SYNOPSIS
A human homologue of the iron-sulfur cluster assembly protein IscA (hIscA1) has been cloned and expressed in Escherichia coli cells. The UV-visible absorption and EPR (electron paramagnetic resonance) measurements reveal that hIscA1 purified from E. coli cells contains a mononuclear iron center and that the iron binding in hIscA1 expressed in E. coli cells can be further modulated by the iron content in the cell growth medium. Additional studies show that purified hIscA1 binds iron with an iron association constant of approx. 2.0 × 1019 M−1, and that the iron-bound hIscA1 is able to provide the iron for the iron-sulfur cluster assembly in a proposed scaffold protein IscU of E. coli in vitro. The complementation experiments indicate that hIscA1 can partially substitute for IscA in restoring the cell growth of E. coli in the M9 minimal medium under aerobic conditions. The results suggest that human IscA1, like E. coli IscA, is an iron binding protein that may act as an iron chaperone for biogenesis of iron-sulfur clusters.
Keywords: Iron-sulfur cluster biogenesis, human IscA homologue, intracellular iron content
INTRODUCTION
IscA is a highly conserved iron-sulfur cluster assembly protein found in prokaryotic and eukaryotic organisms [1–4]. Genetic studies showed that depletion of IscA in Azotobacter vinelandii resulted in a null-growth phenotype in the modified Burks minimal medium under elevated oxygen conditions [5]. In Escherichia coli, in-frame deletion of IscA and its paralog SufA also produced a null-growth phenotype and cellular deficiency of the iron-sulfur cluster assembly in the M9 minimal medium under aerobic conditions [6, 7]. In Saccharomyces cerevisiae, depletion of IscA homologues led to accumulation of iron in mitochondria and dependency on lysine and glutamate for the cell growth under aerobic conditions [8, 9]. In cultured human HeLa cells, RNAi knockdown of the IscA homologue (hIscA1) resulted in decreased activities of iron-sulfur enzymes in both mitochondria and cytosol [10]. These results suggest that IscA and its homologues have a crucial role for biogenesis of iron-sulfur clusters.
IscA and its homologues have previously been characterized as scaffold proteins for the iron-sulfur cluster assembly, as purified proteins can bind transient iron-sulfur clusters and transfer the assembled clusters to target proteins in vitro [11–16]. This hypothesis has gained additional support from recent purification of native iron-sulfur cluster-bound IscA homologues from several organisms under defined growth conditions [17–19]. However, unlike other iron-sulfur cluster assembly scaffold proteins such as IscU [20, 21], IscA purified from E. coli cells has a strong iron binding activity with an iron association constant of 3.0×1019 M−1 [22–24]. Furthermore, the iron center in IscA can be readily mobilized by L-cysteine [25] and transferred for the iron-sulfur cluster assembly in a proposed scaffold IscU in vitro [26, 27]. These results indicate that IscA may act as an iron chaperone for biogenesis of iron-sulfur clusters [6–9, 22–27]. It should be pointed out that IscA has also been proposed as a regulatory protein to control the iron homeostasis and redox stress responses in the cyanobacterium Synechococcus sp. strain PCC 7002 [28], although the underlying mechanism is still not clear.
The human IscA homologue (hIscA1) was initially identified as a putative target of autoimmunity in a patient suffering from Sjogren’s syndrome [29]. The N-terminal amino acids of the full-length hIscA1 appear to be a consensus mitochondrial signal peptide. The rest of the hIscA1 sequence has about 38% identity and 70% similarity to E. coli IscA [29]. To explore whether the iron binding activity is conserved in IscA from other organisms, here we have cloned human IscA homologue gene from a fetal brain cDNA library and expressed the mature form of hIscA1 (without the mitochondrial signal peptide) in E. coli cells. The UV-visible and EPR (electron paramagnetic resonance) measurements reveal that purified hIscA1 contains a mononuclear iron center and that the iron binding in hIscA1 expressed in E. coli cells can be further increased by addition of exogenous iron in the M9 growth medium. Additional studies show that purified hIscA1 has a similar iron binding activity as E. coli IscA, and that the iron-bound hIscA1 can provide the iron for the iron-sulfur cluster assembly in E. coli IscU in vitro. Furthermore, the complementation experiments indicate that hIscA1 can partially substitute for IscA in restoring the cell growth of E. coli in the M9 minimal medium under aerobic conditions. These results led us to propose that human hIscA1, like E. coli IscA, is an iron binding protein that may act as an iron chaperone for biogenesis of iron-sulfur clusters.
EXPERIMENTAL
Cloning and purification of human IscA homologue hIscA1
A cDNA clone (DKFZp547G027Q) containing a full length of the human IscA homologue 1 (hIscA1) was purchased from the German Cancer Research Center. Two primers (primer-1, CCCACCCATATGGCCCTCACCCTGACACCT; primer-2, TGATTTCTGCTGAGCGCCCCAAAG) were designed for amplifying human iscA cDNA in which the mitochondrial signal peptide sequence was left out. The PCR product was digested with Nde1 and Blp1, and ligated to an expression plasmid pET28b+. The constructed plasmid pThIscA1 was introduced into an E. coli strain BL21(DE3) (Stratagene co.). The cloned gene was confirmed by direct sequencing using the T7 promoter. The E. coli cells containing pThIscA1 were grown in the rich LB (Luria-Bertani) medium to O.D. at 600 nm of 0.8 before isopropyl β-d-1-thiogalactopyranoside (0.2 mM) was added to induce the protein expression at 18°C. The E. coli cells containing pThIscA1 were also grown in the M9 minimal medium (containing glucose (0.4%), thiamin (5 µg/ml), and 20 amino acids (each at 10 µg/ml)) supplemented with or without Fe(NH4)2(SO4)2 (50 µM). Recombinant hIscA1 was purified from E. coli cells as described in [26], and purity of purified hIscA1 was over 95% judging from the SDS polyacrylamide gel electrophoresis. The protein concentration of apo-hIscA1 (protein devoid of any iron) was calculated from the absorption amplitude at 280 nm using an extinction coefficient of 4.2 mM−1cm−1. The concentration of monomeric hIscA1 was used throughout text.
Iron binding analysis in purified hIscA1 in vitro
The iron-depleted hIscA1 (apo-hIscA1) was prepared by incubating purified hIscA1 with EDTA (10 mM) and L-cysteine (2 mM) at 37°C for 60 min, followed by re-purification of the protein. For the iron binding experiments, apo-hIscA1 was incubated with various concentrations of Fe(NH4)2(SO4)2 in the presence of dithiothreitol (2 mM) in open-to-air micro-tubes at room temperature for 30 min. The protein samples were then passed through a HiTrap desalting column (GE Healthcare co.) to remove unbound iron and dithiothreitol. For the iron binding competition experiments, the iron-bound hIscA1 was incubated with increasing concentration of sodium citrate in the presence of dithiothreitol (2 mM) at 37°C for 30 min. The protein was then re-purified by passing through a HiTrap desalting column to remove citrate, iron-bound citrate and dithiothreitol. The relative iron binding in hIscA1 was measured from the absorption amplitude at 315 nm as described previously [22]. The amounts of the acid-labile iron and sulphide in the protein samples were analyzed according to the Fischer’s method [30] and the Siegel’s method [31], respectively.
