Abstract
Introduction:
Smoking is the leading preventable cause of morbidity and mortality in the United States, but this burden is not distributed equally among smokers. Women, Blacks, and people with low socioeconomic status are especially vulnerable to the health risks of smoking and are less likely to quit.
Methods:
This research examined cessation rates and treatment response among 2,850 participants (57.2% women, 11.7% Blacks, and 9.0% with less than a high school education) from two large cessation trials evaluating: nicotine patch, nicotine lozenge, bupropion, bupropion + lozenge, and nicotine patch + lozenge.
Results:
Results revealed that women, Blacks, and smokers with less education were less likely to quit smoking successfully than men, Whites, and smokers with more education, respectively. Women did not appear to benefit more from bupropion than from nicotine replacement therapy, but women and smokers with less education benefited more from combination pharmacotherapy than from monotherapy.
Discussion:
Women, Blacks, and smokers with less education are at elevated risk for cessation failure, and research is needed to understand this risk and develop pharmacological and psychosocial interventions to improve their long-term cessation rates.
Introduction
The staggering toll of smoking and tobacco dependence—in terms of both human and economic costs (Centers for Disease Control, 2008)—is not evenly distributed across the smoking population. Women, Blacks, and people with low socioeconomic status (SES) suffer disproportionately from smoking and have been specifically targeted by tobacco companies (Apollonio & Malone, 2005; Carpenter, Wayne, & Connolly, 2005; Hafez & Ling, 2006; Sutton & Robinson, 2004; White, White, Freeman, Gilpin, & Pierce, 2006).
Approximately 17.4% of U.S. women smoke (Centers for Disease Control and Prevention, 2007), and approximately 64% of women smokers die from smoking-related causes (Kenfield, Stampfer, Rosner, & Colditz, 2008). In fact, cigarette smoking accounts for more than 25% of all deaths among U.S. women (Peto, Lopez, Boreham, Thun, & Heath, 1992). The risks of serious smoking-related illnesses are higher for women than for men in part because women smokers experience unique health risks, such as an increased risk of breast cancer and of menopause at an earlier age (Perkins, 2001). Women are more likely than men to try to quit smoking and to seek and engage in smoking cessation treatment (Shiffman, Brockwell, Pillitteri, Gitchell, 2008; Zhu, Melcer, Sun, Rosbrook, & Pierce, 2000) but are less likely to receive smoking cessation pharmacotherapy from their physician (Sherman, Fu, Joseph, Lanto, & Yano, 2005; Steinberg, Akincigil, Delnevo, Crystal, & Carson, 2006). Women may have more difficulty quitting than men do (Bjornson et al., 1995; Cepeda-Benito, Reynoso, & Erath, 2004; Shiffman, Sweeney, & Dresler, 2005; Swan, Jack, & Ward, 1997; Swan et al., 2003; Wetter, Kenford et al., 1999), although this finding is not consistent across studies (Killen, Fortmann, Varady, & Kraemer, 2002; Velicer, Redding, Sun, & Prochaska, 2007). Some research suggests bupropion may close this gender gap (Collins et al., 2004; Gonzales et al., 2002; Scharf & Shiffman, 2004; Smith et al., 2003), perhaps because women may be more responsive to bupropion relative to nicotine replacement (Perkins, 1996; Wetter, Fiore et al., 1999).
Compared with Whites, Blacks smoke at a somewhat lower rate and smoke fewer cigarettes per day (Centers for Disease Control, 2005; O’Connor et al., 2006) but have increased mortality from smoking-related diseases relative to White smokers (e.g., cancer and cardiovascular disease; Harris, Zang, Anderson, & Wynder, 1993; Kurian & Cardarelli, 2007; Yancy, 2007). Evidence suggests that, relative to White smokers, Black smokers are more likely to make a quit attempt (Fiore et al., 1989; Giovino et al., 1994) but are less likely to use smoking cessation treatment during a quit attempt (Shiffman et al., 2008; Zhu et al., 2000) and are less likely to remain abstinent (Cropsey et al., 2009; Fiore et al., 1989; Gilpin & Pierce, 2002; Giovino et al.; King, Polednak, Bendel, Vilsaint, & Nahata, 2004). Despite discrepant smoking outcomes between Whites and Blacks, research has demonstrated the effectiveness of evidence-based treatments for Black smokers (Cropsey et al., 2009; Fiore, Jaen, Baker, Bailey, et al., 2008; Robles, Singh-Franco, & Ghin, 2008).
Smokers with low educational attainment and/or low SES also bear a disproportionate burden from tobacco. In this research, we examined educational attainment, a common proxy for SES, because it is reliable and remains relatively constant in adult samples (Iribarren, Luepker, McGovern, Arnett, & Blackburn, 1997; Kaplan & Keil, 1993). Compared with smokers with higher SES, smokers with low SES smoke at higher rates (Centers for Disease Control and Prevention, 2008) and are at increased risk for smoking-related diseases (Kanjilal et al., 2006), but they have limited access to treatment and are less likely to seek and receive smoking cessation treatment (Connor, Cook, Herbert, Neal, & Williams, 2002; Murphy, Mahoney, Hyland, Higbee, & Cummings, 2005; Shiffman et al., 2008; Shiffman, Di Marino, & Sweeney, 2005), and they are less likely to quit smoking (Giskes, van Lenthe, Turrell, Brug, & Mackenbach, 2006; Velicer et al., 2007). The widening social gradient between smokers and nonsmokers has been documented in both Europe and the United States (Barbeau, Krieger, & Soobader, 2004; Giskes et al., 2005; Kotz & West, 2009).
Given that women, Blacks, and people with low educational attainment have increased health risks from smoking, it is important to identify effective treatments for these populations. While the PHS Clinical Practice Guideline (Fiore, Jaen, Baker, Bailey et al., 2008) suggests that FDA-approved pharmacotherapies are effective in these populations, there was insufficient evidence to conduct meta-analyses of population-specific treatment trials. The present research aims to provide information on smoking cessation and treatment response among these three groups of smokers. The data presented here are a step toward augmenting the small, extant evidence on cessation treatment in specific, vulnerable populations (Fiore, Jaen, & Baker, 2008; Fiore, Jaen, Baker, Bailey et al., 2008; Piper, Fox, Welsch, Fiore, & Baker, 2001).
