Abstract
Background:
Nicotine alters auditory event-related potentials (ERPs) in rodents and humans and is an effective treatment for smoking cessation. Less is known about the effects of the partial nicotine agonist varenicline on ERPs.
Methods:
We measured the effects of varenicline and nicotine on the mouse P20 and varenicline and smoking on the human P50 in a paired-click task. Eighteen mice were tested following nicotine, varenicline, and their combination. One hundred and fourteen current smokers enrolled in a placebo-controlled within-subject crossover study to test the effects of varenicline during smoking and abstinence. Thirty-two subjects participated in the ERP study, with half receiving placebo first and half varenicline first (VP).
Results:
Nicotine and varenicline enhanced mouse P20 amplitude, while nicotine improved P20 habituation by selectively increasing the first-click response. Similar to mice, abstinence reduced P50 habituation relative to smoking by reducing the first-click response. There was no effect of varenicline on P50 amplitude during abstinence across subjects. However, there was a significant effect of medication order on P50 amplitude during abstinence. Subjects in the PV group displayed reduced P50 during abstinence, which was blocked by varenicline. However, subjects in the VP group did not display abstinence-induced P50 reduction.
Conclusions:
Data suggest that smoking improves sensory processing. Varenicline mimics amplitude changes associated with nicotine and smoking but fails to alter habituation. The effect of medication order suggests a possible carryover effect from the previous arm. This study supports the predictive validity of ERPs in mice as a marker of drug effects in human studies.
Introduction
During abstinence, smokers experience deficits in cognition and sensorimotor processing that may contribute to relapse (Hughes, 2007; Jacobsen et al., 2005; Mendrek et al., 2006; Powell, Pickering, Dawkins, West, & Powell, 2004; Rissling, Dawson, Schell, & Nuechterlein, 2007; Ward, Swan, & Jack, 2001). Pharmacotherapies for nicotine dependence (ND) that target these symptoms may enhance quitting success (Markou & Paterson, 2009). Food and Drug Administration-approved smoking cessation therapies including nicotine replacement (NRT), bupropion, and varenicline reduce withdrawal symptoms and smoking urges while improving mood and cognitive function (Patterson et al., 2009; Rahman, Lopez-Hernandez, Corrigall, & Papke, 2008; Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006; Shiffman et al., 2000; West, Baker, Cappelleri, & Bushmakin, 2008). Additionally, there is evidence that bupropion and varenicline can reduce cognitive deficits in animal models of nicotine withdrawal (Paterson, Balfour, & Markou, 2008; Portugal & Gould, 2007; Raybuck, Portugal, Lerman, & Gould, 2008). Varenicline is a newer medication that has superior efficacy relative to NRT, bupropion, and placebo in clinical trials (Aubin et al., 2008; Gonzales et al., 2006; Jorenby et al., 2006; Oncken et al., 2006). Although varenicline is a potent partial agonist at α4β2 receptors, it also has 25-fold lower affinity as a partial agonist at α3β4, α3β2, and α6 nAChRs and 8-fold lower affinity as full agonist at α7 receptors (Mihalak, Carroll, & Luetje, 2006). However, less is known about the effects of varenicline on electrophysiological measures of sensory processing.
In humans, the P50 is a positive voltage deflection in the electroencephalogram (EEG) that occurs approximately 50 ms after onset of an auditory stimulus. When paired auditory stimuli are presented at short interstimulus intervals, the first stimulus (S1) elicits a larger response than the second stimulus (S2). This phenomenon represents habituation of the response to repeated stimuli and is sometimes referred to as habituation in this context (Stevens, Kem, & Freedman, 1999; Stevens, Kem, Mahnir, & Freedman, 1998). Several studies have investigated the effects of nicotine on P50 habituation. Additionally, studies have suggested a role of α7 nicotinic receptors in this function, possibly by influencing cholinergic input to gamma amino butyric acid ergic interneurons (Stevens et al., 1998). Smoking improves P50 habituation in schizophrenic individuals, and nicotine gum is sufficient to achieve similar enhancements in their relatives (Adler, Hoffer, Griffith, Waldo, & Freedman, 1992; Adler, Hoffer, Wiser, & Freedman, 1993). Changes in the ratio of response for the S2:S1 are sometimes used as a measure of habituation. Several studies demonstrate that the S2:S1 ratio is sensitive to changes in S1 rather than S2, suggesting a change in the ability to mount the initial response rather than the ability to habituate the second (Adler, Pang, Gerhardt, & Rose, 1988; Crawford, McClain-Furmanski, Castagnoli, & Castagnoli, 2002; Halene & Siegel, 2008; Maxwell, Kanes, Abel, & Siegel, 2004; Maxwell et al., 2006; Metzger, Maxwell, Liang, & Siegel, 2007; Phillips, Ehrlichman, & Siegel, 2007). For example, nonpsychiatric heavy smokers exhibit greater P50 amplitude and S2:S1 ratio compared with never-smokers (Crawford et al.). However, studies of the acute effects of smoking versus abstinence on P50 responses in normal smokers have yielded mixed results, with some studies demonstrating that the effects of smoking are mediated by S1 response (Adler et al., 1993; Crawford et al.; Domino, 2003; Kishimoto & Domino, 1998).