Iron-sulfur cluster assembly in E. coli IscU
E. coli iron-sulfur cluster assembly scaffold IscU and cysteine desulfurase IscS were prepared as described previously [22]. In typical experiments, IscU (40 µM) was incubated with IscS (1 µM), the iron-bound hIscA1 or E. coli IscA (100 µM), NaCl (200 mM), and Tris (20 mM) (pH 8.0) in the presence of dithiothreitol (2 mM). The reaction solution was pre-incubated at 37°C for 5 min before L-cysteine (1 mM) was added to initiate the iron-sulfur cluster assembly. The amount of the iron-sulfur clusters assembled in IscU was monitored at 456 nm in a Beckman DU-640 UV-Visible spectrometer equipped with a temperature controller [20, 27].
Complementation of hIscA1 in the E. coli iscA−1/sufA−1 double mutant
For the complementation experiments, gene encoding hIscA1 was subcloned into an arabinose-controlled plasmid pBAD (Invitrogene co.). The constructed plasmid pBAD/hiscA1 was introduced into an E. coli mutant in which gene iscA and its paralog sufA were in-frame deleted [6]. The E. coli iscA−1/sufA−1 double mutant was viable in the rich LB medium, but had a null-growth phenotype in the M9 minimal medium under aerobic conditions [6]. The E. coli iscA−1/sufA−1 double mutant containing pBAD/iscA and a vector only [6] was used as the positive and negative control, respectively. About 2×105cells of each E. coli strain were spotted on a M9 minimal medium plate containing glucose (0.2%) and arabinose (0.002%) as described in [6]. The plate was incubated at 37°C under aerobic conditions for 48 hours and photographed.
EPR (Electron Paramagnetic Resonance) measurements
The EPR spectra were recorded at X-band on a Bruker ESR-300 spectrometer using an Oxford Instruments ESR-9 flow cryostat. The routine EPR conditions were: microwave frequency, 9.45 GHz; microwave power, 10 mW; modulation frequency, 100 kHz; modulation amplitude, 2.0 mT; sample temperature, 4.0 K; receive gain, 1.0×105.
RESULTS
Purified human IscA homologue (hIscA1) is an iron binding protein
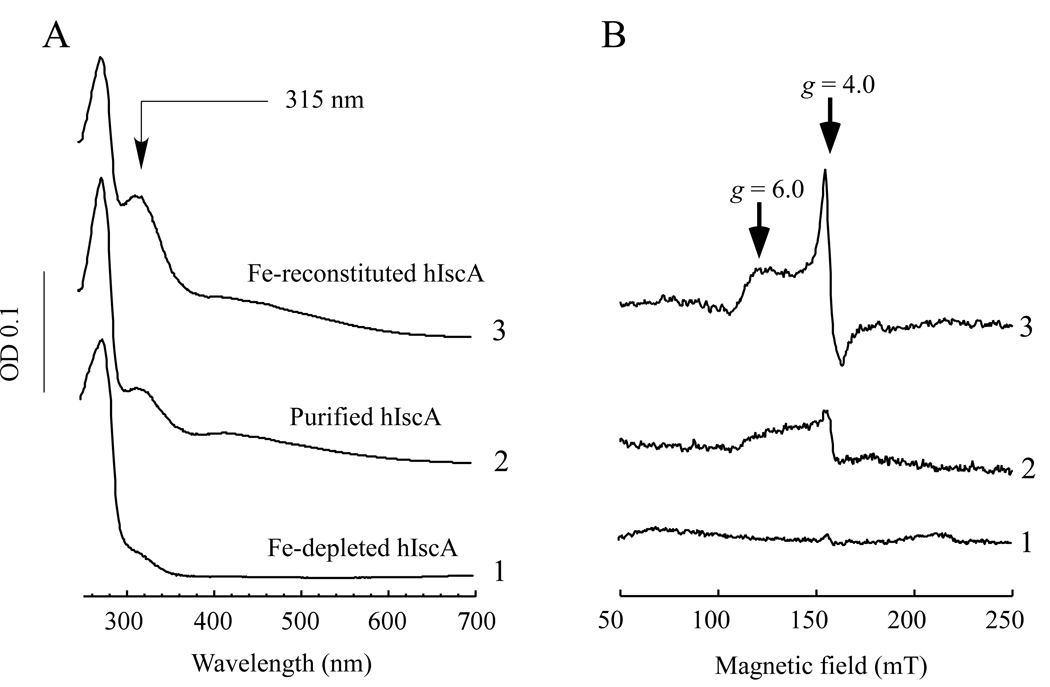
Human IscA homologue (hIscA1) was cloned from a fetal brain cDNA library, expressed in E. coli cells, and purified to a single band on the SDS polyacrylamide gel electrophoresis as described in the Experimental. Figure 1A shows that purified hIscA1 has an absorption peak at 315 nm, indicative of iron binding in the protein [22, 26, 32]. The iron content analysis showed that as-purified hIscA1 contained about 0.1–0.2 iron atoms per hIscA1 dimer (n = 3). The acid-labile sulphide content of purified hIscA1 was less than 0.03 sulphide atoms per hIscA dimer (n = 3). When purified hIscA1 was incubated with L-cysteine and an iron chelator EDTA, the absorption peak at 315 nm of purified hIscA1 was largely eliminated. However, when the iron-depleted hIscA1 was re-incubated with an equivalent amount of ferrous iron in the presence of dithiothreitol, the absorption peak at 315 nm of hIscA1 was fully restored (Figure 1A). The EPR (electron paramagnetic resonance) measurements further indicated that the iron-bound hIscA1 had a unique EPR signal at g = 4–6, reflecting a high-spin S = 3/2 mononuclear iron center in the protein (Figure 1B), which is essentially identical to that of the iron-bound IscA from E. coli [22].
Figure 1. UV-visible and EPR spectra of purified human IscA homologue hIscA1.
HiscA1 was purified from E. coli cells grown in the LB growth medium. A) UV-visible spectra of hIscA1. Purified hIscA1 (50 µM) (spectrum 2) was incubated with EDTA (10 mM) and L-cysteine (2 mM) at 37°C for 60 min (spectrum 1). The iron-depleted hIscA1 (50 µM) was re-incubated with Fe(NH)2(SO4)2 (50 µM) and dithiothreitol (2 mM) (spectrum 3). Each protein was re-purified by passing through a High-Trap Desalting column after incubation. B) EPR spectra of hIscA1. The protein samples were prepared as described in A), except that the protein concentration was ~450 µM.