For each of three groups of smokers (women, Blacks, and smokers with low educational attainment), we examined point prevalence abstinence at 8 weeks and 6 months postquit in two different clinical trials—a highly controlled Efficacy trial and a real-world setting Effectiveness trial—that randomized participants to the same active pharmacotherapies. In addition to examining the main effects of group (e.g., women) on outcome, we combined the datasets from the two trials and examined each group’s response to the five pharmacotherapies tested and to monotherapy versus combination therapy. Each of the two trials found combination therapy to be more effective than monotherapy (Piper et al., 2009; Smith et al., 2009), but the goal of this study was to assess the effectiveness of these treatments in these three specific populations. We also tested whether women were more responsive to bupropion than to nicotine replacement therapy (NRT). Finally, we assessed group characteristics that might be related to cessation outcome.
Method
Efficacy trial
Recruitment
Participants were recruited in Madison and Milwaukee, Wisconsin, through TV, radio, and newspaper advertisements, community flyers, and gained media including radio and TV interviews and press releases (see Piper et al., 2009). The study received human subjects approval from the University of Wisconsin Health Sciences Institutional Review Board (IRB).
Procedure
Participants who passed a phone screen were invited to an information session where they provided written informed consent. Participants then attended the first of the three baseline assessments during which they underwent multiple screenings, including a medical history screening, vital signs measurements, and a carbon monoxide (CO) breath test. Participants also completed demographic, smoking history, and tobacco dependence questionnaires. After the third baseline assessment, eligible participants were randomized to one of the six treatment conditions: bupropion SR (150 mg twice daily for 9 weeks total: 1 week prior to the quit day and 8 weeks starting on the quit day; n = 264); nicotine lozenge (2 or 4 mg based on dependence level as per package instructions, for 12 weeks starting on the quit day; n = 260); nicotine patch (21, 14, and 7 mg; titrated down over the 8 weeks following the quit day; n = 262); nicotine patch + nicotine lozenge (n = 267); bupropion SR + nicotine lozenge (n = 262) or one of five placebo conditions that paralleled the five active pharmacotherapy conditions (n = 189). It should be noted that there were no statistically significant differences among the placebo conditions in 7-day point prevalence outcomes at 1 week, end of treatment or 6 months postquit. Therefore, all analyses in this paper present the placebo conditions as a unified placebo condition. Randomization was conducted in double-blind fashion using a blocked randomization scheme based on gender and race (White/non-White). All participants received six counseling sessions, each lasting between 10 and 20 min, at study visits which occurred 8–15 days before their quit day, on their quit day, and at 1, 2, 4 and 8 weeks after their quit day.
Effectiveness trial
Recruitment
At 12 different primary care clinics, medical assistants (MAs) screened patients for current tobacco use, advised smokers to quit, assessed their willingness to quit, and determined initial eligibility for study participation. MAs documented each clinical encounter in the electronic medical record (EMR), and—for smokers interested in study participation—gave them a copy of the consent form to review, and faxed a Wisconsin Tobacco Quit Line (WTQL) referral form to the study office. Interested patients were then medically evaluated by their physician who documented eligibility on a study Medical Clearance Form listing exclusionary conditions and medications. For patients meeting study criteria, the Medical Clearance Form was faxed to the study office by the clinic cessation coordinator. This study was approved by the Aurora Health Care IRB and the University of Wisconsin Health Sciences IRB.
Procedure
Patients were called within one business day of their clinic visit by a research staff person who explained the study and obtained verbal informed consent from the patient. The staff person then conducted a brief interview that included a smoking history and dependence assessment, obtained the patient’s contact information, and faxed a referral form to the WTQL to arrange for phone-based cessation counseling. The staff person also randomized the patient to treatment, set a quit date with the patient, provided instructions to pick up medication at the clinic pharmacy, faxed a prescription to the clinic pharmacy, and entered the prescription into the EMR. At the clinic pharmacy, the pharmacist witnessed the patient signing the study consent form, collected the consent form, dispensed prepackaged study medication, and faxed the study office verifying that study medication was dispensed to the patient. Patients were randomized to receive one of the five active pharmacotherapy treatments used in the Efficacy trial (bupropion SR [n = 256], nicotine lozenge [n = 261]; nicotine patch [n = 282]; nicotine patch + nicotine lozenge [n = 279]; bupropion SR + nicotine lozenge [n = 268]) at the same dosage and for the same duration.
Measures for both trials
Inclusion/exclusion criteria
Primary inclusion criteria for participation in either study included: (a) being age 18 or older; (b) smoking >9 cigarettes daily for the last 6 months; (c) being motivated to quit smoking; and (d) for women, being willing to use an acceptable method of birth control while on study medication. Primary exclusion criteria for both studies included: (a) a history of seizures or convulsions, bipolar disorder, psychosis, anorexia, or bulimia nervosa; (b) any serious health conditions that would prevent participating in or completing the study; (c) current use of bupropion or an monoamine oxidase inhibitor in the previous 2 weeks; (d) allergy to any study medication; and (e) if women, currently being pregnant, breast feeding, or planning to become pregnant within the next 3 months.
Assessments
A demographics questionnaire assessed characteristics including gender, ethnicity, age, marital status, education level, and employment. A smoking history questionnaire included items such as number of cigarettes smoked per day, age of smoking initiation, and number of prior quit attempts. The Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), a 6-item scale with fair internal consistency (α = .61), measured tobacco dependence. Participants self-reported 7-day point prevalence abstinence at 8 weeks and 6 months postquit, and participants’ self-reported abstinence was biochemically verified (CO < 10) in the Efficacy trial but not in the Effectiveness trial. Participants who did not provide outcome information were assumed to be smoking, using the intent-to-treat principle.
Analytic plan
Analyses were conducted using PASW Statistics 17.0 (SPSS, Chicago, IL). For each trial, we compared smoking cessation outcome and group differences in dependence and other smoking factors for: (a) men (coded as 0) versus women (coded as 1), (b) Whites (coded as 0) versus Blacks (coded as 1; smokers who reported other racial identities were excluded from race analyses), and (c) smokers with less than a high school education (<HS; coded as 1) versus with a high school education only (HS; coded as 2) versus with more than a high school education (>HS; coded as 3). Only the Efficacy trial collected data on medication usage, so only that sample was included in medication adherence analyses.
