Abstract
Introduction:
Widespread tobacco use and high interest in quitting make prisons an ideal environment for smoking cessation interventions; however, little has been done to assist prisoners in their efforts to quit. Valid measurement of tobacco use is a prerequisite to evaluation of cessation programs, yet there has been only one published examination of tobacco use measures among prisoners.
Methods:
Tobacco use interviews were conducted with 200 male prisoners. Three measures of tobacco use, exhaled carbon monoxide (eCO), salivary cotinine measured by enzyme immunoassay (EIA), and salivary cotinine measured by liquid chromatography/tandem mass spectrometry (LC/MS/MS), were evaluated using self-reported tobacco use as the reference. Optimum cutpoints were identified by maximization of the Youden index.
Results:
Carbon monoxide breath testing, though the poorest performing of the three measures examined, still had excellent discrimination (cutpoint ≥ 4 ppm, sensitivity = 88.3%, specificity = 94.9%). Cotinine EIA performed better than eCO (cutpoint ≥ 10 ng/ml, sensitivity = 92.2%, specificity = 94.3%) but poorer than cotinine LC/MS/MS (cutpoint ≥ 9 ng/ml, sensitivity = 98.6%, specificity = 97.8%).
Discussion:
eCO had the poorest performance as a standalone test, though validity of the test may be improved with increased frequency of testing. False-negative results using cotinine EIA limit its utility as a standalone test, however, as part of a two-stage screening process it may reduce the cost of testing. Cotinine LC/MS/MS, while most expensive, was the most accurate standalone measure of prisoners’ tobacco use.
Introduction
Following the release of the first Report on Smoking and Health in 1964, the prevalence of smoking in the United States has decreased steadily from a peak of around 40% to about 20% (Centers for Disease Control and Prevention, 2008; Giovino, 2007). However, declines in smoking have not been equally realized in all segments of the population. One group for whom the prevalence of smoking remains high is prisoners. Despite growing awareness of the harms of tobacco use and its decreased social acceptability, studies have consistently shown that some 60%–80% of incarcerated individuals choose to smoke or use other forms of tobacco (Conklin, Lincoln, & Tuthill, 2000; Cropsey, Eldridge, & Ladner, 2004; National Commission on Correctional Health Care, 2001). There is also evidence that many prisoners wish to quit (Cropsey et al., 2004).
The combination of a high prevalence of smoking and high interest in quitting creates an ideal environment for intervention; however, little work has been done to date to assist prisoners in their efforts to quit. In fact much of the basic research necessary for the design and evaluation of cessation programs has yet to be done in the prison setting. Measuring tobacco consumption is central to understanding patterns of use in populations and evaluating the efficacy of interventions. Much work has been done to determine appropriate measures of tobacco use for the general population (SRNT Subcommittee on Biochemical Verification, 2002); however, it is unclear whether this work is generalizable to the prison setting where greater social acceptability of tobacco use, low wages relative to the high cost of tobacco, and restrictive tobacco policies impact prisoners’ patterns of tobacco use, as these changes may affect the performance of biomarkers and influence the selection of optimal cutpoints. There has been only one published evaluation of self-report and biochemical tobacco use measures among prisoners, and it was limited to female prisoners (Cropsey, Eldridge, Weaver, Villalobos, & Stitzer, 2006).
Asking individuals to self-report tobacco use is the least expensive means of assessment and allows for the collection of detailed information on past tobacco use and variations in current use. Self-reports have generally been found to be accurate; however, they may be prone to recall bias or intentional misreporting (Gorber, Schofield-Hurwitz, Hardt, Levasseur, & Tremblay, 2009). Recall bias is a potential concern in prisons, where drug and alcohol abuse and mental illness are more common than in the general population (Jacobi, 2005; Lo & Stephens, 2000); however, intentional underreporting, observed among groups for whom smoking is perceived as being especially undesirable (Ford, Tappin, Schluter, & Wild, 1997), is unlikely in prisons with limited tobacco restrictions given the high prevalence of smoking among prisoners. There may be specific circumstances that increase the probability of misreporting among prisoners; for example, cessation intervention participants may feel social pressure to underreport smoking, and the threat of punishment may lead to underreporting in facilities with a total tobacco ban.
