Abstract
An echogenic, intravenous drug delivery platform is proposed in which an encapsulated chemotherapeutic can travel to a desired location and drug delivery can be triggered using external, focused ultrasound at the area of interest. Three methods of loading poly lactic acid (PLA) shelled ultrasound contrast agents (UCA) with doxorubicin are presented. Effects on encapsulation efficiency, in vitro enhancement, stability, particle size, morphology and release during UCA rupture are compared by loading method and drug concentration. An agent containing doxorubicin within the shell was selected as an ideal candidate for future hepatocellular carcinoma studies. The agent achieved a maximal drug load of 6.2 mg Dox/g PLA with an encapsulation efficiency of 20.5%, showed a smooth surface morphology and tight size distribution (poly dispersity index = 0.309) with a peak size of 1865 nm. Acoustically, the agent provided 19 dB of enhancement in vitro at a dosage of 10 µg/ml, with a half life of over 15 mins. In vivo, the agent provided ultrasound enhancement of 13.4 ± 1.6 dB within the ascending aorta of New Zealand rabbits at a dose of 0.15 ml/kg. While the drug-incorporated agent is thought to be well suited for future drug delivery experiments, this study has shown that agent properties can be tailored for specific applications based on choice of drug loading method.
Keywords: Ultrasound contrast agents, poly lactic acid, doxorubicin, drug-loaded, echogenicity, double emulsion
Introduction
Success of many traditional chemotherapeutics is tempered by high systemic and organ toxicity producing relatively low drug levels at the area of need. This paper focuses on a relatively hydrophilic chemotherapeutic, doxorubicin (Dox) that has successfully been used to treat liver, breast, ovarian, and lung cancer. However, administration of Dox has also been linked to decreases in white blood cell counts, alopecia and cardiotoxicity including heart arrhythmias, and congestive heart failure, severely limiting its usage [1,2] This lack of specificity has lead to development of targeted or “smart” delivery systems that increase the delivery efficiency of chemotherapeutics. Pegylated liposomal Dox is currently FDA approved (Doxil®; Johnson & Johnson, Langhorne, PA). However despite a lack of specific cardiotoxicity, other limiting effects have been reported including acute infusion-related toxicity, stomatitis, myelosuppression, and dermatologic effects such as palmar-plantar erythrodysesthesia [2,3]. An alternative approach in development is encapsulation of chemotherapeutics within ultrasound (US) sensitive carriers and triggering drug release at a desired location using external, focused US.
Ultrasound contrast agents (UCA) consist of gas bubbles encapsulated with an outer shell for stability. The compressibility and impedance mismatch of the gas within these agents result in acoustic backscatter, increasing the overall contrast of the US image [4]. These agents must be smaller than 8 µm in order to pass through the capillary beds, and have been fabricated using a variety of lipids, surfactants, and polymers, and filled with different gases including air, perfluorocarbons, and sulfur hexafluoride [5]. Various therapeutic strategies for loading phospholipid-based UCA with drugs have been developed and are well reviewed by Unger et al. [6].
A variety of studies have shown encapsulation of Dox to be a more efficient form of delivery. As mentioned above, within the clinic, liposomal encapsulated Dox, Doxil ® has already proven successful in various cancers, showing equivalent efficacy to Dox, while limiting side effects [7–9]. Current research efforts now focus on both encapsulation and controlling the release of Dox. Tan et al. were able to successfully encapsulate Dox within double walled microspheres of both poly lactic acid (PLA) and poly lactic-co-glycolic acid (PLGA), reducing the burst effect and controlling drug release by varying particle size and wall thickness [10]. In terms of US triggered delivery, Dox has been shown to be successfully released from stabilized micelles upon sonication at 70 kHz, at an average intensity of 0.38 W/cm^2 in vitro [11]. Gao et al. showed that Dox loaded, polymeric micelles combined with 20 seconds of US resulted in a 34% decrease in ovarian cancer tumor growth in mice compared to fee Dox [12]. Lentacker et al. formulated Dox-liposome loaded UCA and showed increased melanoma cell nucleic uptake and cell death when insonated in vitro compared to Dox-liposomes alone [13]. Kooiman et al. have reported on encapsulating sudan black (a hydrophobic drug model) using hexadecane oil as a drug-carrier reservoir combined with an air core inside of a polymer shelled UCA [14]. This group has also shown similar agents loaded with paclitaxel (a common, hydrophobic chemotherapeutic) capable of delivering chemotherapeutics in vivo, significantly slowing tumor growth of MC-38 mouse colon adenocarcinomas after sonication at 1 MHz using a mechanical index (MI) of 0.7 [15]. The stability and larger (100–400 nm) shell thickness of these and other polymer shelled agents compared to lipid UCA may be ideal for future drug delivery applications.
PLA UCA have previously been developed within our laboratory [16]. These agents provide over 20 dB enhancement both in vitro and in vivo [16], and have also been conjugated with breast cancer targeted ligands [17]. Additionally, we have shown that these agents significantly reduce in size to below 400 nm (presumably due to US-induced fragmentation) [18]. It is believed these resulting particles have the potential of exiting the leaky tumor vasculature, subsequently providing a sustained, intratumoral release during degradation. This reduction in size is believed to be responsible for the nearly 110% increase in delivery efficiency demonstrated in a VX2 rabbit liver cancel model when the platform was activated with 5 MHz Doppler US at a MI of 1.0 for 20 minutes [18].
This paper compares three methods of loading these agents with Dox. Drug payload, US enhancement, stability, size and morphology, and drug release during US triggered destruction are all considered when selecting an appropriate loading method for future drug delivery studies.
Materials and Methods
Materials
Poly lactic acid (PLA) (100 DL Low IV (low viscosity), MW = 83 KDa) was purchased from Lakeshore Biomaterials (Birmingham, AL). Dox, isopropyl alcohol, dimethyl sulfoxide (DMSO), and camphor were all purchased from Sigma-Aldrich (St.Louis, MO). Ammonium carbonate was purchased from J.T. Baker (Phillipsburg, NJ). Poly (vinyl alcohol) (PVA), 88% mole hydrolyzed, with a MW of 25 KDa was purchased from Polysciences (Warrington, PA). All other chemicals were analytical grade from Fisher Scientific (Springfield, NJ), and used as received.