The topology and habituation of auditory event-related potentials (ERPs) in the mouse closely resemble the human responses but follow a faster time course. The mouse P20, a positive deflection that occurs 20 ms after stimulus onset, is thought to be analogous to the human P50 (Maxwell, Liang, et al., 2004; Siegel et al., 2003; Umbricht et al., 2004). Acute nicotine increases amplitude of the mouse P20 in response to S1 and, as a result, reduces the apparent habituation ratio (S2:S1; Metzger et al., 2007; Phillips et al., 2007). However, it is important to note that such changes reflect altered registration of the initial stimulus rather than improved suppression of the second. Additionally, nicotine has been shown to reverse auditory habituation deficits in mouse models of psychosis (Siegel et al., 2005; Stevens, Meltzer, & Rose, 1995). Previous studies indicate that dihydro-beta-erythroidine (DHβE) blocks nicotine’s effect on amplitude but not habituation of the P20 and P20/N40 auditory ERPs (Siegel, S. et al., Annual Meeting of the American College of Neuropsychopharmacology 2006 and Phillips et al., in review; Kawai, Lazar, & Metherate, 2007; Radek et al., 2006). DHβE blocks α4β2 and α4β4 and, to a lesser extent, α2β2 and α3β2 as well as α2β4 nAChRs (Chavez-Noriega et al., 1997). Therefore, previous studies with DHβE suggest involvement of β2 and/or β4 containing nicotinic receptors in generation of the P20.
In this study, we examined the effects of nicotine and varenicline in mice. In mice, we predicted that nicotine would enhance P20 habituation by increasing S1 response amplitude. Based on the effect of DHβE and varenicline’s relative selectivity for α4β2 nAChRs, we hypothesized that varenicline would increase P20 amplitude without affecting habituation. We also tested the effects of smoking versus abstinence and varenicline versus placebo in human chronic smokers. We hypothesized that smoking would enhance P50 habituation relative to abstinence by increasing S1 response amplitude. Furthermore, we hypothesized that varenicline would attenuate the effects of abstinence on P50 amplitude in human smokers.
Materials and methods
Mouse study
Subjects
Eighteen male wild-type C57BL/6J mice (Jackson Labs, Bar Harbor, ME) between 9 and 11 weeks of age were used for auditory testing. Mice were housed in light- and temperature-controlled animal facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Animals were acclimated to the housing facility for 1 week prior to testing and provided with food and water ad libitum. All protocols were conducted in accordance with University Laboratory Animal Resources guidelines and were approved by the Institutional Animal Care and Use Committee.
Surgery
Mice underwent stereotaxic implantation of electrode assemblies (Plastic Products, Roanoke, VA) for later nonanesthetized recording of auditory ERPs. Mice were anesthetized with isoflurane for the duration of the implantation procedure. Unipolar recording electrodes were placed unilaterally in the CA3 hippocampal region (1.4 mm posterior, 2.65 mm lateral, and 2.75 mm deep relative to bregma) and referenced to the ipsilateral frontal sinus to reflect whole brain electrical activity from these two perspectives. The electrode pedestal was secured to the skull using dental cement (Ortho Jet; Lang Dental, Wheeling, IL) and ethyl cyanoacrylate (Loctite; Henkel KGaA, Duesseldorf, Germany).
Drug conditions
Mice received subcutaneous injections of 1.0 mg/kg nicotine tartrate and 1.2 mg/kg varenicline tartrate (Pfizer, Groton, CT). All drug concentrations are reported as freebase. The mouse study was designed to mimic the human design such that each mouse received each of four conditions as follows: nicotine (similar to smoking), saline (similar to abstinence), nicotine and varenicline (similar to taking varenicline while smoking), and varenicline (similar to taking varenicline during abstinence). These conditions were separated by 48 hr. Animals were divided into two groups and drug conditions were counterbalanced across recording sessions to control for any potential order effects, similar to the human study as noted below (Table 1).
Table 1.
The mouse study was designed to mimic the human design such that each animal received four conditions as follows: nicotine (similar to smoking), saline (similar to abstinence), nicotine and varenicline (similar to taking varenicline while smoking), and varenicline (similar to taking varenicline during abstinence)
| Session | 1 | 2 | 3 | 4 |
| Group 1 | Nicotine | Saline | Nicotine & varenicline | Varenicline |
| Group 2 | Nicotine & varenicline | Varenicline | Nicotine | Saline |
Note. Sessions were separated by 48 hr. Animals were divided into two groups and drug order were counterbalanced across recording sessions to control for any potential order effects, similar to the human study.
Recording
Electrophysiological testing was conducted for 4 days with a washout period of 48 hr between recording sessions and three stimuli presentations per session. The first presentation involved no injection to acclimate animals to the stimuli, the second presentation followed a saline injection, and the third presentation occurred 5 min after injection of the test compound(s). This timing allows for recording of ERPs within 1 serum half-life for nicotine in mouse. Stimuli were generated by Micro1401 hardware and Spike 6 software (Cambridge Electronic Design, Cambridge, UK) and were delivered through speakers attached to the cage top. All recordings were performed in a home cage environment, which was placed in a Faraday cage 15 min before stimulus onset. White noise stimuli were presented at 85-dB intensity, 10-ms duration, and 500-ms interstimulus interval. Stimulus pairs were separated by 8 s, and a total of 50 paired stimuli were presented.