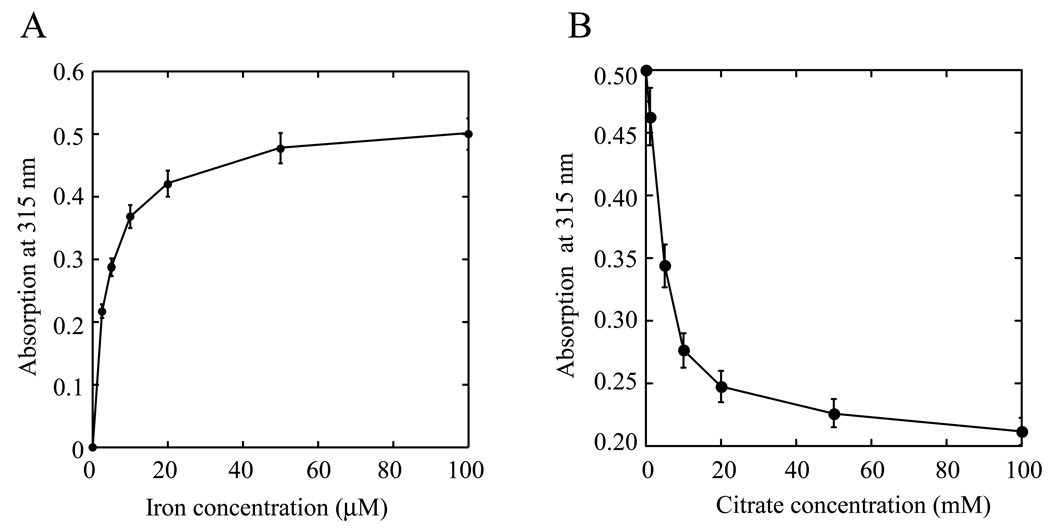
The iron-depleted hIscA1 was also incubated with various concentration of ferrous iron in the presence of dithiothreitol, followed by re-purification of hIscA1. Figure 2A shows that the iron binding in hIscA1 (50 µM) was linearly increased as the iron concentration was increased from 0 to 20 µM, and nearly saturated when the iron concentration was above 25 µM. The iron content analyses of the iron-saturated hIscA1 showed that the ratio of iron to the hIscA1 dimer was about 0.86±0.24 (n = 3), which is similar to that of the iron-saturated E. coli IscA [22]. Again, the acid-labile sulphide in the iron-saturated hIscA1 was less than 0.03 sulphide atoms per hIscA dimer (n = 3).
Figure 2. Iron binding activity of hIscA1 in vitro.
A) Iron binding titration of hIscA1. The iron-depleted hIscA1 (50 µM) was incubated with Fe(NH)2(SO4)2 (0 to 100 µM) in the presence of dithiothreitol (2 mM) at 37°C for 30 min, followed by re-purification of hIscA1. The absorption amplitude at 315 nm of re-purified hIscA1 was plotted as a function of the Fe(NH)2(SO4)2 concentration in the incubation solution. B) Iron binding competition of hIscA1. The iron-saturated hIscA1 (100 µM) was incubated with sodium citrate (0 to 100 mM) in the presence of dithiothreitol (2 mM) at 37°C for 30 min, followed by re-purification of hIscA1. The absorption amplitude at 315 nm of re-purified hIscA1 was plotted as a function of the sodium citrate concentration in the incubation solution. The results are the means ± SD from three independent experiments
Because almost all iron in the incubation solution was bound to apo-hIscA1 before the iron binding was saturated (Figure 2A), it became not possible to obtain the iron binding constant for hIscA1. Therefore, we utilized an iron binding competitor citrate [33] to determine the iron binding affinity of hIscA1 as described previously for E. coli IscA [22]. Citrate is a physiological iron chelator with an iron association constant of approx. 1.0 × 1017 M−1 [33]. In the experiments, the iron-bound hIscA1 was incubated with increasing concentration of sodium citrate in the presence of dithiothreitol. Figure 2B shows that the iron content in the iron-bound hIscA1 was gradually decreased as the concentration of sodium citrate was progressively increased in the incubation solution. About 10 mM sodium citrate was needed to remove half of the iron binding in hIscA1 (100 µM). Even in the presence of 100 mM sodium citrate, about 40% of iron binding still remained in hIscA1 (Figure 2B). Based on these iron competition data, we estimated that the iron association constant of purified hIscA1 will be at least 2.0 × 1019 M−1, which is close to that of human transferrin (4.7 × 1020 M−1) [34].
Human hIscA1 acts as an iron binding protein in E. coli cells
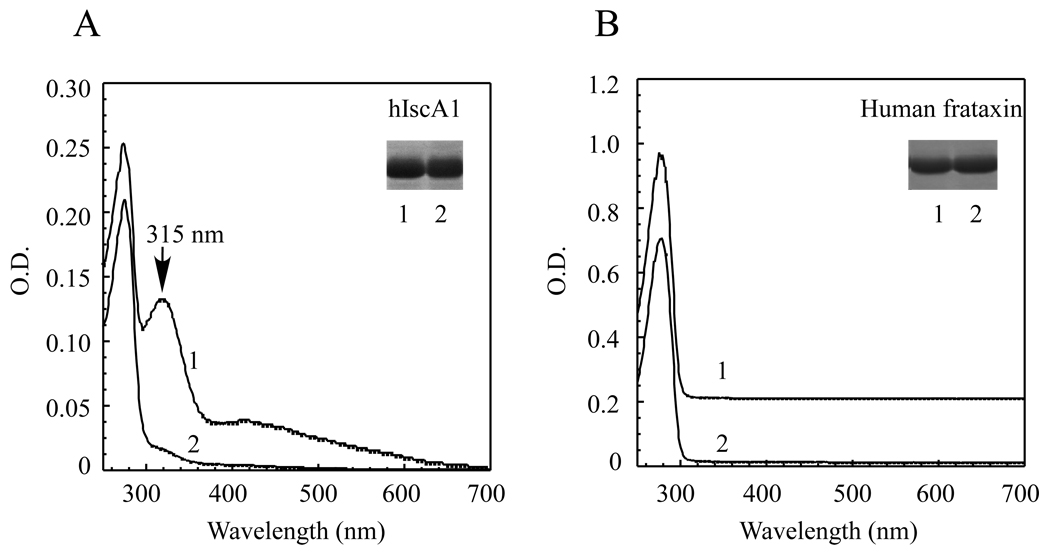
If hIscA1 is an iron binding protein in vivo, we reasoned that iron binding in hIscA1 would be modulated by intracellular iron content. To avoid complex compounds in the rich LB growth medium, we grew the E. coli cells expressing hIscA1 in the M9 minimal growth medium supplemented with or without exogenous iron (50 µM). Before the protein purification, the E. coli cells were washed twice with the protein purification buffer to remove any residual iron in the growth medium. Figure 3A shows that hIscA1 purified from the E. coli cells grown in the M9 minimal medium had only a small absorption peak at 315 nm, indicating that iron could be limited in the M9 minimal medium. When the M9 minimal medium was supplemented with exogenous iron (50 µM), purified hIscA1 had a strong absorption peak at 315 nm (Figure 3A), indicative of the iron binding in the protein. The iron and sulphide content analyses further confirmed that hIscA1 purified from the E. coli cells grown in the M9 minimal medium supplemented with exogenous iron (50 µM) contained about 0.70±0.24 iron atoms and less than 0.1 acid-labile sulphide atoms per hIscA1 dimer (n = 3).