In situations where the validity of self-report data is suspect, biomarkers for tobacco exposure provide an objective measure, though this objectivity comes with several trade-offs. The measurement of biomarkers is more costly than asking participants about their tobacco use and requires specialized equipment. Additionally, biomarkers usually provide a measure of average or cumulative exposure over a period of time and cannot capture variation in exposure during that period.
Exhaled carbon monoxide (eCO) has a half-life of 2–8 hr depending on an individual’s level of physical activity, allowing detection of smoking over a 6–24 hr period (SRNT Subcommittee on Biochemical Verification, 2002). Measurement of eCO is a brief noninvasive procedure that provides immediate results. Following the initial purchase of a carbon monoxide (CO) monitor (current models cost around $1000) testing eCO is relatively inexpensive. The study of eCO among female prisoners, mentioned above, found good agreement between the breath test and self-reported smoking (Cropsey et al., 2006).
The nicotine metabolite cotinine has a half-life of around 16 hr, providing a means of assessing tobacco use over a 3- to 4-day period (SRNT Subcommittee on Biochemical Verification, 2002). Cotinine can be measured in several types of biological specimens, including serum, urine, or saliva (SRNT Subcommittee on Biochemical Verification). Multiple assays exist, with varying degrees of precision and cost. Methods combining chromatography and mass spectrometry allow for a minimum detectable limit of around 1 ng/ml at a cost of approximately $25 per sample. Enzyme immunoassay (EIA) tests are less expensive, costing about $15 per sample, but less precise with a minimum detectable level of 10 ng/ml and may overestimate cotinine concentrations due to cross-reactivity with other nicotine metabolites.
The current study sought to establish the performance of four tobacco use measures among prisoners: (a) prisoner self-report, (b) eCO, (c) salivary cotinine measured by EIA, and (d) salivary cotinine measured by liquid chromatography/tandem mass spectrometry (LC/MS/MS). The performance of the biomarker tests was evaluated using self-reported tobacco use as the reference method. These data are intended to assist prison researchers in selecting appropriate measures of tobacco use for future studies and identifying optimal cutoff points to distinguish smokers from nonsmokers in a prison population.
Methods
Participants and setting
The study was conducted in two low-to-medium security Ohio prisons housing male inmates. At the time of the study, the Department of Correction did not allow any staff or inmates to smoke inside of buildings. Inmates could still purchase tobacco products and smoke when outdoors on the prison grounds.
Consecutively-admitted prisoners were invited to take part in the study, starting with those prisoners entering the prison system 14 weeks prior to the start of interviews. Recruitment continued until 100 participants were obtained in each facility, giving a total of 200 participants for the study. To be eligible, participants had to be 18 years of age or older, speak sufficient English to complete the interview, and be residents of the general population of the facility at the time of the interview (not in solitary confinement, receiving medical care, or off compound for an outside court appearance). Individuals released from prison prior to their invitation to take part in the study were excluded.