Sample Preparation
Drug loaded UCA were prepared based on a previously developed method for producing polymer shelled UCA [16]. Using this double emulsion, 0.5 g of PLA and 0.05 g camphor was dissolved in 10 ml of methylene chloride. After fully dissolving the polymer, 1 ml of 0.4 M ammonium carbonate was added and the mixture sonicated at 20 kHz using 110 Watts of applied power for 30 seconds at 3 seconds on, 1 second off (Misonix Inc. CL4 tapped horn probe with 0.5” tip, Farmingdale, NY) while suspended in an ice bath. The resulting (W/O) emulsion was added to 50 ml of 5 % PVA and homogenized for 5 minutes at 9500 rpm (Brinkmann Instruments, Westbury, NY). After homogenization, the resulting (W/O)/W emulsion was added to 100 mL of 2% isopropyl alcohol. Samples were then continually stirred for 1 hour to evaporate any organic solvent. Following evaporation, UCA were collected using centrifugation (1500 g for 5 mins) and washed three times with 5 mL of hexane. After evaporation of residual hexane the capsules were flash frozen and lyophilized for 48 hours. As the agent undergoes freeze drying, ammonium carbonate and camphor sublime out of the capsule, leaving a void in their place. This hollow core then fills with gas (in this case air) when later exposed to atmospheric pressure.
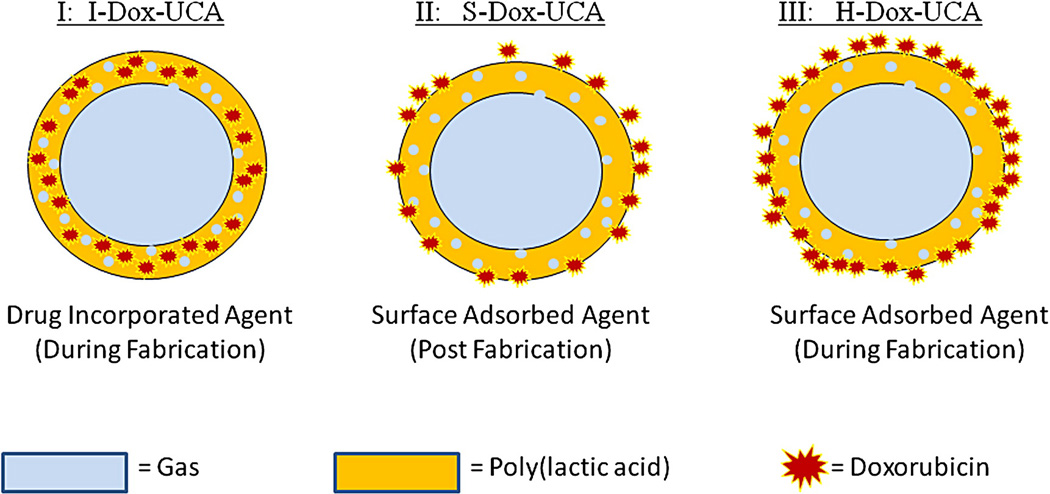
Three methods of drug loading have been developed within our laboratory, resulting in PLA UCA with drug either adsorbed to the surface or incorporated within the shell of the agent. These methods are summarized in Fig. 1. The first method (I) involves addition of Dox during the primary emulsion as the capsules are fabricated, resulting in drug incorporated within the shell of the agent (I-Dox-UCA). The second method (II) results in the addition of Dox to the UCA as the nascent agent is washed with hexane during fabrication (H-Dox-UCA). This agent is then washed in deionized water before being freeze dried as discussed above. The final method of drug loading (III) involved contacting a suspension of pre-fabricated UCA with a solution of free Dox in PBS at 4°C for 24 hours (S-Dox-UCA). After 24 hours, the UCA is again collected by centrifugation, washed with deionized water, and freeze dried. This process has been previously optimized in terms of temperature and contact time and results in surface coated Dox-UCA due to the electrostatic attraction between the drug and polymer shell [19].
Figure 1.
Representation of the three drug loading methods explored: I:drug incorporation during fabrication (I-Dox-UCA); II: surface adsorption post fabrication (S-Dox-UCA); and III: surface adsorption during fabrication (H-Dox-UCA).
Varying loading concentrations of Dox between 0.1 to 4% (weight Dox/weight PLA) were added using each of the three methods described above. All samples were prepared in triplicates and stored until use in a desicator at 4°C and covered in foil to avoid photo bleaching of Dox.
Determination of Drug Payload and Encapsulation Efficiency
Amounts of adsorbed and encapsulated Dox were determined by dissolving dry agent in DMSO and measuring fluorescence. Two mg of dry agent was added to 2 ml DMSO and vortexed for 30 seconds to dissolve the polymer. Fluorescence of the mixture was then read using a Tecan fluorimeter (Männedorf, Switzerland) at an excitation wavelength of 495 nm and an emission wavelength of 585 nm. Dox concentration was then calculated based on a standard curve of known amounts of Dox in DMSO. Encapsulation efficiency was defined as:
| (1) |
Imaging and Particle Sizing
All three drug loaded agents were imaged using an environmental scanning electron microscope (SEM) (FEI XL30, Hillsboro, OR). Dry agent was sputter coated with platinum for 30 seconds prior to imaging. Images were taken at varying magnifications at an accelerating voltage of 10.0 kV, with a working distance of 8.9mm. All SEM imaging was done at the Drexel University Materials Characterization Facility.
Confocal microscopy was performed using an Olympus IX81 microscope run by Olympus Fluorview version 1.7b (Olympus Corporation, Tokyo). Two hundred micrograms of dry agent was suspended in 200 µL of PBS, placed on a glass slide and covered with a cover slip. Dox within the agent was imaged by excitation using a FITC filter and emission using a TRITC filter. Images were obtained using a 100X lens with digital zoom. Proper gain levels were determined automatically using the Fluorview software.
Particle sizing was done using a Malvern Nano ZS (Worcestershire, United Kingdom). One mg of dry agent was suspended in PBS and measured in triplicate. Particle sizes were reported as peak % number.
In vitro Acoustic Testing
Acoustic testing in vitro was performed to determine the agent’s ability to provide US contrast, while also measuring its stability during insonation. A Panametrics (Waltham, MA) 5 MHz, 12.7 mm diameter transducer with −6 dB bandwidth of 91% and focal length of 50.8 mm was held in a 37°C water bath filled with 18.6 MΩ-cm deionized water and focused through the acoustically transparent window of the sample holder. A pulser/receiver (5072 PR Panametrics, Waltham, MA) connected to the transducer was used to generate an acoustic pulse with pulse repetition frequency (PRF) of 100 Hz, resulting in a peak positive pressure amplitude of 0.69 MPa and a peak negative pressure amplitude of 0.45 MPa at the focus (MI=0.20), determined using 0.5 mm polyvinylidene fluoride needle hydrophone (Precision Acoustics, Dorset, UK). Reflected signals were measured using the transducer and amplified 40 dB before being read by an oscilloscope (Lecroy 9350 Chestnut Ridge, NY). Data acquisition and processing was done using LabView 7 Express (National Instruments, Austin, TX). Previous studies have shown similar unloaded agent displays resonance behavior (maximal contrast enhancement) within the 6 dB bandwidth of the 5 MHz transducer [20], and these findings were also consistent with the drug loaded UCA (results not shown).