Figure 3. Iron binding activity of hIscA1 in E. coli cells.
A) The UV-visible absorption spectra of hIscA1 purified from the E. coli cells grown in the M9 minimal medium supplemented with (spectrum 1) or without (spectrum 2) 50 µM ferrous ammonium sulfate. The protein concentration was approx. 50 µM. The insert is a photograph of the SDS/PAGE gel of hIscA1 purified from the E. coli cells grown in the M9 minimal medium supplemented with (lane 1) or without (lane 2) 50 µM ferrous ammonium sulfate. B) The UV-visible absorption spectra of human frataxin purified from the E. coli cells grown in the M9 minimal medium supplemented with (spectrum 1) or without (spectrum 2) 50 µM ferrous ammonium sulfate. The protein concentration was approx. 20 µM. The insert is a photograph of the SDS/PAGE gel of frataxin purified from the E. coli cells grown in the M9 minimal medium supplemented with (lane 1) or without (lane 2) 50 µM ferrous ammonium sulfate. The results are representatives from three independent experiments.
Frataxin, a mitochondrial protein linked to human neurodegenerative disease Friedreich ataxia [35], was also proposed as a possible iron chaperone for biogenesis of iron-sulfur clusters [36, 37]. To explore the iron binding activity of frataxin in E. coli cells, we subcloned the mature form of human frataxin (amino acid residues 56–210) from plasmid pETHF2 (a kind gift from Dr. Grazia Isaya) [38, 39] into the same expression vector as hIscA1, and expressed the protein in E. coli cells grown in the M9 minimal medium. Figure 3B shows that while human frataxin purified from E. coli cells had no iron binding as reported previously [38, 39], addition of exogenous iron (50 µM) in the M9 minimal medium did not increase the iron binding in human frataxin either. These results suggest that unlike human hIscA1, human frataxin is unable to scavenge the accessible iron in E. coli cells grown in the M9 minimal medium under aerobic conditions.
The iron-bound hIscA1 is able to provide iron for the iron-sulfur cluster assembly in E. coli IscU in vitro
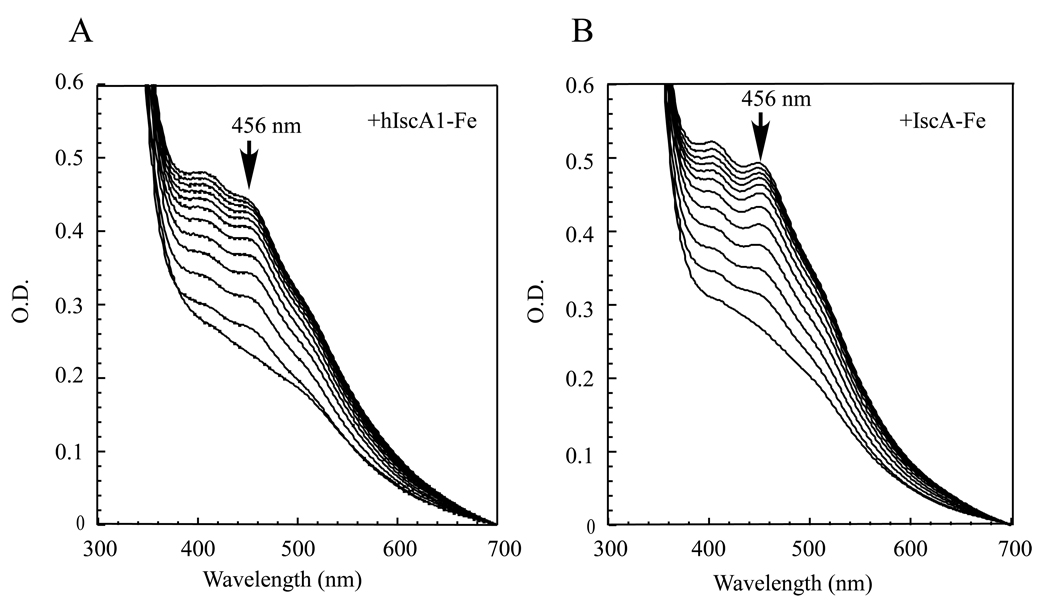
We have shown previously that the iron-bound IscA can provide the iron for the iron-sulfur cluster assembly in a proposed scaffold IscU in vitro [22, 26, 27]. Because human hIscA1 and E. coli IscA share 38% identity and 70% similarity, we postulated that the iron-bound hIscA1 may also provide the iron for the iron-sulfur cluster assembly in E. coli IscU. For the experiments, the freshly prepared iron-bound hIscA1 was incubated with purified E. coli IscU and cysteine desulfurase (IscS) in the presence of dithiothreitol. The iron-sulfur cluster assembly reaction was initiated by adding L-cysteine. Figure 4A shows that the absorption peak at 456 nm of the IscU [2Fe-2S] cluster [20] quickly appeared and reached its maximum after 20 min incubation. Re-purification of human hIscA1 and E. coli IscU from the incubation solution using a Mono-Q column [27] revealed that the iron center in hIscA1 was transferred to the iron-sulfur cluster in E. coli IscU (data not shown). However, compared with the iron-bound E. coli IscA (Figure 4B), the iron-bound human hIscA1 had a relatively slower kinetics for the iron-sulfur cluster assembly in E. coli IscU, indicating that there could be some subtle difference between human hIscA1 and E. coli IscA in delivering the iron for the iron-sulfur cluster assembly in E. coli IscU.
Figure 4. The iron-bound hIscA1 acts as an iron donor for the iron-sulfur cluster assembly in E. coli IscU in vitro.
A) Purified E. coli IscU (50 µM) was incubated with E. coli IscS (1 µM), the iron-bound hIscA1 (100 µM), Tris (20 mM, pH 8.0) and NaCl (200 mM) in the presence of dithiothreitol (2 mM) at 37°C for 5 min. L-cysteine (1 mM) was then added to initiate the iron-sulfur cluster assembly reaction. The absorption peak at 456 nm represents formation of the IscU [2Fe-2S] cluster. Spectra were taken every 2 min for 24 min. B) Same as in A) except the iron-bound hIscA1 was replaced with the iron-bound E. coli IscA in the incubation solution.