The first series of analyses was designed to examine cessation success using three cessation outcomes as the dependent variables: initial cessation (i.e., the ability to remain smoke-free for at least 24 hr during the first 7 days following the target quit day—data collected in the Efficacy sample only) and point prevalence abstinence at 8 weeks and 6 months postquit. For the logistic regression analyses designed to determine whether there were group differences in abstinence (e.g., men vs. women, Whites vs. Blacks), we used treatment condition as a covariate and then included the group of interest (i.e., gender, race, or education) as a predictor. To assess the predictive power of gender, race, and education status, we also examined how well these groups predicted outcome when they were all included simultaneously as predictors in logistic regression models.
To assess treatment response among the specific groups, we combined the datasets from the two trials to increase sample size and statistical power. We first conducted chi-square analyses to assess group differences in 8-week and 6-month cessation outcomes for each of the five treatments. To control for Type 1 error, these analyses were evaluated using a Bonferroni-corrected p = .003 (.05/15 comparisons—five treatment conditions for each of the three groups). Second, we used logistic regression to examine treatment effects (monotherapy vs. combination therapy) for women only, Blacks only, and smokers with less than a high school education. In these regression analyses, in which the Efficacy and Effectiveness samples were combined, study was included as a covariate given the higher abstinence rates in the Efficacy trial and we controlled for study by treatment interactions. We next tested the specific hypothesis that women would be more responsive to bupropion than to NRT. Finally, we examined group differences in specific characteristics that may be related to cessation outcome such as smoking heaviness, tobacco dependence, and cessation medication adherence (assessed using counts of unused medication that participants in the Efficacy trial returned).
Results
Participants
Efficacy sample
Of the 1,504 participants in the Efficacy trial, 58.2% were women. Among the 873 women, 132 (15.1%) self-identified as Black and 48 (5.4%) reported having less than a high school education. The majority of the Efficacy sample self-identified as White (83.9%); 204 (13.6%) self-identified as Black, and 38 (2.6%) self-identified as another race. Of those who self-identified as Black, 32 (15.8%) reported less than a high school education. Approximately 2.8% of participants reported that one of their parents was of Hispanic origin. With respect to education, 84 (5.6%) participants reported having less than a high school education, 353 (23.5%) reported having a high school education/general educational development certificate (GED), and 1,058 (70.3%) reported having more than a high school education. In the Efficacy sample, participants had a mean age of 44.7 (SD = 11.1), smoked 21.4 cigarettes per day (SD = 8.9), and had made 5.7 previous quit attempts (SD = 9.7). There was only one significant correlation between gender, race, and education: White smokers tended to have higher educational attainment (r = −.15, p < .001).
Effectiveness sample
Of the 1,346 participants in the Effectiveness trial, 55.9% were women. Among the women, 81 (10.8%) self-identified as Black and 89 (11.8%) reported having less than a high school education. In the Effectiveness sample, the majority self-identified as White (87.0%), 128 (9.5%) self-identified as Black, and 43 (3.2%) self-identified as another race. Of those who self-identified as Black, 28 (21.8%) reported having less than a high school education. Hispanic ethnicity was reported by 29 (2.1%) participants. With respect to education, 172 (12.8%) participants reported having less than a high school education, 597 (44.4%) reported having a high school education/GED, and 577 (42.9%) reported having more than a high school education. In the Effectiveness sample, participants had a mean age of 44.3 (SD = 12.1), smoked 20.3 cigarettes per day (SD = 8.8), and had made 5.7 previous quit attempts (SD = 9.3). Again there was a small but significant correlation (r = −.07, p < .01) between education and race such that White smokers tended to have higher educational attainment.
Sample differences
Independent samples t test and chi-square analyses suggested that compared with smokers in the Effectiveness sample, smokers in the Efficacy sample smoked significantly more cigarettes per day (mean difference = 1.17; t (2822.29) = −3.54, p < .001), were more likely to be married (χ2 (5, 2844) = 35.34, p < .001), and were more likely to be Black (χ2 (2, 2850) = 13.47, p = .001). There were no differences in age, number of previous quit attempts, gender, or Hispanic ethnicity.
Effects of gender on outcomes
Cessation outcome
There were no gender differences in initial cessation rates in the Efficacy sample or was there a main effect of gender on outcome at 8 weeks postquit (OR = 0.84, p = .11, 95% CI = 0.68–1.04), but at 6 months postquit women were less likely than men to be abstinent (30.6% vs. 36.5%; OR = 0.77, p = .02, 95% CI = 0.62–0.96). In the Effectiveness sample, women were less likely than men to be abstinent at both 8 weeks (31.1% vs. 40.0%; OR = .66, p < .001, 95% CI = 0.53–0.83) and 6 months postquit (18.7% vs. 26.8%; OR = 0.62, p < .001, 95% CI = 0.48–0.81).
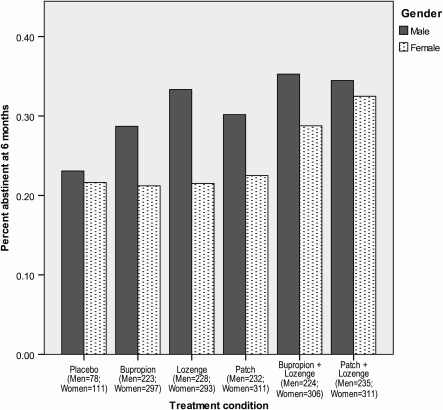
Tables 1 and 2 detail the gender-specific abstinence rates for each treatment group in the Efficacy and Effectiveness studies, respectively. Using the combined dataset, we examined women’s responses to the different treatments. Chi-square analyses for each treatment revealed that in the bupropion + lozenge condition, men were significantly more likely than women to be abstinent at 8 weeks (56.3% vs. 41.8%; p = .001). There was also a significant gender difference (p = .002) in 6-month abstinence rates for the lozenge condition. The gender differences in 6-month abstinence rates for bupropion and patch were both significant at p < .05 but not at the Bonferroni-corrected p < .003 (Figure 1).
Table 1.