Measuring tobacco use
Participants self-reported tobacco use by responding to a modified National Health and Nutrition Examination Survey tobacco use questionnaire. Two versions of the questions were asked. To assess tobacco use before incarceration, the questions were altered to begin “Prior to your arrest … ” while a second set of questions asked about tobacco use since arriving in the prison. Questions separately inquired about cigarettes, cigars, pipes, snuff, chewing tobacco, and other tobacco products. Participants were asked to provide greater detail about their tobacco use since arrival at the facility. For each tobacco product, they were asked if they had used the product, even once, since arriving. Questions about their frequency of indoor tobacco use assessed compliance with prison tobacco policies. Participants also completed the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
At the end of the interview, participants were asked to provide breath and saliva samples. To minimize the risk of coercion, and comply with approved protocols, it was emphasized that provision of the samples was voluntary. eCO was measured using the Micro 4 Smokerlyzer breath analyzer (Bedfont Scientific, USA, Williamsburg, VA). Saliva samples were collected using the Salivette saliva collection device (Sarstedt Inc., Newton, NC). Two samples were collected from each individual to decrease the likelihood that there would be an insufficient volume of saliva for testing. Samples were sent to an external laboratory for analysis (J2 Laboratories, Phoenix, AZ). Laboratory staff were blind to the self-reported smoking status of the participants. Salivary cotinine concentration was measured using two methods, EIA and LC/MS/MS (Bernert, McGuffey, Morrison, & Pirkle, 2000; Diagnostic Products Corporation, 2004).
Statistical analysis
Data analyses were carried out in SAS version 9.2 for Linux (SAS Institute Inc., Cary, NC). Because the widespread use of tobacco products in prisons provides little social pressure to misreport smoking status, self-reported smoking (for eCO) or tobacco use (for cotinine EIA and LC/MS/MS) was used as the reference for evaluating the performance of biomarker tests. Receiver operating characteristic (ROC) curves were constructed to examine test performance across all possible cutpoints using the ROC Macro (SAS Institute, 2007). Maximization of the unweighted Youden index (J) was used to determine the optimum cutpoint for each test (Youden, 1950). Optimal cutpoints were then used to assign a binary classification (user or nonuser) to continuous variables. The SENSPE macro was used to calculate the sensitivity and specificity as well as positive and negative predictive values at the prevalence of smoking observed in the sample (Cha, 2005).
Correlation coefficients were calculated for the eCO and EIA measures relative to individuals’ LC/MS/MS values. Because eCO can only detect smoked tobacco, participants reporting smokeless tobacco use were excluded from the correlation analysis. Concentrations below the lower limit of the EIA test returned a value of “<10 ng/ml.” Given the small range of possible values, these were assigned the central value of 5 ng/ml for the correlation analysis. For some samples with a very high cotinine concentration, the EIA test could only specify the result as “>500 ng/ml.” These points were dropped when calculating the correlation coefficient.
A weighted kappa statistic was used to provide a second assessment of agreement between the EIA and LC/MS/MS measures, which could include these dropped observations (Cohen, 1968). Two methods were used to calculate the kappa statistic. For the first test, results for both cotinine levels were categorized into eight fixed groups <15, 15–49, 50–99, 100–199, 200–299, 300–399, 400–499, and ≥500 ng/ml, allowing for a comparison of absolute agreement. For the second kappa calculation, each measure was categorized into quintiles as a means of gauging relative agreement. A linear weighting scheme was used for both kappa calculations.
Results
Sample characteristics
The interviewer met with 282 eligible prisoners to obtain the target sample size of 200, yielding a response rate of 70.9%. A comparison of study participants with a representative sample of new inmates found that they were of comparable age (mean age: 33.8 vs. 32.3), but somewhat more likely to be White (63.5% vs. 48.7%) and more likely to have at least a high school education (65.5% vs. 58.2%; Bates, Gonzalez, & Muncy, 2008). The final sample of 200 individuals provided self-reported tobacco use information. Of these, 173 individuals (86.5%) provided samples for the eCO, EIA, and LC/MS/MS tests. The remaining individuals were missing one or more values: 16 people (8.0%) declined to take part in either biomarker tests, 8 (4.0%) supplied eCO but declined to provide a saliva sample for cotinine testing, and 3 (1.5%) completed the eCO test and provided a saliva sample; however, insufficient saliva was provided to conduct the LC/MS/MS test. Sample characteristics, including a comparison with those missing one or more values, are presented in Table 1. There were no statistically significant differences in demographic factors between the group of participants who agreed to provide all tobacco measures and those excluded from this analysis.
Table 1.