Complementary activity of hIscA1 in E. coli cells
In addition to IscA, E. coli has three IscA paralogs: SufA [6, 18], NfuA [40], and ErpA [17]. All three proteins have been characterized as alternative scaffold proteins for the iron-sulfur cluster assembly. Among these paralogs, SufA is a member of the gene cluster sufABCDSE [41] which is responsible for the iron-sulfur cluster assembly/repair under oxidative stress [42] or iron starvation conditions [43] in E. coli. Purified E. coli SufA has a similar iron binding activity as IscA in vitro [6]. While deletion of IscA or SufA has only a mild effect on cell growth [44], deletion of both IscA and SufA results in a null-growth phenotype and cellular deficiency of the iron-sulfur cluster assembly in E. coli in the M9 minimal medium under aerobic conditions [6, 7]. Re-introduction of either IscA or SufA fully restores the cell growth of the E. coli iscA−/sufA− double mutant, further demonstrating that IscA and SufA have the complementary roles in biogenesis of iron-sulfur clusters in E. coli cells [7].
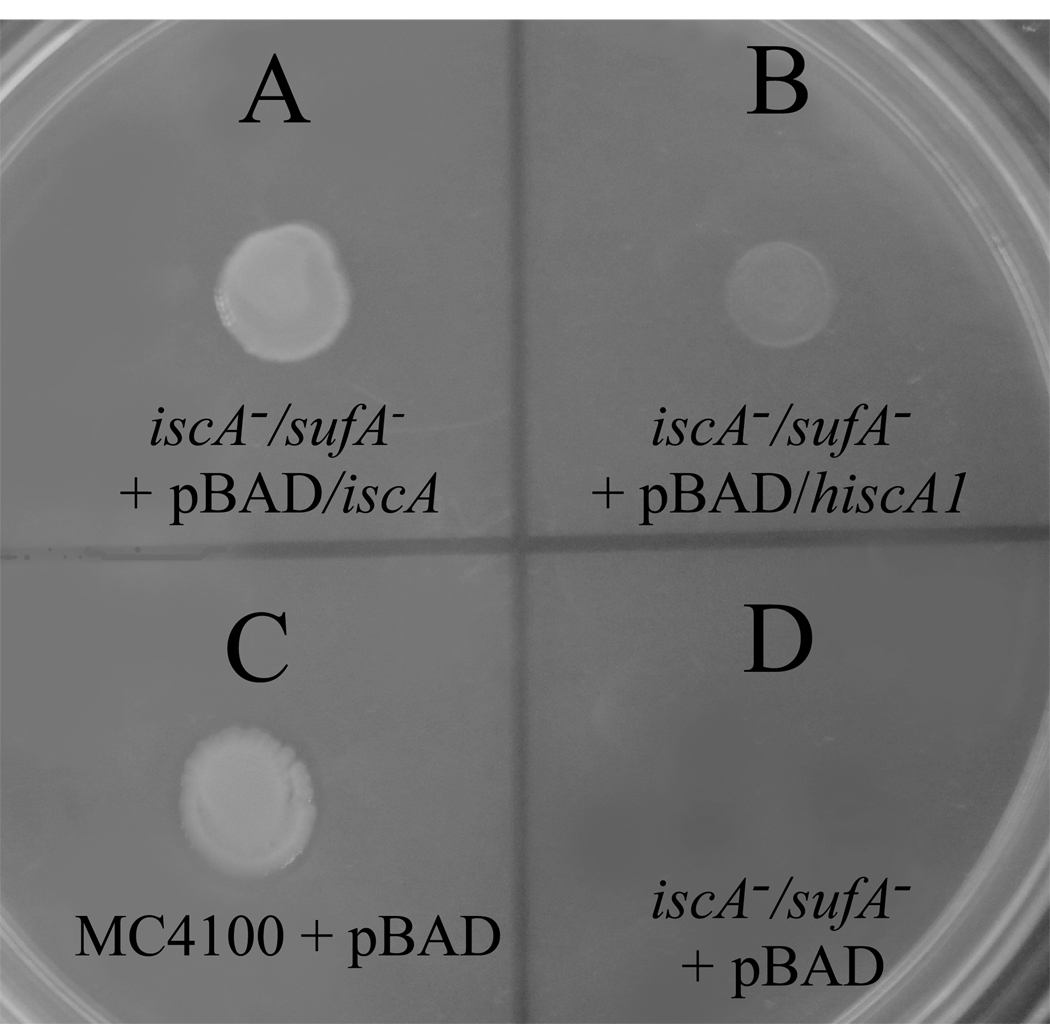
Here we took advantage of the null-growth phenotype of the E. coli iscA−/sufA− double mutant in the M9 minimal medium under aerobic conditions to explore the complementary activity of human hIscA1. Figure 5 shows that expression of hIscA1 did indeed restore the cell growth of the E. coli iscA−/sufA− double mutant in the M9 minimal medium under aerobic conditions. However, compared with E. coli IscA, hIscA1 could not fully restore the cell growth of the E. coli iscA−/sufA− double mutant, suggesting that hIscA1 can only partially substitute for the function of IscA in E. coli cells.
Figure 5. Complementary role of hIscA1 in E. coli cells.
The E. coli iscA−1/sufA−1 double mutant cells containing plasmid expressing E. coli IscA (pBAD/iscA) (A), hIscA1 (pBAD/hiscA1) (B), or the vector only (pBAD) (D), were spotted on the minimal medium plates supplemented with glucose (0.2%) and arabinose (0.002%). The wild-type strain MC4100 cells (C) were also used as control. The plate was incubated at 37°C for 48 hours under aerobic conditions before the photography was taken.
DISCUSSION
Biogenesis of iron-sulfur clusters requires a coordinated delivery of iron and sulphide. While sulphide in iron-sulfur clusters is derived from L-cysteine by cysteine desulfurases [45, 46], iron donor for the iron-sulfur cluster assembly largely remains elusive. Previously, we reported that E. coli IscA is a novel iron binding protein that can provide the iron for the iron-sulfur cluster assembly in a scaffold protein IscU in vitro [22–24]. In the present study, we report that the human IscA homologue, hIscA1, has the similar iron binding activity as E. coli IscA (Figure 2), and that the iron binding in hIscA1 expressed in E. coli cells can be modulated by the accessible iron in the cell growth medium (Figure 3A). We further show that the iron-bound hIscA1 can provide the iron for the iron-sulfur cluster assembly in E. coli IscU in vitro (Figure 4) and partially substitute for IscA in restoring the cell growth of E. coli in the M9 minimal medium under aerobic conditions (Figure 5). Collectively, our results suggest that human hIscA1, like E. coli IscA, is an iron binding protein that may act as an iron chaperone for biogenesis of iron-sulfur clusters.