Percent achieving initial cessation, and 7-day point prevalence abstinence at 8 weeks and 6 months postquit by gender, race, and education across treatment conditions using data from the Efficacy sample
| Placebo |
Bupropion |
Lozenge |
Patch |
Bupropion + lozenge |
Patch + lozenge |
|||||||||||||
| Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | |||||||
| Initial cessation (%) | 70.4 | 68.7 | 84.9 | 80.3 | 82.7 | 80.1 | 87.3 | 88.0 | 83.8 | 84.9 | 90.2 | 92.5 | ||||||
| 8 weeks postquit (%) | 30.8 | 29.7 | 41.8 | 39.0 | 48.6 | 34.4 | 42.2 | 46.4 | 57.4 | 45.5 | 52.6 | 54.2 | ||||||
| 6 months postquit (%) | 23.1 | 21.6 | 34.5 | 29.9 | 44.0 | 25.8 | 34.9 | 34.0 | 36.1 | 31.2 | 42.1 | 38.6 | ||||||
| White | Black | White | Black | White | Black | White | Black | White | Black | White | Black | |||||||
| Initial cessation (%) | 73.4 | 45.0 | 84.0 | 70.6 | 83.5 | 65.7 | 90.6 | 72.7 | 87.4 | 68.4 | 91.6 | 89.5 | ||||||
| 8 weeks postquit (%) | 32.5 | 10.0 | 43.4 | 20.0 | 40.6 | 36.8 | 46.8 | 31.4 | 55.3 | 28.9 | 57.8 | 28.9 | ||||||
| 6 months postquit (%) | 23.8 | 15.0 | 33.9 | 20.0 | 33.2 | 34.2 | 35.9 | 25.7 | 36.9 | 18.4 | 42.2 | 28.9 | ||||||
| <HS | HS | >HS | <HS | HS | <HS | <HS | HS | >HS | <HS | HS | >HS | <HS | HS | >HS | <HS | HS | >HS | |
| Initial cessation (%) | 66.7 | 67.5 | 70.4 | 46.2 | 80.0 | 85.3 | 75.0 | 80.7 | 82.0 | 60.0 | 83.6 | 91.0 | 66.7 | 77.6 | 88.6 | 83.3 | 87.9 | 93.0 |
| 8 weeks postquit (%) | 38.5 | 20.8 | 33.6 | 23.1 | 40.4 | 41.2 | 31.6 | 42.6 | 40.6 | 22.6 | 32.0 | 40.7 | 25.0 | 47.5 | 53.6 | 33.3 | 50.0 | 56.1 |
| 6 months postquit (%) | 30.8 | 16.7 | 24.0 | 0.0 | 26.9 | 35.2 | 21.1 | 36.1 | 33.9 | 17.0 | 25.4 | 27.4 | 18.8 | 34.4 | 33.9 | 33.3 | 39.7 | 40.8 |
Notes. <HS = less than high school diploma/GED (n = 84); HS = high school diploma or GED (n = 353); >HS = more than a high school education (i.e., some college or trade school; n = 1,058).
Table 2.
Percent achieving 7-day point prevalence abstinence at 8 weeks and 6 months postquit by gender, race, and education across treatment conditions using data from the Effectiveness sample
| Bupropion |
Lozenge |
Patch |
Bupropion+Lozenge |
Patch+Lozenge |
|||||||||||
| Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | ||||||
| 8 weeks postquit (%) | 29.2 | 26.6 | 29.4 | 26.8 | 36.3 | 22.2 | 55.2 | 38.2 | 49.6 | 41.1 | |||||
| 6 months postquit (%) | 23.0 | 11.9 | 23.5 | 16.9 | 25.8 | 11.4 | 34.5 | 26.3 | 27.3 | 26.6 | |||||
| White | Black | White | Black | White | Black | White | Black | White | Black | ||||||
| 8 weeks postquit (%) | 29.2 | 13.0 | 26.9 | 34.8 | 26.4 | 34.6 | 45.1 | 57.1 | 47.3 | 28.6 | |||||
| 6 months postquit (%) | 16.8 | 17.4 | 19.4 | 13.0 | 16.5 | 23.1 | 29.8 | 35.7 | 27.8 | 25.0 | |||||
| <HS | HS | >HS | <HS | HS | >HS | <HS | HS | >HS | <HS | HS | >HS | <HS | HS | >HS | |
| 8 weeks postquit (%) | 20.6 | 23.6 | 35.4 | 22.9 | 29.9 | 27.3 | 28.6 | 28.7 | 28.0 | 39.4 | 44.9 | 47.9 | 46.4 | 40.5 | 48.5 |
| 6 months postquit (%) | 8.8 | 13.8 | 23.2 | 17.1 | 22.8 | 17.2 | 19.0 | 23.1 | 12.9 | 36.4 | 25.4 | 32.5 | 28.6 | 20.7 | 32.3 |
Notes. <HS = less than high school diploma/GED (n = 172); HS = high school diploma or GED (n = 597); >HS = more than a high school education (i.e., some college or trade school; n = 577).
Figure 1.
Six-month cessation outcome by treatment for men versus women smokers in the combined Efficacy/Effectiveness sample.
Logistic regression analyses compared 8-week and 6-month abstinence rates among women who used monotherapy versus combination therapy, controlling for study and study by treatment interaction. Analyses revealed that women who received combination therapy rather than monotherapy were more likely to be abstinent at both 8 weeks (OR = 1.96, p < .001, 95% CI = 1.44–2.69) and 6 months postquit (OR = 1.59, p < .001, 95% CI = 1.26–2.01). Study was a significant predictor at both time points such that smokers in the Efficacy study were more likely to quit. There was a significant treatment by study interaction at 6 months such that the improvement in abstinence rates was greater with combination versus monotherapy in the Effectiveness study (26.5% vs. 13.3%) compared with the Efficacy study (34.9% vs. 29.9%).
Group characteristics
In both samples, women smoked fewer cigarettes per day than men (Efficacy mean difference = 3.79 cigarettes; Effectiveness mean difference = 2.61, ps < .001). In the Efficacy sample, women had lower FTND scores than men (mean difference = .29, p = .01) but did not differ on time to first cigarette. In the Effectiveness sample, women and men had equivalent FTND scores (mean difference = .17, p = .15), but women were significantly more likely to smoke within 5 min of waking (45.9% of women) than were men (37.9%; p = .02). In the Efficacy sample, women and men did not differ in their medication adherence rates (77% vs. 78% of medication not returned—i.e., presumably used). For women, the adherence rates ranged from 66% for lozenge to 88% for bupropion, and for men the adherence rates ranged from 69% for lozenge to 87% for patch. There were no significant differences in adherence rates among treatments or was there a significant treatment by gender interaction.