Backscattering enhancement was measured as a function of UCA concentration and used to gauge both the agent’s ability to provide enhancement as well as its sensitivity to US for future drug delivery applications. Three mg of dry UCA was suspended in 800 µl of PBS by vortexing briefly. Samples were then pipetted into the sample holder containing 50 mL of continually stirred PBS (pH 7.4, 37 °C). UCA was allowed to mix for 10 seconds to ensure a homogenous media before measurement. Enhancement in relationship to a baseline reading was then measured for each dosage ranging from 0–16 µg/ml in 1.5 µg/ml increments.
UCA stability under ultrasonic insonation was measured to determine the agent’s ability to provide contrast throughout the duration of an US scan (generally around 15–20 minutes depending on the application). Four µg/ml of UCA was added to the sample holder and continually insonated and stirred with the setup described above. Enhancement was measured over the course of 15 mins and normalized with respect to the initial value to allow for inter sample comparison. All measurements were repeated in triplicate for each of three separate samples (9 readings, n=3).
Determination of Drug Disposition during US Triggered UCA Destruction
Drug release was measured for each agent by suspending 10 mg of agent in 50 ml of phosphate buffered saline (PBS) while stirring. After immediate sampling for zero time release, the solution was insonated using the setup described in the in vitro acoustic testing subsection at a PRF of 100 Hz and peak positive pressure amplitude of 1.68 MPa and peak negative pressure amplitude of 0.94 MPa (MI=0.42) for 20 mins. After 20 mins of insonation, in vitro US enhancement became undetectable, indicating complete microbubble destruction. One ml of the solution was sampled at t = 0 and then every two minutes for 20 mins while stirring. Immediately after sampling, samples were centrifuged at 7500 g for 5 mins to remove polymer and the fluorescence of the supernatant was read. Controls were also performed with no insonation. Free Dox was then calculated using a calibration curve of Dox in PBS, and expressed as a percent of the total in solution.
In vivo Acoustic Testing
The animal studies described here were carried out in an ethical and humane fashion under supervision of a veterinarian, and the Thomas Jefferson University’s Animal Care and Use Committee approved all protocols. In vivo dose response testing was performed on two New Zealand rabbits (mean weight 3 kg). The rabbits were sedated with 35 mg/kg ketamine and 3.5 mg/kg xylazine. Pulsed Doppler US imaging of the ascending aorta was performed using a Sonix RP US scanner with an L14-5 transducer (Ultrasonix, Richmond BC, Canada). Spectral waveforms were obtained using a center frequency of 5.0 MHz, PRF of 3.3 kHz, depth of 2.5 cm, and power of −8 dB and RF data were acquired at a sampling frequency of 40 MHz. Dry agent was suspended in phosphate buffered saline at a concentration of 0.04 g/ml. UCA was injected through an angiography catheter in the left ear vein and flushed with 5 ml of saline. Post injection, the waveform was saved for a 10–20 second period and peak intensities were compared to baseline levels off line.
Power Doppler US images were also obtained from the kidney of a Sprague Dawley rat. The rat (250 g) was sedated with 2–4% isoflurane. Four mg/kg of UCA was injected through the tail vein and flushed with roughly 0.2 cc saline. Power Doppler images were recorded using a Toshiba Aplio 80 scanner (Toshiba America Medical Systems, Tustin, CA). Imaging was performed at a center frequency of 7.5 MHz, PRF of 14.1 kHz.
Statistical Analysis
Statistically significant differences for multiple groups were determined using a one way ANOVA with a Newman-Keuls post test and individual groups were compared using a Student’s t test. All testing was done using Prism 3.0 (GraphPad, San Diego, CA). Statistical significance was determined using α = 0.05. Error bars were displayed as standard error about the mean.
Results
Doxorubicin Payload and Encapsulation Efficiency
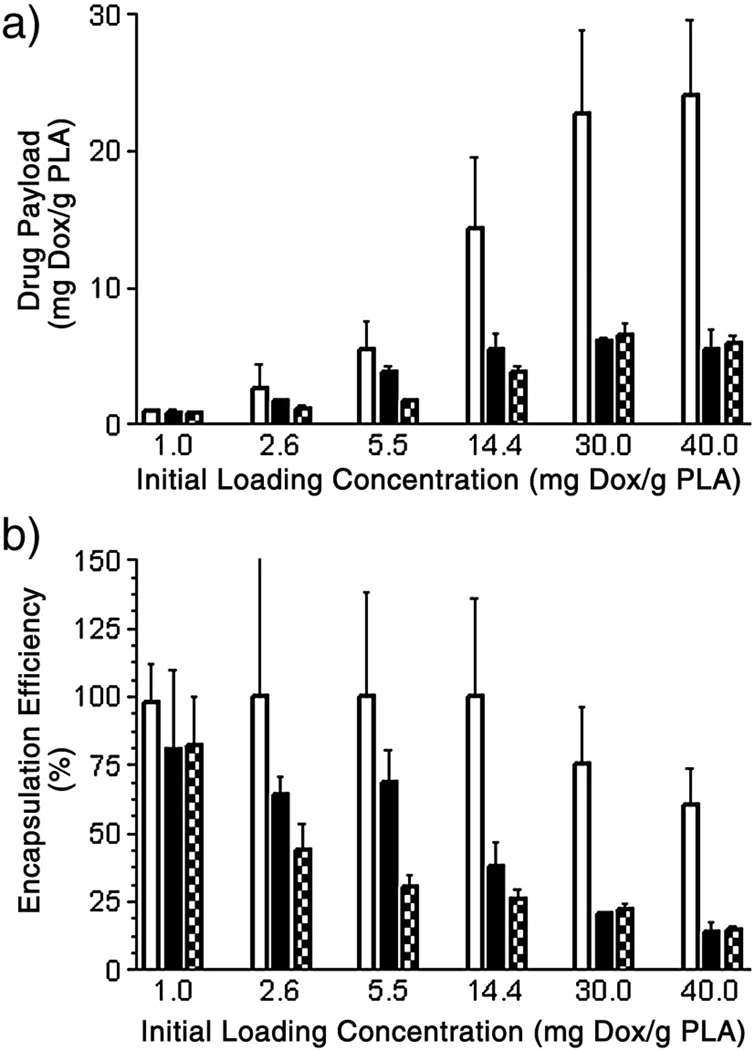
Both final drug payload and encapsulation efficiency are shown below in Fig. 2 for each of the three loading methods over a range of loading concentrations.