Sample demographics and smoking characteristics by availability of biomarker data
| Full biomarker data present (n = 173) | Missing data on ≥1 biomarker (n = 27) | p Valuea | |
| Age in years, M (SD) | 33.4 (10.2) | 36.0 (10.2) | .231 |
| Race, n (%) | .794 | ||
| Non-Hispanic White | 101 (58.4) | 18 (66.7) | |
| Non-Hispanic Black | 56 (32.4) | 7 (25.9) | |
| Hispanic | 5 (2.9) | 0 (0.0) | |
| Other | 11 (6.4) | 2 (7.4) | |
| Education, n (%) | .547 | ||
| Less than HS | 59 (34.1) | 10 (37.0) | |
| G.E.D. | 31 (17.9) | 5 (18.5) | |
| HS Graduate | 44 (25.4) | 9 (33.3) | |
| At least some college | 39 (22.5) | 3 (11.1) | |
| Sentenced for a violent crime | 49 (28.3) | 8 (29.6) | 1.000 |
| Tobacco useb, n (%) | |||
| Never user | 26 (15.0) | 2 (7.4) | .648 |
| Former user | 9 (5.2) | 1 (3.7) | |
| Current user | 138 (79.8) | 24 (88.9) | |
| Smoker characteristicsc | Full biomarker data present (n = 134) | Missing data on ≥1 biomarker (n = 23) | |
| Cigarettes per day, M (SD) | 9.9 (9.0) | 13.8 (17.1) | .109 |
| Age of initiation, M (SD) | 16.8 (15.8) | 16.7 (14.0) | .964 |
| FTNDd score, M (SD) | 3.3 (1.8) | 3.6 (1.8) | .565 |
Note. HS = high school; G.E.D. = General Educational Development.
t Test for continuous variables, Fisher’s exact test for categorical data.
Participants’ self-reported tobacco use status (includes smoking and smokeless use) at time of interview.
Restricted to participants reporting current cigarette use at the time of the interview.
Fagerström Test for Nicotine Dependence (Heatherton et al., 1991).
Exhaled carbon monoxide
The 184 participants who completed the breath test were included in the eCO evaluation. The area under the ROC curve (AUC) for the carbon monoxide breath test was 0.942 (95% CI: 0.910–0.973), indicating excellent discrimination according to the criteria given by Hosmer and Lemeshow (2000). A maximum Youden index (J = 0.831) was obtained with a cutpoint of ≥4 ppm. Using this optimum cutpoint, eCO had a sensitivity of 88.3% (95% CI: 81.9%–93.0%) with 128 of 145 smokers correctly identified. The specificity of the test at the optimum cutpoint was 94.9% (95% CI: 82.7%–99.4%) with 37 of 39 nonsmokers categorized correctly. At the prevalence of tobacco use in the sample, eCO had a positive predictive value of 98.5% (95% CI: 94.6%–99.8%) and a negative predictive value of 68.5% (95% CI: 54.4%–80.5%) with 89.7% of participants classified correctly.
Salivary cotinine—EIA
All 176 participants with EIA test results were used in the evaluation of the test. The AUC for salivary cotinine analyzed using EIA was 0.957 (95% CI: 0.933–0.981), indicating excellent discrimination. There were 44 participants with a result of “<10 ng/ml” of salivary cotinine, the minimum detectable level for the test, including 33 of the 35 participants identifying as nonsmokers. A maximum Youden index (J = 0.865) was obtained when individuals above the detectable limit were classified as tobacco users; however, as the next highest EIA test was 21 ng/ml, it can only be stated that the optimum cutpoint for use of the test in incarcerated populations lies between 10 and 21 ng/ml. Using a cutpoint in this range, the EIA test had a sensitivity of 92.2% (95% CI: 86.5%–96.0%), a specificity of 94.3% (95% CI: 80.8%–99.3%), a positive predictive value of 98.5% (95% CI: 94.6%–99.8%), and negative predictive value of 75.0% (95% CI: 59.7%–86.8%) at the prevalence of tobacco use in the sample. The EIA test correctly classified 92.6% of participants in the sample.