Bupropion hypothesis
We evaluated the hypothesis that women would be more responsive to bupropion than to NRT using smokers who were in the monotherapy conditions (lozenge, patch, and bupropion). In logistic regressions predicting 8-week and 6-month outcome, we found no gender by treatment interactions in either sample. In fact, inspection of the abstinence rates in Table 1 shows women in the Efficacy sample had slightly higher abstinence rates in the nicotine patch condition than in the bupropion condition at both 8 weeks and 6 months postquit; Table 2 shows that in the Effectiveness sample lozenge outperformed bupropion in women at 6 months postquit.
Effects of race on outcomes
Cessation outcome
In the Efficacy sample, Black smokers were less likely to achieve initial cessation than White smokers (64.0% vs. 83.5%; OR = 0.34, p < .001, 95% CI = 0.24–0.50), and less likely to be abstinent at 8 weeks (27.5% vs. 46.7%; OR = 0.41, p < .001, 95% CI = 0.30–0.58) and 6 months postquit (24.5% vs. 34.8%; OR = 0.59, p = .003, 95% CI = 0.42–0.84). There was no main effect of race on outcome in the Effectiveness sample.
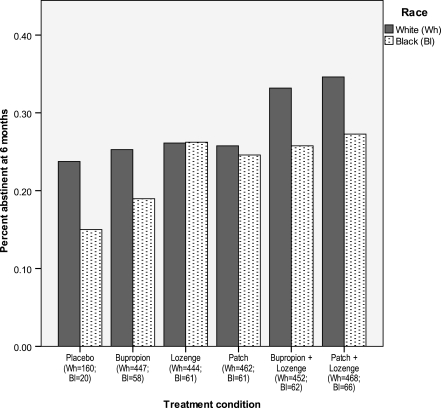
Tables 1 and 2 detail the race-specific abstinence rates for each treatment group in the Efficacy and Effectiveness studies, respectively. In the combined Efficacy/Effectiveness sample, Black smokers in the patch + lozenge condition were significantly less likely to be abstinent at 8 weeks postquit than were White smokers (28.8% vs. 52.4%; p < .001); there was also a difference in bupropion response (p = .004) but it did not meet the Bonferroni-adjusted p value cutoff. There were no significant differences between Black and White smokers in 6-month abstinence rates for the different treatments (ps > .05; Figure 2). Logistic regression analyses revealed no significant difference between Black smokers who received monotherapy versus combination therapy or were there any significant study or study by treatment effects.
Figure 2.
Six-month cessation outcome by treatment for White versus Black smokers in the combined Efficacy/Effectiveness sample.
Group characteristics
White participants smoked more cigarettes per day than Black participants (Efficacy mean difference = 3.11 cigarettes, p < .001; Effectiveness mean difference = 5.51, p < .001). The groups did not differ on FTND score, but, in the Efficacy and Effectiveness samples, significantly more Black smokers reported smoking within 5 min of waking (39.7% and 54.7%, respectively) compared with White smokers (28.0% and 41.1%, respectively). In the Efficacy sample, Black and White smokers both had medication adherence rates of 78% (78% of medications not returned). For Black smokers, adherence rates ranged from 71% in the patch + lozenge condition to 86% in the patch condition, and for White smokers, the adherence rates ranged from 66% in the lozenge condition to 86% in the patch condition. There were no significant differences in adherence rates among treatments or was there a significant treatment by race interaction.
Education effects on outcomes
Cessation outcome
In the Efficacy sample, smokers with less than a high school education (<HS) were less likely to achieve initial cessation relative to smokers with a high school education (HS; p < .05) or more than a high school education (>HS; p < .01). In the Efficacy sample, logistic regression found that at 8 weeks postquit <HS smokers were less likely to be abstinent (26.2%) than HS smokers (40.2% abstinent; OR = 0.51, p = .02, 95% CI = 0.30–0.88) or >HS smokers (46.6% abstinent; OR = 0.41, p = .001, 95% CI = 0.25–0.69). HS smokers were less likely to be abstinent than >HS smokers (OR = 0.78, p = .04, 95% CI = 0.61–0.99). At 6 months postquit, compared with <HS smokers (19.0% abstinent), HS smokers had higher abstinence rates (30.9% abstinent; OR = 1.87, p = .04, 95% CI = 1.04–3.39) as did >HS smokers (35.0% abstinent; OR = 2.24, p = .01, 95% CI = 1.28–3.92), although HS and >HS abstinence rates did not differ. In the Effectiveness sample, there were no main effects of education on cessation outcome.
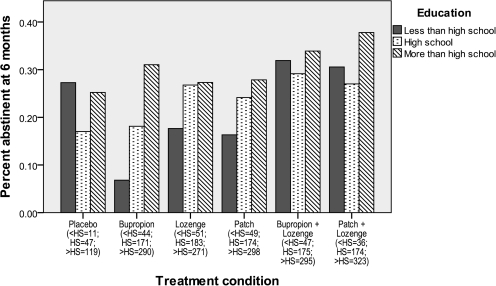
Tables 1 and 2 detail the education-specific abstinence rates for each treatment group in the Efficacy and Effectiveness studies, respectively. In the combined sample, there was a trend for there to be education differences in 8-week abstinence rates in the bupropion (p = .01) and patch (p = .006) conditions, but they did not achieve the Bonferroni-corrected p value cutoff. However, by 6 months postquit, there was a significant difference in abstinence rates in the bupropion condition (<HS = 6.8%; HS = 18.1%; >HS = 31.0%; p < .001; Figure 3). The logistic regression analyses showed that <HS smokers benefited from combination therapy relative to monotherapy at 8 weeks (OR = 2.31, p = .01, 95% CI = 1.19–4.51) and 6 months postquit (OR = 2.70, p = .01, 95% CI = 1.28–5.67). There were no significant effects of study or study by treatment interactions.
Figure 3.
Six-month cessation outcome by treatment by education status in the combined Efficacy/Effectiveness sample.