Figure 2.
Final drug payload as a function of loading method and initial concentration (a), and corresponding encapsulation efficiency (b). H-Dox-UCA =  , I-Dox-UCA =
, I-Dox-UCA =  , S-Dox-UCA =
, S-Dox-UCA =  . H-Dox-UCA approached a maximal drug load of 24.1 mg Dox/g PLA (encapsulation efficiency of 60.2%) at an initial loading concentration of 40.0 mg Dox/g PLA. Both the I-Dox-UCA and S-Dox-UCA samples reached peak drug payloads of 6.2 and 6.5 mg Dox/g PLA (encapsulation efficiencies of 20.5 and 21.9%) respectively at an initial loading concentration of 30.0 mg Dox/g PLA.
. H-Dox-UCA approached a maximal drug load of 24.1 mg Dox/g PLA (encapsulation efficiency of 60.2%) at an initial loading concentration of 40.0 mg Dox/g PLA. Both the I-Dox-UCA and S-Dox-UCA samples reached peak drug payloads of 6.2 and 6.5 mg Dox/g PLA (encapsulation efficiencies of 20.5 and 21.9%) respectively at an initial loading concentration of 30.0 mg Dox/g PLA.
UCA with Dox loaded during the hexane washing process showed significantly higher drug payload and encapsulation efficiency (p=0.0038). H-Dox-UCA samples showed a maximal drug payload of 24.1 mg Dox/g PLA at the highest initial loading concentration (40 mg Dox/g PLA). This corresponds to an encapsulation efficiency of 60.2%. Alternatively, both I-Dox-UCA and S-Dox-UCA samples reached a peak drug payload of 6.2 and 6.5 mg Dox/g PLA respectively, with corresponding encapsulation efficiencies of 20.5 and 21.9%. Thus, in terms of a drug carrier, the hexane adsorbed samples proved superior in both payload and encapsulation efficiency.
Particle Size and Surface Morphology
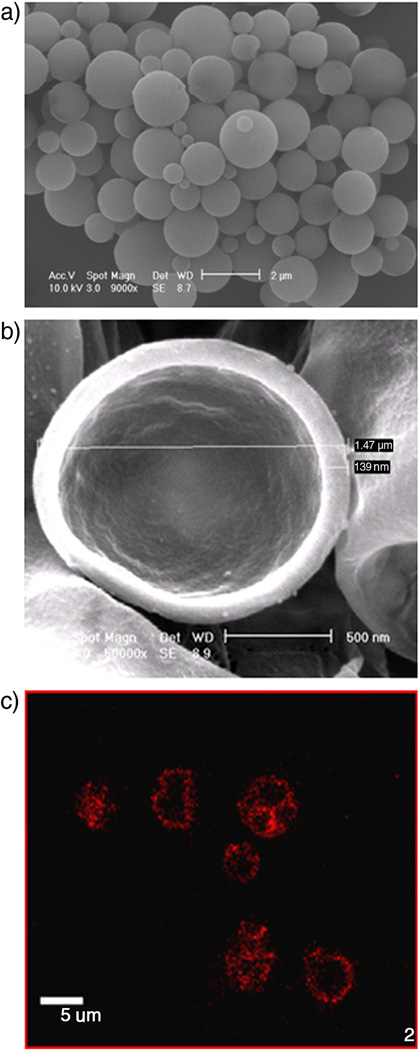
Effects of drug loading methodologies on both the surface morphology and particle size were examined. All three methods resulted in smooth, spherical particles (figure 3a, complete set not shown). When suspended in 37°C PBS, sonicated at 5 MHz, 1.68 MPa positive peak pressure for 20 minutes, and re-freeze dried, ruptured particles displayed a hollow core with shell thickness of roughly 10% of the particle diameter, at less than 1.5 nm when viewed by SEM. Confocal microscopy, which allowed visualization of Dox within larger UCA appeared spherical when viewed at a cross section of the UCA, indicating the presence of Dox within or on the shell. An example of these findings is shown in Fig 3.
Figure 3.
Images of drug loaded agents. a) SEM after fabrication (Mag. = 9000x, Size bar = 2 µm). b) SEM after sample ruptured by sonication (Mag. = 50000X, Size bar = 500 nm). c) Fluorescent confocal micrograph showing Dox within the agent’s shell (Mag=100X, Size bar= 5 µ, (only larger UCA are visible using fluorescent microscopy)). Agent shown is a PLA agent with 3% (g Dox/g PLA) loaded within the shell of the agent. Morphology, core, and shell thicknesses were consistent with all three loading methods and all drug payloads.
Particle size and polydispersity index (PDI) were both measured to determine if Dox loading in the shell of UCA surface significantly altered the size characteristics of the sample. These findings are summarized in Table 1 for 3% (g Dox/g PLA) loading and are consistent with all loading concentrations tested, indicating Dox loading concentration has no effect on either particle size or PDI.
Table 1.
Effects of Dox Loading Method on Particle Size and PDI
| Unloaded UCA | H-Dox-UCA | I-Dox-UCA | S-Dox-UCA | |
|---|---|---|---|---|
| Particle Size (nm) |
1692 +/− 779 | 1734 +/− 1403 | 1865 +/− 1074 | 2206 +/− 2039 |
| PDI | 0.212 +/−0.051 | 0.254 +/−0.104 | 0.309 +/− 0.102 | * 0.412 +/−0.108 |
No statistically significant changes in particle size were detected among loading groups with peak particle sizes ranging from 1.7 µm to 2.2 µm. Both the H-Dox-UCA and I-Dox-UCA groups showed a PDI similar to that of the unloaded agent, indicating no changes in the size distribution had taken place. However, the S-Dox-UCA did show a significantly higher PDI
(*p=0.0441 relative to the unloaded control).