Data analysis
EEG data were inline filtered between 1 and 500 Hz and baseline corrected at stimulus onset. Using Spike 6, individual sweeps were rejected for movement artifact based on a criterion of two times the root mean squared amplitude per mouse. Based on this criterion, 2% of individual sweeps were rejected. The P20 component was selected from each subject’s average ERP by determining the maximum positive deflection between 10 and 30 ms. Data from test sessions were analyzed using repeated measures analyses of variance (ANOVAs) to determine the effects of nicotine, varenicline, stimulus (S1 vs. S2), and treatment order on P20 amplitude. Effects on P20 habituation were assessed as a significant interaction between pharmacological treatment (varenicline or nicotine) and the responses to S1 and S2. We performed repeated measures ANOVAs on baseline daily saline data to control for effects of repeated testing. Significant effects were followed by Fisher’s least significant difference (LSD) post hoc comparisons using Statistica 6.0 (Statsoft, Inc., Tulsa, OK).
Human study
Subjects
Thirty-two healthy smokers were recruited for a randomized, double-blind placebo-controlled study of the effects of varenicline on the P50 ERP (Supplemental Figure 1). Smokers responding to local advertisements for a smoking cessation program were screened for eligibility in September 2006 to August 2007. Eligible smokers were ≥18 years of age and had smoked ≥10 cigarettes/day for the previous 12 months. In order to increase the generalizability of results to the clinical setting, we enrolled treatment-seeking smokers (those planning to quit in the next 3 months; K. Perkins et al., 2008; K. A. Perkins, Stitzer, & Lerman, 2006). Exclusion criteria included: history of seizures, pregnancy, lactation or planning pregnancy, unstable angina, history of heart attack or stroke in previous 6 months, insulin dependent diabetes, current diagnosis or history of DSM-IV Axis I psychiatric disorders or substance abuse, and current use of smoking cessation or contraindicated medications. All subjects provided informed consent in accordance with Institutional Review Board guidelines at the University of Pennsylvania. Participants reported smoking between 10 and 50 cigarettes/day at study onset, with an average of 21.63 (SD = 10.05). The mean score on the Fagerström Test for Nicotine Dependence (FTND) was 5.28 (SD = 2.44). The average age of participants was 41.06 years (SD = 11.75), and they had been smoking for an average of 24.78 years (SD = 12.20). Of the 32 participants, 50% were female, 56.25% were White, 40.63% were Black, and 3.13% were Asian. Carbon monoxide (CO) was measured on the day of testing to confirm abstinence (CO ≤ 10 ppm).
Drug conditions
There were two drug treatment phases during the study and each participant received varenicline in one phase and placebo in the other. Subjects receiving varenicline first and placebo second comprised Group 1; subjects who received placebo first and varenicline second comprised Group 2. Treatment order was counterbalanced between subjects and phases were separated by a nonmedicated 5- to 7-day washout period during which subjects were instructed to smoke as usual. Participants began each phase by smoking as usual for 10 days, which was followed by 3 days of mandatory abstinence. ERPs were obtained on Day 10 (smoking as usual) and Day 12 (second day of abstinence) for both placebo and varenicline phases. This paradigm yielded a total of four possible experimental conditions (placebo + smoking, placebo + abstinence, varenicline + smoking, and varenicline + abstinence). On Day 10 of each phase, subjects smoked one of their own preferred brand cigarettes approximately 35 min before ERP testing. During each day of the mandatory abstinence period, abstinence was biochemically verified by breath CO samples less than 10 parts per million. Varenicline was administered in a manner consistent with clinical titration guidelines: 0.5 mg po Days 1–3, 0.5 mg po bid Days 4–7, and 1.0 mg po bid Days 8–13 (Pfizer, 2007).
Recording
Participants wore a NeuroScan QuickCap (Compumedics, Charlotte, NC) with ground sensors over the mastoid bones and a recording sensor at Cz. Stimuli were presented at 85-dB intensity, 0.1-ms duration, and 580 ms apart and were delivered binaurally. Stimulus pairs were separated by 8 s, and a total of 100 paired stimuli were presented.
Data analysis
EEG data were digitally filtered between 10 and 80 Hz and baseline corrected at stimulus onset using Vision Analyzer (Brain Products Ltd., Gilching, Germany). Individual sweeps were rejected as movement artifact if they exceeded an absolute value of 100 μV. Based on this criterion, 8% of individual sweeps were rejected. The P50 component was selected from each subject’s average ERP by determining the maximum positive deflection between 40 and 75 ms. We analyzed the data using repeated measures ANOVAs to determine the effects of smoking, varenicline, stimulus, and treatment order on P50 amplitude and latency as well as any interaction effects. Effects on P50 habituation were assessed as a significant interaction between pharmacological treatment (varenicline or smoking) and the responses to S1 and S2. We included self-reported baseline cigarette consumption as a covariate in our analysis. Significant effects were followed by Fisher LSD post hoc comparisons using Statistica 6.0.