It has been postulated that frataxin, a mitochondrial protein linked to the human neurodegenerative disease Friedreich ataxia [35], may also act as an iron chaperone for biogenesis of iron-sulfur clusters [36, 37]. Like IscA, frataxin is highly conserved from bacteria to humans. While mature form of human frataxin purified from E. coli cells contained little or no iron (Figure 3B), frataxin was able to bind approx. 10 atoms of iron per frataxin monomer in vitro under aerobic conditions [38]. In addition, the iron-bound frataxin [36] or its bacterial homologue CyaY [37] can provide the iron for the iron-sulfur cluster assembly in proteins in vitro. Furthermore, frataxin has been shown to specifically interact with the iron-sulfur proteins such as aconitase [47] and mitochondrial electron transfer components [48], and with the iron-sulfur cluster assembly proteins IscS [49] and IscU [50]. All these studies suggested that frataxin/CyaY could be directly involved in the iron-sulfur cluster assembly and/or repair. However, other studies indicated that deletion of frataxin/CyaY has little or no effect on biogenesis of iron-sulfur clusters in S. cerevisiae [51], Salmonella enterica [52], and E. coli [53], and that expression of frataxin in cytosol [54] or addition of manganese [55] in cultured mammalian cells is sufficient to substitute for frataxin in protecting iron-sulfur enzymes in mitochondria. In addition, it has been shown that frataxin/CyaY has a fairly weak iron binding activity under physiologically relevant conditions in vitro [56, 57]. Here we find that unlike human hIscA1, human frataxin fails to bind any accessible iron in E. coli cells grown in the M9 minimal medium (Figure 3B), further indicating that frataxin has a weak iron binding activity in vivo under normal physiological conditions. Without a strong iron binding activity in cells, it is arguable whether frataxin/CyaY could act as an iron donor for biogenesis of iron-sulfur clusters.
It should be emphasized that the physiological function of IscA in biogenesis of iron-sulfur clusters still remains controversial. Our results presented here and previously [6, 7, 22–27] could not exclude the possibility that IscA may act as an alternative scaffold protein for biogenesis of iron-sulfur clusters as proposed by others [11–19]. Recent purification of the native iron-sulfur cluster-bound SufA from E. coli cells by co-expressing SufA with the entire gene cluster sufABCDSE clearly indicated that SufA is able to bind an iron-sulfur cluster in vivo [18]. On the other hand, since the iron-sulfur cluster assembly will require a concert delivery of stoichiometric amounts of iron and sulphide, it is conceivable that if cells have more available iron than sulphide, an excess of iron must be transiently stored. Under this scenario, IscA may act as an iron chaperone to scavenge excess iron and deliver the iron for the subsequent iron-sulfur cluster assembly. In this context, we propose that IscA could be a dual functional protein that is capable of binding either a mononuclear iron or an iron-sulfur cluster depending on accessible iron and sulphide contents in cells. The dual functional model for IscA is consistent with the x-ray crystal structures of E. coli IscA [58, 59] and its paralog SufA [60] which reveal that the conserved “cysteine pocket” in IscA/SufA can readily accommodate a mononuclear iron or an iron-sulfur cluster without significant re-arrangements of protein structure. The dynamic alteration between the mononuclear iron binding mode and the iron-sulfur cluster binding mode in IscA is currently under investigation.
ACKNOWLEDGEMENTS
We would like to thank Dr. Grazia Isaya (Mayo Clinic) for kindly providing the human frataxin expression plasmid pETHF2, and Ms. Liana Coleman for technical assistance in the early stage of this study. This work was supported in part by the Public Health Service Grant (CA107494) from the National Cancer Institute (NIH), the Chinese National Natural Science Foundation Grant (30770448), the Science and Technology Key Program of Zhejiang Province Grant (2006C14025), and the Natural Science Foundation of Zhejiang Province Grant (Y2081075).
Abbreviations
- EPR
electron paramagnetic resonance
- hIscA1
human IscA homologue
Footnotes
Author Contributions:
All authors contributed to the experimental design and data collections. J.L and H.D. wrote the paper.
REFERENCES
- 1.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi Y, Nakamura M. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster involved in the assembly of Fe-S clusters in Escherichia coli. J. Biochem. (Tokyo) 1999;126:917–926. doi: 10.1093/oxfordjournals.jbchem.a022535. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz CJ, Giel JL, Patschkowski T, Luther C, Ruzicka FJ, Beinert H, Kiley PJ. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc. Natl. Acad. Sci. U S A. 2001;98:14895–14900. doi: 10.1073/pnas.251550898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F. Iron-sulfur (Fe/S) protein biogenesis: phylogenomic and genetic studies of A-type carriers. PLoS Genet. 2009;5:e1000497. doi: 10.1371/journal.pgen.1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DC, Unciuleac MC, Dean DR. Controlled expression and functional analysis of iron-sulfur cluster biosynthetic components within Azotobacter vinelandii. J. Bacteriol. 2006;188:7551–7561. doi: 10.1128/JB.00596-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, Yang J, Tan G, Ding H. Complementary roles of SufA and IscA in the biogenesis of iron-sulfur clusters in Escherichia coli. Biochem. J. 2008;409:535–543. doi: 10.1042/BJ20071166. [DOI] [PubMed] [Google Scholar]
- 7.Tan G, Lu J, Bitoun JP, Huang H, Ding H. IscA/SufA paralogs are required for the [4Fe-4S] cluster assembly in enzymes of multiple physiological pathways in Escherichia coli under aerobic growth conditions. Biochem. J. 2009;420:463–472. doi: 10.1042/BJ20090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen LT, Culotta VC. Role of Saccharomyces cerevisiae ISA1 and ISA2 in iron homeostasis. Mol. Cell. Biol. 2000;20:3918–3927. doi: 10.1128/mcb.20.11.3918-3927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaut A, Lange H, Diekert K, Kispal G, Lill R. Isa1p is a component of the mitochondrial machinery for maturation of cellular iron-sulfur proteins and requires conserved cysteine residues for function. J. Biol. Chem. 2000;275:15955–15961. doi: 10.1074/jbc.M909502199. [DOI] [PubMed] [Google Scholar]