Salivary cotinine—LC/MS/MS
LC/MS/MS results were available for 173 individuals. The AUC for salivary cotinine analyzed using LC/MS/MS was 0.990 (95% CI: 0.977–1.000), indicating excellent discrimination. A cutpoint of ≥9 yielded the maximum Youden index (J = 0.900). At the optimum, the LC/MS/MS test had a sensitivity of 98.6% (95% CI: 94.9%–99.8%), a specificity of 91.4% (95% CI: 76.9%–98.2%), a positive predictive value of 97.8% (95% CI: 93.8%–99.6%), and negative predictive value of 94.1% (95% CI: 80.3%–99.3%) at the prevalence of tobacco use in the sample. The LC/MS/MS test correctly classified 97.1% of participants in the sample.
Correlation of biomarker tests
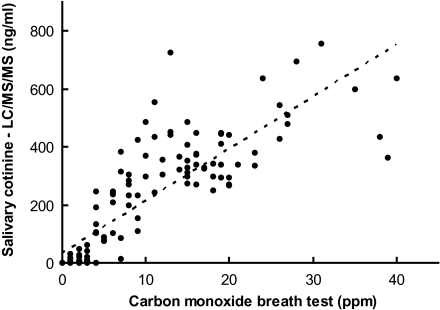
Data from the 122 individuals who did not report using smokeless tobacco were used to calculate the correlation between eCO and LC/MS/MS salivary cotinine measures. Figure 1 compares eCO with salivary cotinine as measured by LC/MS/MS. The correlation coefficient was .8310.
Figure 1.
Scatter plot of exhaled carbon monoxide concentrations versus salivary cotinine concentrations measured by liquid chromatography/tandem mass spectrometry (LC/MS/MS) for incarcerated men excluding users of smokeless tobacco (r = .8310).
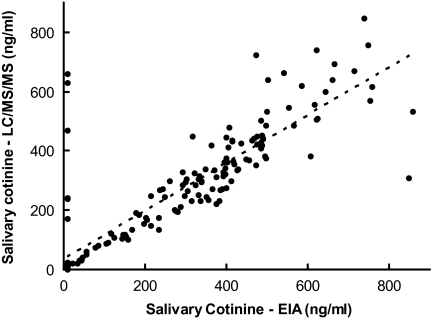
Data from 164 individuals were used to calculate the correlation between the EIA and LC/MS/MS salivary cotinine results; 9 individuals with EIA readings reported as “>500 ng/ml” were excluded. The scatter plot comparing EIA and LC/MS/MS measures of salivary cotinine is shown in Figure 2. The correlation coefficient was .8602.
Figure 2.
Scatter plot of salivary cotinine concentrations measured by enzyme immunoassay (EIA) versus salivary cotinine concentrations measured by liquid chromatography/tandem mass spectrometry (LC/MS/MS; r = .8602).
The kappa calculations yielded similar results whether using absolute or relative categories. For the absolute categories, there was 92.65% agreement, well above the 59.38% expected from chance (κ = .8191, p < .0001). For the relative categories, there was 92.05% agreement, with 59.34% agreement expected by chance (κ = .8045, p < .0001).
Discussion
Acceptability of biological testing
This study provides evidence that many incarcerated individuals are willing to supply minimally invasive biological samples for research purposes. Even with a separate assent process and clear indications that provision of samples was voluntary, a strong majority of prisoners provided samples. A few participants (4.0%) agreed to the breath test but declined to supply a saliva sample, and a minority (8.0%) declined to take part in either test. Motivation for declining the tests was not assessed; however, it is possible that some prisoners may have had concerns about forensic uses of samples, despite assurances that they would only be used to measure tobacco use.