Results
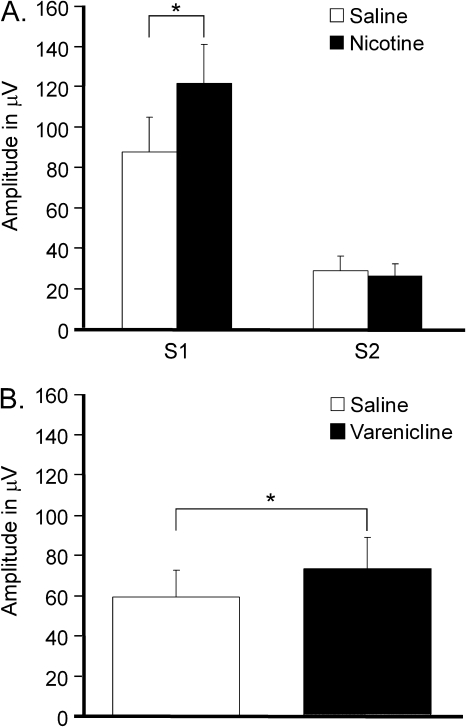
For the mouse study, the second P20 response was significantly reduced relative to the first (S1 = 104.68 μV ± 24.43, S2 = 27.53 ± 6.61, p < .001; Figure 1A, Table 2). Nicotine increased P20 amplitude (p = .009), and an interaction between nicotine and stimulus (p < .001) indicated that nicotine enhanced habituation (Figure 2A). Post hoc analyses revealed an increased response to S1 (p < .001) without a change in the response to S2 (p = .702). Figure 2B shows that varenicline increased overall P20 amplitude (p = .019), but there was no significant interaction with stimulus (p = .088). It is possible that increased statistical power could reveal a significant effect of varenicline on P20 habituation, but it is important to note that the study size was sufficient to reveal a highly significant effect of nicotine on P20 habituation. Acute drug exposure did not alter P20 responses on subsequent testing days since there were no effects of treatment order and baseline saline data revealed no differences across treatment conditions prior to drug exposure.
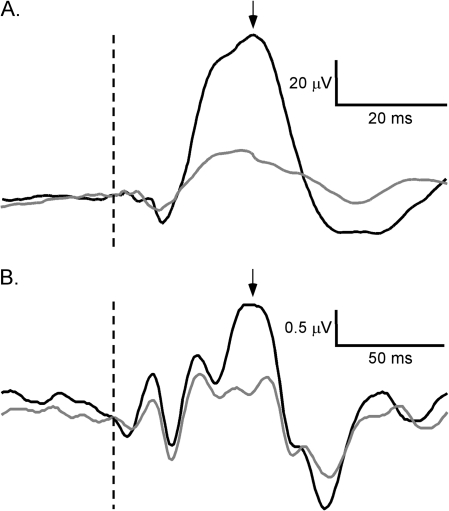
Figure 1.
Grand averages of mouse (A) and human (B) event-related potentials showing the responses to S1 (black) and S2 (gray). Maximum positive deflections in (A) and (B) represent the P20 and P50 components, respectively. Dotted lines indicate stimulus onset and arrows indicate P20 and P50 components.
Table 2.
Mean and SD for the P20 amplitude in mice and P50 amplitude in humans for both S1 and S2 responses
| Species | Drug condition | Stimulus condition | M | SD |
| Mouse | Saline + saline | S1 | 69.65 | 13.99 |
| S2 | 25.66 | 5.33 | ||
| Nicotine + saline | S1 | 105.89 | 13.76 | |
| S2 | 31.66 | 8.49 | ||
| Saline + varenicline | S1 | 117.76 | 12.49 | |
| S2 | 24.12 | 4.12 | ||
| Nicotine + varenicline | S1 | 125.41 | 15.85 | |
| S2 | 28.66 | 6.59 | ||
| Human | Abstinence + placebo | S1 | 2.45 | 1.52 |
| S2 | 1.44 | 1.12 | ||
| Smoking + placebo | S1 | 3.06 | 1.87 | |
| S2 | 1.83 | 1.70 | ||
| Abstinence + varenicline | S1 | 2.87 | 1.90 | |
| S2 | 2.06 | 1.33 | ||
| Smoking + varenicline | S1 | 3.15 | 1.64 | |
| S2 | 1.96 | 1.70 |
Figure 2.
Effects of nicotine and varenicline on the mouse P20. (A) Nicotine enhances P20 habituation by selectively increasing the response to S1. Amplitudes are averaged across varenicline conditions. This means that nicotine increases the S1 response of the P20 regardless of varenicline condition. (B) There was a main effect of varenicline, regardless of nicotine condition (nicotine or saline) or stimulus condition (S1 and S2). Varenicline increases P20 amplitude. Amplitudes are averaged across nicotine and stimulus conditions (S1 and S2). Data are presented as mean ± SEM, and collapsed across variables for which no statistically significant interaction effects were found. *p < .050 (Fisher’s LSD post hoc test).
For the human study, there was significant habituation of the second P50 response relative to the first (S1 = 2.91 μV ± 0.39, S2 = 1.83 ± 0.29, p = .010; Figure 1B). There was no main effect of abstinence on P50 amplitude (p = .584), but an interaction with stimulus (p = .041) indicated that abstinence reduced habituation relative to smoking. Post hoc analyses showed that abstinence decreased S1 response amplitude (p = .004) but had no effect on S2 response amplitude (p = .308; Figure 3A). Neither baseline number of daily cigarettes (p = .296) nor FTND (p = .751) were significantly associated with the effect of smoking on auditory habituation.
