi: 10.1161/CIRCULATIONAHA.105.540500. [DOI] [PubMed] [Google Scholar]
- 2.Bremer YA, Salloum F, Ockaili R, Chou E, Moskowitz WB, Kukreja RC. Sildenafil citrate (viagra) induces cardioprotective effects after ischemia/reperfusion injury in infant rabbits. Pediatr Res. 2005;57:22–27. doi: 10.1203/01.PDR.0000147736.27672.15. [DOI] [PubMed] [Google Scholar]
- 3.Brixius K, Bloch W, Ziskoven C, Bolck B, Napp A, Pott C, Steinritz D, Jiminez M, Addicks K, Giacobino JP, Schwinger RH. Beta3-adrenergic eNOS stimulation in left ventricular murine myocardium. Can J Physiol Pharmacol. 2006;84:1051–1060. doi: 10.1139/y06-033. [DOI] [PubMed] [Google Scholar]
- 4.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dostmann WR, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge WJ. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc Natl Acad Sci USA. 2000;97:14772–14777. doi: 10.1073/pnas.97.26.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 8.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardio-myocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, Bril A, Balligand JL, Le Marec H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest. 1998;102:1377–1384. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauthier C, Seze-Goismier C, Rozec B. Beta 3-adrenoceptors in the cardiovascular system. Clin Hemorheol Microcirc. 2007;37:193–204. [PubMed] [Google Scholar]
- 11.Kobayashi T, Jin L, de Tombe PP. Cardiac thin filament regulation. Pflugers Arch. 2008;457:37–46. doi: 10.1007/s00424-008-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi T, Yang X, Walker LA, Van Breemen RB, Solaro RJ. A non-equilibrium isoelectric focusing method to determine states of phosphorylation of cardiac troponin I: identification of Ser-23 and Ser-24 as significant sites of phosphorylation by protein kinase C. J Mol Cell Cardiol. 2005;38:213–218. doi: 10.1016/j.yjmcc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Kooij V, Boontje N, Zaremba R, Jaquet K, Dos RC, Stienen GJ, van der Velden Protein kinase C alpha and epsilon phosphorylation of troponin and myosin binding protein C reduce Ca(2+) sensitivity in human myocardium. Basic Res Cardiol. 2009 doi: 10.1007/s00395-009-0053-z. doi:10.1007/s00395-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leroy J, Abi-Gerges A, Nikolaev VO, Richter W, Lechene P, Mazet JL, Conti M, Fischmeister R, Vandecasteele G. Spatiotemporal dynamics of beta-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: role of phosphodiesterases. Circ Res. 2008;102:1091–1100. doi: 10.1161/CIRCRESAHA.107.167817. [DOI] [PubMed] [Google Scholar]
- 16.Marston SB, de Tombe PP. Troponin phosphorylation and myofilament Ca2+-sensitivity in heart failure: increased or decreased? J Mol Cell Cardiol. 2008;45:603–607. doi: 10.1016/j.yjmcc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez SE, Wu AY, Glavas NA, Tang XB, Turley S, Hol WG, Beavo JA. The two GAF domains in phosphodiesterase 2A have distinct roles in dimerization and in cGMP binding. Proc Natl Acad Sci USA. 2002;99:13260–13265. doi: 10.1073/pnas.192374899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massion PB, Dessy C, Desjardins F, Pelat M, Havaux X, Belge C, Moulin P, Guiot Y, Feron O, Janssens S, Balligand JL. Cardiomyocyte-restricted overexpression of endothelial nitric oxide synthase (NOS3) attenuates beta-adrenergic stimulation and reinforces vagal inhibition of cardiac contraction. Circulation. 2004;110:2666–2672. doi: 10.1161/01.CIR.0000145608.80855.BC. [DOI] [PubMed] [Google Scholar]
- 19.Moens AL, Leyton-Mange JS, Niu X, Yang R, Cingolani O, Arkenbout EK, Champion HC, Bedja D, Gabrielson KL, Chen J, Xia Y, Hale AB, Channon KM, Halushka MK, Barker N, Wuyts FL, Kaminski PM, Wolin MS, Kass DA, Barouch LA. Adverse ventricular remodeling and exacerbated NOS uncoupling from pressure-overload in mice lacking the beta3-adreno-receptor. J Mol Cell Cardiol. 2009;47:576–585. doi: 10.1016/j.yjmcc.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 21.Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation. 2001;103:1649–1655. doi: 10.1161/01.cir.103.12.1649. [DOI] [PubMed] [Google Scholar]