Self-reported tobacco use
Since self-reported tobacco use was treated as the reference for evaluating the other measures, it is important to consider the validity of the self-report data. Concerns with intentional misreporting are usually focused on underreporting of tobacco use. Only five individuals reported not smoking but had a positive result on one or more of the biomarker tests. Four of these reported smoking at some point during their incarceration, but three said that they had quit smoking since arriving and one reported only smoking twice during incarceration. The positive test results may be due to unreported lapses during the current quit attempt. In future studies, the validity of self-reported tobacco use may be improved by including specific questions about smoking lapses for prisoners reporting tobacco cessation. These “one-time” events could be viewed as unimportant by prisoners and might otherwise go unreported.
Generally, self-reported tobacco use appears, under the study conditions, to provide an accurate measure of tobacco use in prisons with an indoor smoking ban. Several factors could explain the accuracy of self-reported tobacco use. In populations with strong social pressure to underreport tobacco use, the knowledge that a biological sample will be collected to confirm self-reports has been observed to increase the accuracy of reporting in what is known as the bogus pipeline effect (Evans, Hansen, & Mittelmark, 1977). In the current study, participants were informed during the consent process that collection of biomarkers was one aspect of the study but that providing the samples was optional and they could take part in the rest of the study without doing so. The knowledge that their responses could be checked against an objective measure may have promoted more accurate reporting; however, with the option of refusing the biomarker tests, this effect was probably minimal. A second, and likely dominant, contributor to the observed high validity was the lack of social pressure to misreport tobacco use. In the facilities under study, prisoners could purchase tobacco products through the commissary and freely smoke outside of buildings. Furthermore, smoking may be regarded as normative in a facility where more than three quarters of residents report smoking. In such an environment, most prisoners would have little reason to intentionally misreport their tobacco use.
Further work is needed to determine the validity of self-reported tobacco use in the increasing number of correctional facilities with a total smoking bans (Kauffman, Ferketich, & Wewers, 2008). Prisoners may be reluctant to provide information that they believe could result in punishment, despite assurances of confidentiality. Informing participants of the intention to use biomarker confirmation may have a greater impact on the validity of self-reported tobacco use in prisons with a total ban. It should be noted that participants were asked about indoor smoking (in violation of prison policy) during the current study. The fact that a majority of smokers admitted to violating the policy indicates openness among prisoners to share information with researchers, even about sensitive topics.
Exhaled carbon monoxide
Though the test still showed excellent discrimination, carbon monoxide breath testing was the least effective of the tested methods for measuring tobacco use when used as a one-time test. In the current sample, the optimum eCO cutpoint for differentiating smoking status was ≥4 ppm, well below the range of 8–10 ppm recommended for studies in the general population (SRNT Subcommittee on Biochemical Verification, 2002). This mirrors findings from a study among female prisoners, carried out in a facility without an indoor smoking ban, which found that using a low cutpoint (≥3 ppm) provided the best performance (Cropsey et al., 2006). Cropsey et al. reported better performance of eCO than that we found (sensitivity: 98.1% vs. 88.3%, specificity: 95.8% vs. 94.9%). The difference in test performance may be attributable to differential patterns of use under the prisons’ tobacco policies. The high cost of tobacco and limitations on when and where inmates are allowed to smoke contributes to irregular patterns of tobacco use in the present sample. Consequently, eCO, with its relatively short half-life, is not an ideal indicator of tobacco smoking in prison when employed as a one-time test. Since the half-life of CO is impacted by physical activity, problems with detecting irregular smoking may be exacerbated among the subgroup of prisoners who use exercise as their primary means of passing time.
These limitations do not, however, eliminate the utility of eCO in prison research. Breath testing is inexpensive and noninvasive, and the regression analysis showed a strong correlation between cotinine and eCO values. Rapid clearance of CO from the body can be countered by more frequent testing. Though unsuitable for epidemiological studies gathering information during a single visit, eCO testing could offer a valuable means of monitoring abstinence from smoking during cessation trials with regular follow-up. When repeated measures are desirable, the low cost and instantaneous feedback offered by eCO may outweigh its disadvantages.