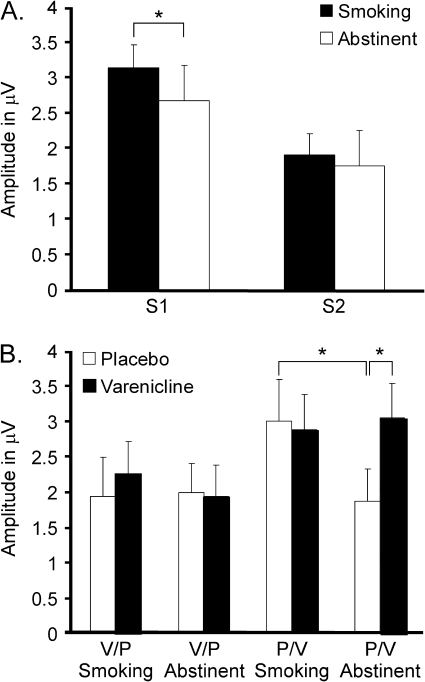
Figure 3.
Effects of smoking and varenicline on human P50. (A) Smoking enhances P50 habituation by selectively increasing the response to S1. Amplitudes are averaged across varenicline conditions. This shows the main effect of smoking when collapsing across other variables. (B) Drug treatments were counterbalanced across study phases. Group 1 received varenicline during Phase 1 and placebo during Phase 2, while Group 2 received placebo during Phase 1 and varenicline during Phase 2. Group 1 did not show any changes in P50 amplitude across drug conditions, but Group 2 exhibited reduced P50 amplitude during abstinence on placebo. Amplitudes are averaged across S1 and S2 because there was no statistical interaction that included stimulus condition. Data are presented as mean ± SEM, and collapsed across variables for which no statistically significant interaction effects were found. *p < .050 (Fisher’s LSD post hoc test).
There was no effect of varenicline on P50 amplitude (p = .579) or habituation (p = .191) when averaged across treatment orders (Group 1 and Group 2). However, there was a significant effect of treatment order on P50 amplitude (p = .043). Subjects who received placebo first and varenicline second (Group 2; Figure 3B) had higher P50 amplitude than subjects receiving varenicline first and placebo second (Group 1; Figure 3B). Furthermore, there was a significant interaction between varenicline, smoking, and treatment order (p = .037), indicating that pharmacological effects differed based on treatment order. Subjects receiving placebo first exhibited decreased P50 amplitude during abstinence (p = .004), which was attenuated by varenicline. Subjects receiving varenicline first followed by placebo did not have a similar effect (p = .862). P50 amplitude during abstinence was significantly higher on varenicline than on placebo (p = .003) for subjects who received placebo first (p = .818) without similar effect in subjects who received varenicline first (Figure 3B).
There was no significant main effect of varenicline on latency when averaged across smoking conditions (smoking or abstinent) and stimulus type (S1 or S2; p = .414). Abstinence caused a significant increase in P50 latency relative to smoking across all other conditions (p < .001). S2 response latency was significantly higher than that for S1 (p < .001). Varenicline did not modify the effects of smoking or stimulus type (no interaction between varenicline and smoking or varenicline and stimulus p > .05 for both comparisons). However, there was a significant interaction between smoking and stimulus condition such that abstinence caused an increase in S2 latency (p < .01) without significant changes on S1.
Discussion
There are two main novel findings in the current study. Although varenicline did not increase P50 across all subjects and conditions, data indicate that it acts electrophysiologically as a functional agonist to replace nicotine during periods of smoking cessation. Second, acute nicotine has the same effects on the mouse P20 as smoking does on the P50 in humans. This is a crucial translational link for studies that make such an assumption without direct data. Similarly, varenicline has the same effect on the mouse P20 as it does on the human P50. There has been much debate regarding how ERP components in mouse and human align. This study provides very strong support that the mouse P20 is the appropriate correlate of the human P50.
We found that nicotine in mice and smoking (vs. abstinence) in humans enhanced habituation of the P20 and P50, respectively. In both species, enhancement of habituation involved an increased response to S1 without a change in the response to S2. This finding is consistent with previous reports that S1 amplitude changes, in the absence of S2 amplitude changes, can be observed following cholinergic modulation of auditory habituation (Crawford et al., 2002; Metzger et al., 2007; Rudnick, Koehler, Picciotto, & Siegel, 2009). Varenicline increased P20 amplitude in mice without an effect on habituation. In the human crossover study, subjects receiving placebo during Phase 1 exhibited decreased P50 amplitude during abstinence, which was attenuated by varenicline during Phase 2. Subjects receiving varenicline during Phase 1 followed by placebo during Phase 2 exhibited no change in P50 amplitude during either treatment phase. Although we cannot offer a definitive explanation for the effect of treatment order, a pharmacologic carryover effect cannot be ruled out. In summary, these findings support the hypothesis that varenicline can modulate amplitude of the P20 and P50 components (Table 3).
Table 3.
Consolidated results from mouse and human experiments demonstrate translational validity of event-related potentials technique
| Mouse P20 | Human P50 | |
| Stimulus | S1 > S2 | S1 > S2 |
| Nicotine/smoking | ↑S1 | ↑S1 |
| Varenicline | ↑(S1, S2) | ↑(S1, S2)a |
Note. In both species, habituation of the second stimulus relative to the first can be observed. Nicotine in mice selectively increases P20 response to S1, just as smoking in human selectively increases P50 response to S1. Last, varenicline increases overall ERP amplitude in both species.