- 10.Song D, Tu Z, Lee FS. Human IscA1 interacts with IOP1/NARFL and functions in both cytosolic and mitochondrial iron-sulfur protein biogenesis. J. Biol. Chem. 2009;284:35297–35307. doi: 10.1074/jbc.M109.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ollagnier-de-Choudens S, Mattioli T, Takahashi Y, Fontecave M. Iron-sulfur cluster assembly: characterization of IscA and evidence for a specific and functional complex with ferredoxin. J. Biol. Chem. 2001;276:22604–22607. doi: 10.1074/jbc.M102902200. [DOI] [PubMed] [Google Scholar]
- 12.Krebs C, Agar JN, Smith AD, Frazzon J, Dean DR, Huynh BH, Johnson MK. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry. 2001;40:14069–14080. doi: 10.1021/bi015656z. [DOI] [PubMed] [Google Scholar]
- 13.Wu SP, Cowan JA. Iron-sulfur cluster biosynthesis. A comparative kinetic analysis of native and Cys-substituted ISA-mediated [2Fe-2S]2+ cluster transfer to an apoferredoxin target. Biochemistry. 2003;42:5784–5791. doi: 10.1021/bi026939+. [DOI] [PubMed] [Google Scholar]
- 14.Wollenberg M, Berndt C, Bill E, Schwenn JD, Seidler A. A dimer of the FeS cluster biosynthesis protein IscA from cyanobacteria binds a [2Fe2S] cluster between two protomers and transfers it to [2Fe2S] and [4Fe4S] apo proteins. Eur. J. Biochem. 2003;270:1662–1671. doi: 10.1046/j.1432-1033.2003.03522.x. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto K, Yamashita E, Kondou Y, Lee SJ, Arisaka F, Tsukihara T, Nakai M. The asymmetric IscA homodimer with an exposed [2Fe-2S] cluster suggests the structural basis of the Fe-S cluster biosynthetic scaffold. J. Mol. Biol. 2006;360:117–132. doi: 10.1016/j.jmb.2006.04.067. [DOI] [PubMed] [Google Scholar]
- 16.Chahal HK, Dai Y, Saini A, Ayala-Castro C, Outten FW. The SufBCD Fe-S scaffold complex interacts with SufA for Fe-S cluster transfer. Biochemistry. 2009;48:10644–10653. doi: 10.1021/bi901518y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loiseau L, Gerez C, Bekker M, Ollagnier-de Choudens S, Py B, Sanakis Y, Teixeira de Mattos J, Fontecave M, Barras F. ErpA, an iron sulfur (Fe S) protein of the A-type essential for respiratory metabolism in Escherichia coli. Proc. Natl. Acad. Sci. U S A. 2007;104:13626–13631. doi: 10.1073/pnas.0705829104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta V, Sendra M, Naik SG, Chahal HK, Huynh BH, Outten FW, Fontecave M, Ollagnier de Choudens S. Native Escherichia coli SufA, coexpressed with SufBCDSE, purifies as a [2Fe-2S] protein and acts as an Fe-S transporter to Fe-S target enzymes. J. Am. Chem. Soc. 2009;131:6149–6153. doi: 10.1021/ja807551e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng J, Geng M, Jiang H, Liu Y, Liu J, Qiu G. The IscA from Acidithiobacillus ferrooxidans is an iron-sulfur protein which assemble the [Fe4S4] cluster with intracellular iron and sulfur. Arch. Biochem. Biophys. 2007;463:237–244. doi: 10.1016/j.abb.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39:7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- 21.Raulfs EC, O'Carroll IP, Dos Santos PC, Unciuleac MC, Dean DR. In vivo iron-sulfur cluster formation. Proc. Natl. Acad. Sci. U S A. 2008;105:8591–8596. doi: 10.1073/pnas.0803173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding H, Clark RJ. Characterization of iron binding in IscA, an ancient iron-sulphur cluster assembly protein. Biochem. J. 2004;379:433–440. doi: 10.1042/BJ20031702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding H, Harrison K, Lu J. Thioredoxin reductase system mediates iron binding in IscA and iron delivery for the iron-sulfur cluster assembly in IscU. J. Biol. Chem. 2005;280:30432–30437. doi: 10.1074/jbc.M504638200. [DOI] [PubMed] [Google Scholar]
- 24.Bitoun JP, Wu G, Ding H. Escherichia coli FtnA acts as an iron buffer for re-assembly of iron-sulfur clusters in response to hydrogen peroxide stress. Biometals. 2008;21:693–703. doi: 10.1007/s10534-008-9154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding B, Smith ES, Ding H. Mobilization of the iron centre in IscA for the iron-sulphur cluster assembly in IscU. Biochem. J. 2005;389:797–802. doi: 10.1042/BJ20050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding H, Clark RJ, Ding B. IscA mediates iron delivery for assembly of iron-sulfur clusters in IscU under the limited accessible free iron conditions. J. Biol. Chem. 2004;279:37499–37504. doi: 10.1074/jbc.M404533200. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Bitoun JP, Ding H. Interplay of IscA and IscU in biogenesis of iron-sulfur clusters. J. Biol. Chem. 2006;281:27956–27963. doi: 10.1074/jbc.M601356200. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanian R, Shen G, Bryant DA, Golbeck JH. Regulatory roles for IscA and SufA in iron homeostasis and redox stress responses in the cyanobacterium Synechococcus sp. strain PCC 7002. J. Bacteriol. 2006;188:3182–3191. doi: 10.1128/JB.188.9.3182-3191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cozar-Castellano I, Del Valle Machargo M, Trujillo E, Arteaga MF, Gonzalez T, Martin-Vasallo P, Avila J. hIscA: a protein implicated in the biogenesis of iron-sulfur clusters. Biochim. Biophys. Acta. 2004;1700:179–188. doi: 10.1016/j.bbapap.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Fischer DS. A method for the rapid detection of acute iron toxicity. Clin. Chem. 1967;13:6–11. [PubMed] [Google Scholar]
- 31.Siegel LM. A Direct Microdetermination of Sulphide. Anal. Biochem. 1965;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- 32.Bennett DE, Johnson MK. The electronic and magnetic properties of rubredoxin: a low-temperature magnetic circular dichroism study. Biochim. Biophys. Acta. 1987;911:71–80. doi: 10.1016/0167-4838(87)90272-x. [DOI] [PubMed] [Google Scholar]
- 33.Crichton RR. Proteins of iron storage and transport. Adv. Protein. Chem. 1990;40:281–363. doi: 10.1016/s0065-3233(08)60288-0. [DOI] [PubMed] [Google Scholar]