- 22.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson K, Takimoto E, Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy due to pressure-overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagayama T, Zhang M, Hsu S, Takimoto E, Kass DA. Sustained soluble guanylate cyclase stimulation offsets nitric-oxide synthase inhibition to restore acute cardiac modulation by sildenafil. J Pharmacol Exp Ther. 2008;326:380–387. doi: 10.1124/jpet.108.137422. [DOI] [PubMed] [Google Scholar]
- 24.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 25.Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol. 2006;128:3–14. doi: 10.1085/jgp.200509403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pokreisz P, Vandenwijngaert S, Bito V, Van den BA, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Recchia FA, McConnell PI, Bernstein RD, Vogel TR, Xu X, Hintze TH. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog. Circ Res. 1998;83:969–979. doi: 10.1161/01.res.83.10.969. [DOI] [PubMed] [Google Scholar]
- 28.Rozec B, Gauthier C. beta3-adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther. 2006;111:652–673. doi: 10.1016/j.pharmthera.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi I, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) Attenuates Ischemic Cardiomyopathy and Improves Left VentricularFunction in Mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–H1406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 30.Satoh S, Makino N. Intracellular mechanisms of cGMP-mediated regulation of myocardial contraction. Basic Res Cardiol. 2001;96:652–658. doi: 10.1007/s003950170018. [DOI] [PubMed] [Google Scholar]
- 31.Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 32.Shah AM, Spurgeon HA, Sollott SJ, Talo A, Lakatta EG. 8-bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ Res. 1994;74:970–978. doi: 10.1161/01.res.74.5.970. [DOI] [PubMed] [Google Scholar]
- 33.Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res. 2007;101:811–818. doi: 10.1161/CIRCRESAHA.107.154229. [DOI] [PubMed] [Google Scholar]
- 34.Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115:2159–2167. doi: 10.1161/CIRCULATIONAHA.106.643536. [DOI] [PubMed] [Google Scholar]
- 35.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ, Kass DA. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 36.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 37.Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122:216–238. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varghese P, Harrison RW, Lofthouse RA, Georgakopoulos D, Berkowitz DE, Hare JM. beta(3)-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. J Clin Invest. 2000;106:697–703. doi: 10.1172/JCI9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ Res. 1999;84:1020–1031. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Kohr MJ, Traynham CJ, Ziolo MT. Phosphodiesterase 5 restricts NOS3/Soluble guanylate cyclase signaling to L-type Ca2+ current in cardiac myocytes. J Mol Cell Cardiol. 2009;47:304–314. doi: 10.1016/j.yjmcc.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wattanapermpool J, Guo X, Solaro RJ. The unique amino-terminal peptide of cardiac troponin I regulates myofibrillar activity only when it is phosphorylated. J Mol Cell Cardiol. 1995;27:1383–1391. doi: 10.1006/jmcc.1995.0131. [DOI] [PubMed] [Google Scholar]
- 42.Wolska BM, Vijayan K, Arteaga GM, Konhilas JP, Phillips RM, Kim R, Naya T, Leiden JM, Martin AF, de Tombe PP, Solaro RJ. Expression of slow skeletal troponin I in adult transgenic mouse heart muscle reduces the force decline observed during acidic conditions. J Physiol. 2001;536:863–870. doi: 10.1111/j.1469-7793.2001.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu AY, Tang XB, Martinez SE, Ikeda K, Beavo JA. Molecular determinants for cyclic nucleotide binding to the regulatory domains of phosphodiesterase 2A. J Biol Chem. 2004;279:37928–37938. doi: 10.1074/jbc.M404287200. [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101:465–474. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circ Res. 2007;101:377–386. doi: 10.1161/CIRCRESAHA.106.145557. [DOI] [PubMed] [Google Scholar]
- 46.Zaccolo M. Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol. 2006;85:693–697. doi: 10.1016/j.ejcb.2006.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.