Note that some subjects did not show differences between placebo and varenicline due to a possible carryover effect.
We show an acute effect of smoking status on P50 habituation in healthy current smokers. While previous studies employed brief periods of abstinence (6–15 hr), we employed a longer period of abstinence (3 days), which was confirmed by exhaled CO (Adler et al., 1993; Crawford et al., 2002; Domino, 2003; Domino & Kishimoto, 2002). Therefore, our paradigm may have been more sensitive to effects of smoking and abstinence compared with studies with a shorter duration of abstinence. In vivo radiotracer experiments suggest that nicotine can take several days to clear high-affinity binding sites (i.e., α4β2 nAChRs) in humans and nonhuman primates (Staley et al., 2006). Therefore, the effects of abstinence may follow a protracted time course. One potential drawback of our approach is that each subject started from a different level of baseline smoking. We controlled for this possibility by including self-reported baseline cigarette consumption as a covariate in our analyses.
Auditory habituation depends on the responses to S1 and S2, and frequently, these amplitudes are condensed into a single ratio. This approach can obscure the mechanism of habituation enhancements, which may depend on an increased response to S1, decreased response to S2, or both. It has been proposed that nicotine inhibits the response to S2 by activating α7 nAChRs interneurons in the CA3 region of the hippocampus (Adler et al., 1998; Stevens et al., 1998). However, this mechanism alone is not sufficient to explain our data because nicotine and smoking increased the amplitude of the S1 response without significantly affecting the amplitude of the S2 response. Previous studies in rodents and humans show similar changes in the response to S1 but not S2 (Crawford et al., 2002; Cromwell & Woodward, 2007; Metzger et al., 2007; Phillips et al., 2007). Varenicline, a relatively selective α4β2 nAChRs partial agonist (Mihalak et al., 2006), increased amplitude but not habituation of auditory ERPs. Therefore, it is likely that brain regions rich in α4β2 nAChRs, the target of varenicline, contribute to the amplifying effect of nicotine on the S1 response. Consistent with this hypothesis, nicotine enhances action potential propagation and synaptic release in thalamocortical circuits via DHβE-sensitive nAChRs (Kawai et al., 2007; Lambe, Picciotto, & Aghajanian, 2003). These circuits are an obligate stage of auditory processing and participate in generation of the midlatency ERP components (Hinman & Buchwald, 1983; McGee, Kraus, Comperatore, & Nicol, 1991). Even if nicotine increases the response to S1 by enhancing thalamic transmission, it must also activate inhibitory networks in order to prevent the response to S2 from increasing in amplitude as well. Therefore, it is likely that both α7 nAChRs- and α4β2 nAChRs-expressing brain regions are involved in auditory habituation. Our initial hypothesis was that varenicline would attenuate the effects of nicotine in the presence of nicotine but mimic the effects of a full agonist when given alone, consistent with activity as a partial agonist. Data support that varenicline acted as a functional agonist when given alone. The combination of varenicline and smoking were similar to either smoking or varenicline alone.
Varenicline increased P20 amplitude in mice and P50 amplitude during abstinence in humans. Changes in EEG power and ERP amplitude are thought to reflect changes in arousal (Kishimoto & Domino, 1998; Pickworth, Herning, & Henningfield, 1989). Because decreased arousal is a symptom of nicotine withdrawal, varenicline’s effects on sensory habituation may contribute to its therapeutic efficacy. However, nonspecific increases in arousal may also contribute to sleep disturbances observed in some clinical studies (Gonzales et al., 2006; Oncken et al., 2006). Nonetheless, the aforementioned connection between ERP amplitude and arousal remains speculative because stimulants such as amphetamine can decrease amplitude (Maxwell, Kanes, et al., 2004). Interestingly, the smoking cessation medication bupropion reduces the amplitude of ERPs in mice, similar to amphetamine (Siegel et al., 2005). To the best of our knowledge, the effects of bupropion on human ERPs are not known. Although alpha-7 nAChRs agonists such as 3-(2,4)-dimethoxybenzylidine anabaseine and tropisetron reverse the effects of cocaine or amphetamine on ERPs, their effects on P50 or smoking status in humans are not known (Hashimoto, Iyo, Freedman, & Stevens, 2005; Stevens et al., 1999). The most direct interpretation of increased ERP amplitude may simply be that it reflects a greater degree of phase synchrony in ongoing EEG rhythms (Jansen, Agarwal, Hegde, & Boutros, 2003; Makeig et al., 2002).
Our human data reveal that treatment order significantly modulated the effects of abstinence and varenicline on P50 amplitude. It is possible that subjects receiving varenicline prior to placebo failed to undergo a reduction in P50 amplitude during the placebo phase because varenicline had long-lasting effects in the brain, despite the 5- to 7-day washout period and 17-hr half-life of varenicline (Obach et al., 2006). An alternative explanation for the order effect may be that abstinence failed to reduce P50 amplitude because of a floor effect. Overall P50 amplitudes also differed by treatment order, suggesting that there may have been baseline asymmetries in sensory processing between subjects randomized to different treatment orders; however, in the absence of baseline ERP data, we cannot examine this directly. To simplify interpretation of results, future within-subject designs may benefit from extending the washout period to 2 weeks.