Salivary cotinine
Cotinine is a good measure of average tobacco use over a 3- to 4-day period; however, it cannot assess use outside of that window. In prisons, the high cost of tobacco relative to prisoner income leads many prisoners to reduce tobacco consumption. For some prisoners who smoke, this means abstaining from smoking for several days at a time. To avoid false-negative results when using cotinine to detect tobacco use, it may be desirable to collect two samples several days apart. This could be done without increasing testing costs by pooling an individual’s samples and running a single test. Pooling saliva samples for cotinine testing has been used in other contexts to good results (Bell & Ellickson, 1989). Another strategy for reducing false-negative tests is to collect samples within 3 days of the time a prisoner visits the commissary. At many facilities, prisoners have limited opportunities to make purchases, with a single scheduled visit to the commissary once every 1–2 weeks based on their dorm or cell block of residence. Incarcerated individuals, especially those with limited financial resources, are most likely to have access to and use tobacco around that time.
The two cotinine tests were strongly correlated and consistently showed good agreement; however, the current study suggests that there are some differences in their performance. Using LC/MS/MS to measure salivary cotinine concentrations was not only the most expensive biomarker tested but also the most accurate. While the EIA test performed well overall, correctly classifying 92.6% of participants, there was a problem with the test producing false-negative values. Of 137 individuals with LC/MS/MS values above 15 ng/ml, 8 people (5.8%) had EIA tests with the minimum value of <10 ng/ml. All but one of these participants reported using tobacco products during their interviews, supporting the LC/MS/MS results.
When testing interventions, researchers are most often concerned with confirming abstinence. The negative predictive value of the EIA test (75.0%) may not be sufficient for these purposes; however, in large epidemiological studies where the approach involves biological confirmation of tobacco use status for smokers and nonsmokers alike, the high sensitivity and overall accuracy of the test may provide a means of biomarker confirmation that is more economically feasible than LC/MS/MS testing. In populations where the prevalence of smoking exceeds 63%, a common reality in the prison setting, using a two-stage screening strategy (EIA first, retesting negative results with LC/MS/MS), would save money over a one-stage strategy using the LC/MS/MS test on all samples while providing a greater negative predictive value (99.8%).
General conclusions
Strong agreement between self-reports and multiple biomarkers indicates that directly asking prisoners about their tobacco use is an accurate means of assessing tobacco use in prisons with indoor tobacco bans. This approach is not only less expensive than biomarkers but also provides researchers with detailed information about variations in tobacco use that cannot be obtained using traditional biological indicators. These variations are especially important in the prison population where the high cost of tobacco relative to prisoner pay may lead to erratic patterns of tobacco use, which may negatively impact the validity of biomarker tests, especially those with shorter half-lives, such as eCO.
When circumstances call for biological confirmation of self-reported tobacco use, our findings indicate that, for a test conducted at a single point in time, cotinine testing using LC/MS/MS is the best option when economically viable. eCO had the poorest performance as a standalone test, though the validity of the test may be able to be improved by increasing the frequency of testing. False-negative results from the EIA test limit its utility as a standalone test; however, as a component of a two-stage screening process, EIA may offer a means of increasing the affordability of biomarker confirmation. This study has described the performance of key measures of tobacco use in prisons with indoor tobacco bans. Given current policy trends in U.S. prison systems, there is a need for similar work to be conducted in facilities with total tobacco bans.
Funding
Design of the study was supported by the Behavioral Cooperative Oncology Group of the Mary Margaret Walther Program, Walther Cancer Institute, Indianapolis, Indiana. This work was supported by the Centers for Disease Control and Prevention (CDC; grant number 1R36DP001167-01) and the National Cancer Institute (NCI) at the National Institutes of Health (grant number R25-CA117865-01A1).
Declaration of Interests
None declared.
Acknowledgments
The views expressed in this publication are the authors’ and do not necessarily represent the official views of the CDC or NCI.