- 34.Aisen P, Leibman A, Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J. Biol. Chem. 1978;253:1930–1937. [PubMed] [Google Scholar]
- 35.Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Canizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 36.Yoon T, Cowan JA. Iron-sulfur cluster biosynthesis. Characterization of frataxin as an iron donor for assembly of [2Fe-2S] clusters in ISU-type proteins. J. Am. Chem. Soc. 2003;125:6078–6084. doi: 10.1021/ja027967i. [DOI] [PubMed] [Google Scholar]
- 37.Layer G, Ollagnier-de Choudens S, Sanakis Y, Fontecave M. Iron-sulfur cluster biosynthesis: characterization of Escherichia coli CYaY as an iron donor for the assembly of [2Fe-2S] clusters in the scaffold IscU. J. Biol. Chem. 2006;281:16256–16263. doi: 10.1074/jbc.M513569200. [DOI] [PubMed] [Google Scholar]
- 38.Cavadini P, Gellera C, Patel PI, Isaya G. Human frataxin maintains mitochondrial iron homeostasis in Saccharomyces cerevisiae. Hum Mol Genet. 2000;9:2523–2530. doi: 10.1093/hmg/9.17.2523. [DOI] [PubMed] [Google Scholar]
- 39.Cavadini P, Adamec J, Taroni F, Gakh O, Isaya G. Two-step processing of human frataxin by mitochondrial processing peptidase. Precursor and intermediate forms are cleaved at different rates. J. Biol. Chem. 2000;275:41469–41475. doi: 10.1074/jbc.M006539200. [DOI] [PubMed] [Google Scholar]
- 40.Angelini S, Gerez C, Ollagnier-de Choudens S, Sanakis Y, Fontecave M, Barras F, Py B. NfuA, a new factor required for maturing Fe/S proteins in Escherichia coli under oxidative stress and iron starvation conditions. J. Biol. Chem. 2008;283:14084–14091. doi: 10.1074/jbc.M709405200. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J. Biol. Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Yeo WS, Roe JH. Induction of the sufA operon encoding Fe-S assembly proteins by superoxide generators and hydrogen peroxide: involvement of OxyR, IHF and an unidentified oxidant-responsive factor. Mol. Microbiol. 2004;51:1745–1755. doi: 10.1111/j.1365-2958.2003.03946.x. [DOI] [PubMed] [Google Scholar]
- 43.Outten FW, Djaman O, Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol. Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 44.Djaman O, Outten FW, Imlay JA. Repair of oxidized iron-sulfur clusters in Escherichia coli. J Biol Chem. 2004;279:44590–44599. doi: 10.1074/jbc.M406487200. [DOI] [PubMed] [Google Scholar]
- 45.Smith AD, Agar JN, Johnson KA, Frazzon J, Amster IJ, Dean DR, Johnson MK. Sulfur transfer from IscS to IscU: the first step in iron-sulfur cluster biosynthesis. J. Am. Chem. Soc. 2001;123:11103–11104. doi: 10.1021/ja016757n. [DOI] [PubMed] [Google Scholar]
- 46.Cupp-Vickery JR, Urbina H, Vickery LE. Crystal structure of IscS, a cysteine desulfurase from Escherichia coli. J. Mol. Biol. 2003;330:1049–1059. doi: 10.1016/s0022-2836(03)00690-9. [DOI] [PubMed] [Google Scholar]
- 47.Bulteau AL, O'Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science. 2004;305:242–245. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Cabo P, Vazquez-Manrique RP, Garcia-Gimeno MA, Sanz P, Palau F. Frataxin interacts functionally with mitochondrial electron transport chain proteins. Hum. Mol. Genet. 2005;14:2091–2098. doi: 10.1093/hmg/ddi214. [DOI] [PubMed] [Google Scholar]
- 49.Adinolfi S, Iannuzzi C, Prischi F, Pastore C, Iametti S, Martin SR, Bonomi F, Pastore A. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat. Struct. Mol. Biol. 2009;16:390–396. doi: 10.1038/nsmb.1579. [DOI] [PubMed] [Google Scholar]
- 50.Gerber J, Muhlenhoff U, Lill R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 2003;4:906–911. doi: 10.1038/sj.embor.embor918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duby G, Foury F, Ramazzotti A, Herrmann J, Lutz T. A non-essential function for yeast frataxin in iron-sulfur cluster assembly. Hum Mol Genet. 2002;11:2635–2643. doi: 10.1093/hmg/11.21.2635. [DOI] [PubMed] [Google Scholar]
- 52.Vivas E, Skovran E, Downs DM. Salmonella enterica strains lacking the frataxin homolog CyaY show defects in Fe-S cluster metabolism in vivo. J. Bacteriol. 2006;188:1175–1179. doi: 10.1128/JB.188.3.1175-1179.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li DS, Ohshima K, Jiralerspong S, Bojanowski MW, Pandolfo M. Knock-out of the cyaY gene in Escherichia coli does not affect cellular iron content and sensitivity to oxidants. FEBS Lett. 1999;456:13–16. doi: 10.1016/s0014-5793(99)00896-0. [DOI] [PubMed] [Google Scholar]
- 54.Condo I, Ventura N, Malisan F, Tomassini B, Testi R. A pool of extramitochondrial frataxin that promotes cell survival. J. Biol. Chem. 2006;281:16750–16756. doi: 10.1074/jbc.M511960200. [DOI] [PubMed] [Google Scholar]
- 55.Irazusta V, Cabiscol E, Reverter-Branchat G, Ros J, Tamarit J. Manganese is the link between frataxin and iron-sulfur deficiency in the yeast model of Friedreich ataxia. J. Biol. Chem. 2006;281:12227–12232. doi: 10.1074/jbc.M511649200. [DOI] [PubMed] [Google Scholar]
- 56.Bou-Abdallah F, Adinolfi S, Pastore A, Laue TM, Dennis Chasteen N. Iron Binding and Oxidation Kinetics in Frataxin CyaY of Escherichia coli. J. Mol. Biol. 2004;341:605–615. doi: 10.1016/j.jmb.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 57.Ding H, Yang J, Coleman LC, Yeung S. Distinct iron binding property of two putative iron donors for the iron-sulfur cluster assembly: IscA and the bacterial frataxin ortholog CyaY under physiological and oxidative stress conditions. J. Biol. Chem. 2007;282:7997–8004. doi: 10.1074/jbc.M609665200. [DOI] [PubMed] [Google Scholar]
- 58.Bilder PW, Ding H, Newcomer ME. Crystal structure of the ancient, Fe-S scaffold IscA reveals a novel protein fold. Biochemistry. 2004;43:133–139. doi: 10.1021/bi035440s. [DOI] [PubMed] [Google Scholar]
- 59.Cupp-Vickery JR, Silberg JJ, Ta DT, Vickery LE. Crystal Structure of IscA, an Iron-sulfur Cluster Assembly Protein from Escherichia coli. J. Mol. Biol. 2004;338:127–137. doi: 10.1016/j.jmb.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 60.Wada K, Hasegawa Y, Gong Z, Minami Y, Fukuyama K, Takahashi Y. Crystal structure of Escherichia coli SufA involved in biosynthesis of iron-sulfur clusters: Implications for a functional dimer. FEBS Lett. 2005;579:6543–6548. doi: 10.1016/j.febslet.2005.10.046. [DOI] [PubMed] [Google Scholar]