Group characteristics
A one-way analysis of variance revealed significant education effects for cigarettes smoked per day and FTND score in both the Efficacy and Effectiveness samples. In both samples, there was a trend for more education to be related to smoking fewer cigarettes per day, having lower FTND scores, and delaying morning smoking. However, in each sample, the only significant difference in cigarettes smoked per day was between HS smokers and >HS smokers (ps < .05). This may have been due to the small size of the <HS group. In each sample, <HS and HS smokers had significantly higher FTND scores relative to >HS smokers, but the difference between <HS and HS was only significant in the Effectiveness sample. There were no differences in medication adherence rates among the education groups in the Efficacy trial (<HS = 77%; HS = 78%; >HS = 78%). Adherence rates by education were as follows: <HS smokers ranged from 69% in the patch + lozenge condition to 91% in the patch condition; HS smokers ranged from 66% for lozenge to 87% for patch; >HS smokers ranged from 67% for lozenge to 86% for bupropion. There were no significant differences in adherence rates among treatments or was there a significant treatment by education interaction.
Combined model
Using the combined dataset, we included study, treatment, gender, race, and education in logistic regression models predicting 8-week and 6-month abstinence. All the independent variables were predictive of outcome at 8 weeks (p < .02), but only study, treatment, and gender were significant predictors (p < .001) at 6 months, although the education effect approached significance (p = .06).
Discussion
This research presents abstinence rates for three vulnerable populations from two independent cessation trials each of which evaluated the same five pharmacotherapy treatments. The results suggest that women, Blacks, and smokers with less than a high school education are less likely to quit smoking successfully than are men, Whites, and smokers with more than a high school education, respectively, despite receiving efficacious pharmacotherapy and despite there being no group differences in amount of medication used. These results support previous findings that these populations have disproportionate difficulty maintaining abstinence. Our combined model also showed that each of these factors—gender, race, and education—are uniquely related to quitting success in the short-term. However, only gender was a significant predictor of long-term abstinence. It may be that women are particularly vulnerable to long-term or posttreatment relapse. Identifying the nature of this vulnerability is an important area for further research so that effective treatments, such as long-term pharmacotherapy, can be developed and/or applied appropriately.
While these groups had lower abstinence rates across the board, one notable finding was that women and <HS smokers appeared to benefit specifically from combination pharmacotherapy, relative to monotherapy. This finding is promising for treating tobacco dependence in women and <HS smokers, but overall this research underscores the need to develop new treatments—including novel psychosocial interventions—that address cessation difficulties among women, Blacks, and smokers with less education.
Our results did not support the hypothesis that women are particularly responsive to bupropion. Rather, women appeared to be most responsive to combination pharmacotherapy. We examined gender differences in abstinence rates for each treatment condition and found that the difference between men and women was smallest for the patch condition in the Efficacy sample and in the patch + lozenge condition in the Effectiveness sample. In the combined sample, there was actually an opposite effect such that men who received bupropion combined with lozenge had significantly higher 8-week abstinence rates than did women in that condition. The overall findings suggest that women indeed benefit from NRT, but women may need higher doses than previously thought as they had the highest abstinence rates in the combination nicotine patch + lozenge condition. This finding needs to be replicated and explored as it contradicts logic that women should require less nicotine replacement since they smoke fewer cigarettes per day than men, and therefore, are less dependent than men. However, these findings do fit with research showing that women have significantly higher rates of nicotine metabolism than do men, particularly when using oral contraceptives (Benowitz, Lessov-Schlaggar, Swan, & Jacob, 2006); therefore, women require more nicotine to maintain a steady state of nicotine in the blood.
With respect to race, Blacks were less likely to quit, overall, and did not appear particularly responsive to combination therapy. This may be related to the finding that Black smokers appear to have slower rates of nicotine metabolism than do White smokers (Benowitz et al., 1999; Perez-Stable, Herrera, Jacob, & Benowitz, 1998) and therefore they do not receive significant benefit from extra nicotine. However, there were some treatment conditions that may be promising, although these findings were not consistent across the samples. For instance, in the Efficacy sample, the nicotine lozenge and the nicotine patch + lozenge conditions had the highest abstinence rates and the bupropion and bupropion + lozenge condition produced the lowest abstinence rates. It may be that Black smokers, 90.6% of whom reported smoking menthol cigarettes (compared with 35.9% of White smokers), found the mint-flavored lozenges more palatable or reinforcing (although the lozenge in the bupropion + lozenge condition was not particularly effective). The 90.6% rate of menthol cigarette use is higher than has been previously reported among Blacks (Giovino et al., 2004; Okuyemi, Faseru, Sanderson Cox, Bronars, & Ahluwalia, 2007). Conversely, in the Effectiveness sample among Black smokers, bupropion + lozenge produced the highest abstinence rates and the lozenge alone produced the lowest abstinence rates. Future research is needed to determine optimal pharmacotherapy for Black smokers and smokers of menthol cigarettes.
This research is consistent with other findings that low SES smokers are less likely to quit (Giskes et al., 2006; Velicer et al., 2007). However, combination pharmacotherapy for smokers with less than a high school education more than doubled their abstinence rates relative to monotherapies. In fact, combination pharmacotherapy appeared to minimize the differences between educational attainment groups. This is consistent with the finding that <HS smokers smoked at the highest rates of the three education groups, suggesting that <HS smokers may be more dependent and therefore less likely to quit and perhaps more likely to benefit from greater levels of nicotine replacement. However, additional analyses (not shown) revealed that, after controlling for dependence, education continued to predict 8-week, but not 6-month, outcome. This suggests that the difference in abstinence rates by education, at least early in the quit attempt, is not solely due to higher levels of dependence. While these results suggest that smokers with low educational attainment should receive combination pharmacotherapy, economically disadvantaged smokers may need assistance to pay for the more expensive combination therapy for this pharmacological treatment to have a true public health impact.
This study’s findings need to be interpreted within the context of certain limitations. First, this is a secondary analysis of two studies—one was a longitudinal study, which may have selected for participants with greater motivation to quit than smokers in the general population, and the other occurred in a primary care setting and offered limited psychosocial counseling. However, there was only one Treatment × Study interaction, suggesting that the treatments performed similarly across the two studies although combination pharmacotherapy appeared to be particularly advantageous in the Effectiveness context, relative to the Efficacy context. Second, despite having two large samples, when the samples were broken down into subgroups (e.g., Black smokers, smokers with less than a high school education) and then further divided into the five treatment conditions, the individual group sample sizes were small (e.g., 36 <HS smokers received patch + lozenge; 58 Black smokers received bupropion alone). Therefore, the stability of the estimates is reduced and the power to detect smaller effects may have been compromised.