References
- Bates J, Gonzalez C, Muncy V. Ohio Department of Rehabilitation and Correction: 2007 Intake Study. Columbus, OH: Ohio Department of Rehabilitation and Correction; 2008. Retrieved 16 October 2009, from http://www.drc.state.oh.us/web/Reports/intake/Intake%202007.pdf. [Google Scholar]
- Bell RM, Ellickson PL. Does pooling saliva for cotinine testing save money without losing information? Journal of Behavioral Medicine. 1989;12:503–507. doi: 10.1007/BF00844881. [DOI] [PubMed] [Google Scholar]
- Bernert JT, Jr., McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. Journal of Analytical Toxicology. 2000;24:333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2007. MMWR. Morbidity and Mortality Weekly Report. 2008;57:1221–1226. [PubMed] [Google Scholar]
- Cha S. SenSpe SAS Macro. 2005. Retrieved 27 January 2010, from http://mayoresearch.mayo.edu/biostat/upload/senspe.sas. [Google Scholar]
- Cohen J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychological Bulletin. 1968;70:213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- Conklin TJ, Lincoln T, Tuthill RW. Self-reported health and prior health behaviors of newly admitted correctional inmates. American Journal of Public Health. 2000;90:1939–1941. doi: 10.2105/ajph.90.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey K, Eldridge GD, Ladner T. Smoking among female prisoners: An ignored public health epidemic. Addictive Behaviors. 2004;29:425–431. doi: 10.1016/j.addbeh.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Eldridge GD, Weaver MF, Villalobos GC, Stitzer ML. Expired carbon monoxide levels in self-reported smokers and nonsmokers in prison. Nicotine & Tobacco Research. 2006;8:653–659. doi: 10.1080/14622200600789684. [DOI] [PubMed] [Google Scholar]
- Diagnostic Products Corporation. IMMULITE 2000 nicotine metabolite. 2004. Retrieved 4 March 2009, from http://www.crlcorp.com/getDocument.cfm?documentID=63. [Google Scholar]
- Evans RI, Hansen WB, Mittelmark MB. Increasing the validity of self-reports of smoking behavior in children. Journal of Applied Psychology. 1977;62:521–523. [PubMed] [Google Scholar]
- Ford RP, Tappin DM, Schluter PJ, Wild CJ. Smoking during pregnancy: How reliable are maternal self reports in New Zealand? Journal of Epidemiology and Community Health. 1997;51:246–251. doi: 10.1136/jech.51.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino GA. The tobacco epidemic in the United States. American Journal of Preventive Medicine. 2007;33(6 Suppl):S318–S26. doi: 10.1016/j.amepre.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine & Tobacco Research. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Assessing the fit of the model. Applied logistic regression. 2nd ed. New York: John Wiley & Sons; 2000. pp. 143–202. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jacobi JV. Prison health, public health: Obligations and opportunities. American Journal of Law & Medicine. 2005;3:447–478. doi: 10.1177/009885880503100403. [DOI] [PubMed] [Google Scholar]
- Kauffman RM, Ferketich AK, Wewers ME. Tobacco policy in American prisons, 2007. Tobacco Control. 2008;17:357–360. doi: 10.1136/tc.2007.024448. [DOI] [PubMed] [Google Scholar]
- Lo CC, Stephens RC. Drugs and prisoners: Treatment needs on entering prison. American Journal of Drug and Alcohol Abuse. 2000;26:229–245. doi: 10.1081/ada-100100602. [DOI] [PubMed] [Google Scholar]
- National Commission on Correctional Health Care. National Commission on Correctional Health Care Clinical Guideline for Correctional Facilities: Treatment of high blood cholesterol. Journal of Correctional Health Care. 2001;8:123–130. [Google Scholar]
- SAS Institute. ROC SAS Macro. SAS Knowledge Base. 2007. Retrieved 28 January 2010, from http://support.sas.com/kb/25/addl/fusion_25017_6_roc.sas.txt. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]