Previous studies indicate that nicotine in rodents produces similar biological and behavioral effects as smoking in humans, and this study further supports the face validity of mouse models (Blendy et al., 2005; Corrigall, 1999; Lerman et al., 2007; Liu et al., 2003; Slawecki, Gilder, Roth, & Ehlers, 2003). However, there are limitations to this approach. Besides nicotine, cigarettes contain psychoactive compounds such as monoamine oxidase inhibitors that may play a role in dependence and auditory habituation (Crawford et al., 2002; Guillem et al., 2005; Siegel et al., 2005). Furthermore, our mouse study used acute doses of nicotine, which likely fail to produce the types of nAChRs upregulation associated with chronic smoking. Although previous studies in mice have shown biological effects at the dose of varenicline used in the present study, the lack of a dose–response relationship for ERPs is a potential limitation since we cannot determine the effects of higher doses, which may have increased P50 amplitude in the VP group (Raybuck et al., 2008; Rollema et al., 2009). Despite these caveats, the extensive interspecies overlap in our results suggests that acute doses of cholinergic agents approximate chronic exposure in humans. Thus, EEG in mice allows for rapid screening of novel treatments for ND that may ameliorate sensory deficits associated with abstinence (Lerman et al.).
Supplementary Material
Supplementary Figure 1 can be found at Nicotine and Tobacco Research online (http://www.ntr.oxfordjournals.org/).
Funding
This research was supported by the AstraZeneca, the National Cancer Institute and National Institutes on Drug Abuse (P50084718, CL, Principal Investigator), and a fellowship from the Irene and Eric Simon Brain Research Foundation to NDR and SJS; Pfizer generously provided varenicline for the mouse study.
Declaration of Interests
NDR has no potential conflicts of interest to disclose. AAS has no potential conflicts of interest to disclose. JMP has no potential conflicts of interest to disclose. CJ has no potential conflicts of interest to disclose. FP has no potential conflicts of interest to disclose. JMF is an employee of AstraZeneca. BIT receives unrelated research grant support from AstraZeneca and Pfizer pharmaceutical companies. CL has received compensation and research funding from Pfizer, AstraZeneca, and GlaxoSmith Kline. SJS has received unrelated research funding from AstraZeneca, unrelated research funding and compensation from NuPathe, and compensation from the Network for Continuing Medical Education.
Supplementary Material
References
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. American Journal of Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biological Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia Bulletin. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pang K, Gerhardt G, Rose GM. Modulation of the gating of auditory evoked potentials by norepinephrine: Pharmacological evidence obtained using a selective neurotoxin. Biological Psychiatry. 1988;24:179–190. doi: 10.1016/0006-3223(88)90273-9. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr., Gong J, et al. Varenicline versus transdermal nicotine patch for smoking cessation: Results from a randomised, open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, et al. Reduced nicotine reward in obesity: Cross-comparison in human and mouse. Psychopharmacology. 2005;180:306–315. doi: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. Journal of Pharmacology and Experimental Therapeutics. 1997;280:346–356. [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine & Tobacco Research. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Crawford HJ, McClain-Furmanski D, Castagnoli N, Jr., Castagnoli K. Enhancement of auditory sensory gating and stimulus-bound gamma band (40 Hz) oscillations in heavy tobacco smokers. Neuroscience Letters. 2002;317:151–155. doi: 10.1016/s0304-3940(01)02454-5. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Woodward DJ. Inhibitory gating of single unit activity in amygdala: Effects of ketamine, haloperidol, or nicotine. Biological Psychiatry. 2007;61:880–889. doi: 10.1016/j.biopsych.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Domino EF. Effects of tobacco smoking on electroencephalographic, auditory evoked and event related potentials. Brain and Cognition. 2003;53:66–74. doi: 10.1016/s0278-2626(03)00204-5. [DOI] [PubMed] [Google Scholar]
- Domino EF, Kishimoto T. Tobacco smoking increases gating of irrelevant and enhances attention to relevant tones. Nicotine & Tobacco Research. 2002;4:71–78. doi: 10.1080/14622200110098400. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. Journal of Neuroscience. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene TB, Siegel SJ. Antipsychotic-like properties of phosphodiesterase 4 inhibitors: Evaluation of 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (RO-20-1724) with auditory event-related potentials and prepulse inhibition of startle. Journal of Pharmacology and Experimental Therapeutics. 2008;326:230–239. doi: 10.1124/jpet.108.138586. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Iyo M, Freedman R, Stevens KE. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: Role of alpha 7 nicotinic acetylcholine receptors. Psychopharmacology. 2005;183:13–19. doi: 10.1007/s00213-005-0142-0. [DOI] [PubMed] [Google Scholar]