In vitro Acoustic Stability and Back Scattering Enhancement
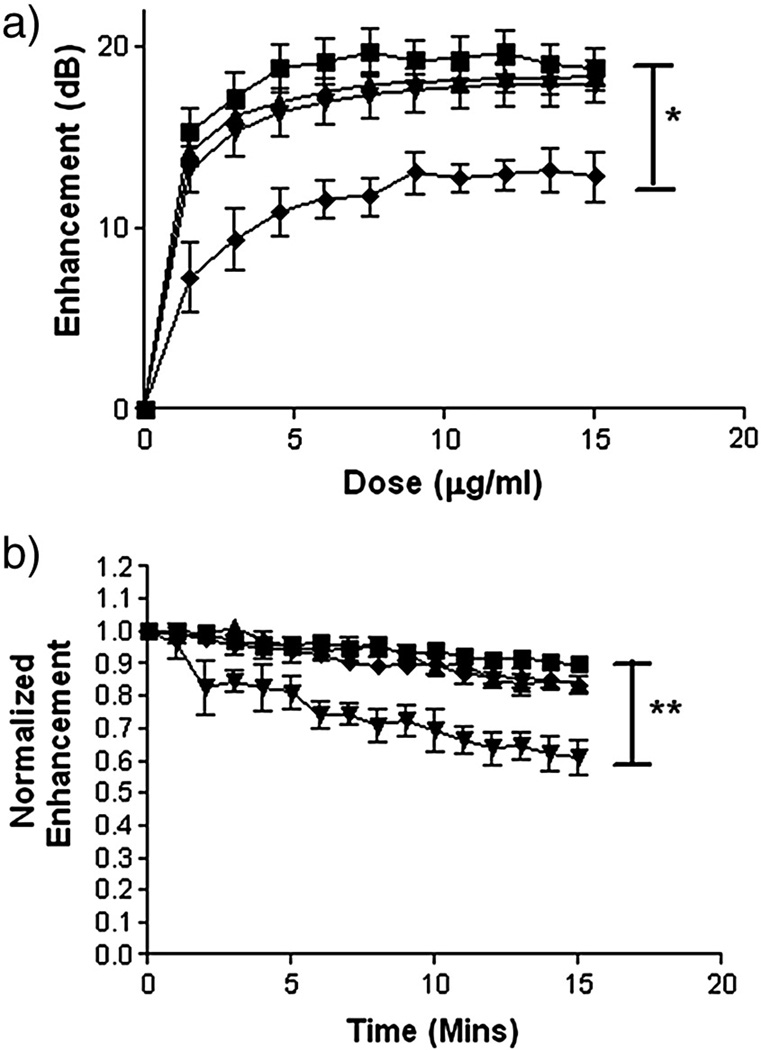
Effects of both Dox loading method and concentration on the agent’s acoustic properties were examined. Fig 4 shows the results of both backscattering enhancement as a function of UCA dose (a) and acoustic stability over time (b). Data is presented for 3% loading (weight Dox/weight PLA), however results were consistent for all loading concentrations (results not shown).
Figure 4.
Effects of 3% Dox loading on back scattering enhancement (a) and stability (b) in vitro by each of the three drug loading methods (-■- unloaded UCA, -▲- H-Dox-UCA, -▼- I-Dox-UCA, -♦- S-Dox-UCA). A significant decrease in in vitro enhancement was seen in S-Dox-UCA relative to the unloaded control (* p=0.0062), while a significant decrease in stability was measured for I-Dox-UCA relative to the unloaded control (** p<0.0001).
No statistically significant changes in backscattering (a) were measured for either H-Dox-UCA or I-Dox-UCA relative to the unloaded control, with all three agents reaching enhancements of 18–19 dB at doses of 7.5 µg/ml and above. However a significant decrease in enhancement was seen in S-Dox-UCA relative to the unloaded control (p=0.0062), with samples reaching a maximal enhancement of 12.5 dB at a dose of 9.0 µg/ml.
Effects of drug loading on the agent’s stability during insonation were measured and are shown in Fig 4 (b). No significant decreases in stability were seen for either adsorption method relative to the unloaded control, only losing roughly 15% of the agent’s original enhancement after 15 mins. However, a statistically significant (p<0.0001) decrease was seen in stability of the I-Dox-UCA relative to the unloaded control, with the incorporated agent losing roughly 40% of its original enhancement after 15 mins of insonation.
Effect of loading method on Drug Release during US Triggered UCA Destruction
Dox loaded UCA were suspended in stirred PBS to determine the effect of loading method on the degree of release during UCA destruction. After an initial burst upon introduction into the release medium, all samples showed no statistically significant release of Dox during UCA destruction (p>0.05 relative to the corresponding uninsonated controls). These results are shown in Fig 5. While US triggered release may be desirable for some delivery applications, this lack of release is ideal for a platform designed to deposit sustained release drug-polymer fragments at a desired release site.
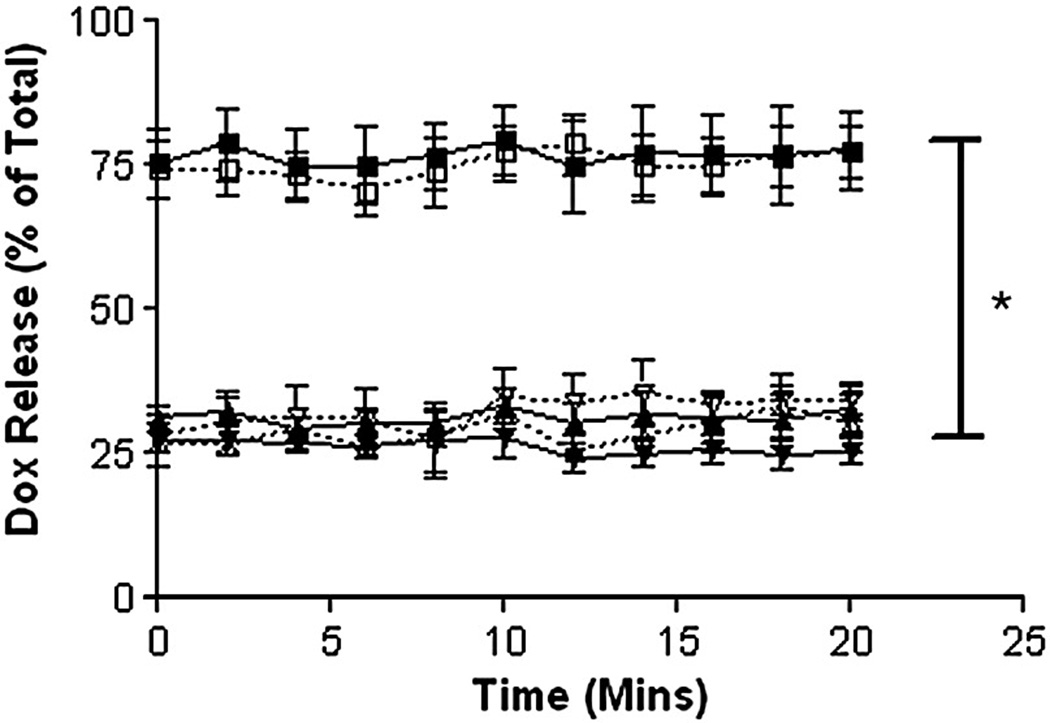
Figure 5.