Conclusion
The results from two independent, parallel randomized smoking cessation trials revealed that—in the context of both an intensive Efficacy trial and a more real-world Effectiveness trial—women, Blacks, and smokers with low educational attainment are at elevated risk for cessation failure. Women and smokers with less than a high school education appeared to be most aided by combination pharmacotherapy compared with monotherapy, but there were no consistent findings regarding treatment response among Black smokers. These findings illustrate the need for further research into the cessation process for these groups, the need to identify cessation process factors that may interfere with cessation (e.g., increased withdrawal and lack of social support), and the need to develop novel interventions—both pharmacological and counseling—to increase long-term cessation rates among these vulnerable groups.
Funding
This research was conducted at the University of Wisconsin, Madison and was supported by grant P50-DA0197 from National Institutes of Health (NIH)/National Institute on Drug Abuse and by grant M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources, NIH. MEP was supported by an Institutional Clinical and Translational Science Award (UW-Madison; KL2 grant 1KL2RR025012-01). JWC was supported by K08DA021311. W-YL was partially supported by the U.S. Army Research Laboratory and the U.S. Army Research Office under grant W911NF-09-1-0205.
Declaration of Interests
MEP, JWC, TRS, DMB, and W-YL report no potential conflicts of interest for the past 5 years. SSS has received research support from GlaxoSmithKline and Elan Corporation, plc. DEJ has received research support from the National Institute on Drug Abuse, the National Cancer Institute, Pfizer, Inc., Sanofi-Synthelabo, and Nabi Biopharmaceuticals. He has received support for educational activities from the National Institute on Drug Abuse and the Veterans Administration, and consulting fees from Nabi Biopharmaceuticals. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Medication, including placebo, was provided to patients at no cost under a research agreement with GlaxoSmithKline (GSK); no part of this manuscript was written or edited by anyone employed by GSK. The authors are solely responsible for the analyses, content, and writing of this article.
References
- Apollonio DE, Malone RE. Marketing to the marginalised: Tobacco industry targeting of the homeless and mentally ill. Tobacco Control. 2005;14:409–415. doi: 10.1136/tc.2005.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau EM, Krieger N, Soobader MJ. Working class matters: Socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. American Journal of Public Health. 2004;94:269–278. doi: 10.2105/ajph.94.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., III Female sex and oral contraceptive use accelerate nicotine metabolism. Clinical Pharmacology and Therapeutics. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., III Ethnic differences in N-glucuronidation of nicotine and cotinine. Journal of Pharmacology and Experimental Therapeutics. 1999;291:1196–1203. [PubMed] [Google Scholar]
- Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, et al. Gender differences in smoking cessation after 3 years in the Lung Health Study. American Journal of Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CM, Wayne GF, Connolly GN. Designing cigarettes for women: New findings from the tobacco industry documents. Addiction. 2005;100:837–851. doi: 10.1111/j.1360-0443.2005.01072.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Cigarette smoking among adults—United States, 2004. Morbidity and Mortality Weekly Report. 2005;54:509–513. [PubMed] [Google Scholar]
- Centers for Disease Control. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2006. Morbidity and Mortality Weekly Report. 2007;56:1157–1161. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2007. Morbidity and Mortality Weekly Report. 2008;57:1221–1226. [PubMed] [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: Differences between men and women. Journal of Consulting and Clinical Psychology. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Collins BN, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Kaufmann V, et al. Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine & Tobacco Research. 2004;6:27–37. doi: 10.1080/14622200310001656830. [DOI] [PubMed] [Google Scholar]
- Connor SE, Cook RL, Herbert MI, Neal SM, Williams JT. Smoking cessation in a homeless population: There is a will, but is there a way? Journal of General Internal Medicine. 2002;17:369–372. doi: 10.1046/j.1525-1497.2002.10630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Weaver MF, Eldridge GD, Villalobos GC, Best AM, Stitzer ML. Differential success rates in racial groups: Results of a clinical trial of smoking cessation among female prisoners. Nicotine & Tobacco Research. 2009;11:690–697. doi: 10.1093/ntr/ntp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. American Journal of Preventive Medicine. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- Fiore MC, Novotny TE, Pierce JP, Hatziandreu EJ, Patel KM, Davis RM. Trends in cigarette smoking in the United States. The changing influence of gender and race. JAMA. 1989;261:49–55. [PubMed] [Google Scholar]
- Gilpin E, Pierce J. Demographic differences in patterns in the incidence of smoking cessation: United States 1950-1990. Annals of Epidemiology. 2002;12:141–150. doi: 10.1016/s1047-2797(01)00266-6. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Schooley MW, Zhu BP, Chrismon JH, Tomar SL, Peddicord JP, et al. Surveillance for selected tobacco-use behaviors—United States, 1900-1994. Morbidity and Mortality Weekly Report. 1994;43:1–43. [PubMed] [Google Scholar]
- Giovino GA, Sidney S, Gfroerer JC, O’Malley PM, Allen JA, Richter PA, et al. Epidemiology of menthol cigarette use. Nicotine & Tobacco Research. 2004;6(Suppl. 1):S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- Giskes K, Kunst AE, Benach J, Borrell C, Costa G, Dahl E, et al. Trends in smoking behaviour between 1985 and 2000 in nine European countries by education. Journal of Epidemiology and Community Health. 2005;59:395–401. doi: 10.1136/jech.2004.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giskes K, van Lenthe FJ, Turrell G, Brug J, Mackenbach JP. Smokers living in deprived areas are less likely to quit: a longitudinal follow-up. Tobacco Control. 2006;15:485–488. doi: 10.1136/tc.2006.015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Bjornson W, Durcan MJ, White JD, Johnston JA, Buist AS, et al. Effects of gender on relapse prevention in smokers treated with bupropion SR. American Journal of Preventive Medicine. 2002;22:234–239. doi: 10.1016/s0749-3797(02)00419-1. [DOI] [PubMed] [Google Scholar]