- Hinman CL, Buchwald JS. Depth evoked potential and single unit correlates of vertex midlatency auditory evoked responses. Brain Research. 1983;264:57–67. doi: 10.1016/0006-8993(83)91120-4. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jansen BH, Agarwal G, Hegde A, Boutros NN. Phase synchronization of the ongoing EEG and auditory EP generation. Clinical Neurophysiology. 2003;114:79–85. doi: 10.1016/s1388-2457(02)00327-9. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nature Neuroscience. 2007;10:1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Domino EF. Effects of tobacco smoking and abstinence on middle latency auditory evoked potentials. Clinical Pharmacology and Therapeutics. 1998;63:571–579. doi: 10.1016/S0009-9236(98)90108-4. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nature Reviews Drug Discovery. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Liu X, Koren AO, Yee SK, Pechnick RN, Poland RE, London ED. Self-administration of 5-iodo-A-85380, a beta2-selective nicotinic receptor ligand, by operantly trained rats. Neuroreport. 2003;14:1503–1505. doi: 10.1097/00001756-200308060-00020. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. Multiple motivational forces contribute to nicotine dependence. Nebraska Symposium of Motivation. 2009;55:65–89. doi: 10.1007/978-0-387-78748-0_5. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, et al. Ketamine produces lasting disruptions in encoding of sensory stimuli. Journal of Pharmacology and Experimental Therapeutics. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Kanes SJ, Abel T, Siegel SJ. Phosphodiesterase inhibitors: A novel mechanism for receptor-independent antipsychotic medications. Neuroscience. 2004;129:101–107. doi: 10.1016/j.neuroscience.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, et al. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004;29:739–746. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- McGee T, Kraus N, Comperatore C, Nicol T. Subcortical and cortical components of the MLR generating system. Brain Research. 1991;544:211–220. doi: 10.1016/0006-8993(91)90056-2. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, et al. Working memory in cigarette smokers: Comparison to non-smokers and effects of abstinence. Addictive Behaviors. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger KL, Maxwell CR, Liang Y, Siegel SJ. Effects of nicotine vary across two auditory evoked potentials in the mouse. Biological Psychiatry. 2007;61:23–30. doi: 10.1016/j.biopsych.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metababolism and Disposition. 2006;34:121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine & Tobacco Research. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K, Lerman C, Stitzer M, Fonte C, Briski J, Scott J, et al. Development of procedures for early screening of smoking cessation medications in humans. Clinical Pharmacology and Therapeutics. 2008;84:216–221. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: A proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Pfizer. Chantix prescribing information. 2007. [Investigator Brochure]. Retrieved from http://www.pfizer.com/files/products/uspi_chantix.pdf. [Google Scholar]
- Phillips JM, Ehrlichman RS, Siegel SJ. Mecamylamine blocks nicotine-induced enhancement of the P20 auditory event-related potential and evoked gamma. Neuroscience. 2007;144:1314–1323. doi: 10.1016/j.neuroscience.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Herning RI, Henningfield JE. Spontaneous EEG changes during tobacco abstinence and nicotine substitution in human volunteers. Journal of Pharmacology and Experimental Therapeutics. 1989;251:976–982. [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacology Biochemistry and Behavior. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addictive Behaviors. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Radek RJ, Miner HM, Bratcher NA, Decker MW, Gopalakrishnan M, Bitner RS. Alpha4beta2 nicotinic receptor stimulation contributes to the effects of nicotine in the DBA/2 mouse model of sensory gating. Psychopharmacology. 2006;187:47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- Rahman S, Lopez-Hernandez GY, Corrigall WA, Papke RL. Neuronal nicotinic receptors as brain targets for pharmacotherapy of drug addiction. CNS & Neurological Disorders Drug Targets. 2008;7:422–441. doi: 10.2174/187152708786927831. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behavioral Neuroscience. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling AJ, Dawson ME, Schell AM, Nuechterlein KH. Effects of cigarette smoking on prepulse inhibition, its attentional modulation, and vigilance performance. Psychophysiology. 2007;44:627–634. doi: 10.1111/j.1469-8986.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, et al. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline’s effect. European Journal of Pharmacology. 2009;605:114–116. doi: 10.1016/j.ejphar.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick ND, Koehler C, Picciotto MR, Siegel SJ. Role of beta2-containing nicotinic acetylcholine receptors in auditory event-related potentials. Psychopharmacology. 2009;202:745–751. doi: 10.1007/s00213-008-1358-6. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, et al. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Maxwell CR, Majumdar S, Trief DF, Lerman C, Gur RE, et al. Monoamine reuptake inhibition and nicotine receptor antagonism reduce amplitude and gating of auditory evoked potentials. Neuroscience. 2005;133:729–738. doi: 10.1016/j.neuroscience.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacology Biochemistry and Behavior. 2003;75:355–361. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. Journal of Neuroscience. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Freedman R. Selective alpha 7 nicotinic receptor stimulation normalizes chronic cocaine-induced loss of hippocampal sensory inhibition in C3H mice. Biological Psychiatry. 1999;46:1443–1450. doi: 10.1016/s0006-3223(99)00200-0. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology. 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Meltzer J, Rose GM. Nicotinic cholinergic normalization of amphetamine-induced loss of auditory gating in freely moving rats. Psychopharmacology. 1995;119:163–170. doi: 10.1007/BF02246157. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotky D, Latanov A, Nitsch R, Brambilla R, D’Adamo P, et al. Midlatency auditory event-related potentials in mice: Comparison to midlatency auditory ERPs in humans. Brain Research. 2004;1019:189–200. doi: 10.1016/j.brainres.2004.05.097. [DOI] [PubMed] [Google Scholar]
- Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addictive Behaviors. 2001;26:311–327. doi: 10.1016/s0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.