Effects of loading method and insonation on drug release (-■- H-Dox-UCA, -▲- S-Dox-UCA, -▼- I-Dox-UCA, -□- H-Dox-UCA + US, -△- S-Dox-UCA + US, -▽- I-Dox-UCA + US,). H-Dox-UCA samples showed significantly more burst (*p<0.0001) relative to the S-Dox-UCA or I-Dox-UCA samples (78% vs. 27% of the total Dox within the UCA). No samples showed any significant release when insonated (p>0.05). Data shown is for 3% Dox loading, but consistent for all drug loading concentrations (n=3, error bars=SEAM).
Both S-Dox-UCA and I-Dox-UCA showed an immediate burst of roughly 27% of the total encapsulated Dox and no further release was detected over the 20 minute insonation period. However, the H-Dox-UCA showed a significantly higher burst release (*p<0.0001) than the incorporated or cold-adsorbed samples, immediately releasing 78% of the total Dox and remaining constant.
In vivo Imaging of Agent
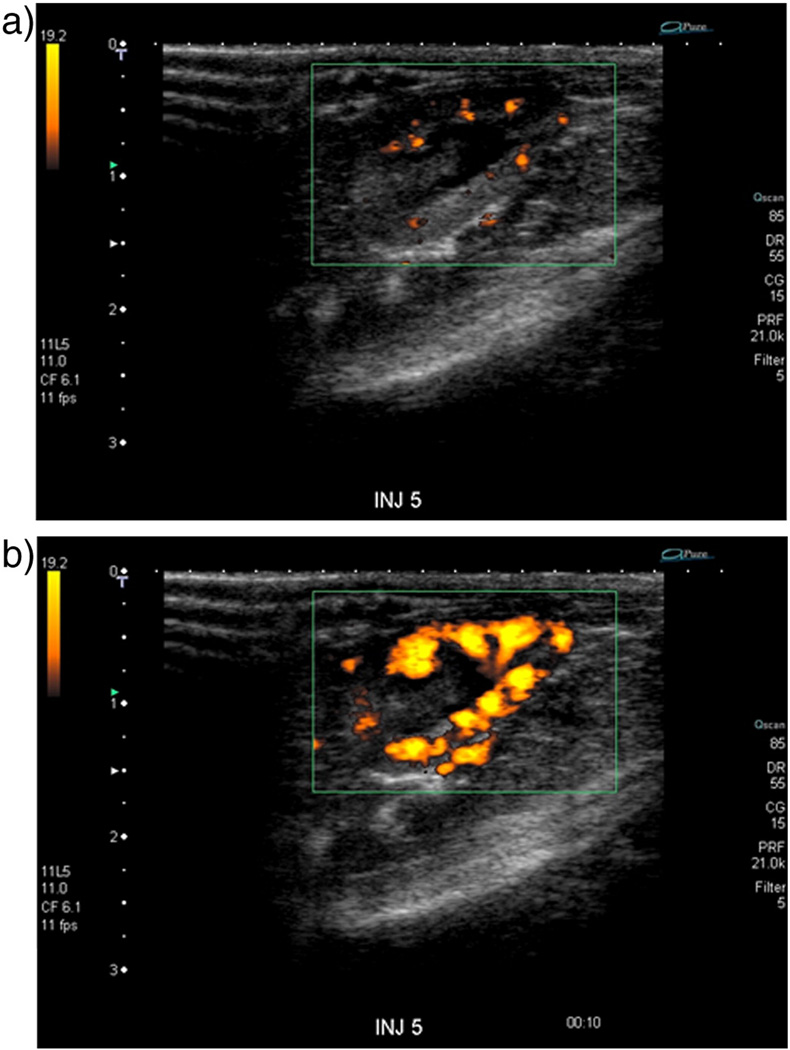
The I-Dox-UCA agent was chosen for in vivo imaging experiments due to its high enhancement and low burst effect in vitro. Figure 6 shows an example of a Power Doppler image of a rat kidney prior to (a) and 8 sec post injection (b) of 0.1 ml/kg of I-Dox-UCA. The agent is clearly detectable and provides adequate contrast of the rat kidney using Power Doppler Imaging for roughly 45 seconds post injection.
Figure 6.
Doppler US scans of a rat kidney (a) pre injection, and (b) 8 sec post injection of 0.1 ml/kg of I-Dox-UCA (3% Dox loading). The agent was clearly detectable in vivo and provides image enhancement for roughly 45 seconds.
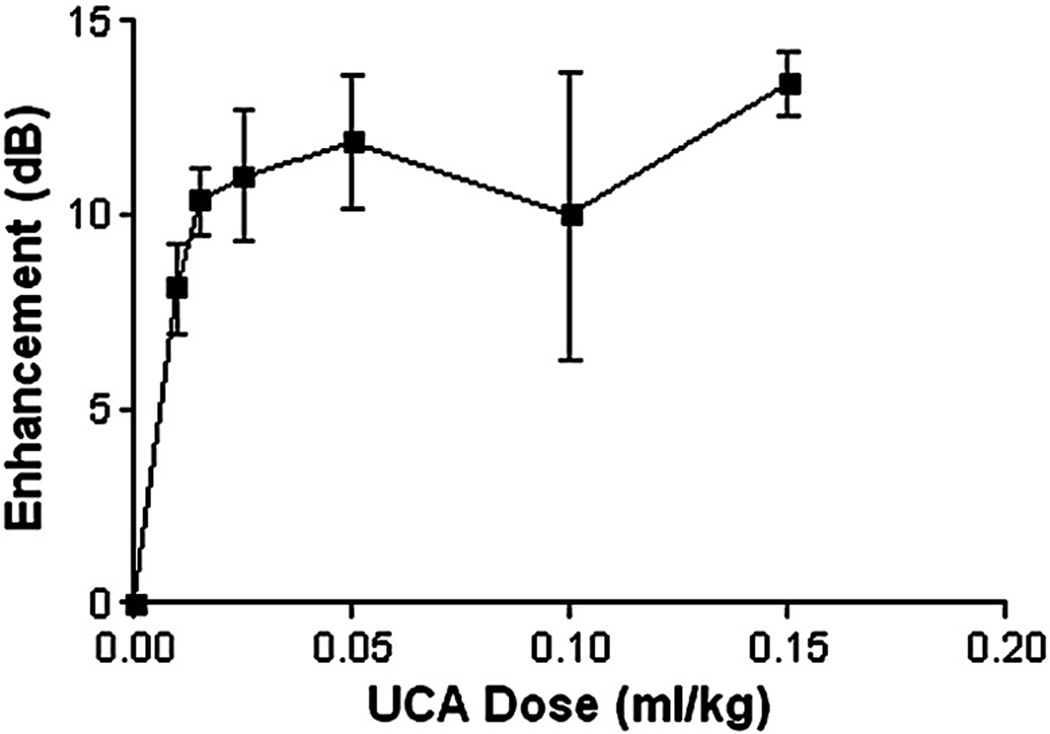
Results of in vivo dose response curves monitored over the ascending aorta of a New Zealand rabbit are shown in Fig 7. UCA with Dox incorporated within the shell showed improved enhancement within the ascending aorta of a New Zealand rabbit at all doses, with a peak enhancement of 13.4 dB at a dosage of 0.15 ml/kg. Peak enhancement in vivo was 4.6 dB lower than in vitro results (Fig. 4), and this was found to be statistically significant (p=0.0116).