- Hafez N, Ling PM. Finding the Kool Mixx: how Brown & Williamson used music marketing to sell cigarettes. Tobacco Control. 2006;15:359–366. doi: 10.1136/tc.2005.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Zang EA, Anderson JI, Wynder EL. Race and sex differences in lung cancer risk associated with cigarette smoking. International Journal of Epidemiology. 1993;22:592–599. doi: 10.1093/ije/22.4.592. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Iribarren C, Luepker RV, McGovern PG, Arnett DK, Blackburn H. Twelve-year trends in cardiovascular disease risk factors in the Minnesota Heart Survey. Are socioeconomic differences widening? Archives of Internal Medicine. 1997;157:873–881. [PubMed] [Google Scholar]
- Kanjilal S, Gregg EW, Cheng YJ, Zhang P, Nelson DE, Mensah G, et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971-2002. Archives of Internal Medicine. 2006;166:2348–2355. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: A review of the literature. Circulation. 1993;88(Pt 1):1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Varady A, Kraemer HC. Do men outperform women in smoking cessation trials? Maybe, but not by much. Experimental and Clinical Psychopharmacology. 2002;10:295–301. doi: 10.1037//1064-1297.10.3.295. [DOI] [PubMed] [Google Scholar]
- King G, Polednak A, Bendel RB, Vilsaint MC, Nahata SB. Disparities in smoking cessation between african americans and whites: 1990-2000. American Journal of Public Health. 2004;94:1965–1971. doi: 10.2105/ajph.94.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz D, West R. Explaining the social gradient in smoking cessation: It’s not in the trying, but in the succeeding. Tobacco Control. 2009;18:43–46. doi: 10.1136/tc.2008.025981. [DOI] [PubMed] [Google Scholar]
- Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: A systematic review. Ethnic Disparities. 2007;17:143–152. [PubMed] [Google Scholar]
- Murphy JM, Mahoney MC, Hyland AJ, Higbee C, Cummings KM. Disparity in the use of smoking cessation pharmacotherapy among Medicaid and general population smokers. Journal of Public Health Management and Practice. 2005;11:341–345. doi: 10.1097/00124784-200507000-00013. [DOI] [PubMed] [Google Scholar]
- O’Connor RJ, Giovino GA, Kozlowski LT, Shiffman S, Hyland A, Bernert JT, et al. Changes in nicotine intake and cigarette use over time in two nationally representative cross-sectional samples of smokers. American Journal of Epidemiology. 2006;164:750–759. doi: 10.1093/aje/kwj263. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102:1979–1986. doi: 10.1111/j.1360-0443.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Experimental and Clinical Psychopharmacology. 1996;4:166–177. [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, Heath C., Jr. Mortality from tobacco in developed countries: Indirect estimation from national vital statistics. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- Piper ME, Fox BJ, Welsch SK, Fiore MC, Baker TB. Gender and racial/ethnic differences in tobacco-dependence treatment: A commentary and research recommendations. Nicotine & Tobacco Research. 2001;3:291–297. doi: 10.1080/14622200110050448. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D. Efficacy of three single and two combination pharmacotherapies among daily smokers: A randomized placebo-controlled clinical trial. (2009, April) doi: 10.1001/archgenpsychiatry.2009.142. Paper presented at the Society for Research on Nicotine and Tobacco, Dublin, Ireland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles GI, Singh-Franco D, Ghin HL. A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations. Clinical Therapeutics: The International Peer-Reviewed Journal of Drug Therapy. 2008;30:800–812. doi: 10.1016/j.clinthera.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99:1462–1469. doi: 10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- Sherman SE, Fu SS, Joseph AM, Lanto AB, Yano EM. Gender differences in smoking cessation services received among veterans. Women's Health Issues. 2005;15:126–133. doi: 10.1016/j.whi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. American Journal of Preventive Medicine. 2008;34:102–111. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Di Marino ME, Sweeney CT. Characteristics of selectors of nicotine replacement therapy. Tobacco Control. 2005;14:346–355. doi: 10.1136/tc.2004.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Sweeney CT, Dresler CM. Nicotine patch and lozenge are effective for women. Nicotine & Tobacco Research. 2005;7:119–127. doi: 10.1080/14622200412331328439. [DOI] [PubMed] [Google Scholar]
- Smith SS, Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, et al. Targeting smokers at increased risk for relapse: Treating women and those with a history of depression. Nicotine & Tobacco Research. 2003;5:99–109. doi: 10.1080/1462220021000060437. [DOI] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich S, Christiansen B, Piper ME, Jorenby DE, et al. Comparative effectiveness trial of five smoking cessation pharmacotherapies in primary care clinics. Archives of Internal Medicine. 2009;169:2148–2155. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MB, Akincigil A, Delnevo CD, Crystal S, Carson JL. Gender and age disparities for smoking-cessation treatment. American Journal of Preventive Medicine. 2006;30:405–412. doi: 10.1016/j.amepre.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Sutton CD, Robinson RG. The marketing of menthol cigarettes in the United States: populations, messages, and channels. Nicotine & Tobacco Research. 2004;6(Suppl. 1):S83–S91. doi: 10.1080/14622203310001649504. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction. 1997;92:207–217. [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: A randomized trial. Archives of Internal Medicine. 2003;163:2337–2344. doi: 10.1001/archinte.163.19.2337. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Redding CA, Sun X, Prochaska JO. Demographic variables, smoking variables, and outcome across five studies. Health Psychology. 2007;26:278–287. doi: 10.1037/0278-6133.26.3.278. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Fiore MC, Young TB, McClure JB, de Moor CA, Baker TB. Gender differences in response to nicotine replacement therapy: Objective and subjective indexes of tobacco withdrawal. Experimental and Clinical Psychopharmacology. 1999;7:135–144. doi: 10.1037//1064-1297.7.2.135. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- White VM, White MM, Freeman K, Gilpin EA, Pierce JP. Cigarette promotional offers: Who takes advantage? American Journal of Preventive Medicine. 2006;30:225–231. doi: 10.1016/j.amepre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Yancy CW. Executive summary of the African-American Initiative. Medscape General Medicine. 2007;9:28. [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Melcer T, Sun J, Rosbrook B, Pierce JP. Smoking cessation with and without assistance: A population-based analysis. American Journal of Preventive Medicine. 2000;18:305–311. doi: 10.1016/s0749-3797(00)00124-0. [DOI] [PubMed] [Google Scholar]