Figure 7.
In vivo dose response of 3% I-Dox-UCA in the ascending aorta of two New Zealand rabbits. The agent showed marked enhancement at all dosages, with a peak US enhancement of 13.4 dB at a dose of 0.15 ml/kg and lasting roughly 5 mins.
Discussion
In their recent review of drug loaded UCA, Lentacker et al. [22] identified the first report of the use of US in drug delivery as 1985 by Miyazaki and co workers [23]. Since that time, interactions between US and UCA to both influence drug uptake [24], and target gene and drug delivery has become increasingly studied and extensively reviewed [22, 25]. The prevailing methodology is to load a drug into a microbubble possessing a self-assembled, usually phospholipid surfactant-based shell [26]. Upon interaction with US these vesicles rupture, instantly releasing all their contents. Our approach has been to load drug into a polymeric shelled microbubble, where drug-polymer, and US-polymer interactions are distinctly different from those encountered in self-assembled vesicles. Here, we investigate the interplay between drug loading methods and UCA performance in the areas of both echogenicity (ability to provide good image enhancement), and triggered drug delivery.
In terms of a drug carrier, the H-Dox-UCA proved superior in both payload and encapsulation efficiency. By adding drug in the hexane wash, the drug has the opportunity to adhere to the capsule surface before it has completely hardened. This could account for the high payload and encapsulation efficiency. In terms of how this method affects the final capsule population, it is not surprising that the mean size, and PDI are very similar to values found for capsules made by incorporation of drug during the emulsion steps and to drug-free control, since very minor process modifications have been employed. However, the S-Dox-UCA did show a significantly higher PDI (p=0.0441 relative to the unloaded control). While the mechanism of this change is not fully understood, they are believed to be due to both UCA swelling and hydrolytic degradation in the aqueous phase during drug adsorption and also the necessity for a second lyophilization step with the attendant expansion of the suspending liquid during freezing and subsequent exposure to low pressures. The possibility of bubble-bubble attachment with Dox as a linker was examined using microscopy, but bubbles remained unattached after resuspension for all three loading methods. There was also no visible distress upon injection into rabbits, indicating that the mean bubble size did not increase beyond the limits of the pulmonary bed (6–8µm).
The pattern continues into the acoustic properties of the variously loaded UCA. The drop off in enhancement exhibited by S-Dox-UCA is again believed to be the result of hydrolytic damage during the adsorption phase and the second freeze drying process. Additional freeze drying is believed to destroy some UCA and alter the shell properties of others, resulting in lower overall enhancement. These results are consistent throughout Table 1.
The results obtained when plotting the normalized stability (Fig. 4b) have led us to our concept of drug delivery via US initiated nano shards [18]. Once normalized to account for initial differences in enhancement values, the results revealed that the stability of I-Dox-UCA in an US beam differed significantly (p < 0.0001) from that of the other three preparations (unloaded, H-Dox-UCA and S-Dox-UCA). This decrease in UCA stability is believed to be due to the introduction of additional wall defects into the shell of the UCA, making it more susceptible to both hydrolysis and US-mediated destruction. While this decrease in stability during insonation may inhibit the agent’s ability to provide sustained contrast, it may also prove advantageous in future drug delivery situations.
In vivo enhancement of I-Dox-UCA was found to be significantly lower than values measured in vitro. Additionally, peak enhancement was roughly 6 dB lower than a comparative study in which in vivo enhancement of unloaded PLA UCA were measured in the distal aorta below the renal arteries in New Zealand rabbits [21]. This decrease in enhancement is believed to be due to agent’s decreased stability as shown in fig 4. Decreased stability of the agent due to creation of additional voids and point defects would result in an agent that is more susceptible to destruction by in vivo forces during circulation. Thus as a result of this loss in stability, fewer UCA may reach the imaging location intact compared to an unloaded agent or within an in vitro setup, reducing overall contrast enhancement. However, it is important to note that the agent is still clearly detectable at all dosages and reactive to US for future triggering applications.
All the loading methods resulted in a burst of drug release upon suspension in 37°C PBS. Since the various preparations were all washed extensively prior to freeze drying, this burst must be caused by movement of the drug towards the surface of the capsule as the various components (water camphor and ammonium carbonate) sublime off under vacuum. While even the low levels of initially released Dox are not ideal, it is important to note that the resulting level of free drug is still substantially lower than traditional chemotherapy. In a preliminary delivery experiment using I-Dox-UCA in vivo, peak serum levels reached 3.9 pM and became undetectable 15 minutes after administration [18]. Further, any free drug will most likely be preferentially uptaken by the tumor via US-assisted drug uptake [27].
These results highlight the importance, when dealing with multiple parameters, of the tradeoff that may be required when balancing excellent properties in one parameter against poorer outcomes in another. The loading method which had shown the highest encapsulation efficiency and smallest loss in acoustic performance (H-Dox-UCA) also resulted in a burst effect three times greater than the other methods. In the case of loading at the hexane wash stage, the capsules are not completely hardened, but it would appear that the Dox does not have time to penetrate deeply into the shell of the nascent capsules. This results in the weak interactions between the Dox and UCA. This method would be chosen in situations where an initial input of drug (75% of the payload) is desired followed by a sustained release (the remaining 25%) as the polymer biodegrades. However, this was not our emphasis and as a result, incorporation of Dox within the shell of the UCA was selected for future work. This method results in highly echogenic, drug loaded capsules that maintains the majority of the drug payload for future US triggered delivery studies.
We have recently shown that US triggered destruction of this agent results in the in situ generation of Dox loaded nanoparticles, capable of exiting the tumor vasculature into the interstia [18]. Additionally, we have shown this platform to be capable of inducing cell death in vitro after sonication [28]. Thus, in vivo Dox-UCA destruction results in generation of particles capable of exiting tumor vasculature and subsequently killing cells. Future work will investigate biodistribution of Dox after platform administration as well as overall efficacy.
Conclusions
Several methods of loading polymer shelled UCA with Dox have been developed. These methods include two forms of surface coating, and one form of drug incorporation within the shell of the agent. The agent with Dox incorporated within the shell of the agent was selected due to its optimal mix of high in vitro enhancement (19 dB), tighter size distribution (PDI=0.309), and low burst effect (27%) relative to the alternative methods. While this agent does show significantly less stability relative to the alternative methods (p<0.0001), this may be ideal for future drug delivery experiments. Future work will examine acoustic parameters and methods for US triggered drug delivery in vitro and in vivo.
Acknowledgments
The authors would like to thank Traci B. Fox and Dan Merton for assistance obtaining the Power Doppler Images and in vivo dose response data. Confocal microscopy was performed with assistance from Steven Kemeny, Drexel University, Dept. Mech. Eng. The authors would also like to thank Toshiba America Medical Systems for equipment support. Funding for this work was provided by The Coulter Foundation and by NIH HLB 52901.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J.R. Eisenbrey, Email: jeisenbrey@gmail.com.
O. Mualem Burstein, Email: odelmb@gmail.com.
R. Kambhampati, Email: rekhakamb@gmail.com.
F. Forsberg, Email: flemming.forsberg@jefferson.edu.
J-B. Liu, Email: ji-bin.liu@jefferson.edu.
M.A. Wheatley, Email: wheatley@coe.drexel.edu.
References
- 1.Singal P, Iliskovic N. Doxorubicin-induced cardiomyopathy. New Engl. J. Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 2.Alberts DS, Garcia DJ. Safety aspects of pegylated liposomal doxorubicin in patients with cancer. Drugs. 1997;54:30–35. doi: 10.2165/00003495-199700544-00007. [DOI] [PubMed] [Google Scholar]
- 3.Goram AL, Richmond PL. Pegylated liposomal doxorubicin: tolerability and toxicity. Pharmacotherapy. 2001;21:751–763. doi: 10.1592/phco.21.7.751.34572. [DOI] [PubMed] [Google Scholar]
- 4.Hoff L. Acoustic properties of ultrasonic contrast agents. Ultrasonics. 1996;34:591–593. [Google Scholar]
- 5.Goldberg BB, Raichlen JS, Forsberg F. Ultrasound contrast agents: basic principles and clinical applications. second edition. London: Martin Dunitz; 2001. [Google Scholar]
- 6.Unger EC, Matsunaga TO, McCreery T, Schumann P, Sweitzer R, Quigley R. Therapeutic applications of microbubbles. Eur. J. Radiol. 2002;42:160–168. doi: 10.1016/s0720-048x(01)00455-7. [DOI] [PubMed] [Google Scholar]
- 7.Judson I, Radford JA, Harris M, et al. Randomized phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer. 2001;37:870–877. doi: 10.1016/s0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 9.Muggia FM, Hainsworth JD, Jeffers S, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J. Clin. Oncol. 1997;15:987–993. doi: 10.1200/JCO.1997.15.3.987. [DOI] [PubMed] [Google Scholar]
- 10.Tan EC, Lin R, Wang CH. Fabrication of double-walled microspheres for the sustained release of doxorubicin. J. Colloid Interface Sci. 2003;291:135–143. doi: 10.1016/j.jcis.2005.04.089. [DOI] [PubMed] [Google Scholar]
- 11.Husseini GA, de la Rosa MAD, Gabuji T, et al. Release of doxorubicin from unstabilized and stabilized micelles under the action of ultrasound. J. Nanosci. Nanotechnol. 2007;7:1028–1033. doi: 10.1166/jnn.2007.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J. Control. Release. 2005;102:203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Lentacker I, Geers B, Demeester J, De Smedt SC, Sanders NN. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Molecular Therapy. doi: 10.1038/mt.2009.160. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kooiman K, Böhmer MR, Emmer M, et al. Oil-filled polymer microcapsules for ultrasound-mediated delivery of lipophilic drugs. J. Control. Release. 2009;133:109–118. doi: 10.1016/j.jconrel.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 15.Shi WT, Böhmer M, van Wamel A, et al. Ultrasound therapy with drug loaded microcapsules. IEEE Ultrasonics Symposium. 2007:773–776. [Google Scholar]
- 16.El-Sherif DM, Wheatley MA. Development of a novel method for synthesis of a polymeric ultrasound contrast agent. J. Biomed. Mater. Res. A. 2003;66:347–355. doi: 10.1002/jbm.a.10586. [DOI] [PubMed] [Google Scholar]
- 17.Wheatley MA, Lathia JD, Oum KL. Polymeric ultrasound contrast agents targeted to integrins: importance of process methods and surface density of ligands. Biomacromolecules. 2007;8:516–522. doi: 10.1021/bm060659i. [DOI] [PubMed] [Google Scholar]
- 18.Eisenbrey JR, Soulen MC, Wheatley MA. Delivery of Encapsulated Doxorubicin by Ultrasound Mediated Size Reduction of Drug Loaded Polymer Contrast Agents. IEEE TBME Letters. 2009 doi: 10.1109/TBME.2009.2030497. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Eisenbrey JR, Huang PN, Soulen MC, et al. Doxorubicin Loaded Contrast Agents for Ultrasound Triggered Drug Delivery: Importance of Process Parameters. Pharm. Eng. 2008;28:70–78. [Google Scholar]
- 20.Wheatley MA, Forsberg F, Oum KL, et al. Comparison of in vitro and in vivo acoustic response of a novel 50:50 PLGA contrast agent. Ultrasonics. 2006;44:360–367. doi: 10.1016/j.ultras.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Forsberg F, Lathia JD, Merton DA, et al. Effect of shell type on the in vivo backscatter from polymer-encapsulated microbubbles. Ultrasound in Med. & Biol. 2004;30:1281–1287. doi: 10.1016/j.ultrasmedbio.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Lentacker I, De Smedt SC, Sander NN. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter. 2009;5:2161–2170. [Google Scholar]
- 23.Miyazaki S, Hou WM, Takada M. Controlled drug release by ultrasound irradiation. Chem. Pharm. Bull. 1985;33:428–431. doi: 10.1248/cpb.33.428. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Nyborg WL. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Del. Rev. 2008;60:1103–1116. doi: 10.1016/j.addr.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv. Drug Del. Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postema M, Schmitz G. Ultrasonic bubbles in medicine: Influence of the shell. Ultrason. Sonochem. 2007;15:438–444. doi: 10.1016/j.ultsonch.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 28.Eisenbrey JR, Huang PN, Hsu J, et al. Ultrasound triggered cell death in vitro with doxorubicin loaded poly lactic-acid contrast agents. Ultrasonics. 2009;49:628–633. doi: 10.1016/j.ultras.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]